Basic Properties of MgAl-Mixed Oxides in CO2 Adsorption at High Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Layered Double Hydroxides
2.2. Physico-Chemical Characterization Techniques
2.3. Basicity Measurement by SO2 Adsorption Calorimetry
2.4. CO2 Adsorption Tests
3. Results and Discussion
3.1. Characterization of the Hydrotalcite Precursors
3.2. Characterization of the Hydrotalcite-Derived Mixed Oxides
3.2.1. SO2 Adsorption Calorimetry
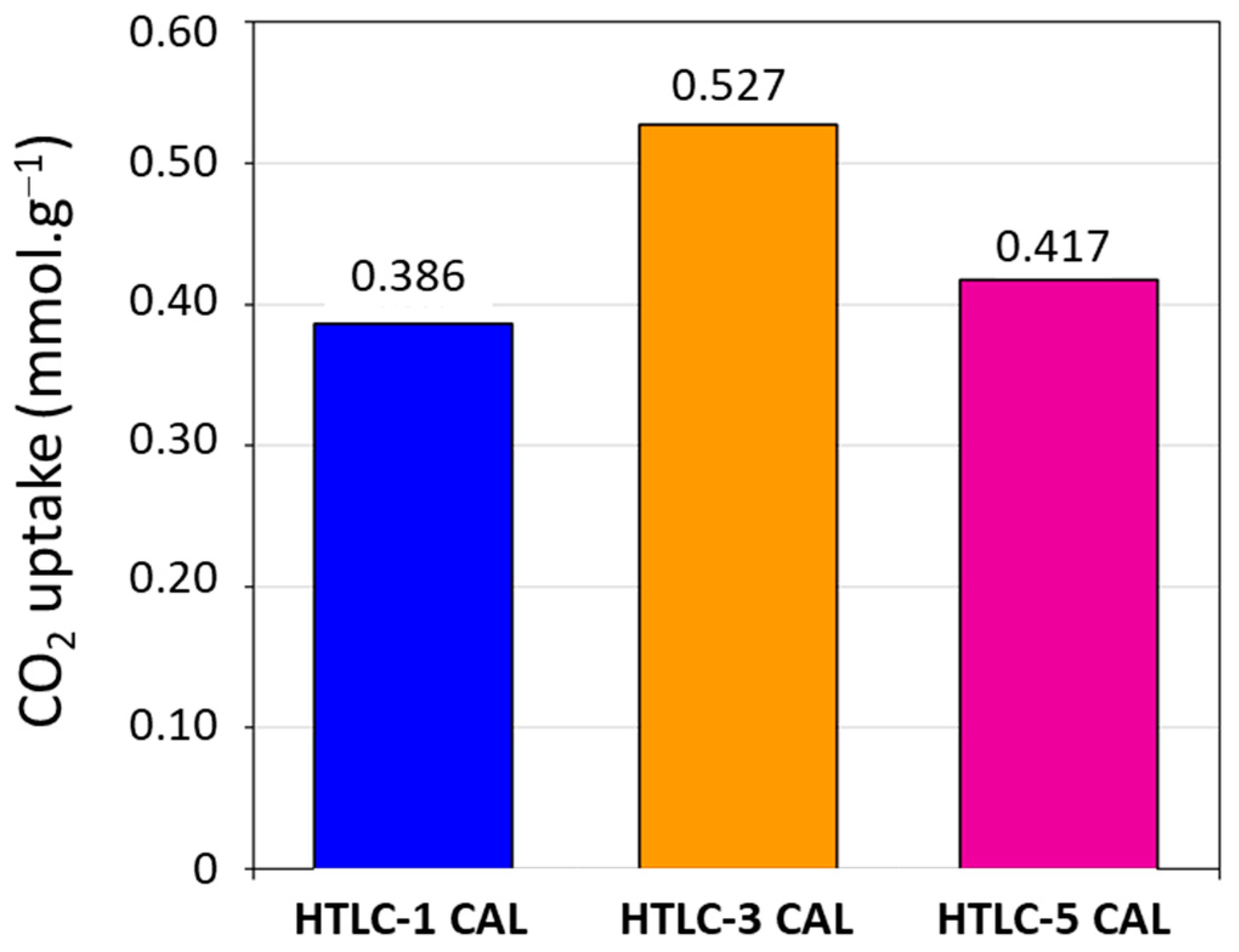
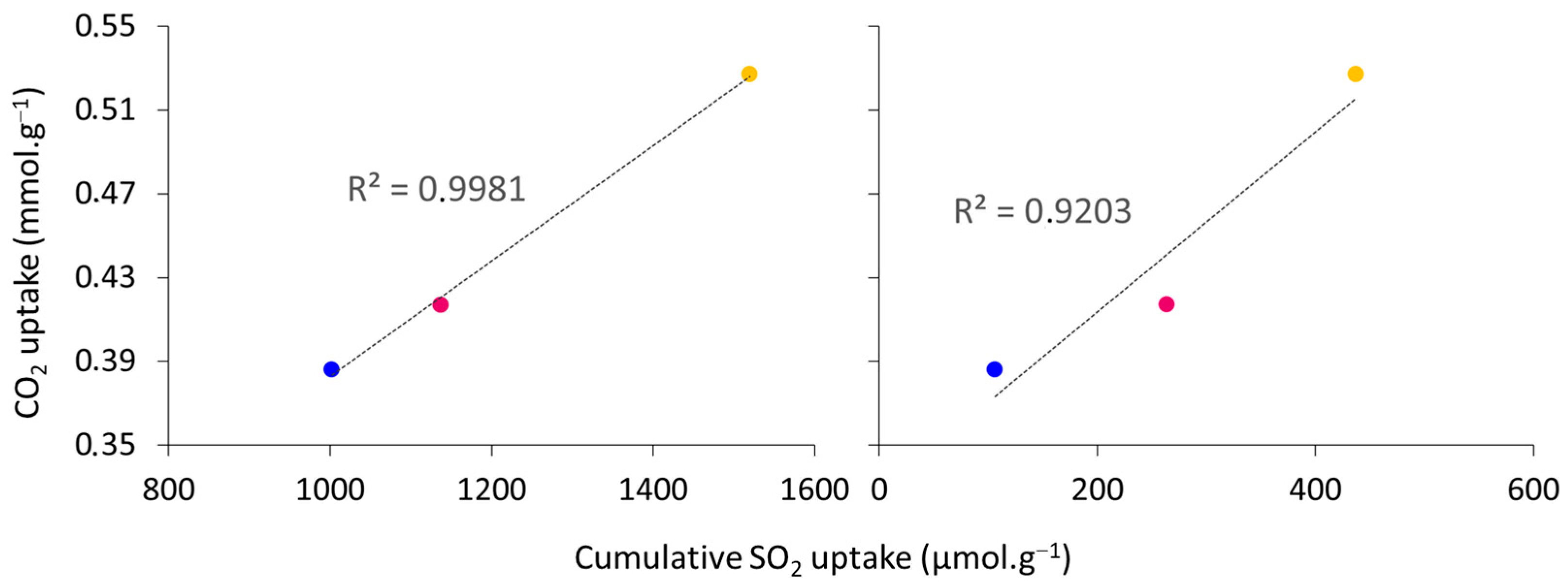
3.2.2. CO2 Adsorption Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Du, T.; Liu, L.; Che, S.; Song, Y.; Fang, X. A Review of Layered Double Hydroxides as Intermediate-Temperature CO2 Adsorbents. In Proceedings of the 2017 6th International Conference on Energy, Environment and Sustainable Development (ICEESD 2017), Zhuhai, China, 11–12 March 2017; pp. 688–692. [Google Scholar]
- Marquevich, M.; Medina, F.; Montané, D. Hydrogen Production via Steam Reforming of Sunflower Oil over Ni/Al Catalysts from Hydrotalcite Materials. Catal. Commun. 2001, 2, 119–124. [Google Scholar] [CrossRef]
- Ashok, J.; Subrahmanyam, M.; Venugopal, A. Hydrotalcite Structure Derived Ni–Cu–Al Catalysts for the Production of H2 by CH4 Decomposition. Int. J. Hydrogen Energy 2008, 33, 2704–2713. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Koike, M.; Nakagawa, Y.; Tomishige, K. Steam Reforming of Tar from Pyrolysis of Biomass over Ni/Mg/Al Catalysts Prepared from Hydrotalcite-like Precursors. Appl. Catal. B Environ. 2011, 102, 528–538. [Google Scholar] [CrossRef]
- Yong, Z.; Mata, V.; Rodriguez, A. Adsorption of Carbon Dioxide at High Temperature—A Review. Sep. Purif. Technol. 2002, 26, 195–205. [Google Scholar] [CrossRef]
- León, M.; Díaz, E.; Bennici, S.; Vega, A.; Ordóñez, S.; Auroux, A. Adsorption of CO2 on Hydrotalcite-Derived Mixed Oxides: Sorption Mechanisms and Consequences for Adsorption Irreversibility. Ind. Eng. Chem. Res. 2010, 49, 3663–3671. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, J.; Zeng, G.; Zhang, H.; Tan, X.; Ma, C.; Li, X.; Li, Z.; Zhang, C. A Review on Strategies to LDH-Based Materials to Improve Adsorption Capacity and Photoreduction Efficiency for CO2. Coord. Chem. Rev. 2019, 386, 154–182. [Google Scholar] [CrossRef]
- Molinard, A.; Vansant, E.F. Controlled Gas Adsorption Properties of Various Pillared Clays. Adsorption 1995, 1, 49–59. [Google Scholar] [CrossRef]
- Venaruzzo, J.; Volzone, C.; Rueda, M.; Ortiga, J. Modified Bentonitic Clay Minerals as Adsorbents of CO, CO2 and SO2 Gases. Microporous Mesoporous Mater. 2002, 56, 73–80. [Google Scholar] [CrossRef]
- Moura, K.O.; Pastore, H.O. Comparative Adsorption of CO2 by Mono-, Di-, and Triamino-Organofunctionalized Magnesium Phyllosilicates. Environ. Sci. Technol. 2013, 47, 12201–12210. [Google Scholar] [CrossRef]
- Sarker, A.I.; Aroonwilas, A.; Veawab, A. Equilibrium and Kinetic Behaviour of CO2 Adsorption onto Zeolites, Carbon Molecular Sieve and Activated Carbons. Energy Procedia 2017, 114, 2450–2459. [Google Scholar] [CrossRef]
- Coudert, F.-X.; Kohen, D. Molecular Insight into CO2 “Trapdoor” Adsorption in Zeolite Na-RHO. Chem. Mater. 2017, 29, 2724–2730. [Google Scholar] [CrossRef]
- Mao, G. Synthesis and CO2 Adsorption Features of a Hydrotalcite-Like Compound of the Mg2+-Al3+-Fe(CN)64- System with High Layer-Charge Density. Clays Clay Miner. 1993, 41, 731–737. [Google Scholar] [CrossRef]
- Tsuji, M.; Mao, G.; Yoshida, T.; Tamaura, Y. Hydrotalcites with an Extended Al3+-Substitution: Synthesis, Simultaneous TG-DTA-MS Study, and Their CO2 Adsorption Behaviors. J. Mater. Res. 1993, 8, 1137–1142. [Google Scholar] [CrossRef]
- Dantas, T.C.M.; Junior, V.J.F.; dos Santos, A.P.B.; Bezerra, F.A.; Araujo, A.S.; Alves, A.P.M. CO2 Adsorption on Modified Mg–Al-Layered Double Hydroxides. Adsorpt. Sci. Technol. 2015, 33, 165–173. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, J.; Xiao, P.; He, Y.; Zhao, Q.; Chu, Z.; Liu, Y.; Li, Z.; Webley, P.A. Simultaneous Biogas Purification and CO2 Capture by Vacuum Swing Adsorption Using Zeolite NaUSY. Chem. Eng. J. 2018, 334, 2593–2602. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Krishna, R.; Liu, Y.; Cui, Y.; Du, J.; Liang, Z.; Song, X.; Yu, J. Enhancing CO2 Adsorption and Separation Properties of Aluminophosphate Zeolites by Isomorphous Heteroatom Substitutions. ACS Appl. Mater. Interfaces 2018, 10, 43570–43577. [Google Scholar] [CrossRef]
- Bhatta, L.K.G.; Subramanyam, S.; Chengala, M.D.; Bhatta, U.M.; Venkatesh, K. Enhancement in CO2 Adsorption on Hydrotalcite-Based Material by Novel Carbon Support Combined with K2CO3 Impregnation. Ind. Eng. Chem. Res. 2015, 54, 10876–10884. [Google Scholar] [CrossRef]
- Moreira, R.F.P.M.; Soares, J.L.; Casarin, G.L.; Rodrigues, A.E. Adsorption of CO2 on Hydrotalcite-like Compounds in a Fixed Bed. Sep. Sci. Technol. 2006, 41, 341–357. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.; Wu, J.; Yi, X.; Zheng, A.; Umar, A.; O’Hare, D.; Wang, Q. Comprehensive Investigation of CO2 Adsorption on Mg–Al–CO3 LDH-Derived Mixed Metal Oxides. J. Mater. Chem. A 2013, 1, 12782. [Google Scholar] [CrossRef]
- Radha, S.; Navrotsky, A. Energetics of CO2 Adsorption on Mg–Al Layered Double Hydroxides and Related Mixed Metal Oxides. J. Phys. Chem. C 2014, 118, 29836–29844. [Google Scholar] [CrossRef]
- Colonna, S.; Bastianini, M.; Sisani, M.; Fina, A. CO2 Adsorption and Desorption Properties of Calcined Layered Double Hydroxides. J. Therm. Anal. Calorim. 2018, 133, 869–879. [Google Scholar] [CrossRef]
- Hutson, N.D.; Attwood, B.C. High Temperature Adsorption of CO2 on Various Hydrotalcite-like Compounds. Adsorption 2008, 14, 781–789. [Google Scholar] [CrossRef]
- Tang, N.; He, T.; Liu, J.; Li, L.; Shi, H.; Cen, W.; Ye, Z. New Insights into CO2 Adsorption on Layered Double Hydroxide (LDH)-Based Nanomaterials. Nanoscale Res. Lett. 2018, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Bingre, R.; Huang, L.; Lutzweiler, G.; Wang, Q.; Louis, B. CO2 Adsorption Capacities in Zeolites and Layered Double Hydroxide Materials. Front. Chem. 2019, 7, 551. [Google Scholar] [CrossRef]
- Ram Reddy, M.K.; Xu, Z.P.; Diniz da Costa, J.C. Influence of Water on High-Temperature CO2 Capture Using Layered Double Hydroxide Derivatives. Ind. Eng. Chem. Res. 2008, 47, 2630–2635. [Google Scholar] [CrossRef]
- Bellotto, M.; Rebours, B.; Clause, O.; Lynch, J.; Bazin, D.; Elkaïm, E. Hydrotalcite Decomposition Mechanism: A Clue to the Structure and Reactivity of Spinel-like Mixed Oxides. J. Phys. Chem. 1996, 100, 8535–8542. [Google Scholar] [CrossRef]
- Tichit, D.; Bennani, M.N.; Figueras, F.; Ruiz, J.R. Decomposition Processes and Characterization of the Surface Basicity of Cl− and CO32- Hydrotalcites. Langmuir 1998, 14, 2086–2091. [Google Scholar] [CrossRef]
- Kagunya, W.; Hassan, Z.; Jones, W. Catalytic Properties of Layered Double Hydroxides and Their Calcined Derivatives. Inorg. Chem. 1996, 35, 5970–5974. [Google Scholar] [CrossRef]
- Yan, K.; Liu, Y.; Lu, Y.; Chai, J.; Sun, L. Catalytic Application of Layered Double Hydroxide-Derived Catalysts for the Conversion of Biomass-Derived Molecules. Catal. Sci. Technol. 2017, 7, 1622–1645. [Google Scholar] [CrossRef]
- Chisem, I.C.; Jones, W.; Martín, I.; Martín, C.; Rives, V. Probing the Surface Acidity of Lithium Aluminium and Magnesium Aluminium Layered Double Hydroxides. J. Mater. Chem. 1998, 8, 1917–1925. [Google Scholar] [CrossRef]
- Prinetto, F.; Ghiotti, G.; Durand, R.; Tichit, D. Investigation of Acid−Base Properties of Catalysts Obtained from Layered Double Hydroxides. J. Phys. Chem. B 2000, 104, 11117–11126. [Google Scholar] [CrossRef]
- Kuśtrowski, P.; Chmielarz, L.; Bożek, E.; Sawalha, M.; Roessner, F. Acidity and Basicity of Hydrotalcite Derived Mixed Mg–Al Oxides Studied by Test Reaction of MBOH Conversion and Temperature Programmed Desorption of NH3 and CO2. Mater. Res. Bull. 2004, 39, 263–281. [Google Scholar] [CrossRef]
- Lei, X.; Zhang, F.; Yang, L.; Guo, X.; Tian, Y.; Fu, S.; Li, F.; Evans, D.G.; Duan, X. Highly Crystalline Activated Layered Double Hydroxides as Solid Acid-Base Catalysts. AIChE J. 2007, 53, 932–940. [Google Scholar] [CrossRef]
- Pérez-Barrado, E.; Pujol, M.C.; Aguiló, M.; Llorca, J.; Cesteros, Y.; Díaz, F.; Pallarès, J.; Marsal, L.F.; Salagre, P. Influence of Acid–Base Properties of Calcined MgAl and CaAl Layered Double Hydroxides on the Catalytic Glycerol Etherification to Short-Chain Polyglycerols. Chem. Eng. J. 2015, 264, 547–556. [Google Scholar] [CrossRef]
- Parida, K.; Das, J. Mg/Al Hydrotalcites: Preparation, Characterisation and Ketonisation of Acetic Acid. J. Mol. Catal. A Chem. 2000, 151, 185–192. [Google Scholar] [CrossRef]
- Pavel, O.D.; Tichit, D.; Marcu, I.-C. Acido-Basic and Catalytic Properties of Transition-Metal Containing Mg–Al Hydrotalcites and Their Corresponding Mixed Oxides. Appl. Clay Sci. 2012, 61, 52–58. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Li, P.; Yu, J.-G.; Cunha, A.F.; Rodrigues, A.E. K-Promoted Hydrotalcites for CO2 Capture in Sorption Enhanced Reactions. Chem. Eng. Technol. 2013, 36, 567–574. [Google Scholar] [CrossRef]
- Hanif, A.; Dasgupta, S.; Divekar, S.; Arya, A.; Garg, M.O.; Nanoti, A. A Study on High Temperature CO2 Capture by Improved Hydrotalcite Sorbents. Chem. Eng. J. 2014, 236, 91–99. [Google Scholar] [CrossRef]
- Coenen, K.; Gallucci, F.; Mezari, B.; Hensen, E.; van Sint Annaland, M. An In-Situ IR Study on the Adsorption of CO2 and H2O on Hydrotalcites. J. CO2 Util. 2018, 24, 228–239. [Google Scholar] [CrossRef]
- Lopez, T.; Bosch, P.; Ramos, E.; Gomez, R.; Novaro, O.; Acosta, D.; Figueras, F. Synthesis and Characterization of Sol−Gel Hydrotalcites. Structure and Texture. Langmuir 1996, 12, 189–192. [Google Scholar] [CrossRef]
- Bolognini, M.; Cavani, F.; Scagliarini, D.; Flego, C.; Perego, C.; Saba, M. Mg/Al Mixed Oxides Prepared by Coprecipitation and Sol–Gel Routes: A Comparison of Their Physico-Chemical Features and Performances in m-Cresol Methylation. Microporous Mesoporous Mater. 2003, 66, 77–89. [Google Scholar] [CrossRef]
- Othman, M.R.; Rasid, N.M.; Fernando, W.J.N. Effects of Thermal Treatment on the Micro-Structures of Co-Precipitated and Sol–Gel Synthesized (Mg–Al) Hydrotalcites. Microporous Mesoporous Mater. 2006, 93, 23–28. [Google Scholar] [CrossRef]
- Prince, J.; Montoya, A.; Ferrat, G.; Valente, J.S. Proposed General Sol−Gel Method to Prepare Multimetallic Layered Double Hydroxides: Synthesis, Characterization, and Envisaged Application. Chem. Mater. 2009, 21, 5826–5835. [Google Scholar] [CrossRef]
- Smalenskaite, A.; Vieira, D.E.L.; Salak, A.N.; Ferreira, M.G.S.; Katelnikovas, A.; Kareiva, A. A Comparative Study of Co-Precipitation and Sol-Gel Synthetic Approaches to Fabricate Cerium-Substituted Mg Al Layered Double Hydroxides with Luminescence Properties. Appl. Clay Sci. 2017, 143, 175–183. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-Type Anionic Clays: Preparation, Properties and Applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Ardanuy, M.; Velasco, J.I. Mg–Al Layered Double Hydroxide Nanoparticles. Appl. Clay Sci. 2011, 51, 341–347. [Google Scholar] [CrossRef]
- Mondal, S.; Dasgupta, S.; Maji, K. MgAl- Layered Double Hydroxide Nanoparticles for Controlled Release of Salicylate. Mater. Sci. Eng. C 2016, 68, 557–564. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kushwaha, P.K.; Srivastava, V.K.; Bhatt, S.D.; Jasra, R. V Effect of Hydrothermal Conditions on Structural and Textural Properties of Synthetic Hydrotalcites of Varying Mg/Al Ratio. Ind. Eng. Chem. Res. 2007, 46, 4856–4865. [Google Scholar] [CrossRef]
- Klemkaite, K.; Prosycevas, I.; Taraskevicius, R.; Khinsky, A.; Kareiva, A. Synthesis and Characterization of Layered Double Hydroxides with Different Cations (Mg, Co, Ni, Al), Decomposition and Reformation of Mixed Metal Oxides to Layered Structures. Open Chem. 2011, 9, 275–282. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Balsamo, N.; Mendieta, S.; Oliva, M.; Eimer, G.; Crivello, M. Synthesis and Characterization of Metal Mixed Oxides from Layered Double Hydroxides. Procedia Mater. Sci. 2012, 1, 506–513. [Google Scholar] [CrossRef]
- Reyero, I.; Velasco, I.; Sanz, O.; Montes, M.; Arzamendi, G.; Gandía, L.M. Structured Catalysts Based on Mg–Al Hydrotalcite for the Synthesis of Biodiesel. Catal. Today 2013, 216, 211–219. [Google Scholar] [CrossRef]
- Kurnia Julianti, N.; Kusuma Wardani, T.; Gunardi, I.; Roesyadi, A. Effect of Calcination at Synthesis of Mg-Al Hydrotalcite Using Co-Precipitation Method. J. Pure Appl. Chem. Res. 2017, 6, 7–13. [Google Scholar] [CrossRef]
- Auroux, A. Acidity Characterization by Microcalorimetry and Relationship with Reactivity. Top. Catal. 1997, 4, 71–89. [Google Scholar] [CrossRef]
- Bennici, S.; Auroux, A. Thermal analysis and calorimetric methods. In Metal Oxide Catalysis; Hargreaves, S.J., Jackson, S.D., Eds.; WILEY-VCH Verlag GmbH: Weinheim, Germany, 2009; Volume 1, pp. 391–441. ISBN 978-3-527-31815-5. [Google Scholar]
- Occelli, M.L.; Olivier, J.P.; Auroux, A.; Kalwei, M.; Eckert, H. Basicity and Porosity of a Calcined Hydrotalcite-Type Material from Nitrogen Porosimetry and Adsorption Microcalorimetry Methods. Chem. Mater. 2003, 15, 4231–4238. [Google Scholar] [CrossRef]
- Yang, J.-I.; Kim, J.-N. Hydrotalcites for Adsorption of CO2 at High Temperature. Korean J. Chem. Eng. 2006, 23, 77–80. [Google Scholar] [CrossRef]
- Ram Reddy, M.K.; Xu, Z.P.; (Max) Lu, G.Q.; Diniz da Costa, J.C. Layered Double Hydroxides for CO2 Capture: Structure Evolution and Regeneration. Ind. Eng. Chem. Res. 2006, 45, 7504–7509. [Google Scholar] [CrossRef]
- Hutson, N.D.; Speakman, S.A.; Payzant, E.A. Structural Effects on the High Temperature Adsorption of CO2 on a Synthetic Hydrotalcite. Chem. Mater. 2004, 16, 4135–4143. [Google Scholar] [CrossRef]
- Yong, Z.; Mata; Rodrigues, A.E. Adsorption of Carbon Dioxide onto Hydrotalcite-like Compounds (HTlcs) at High Temperatures. Ind. Eng. Chem. Res. 2001, 40, 204–209. [Google Scholar] [CrossRef]
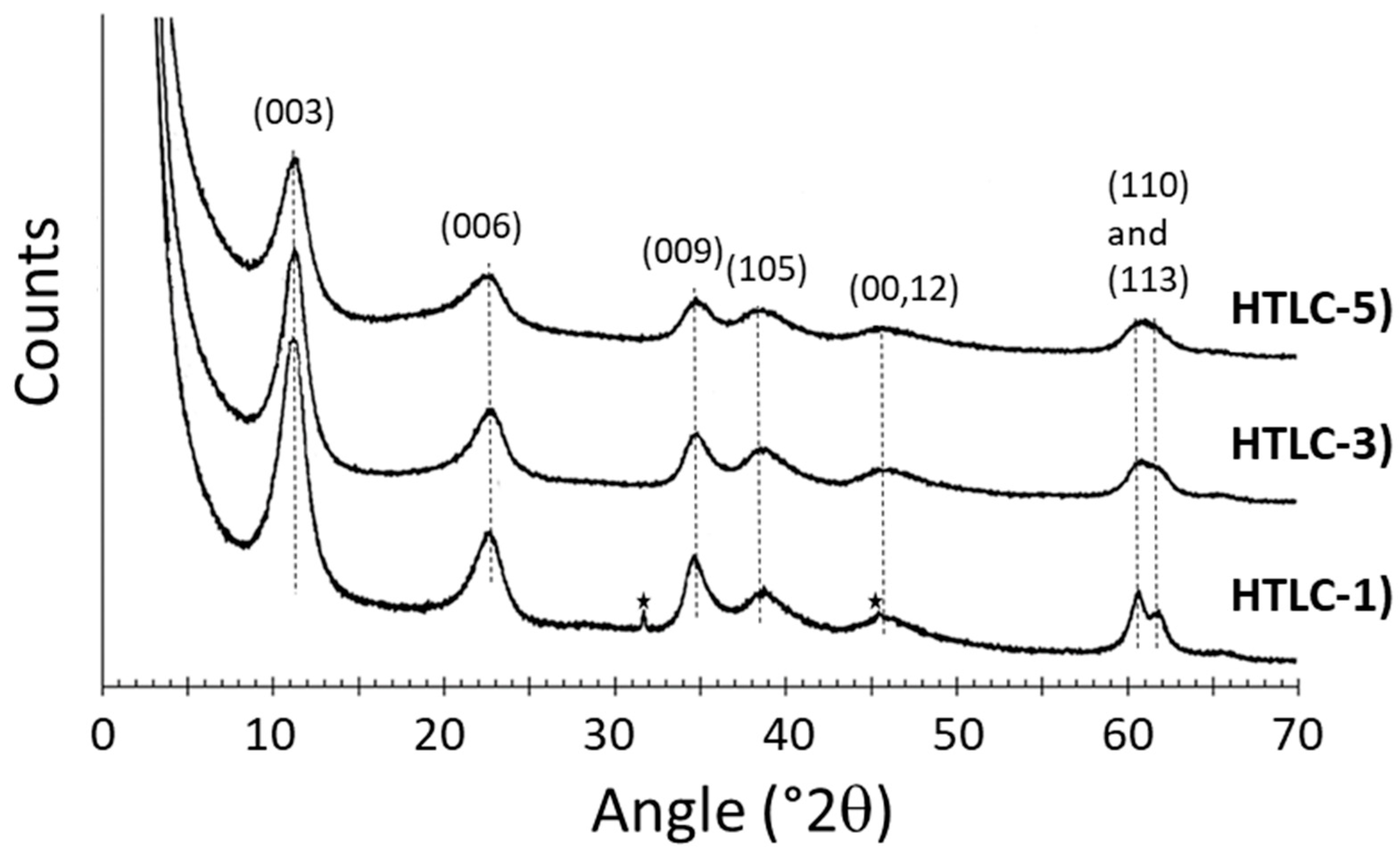
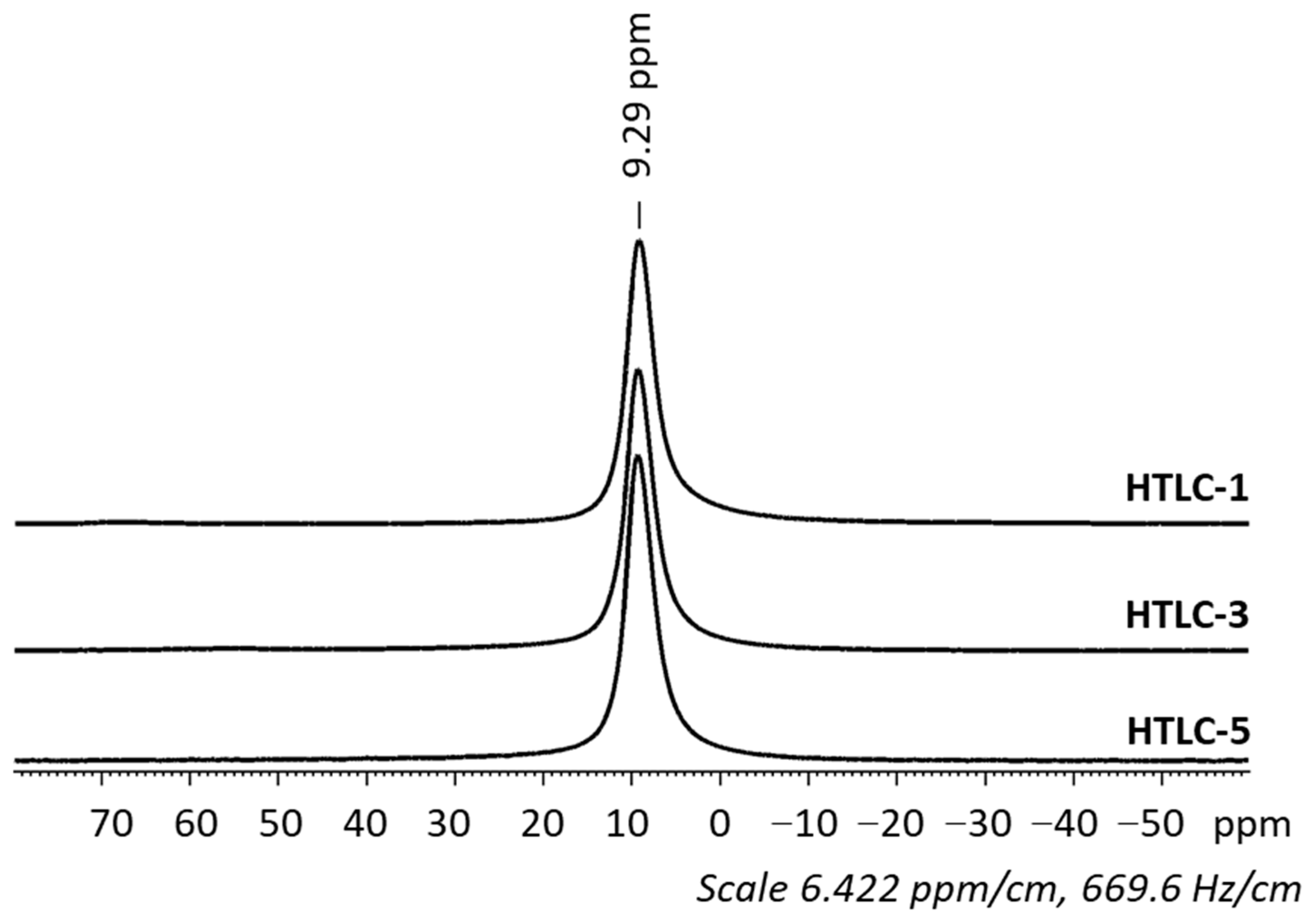

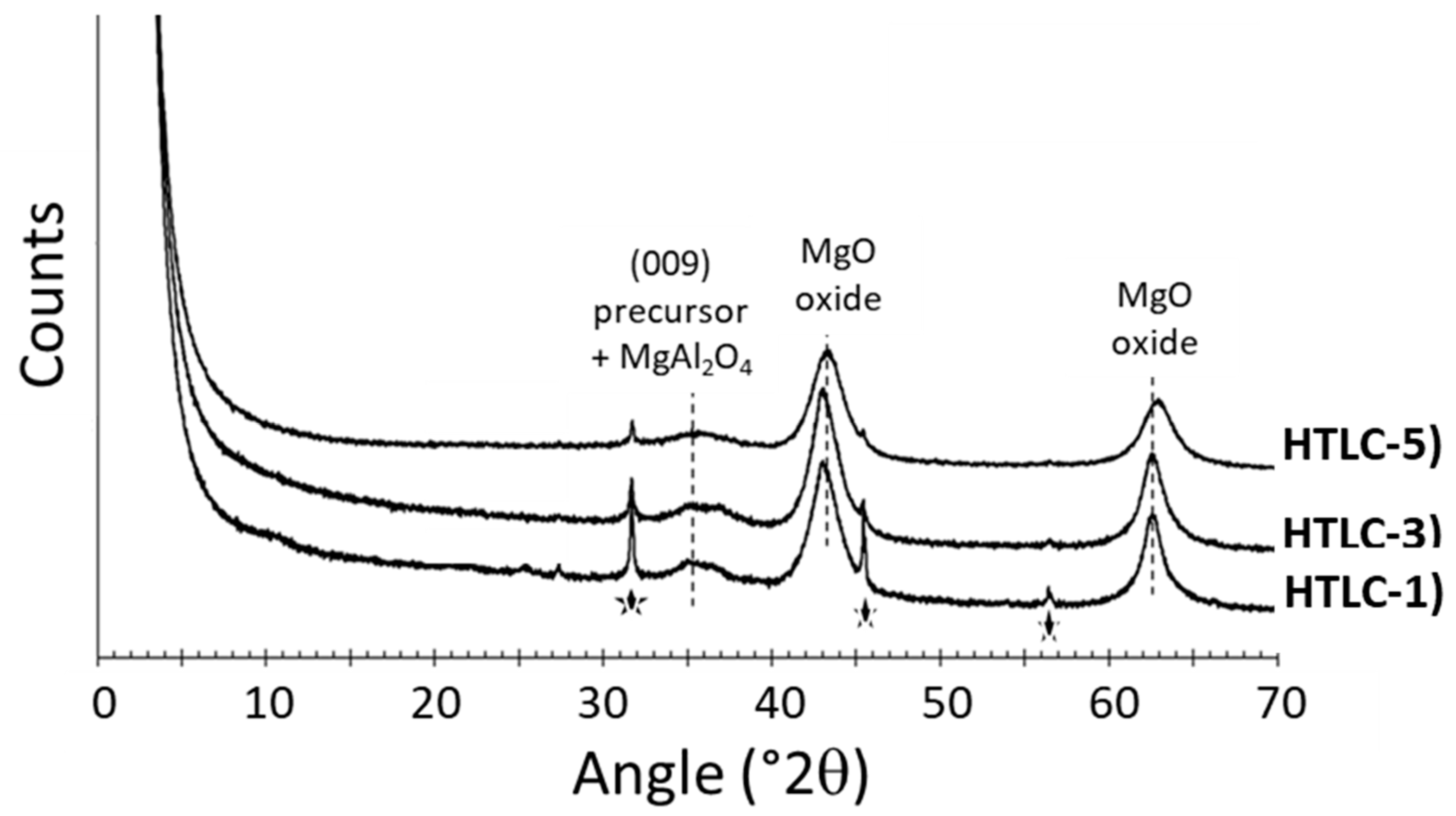
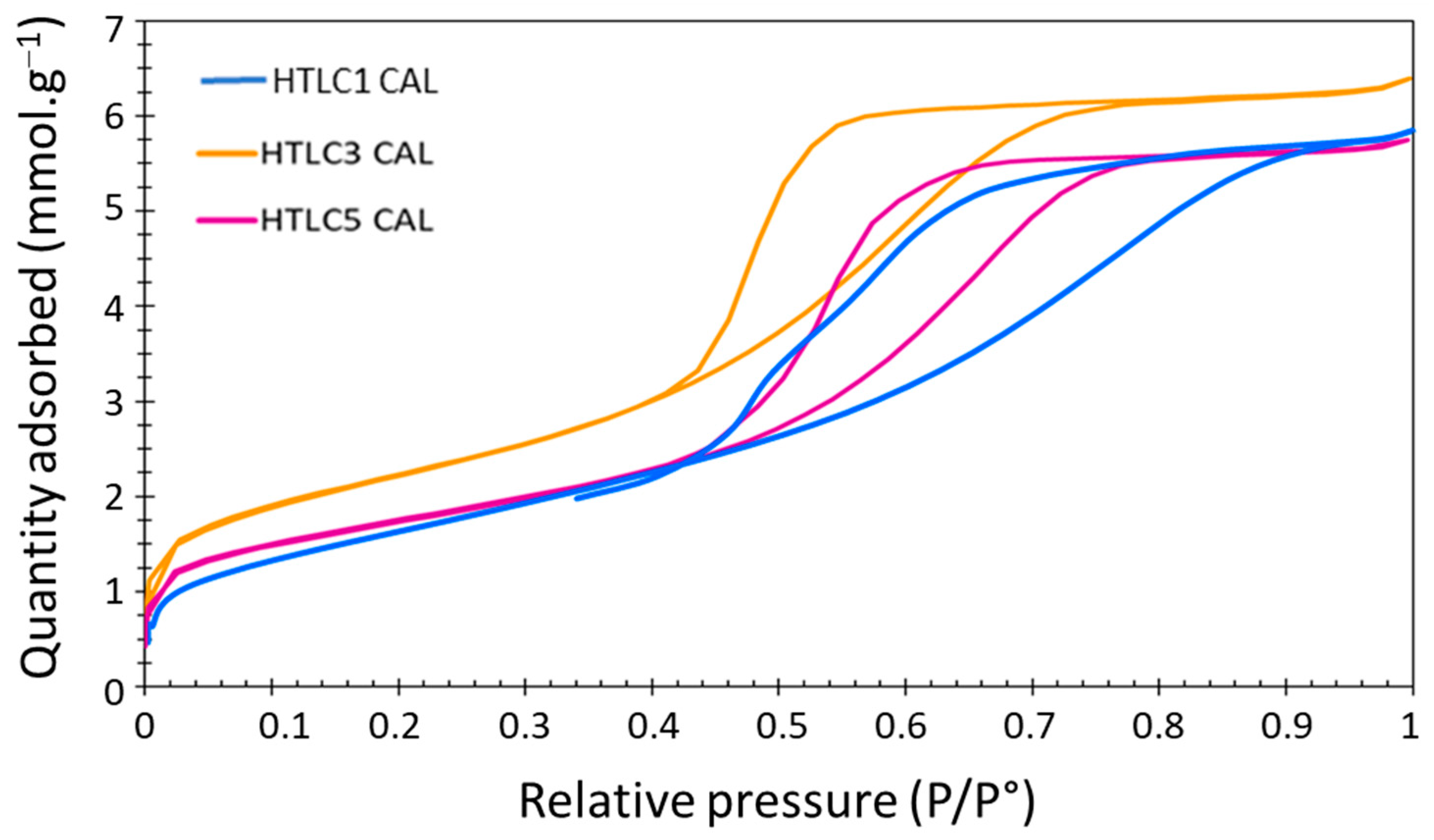
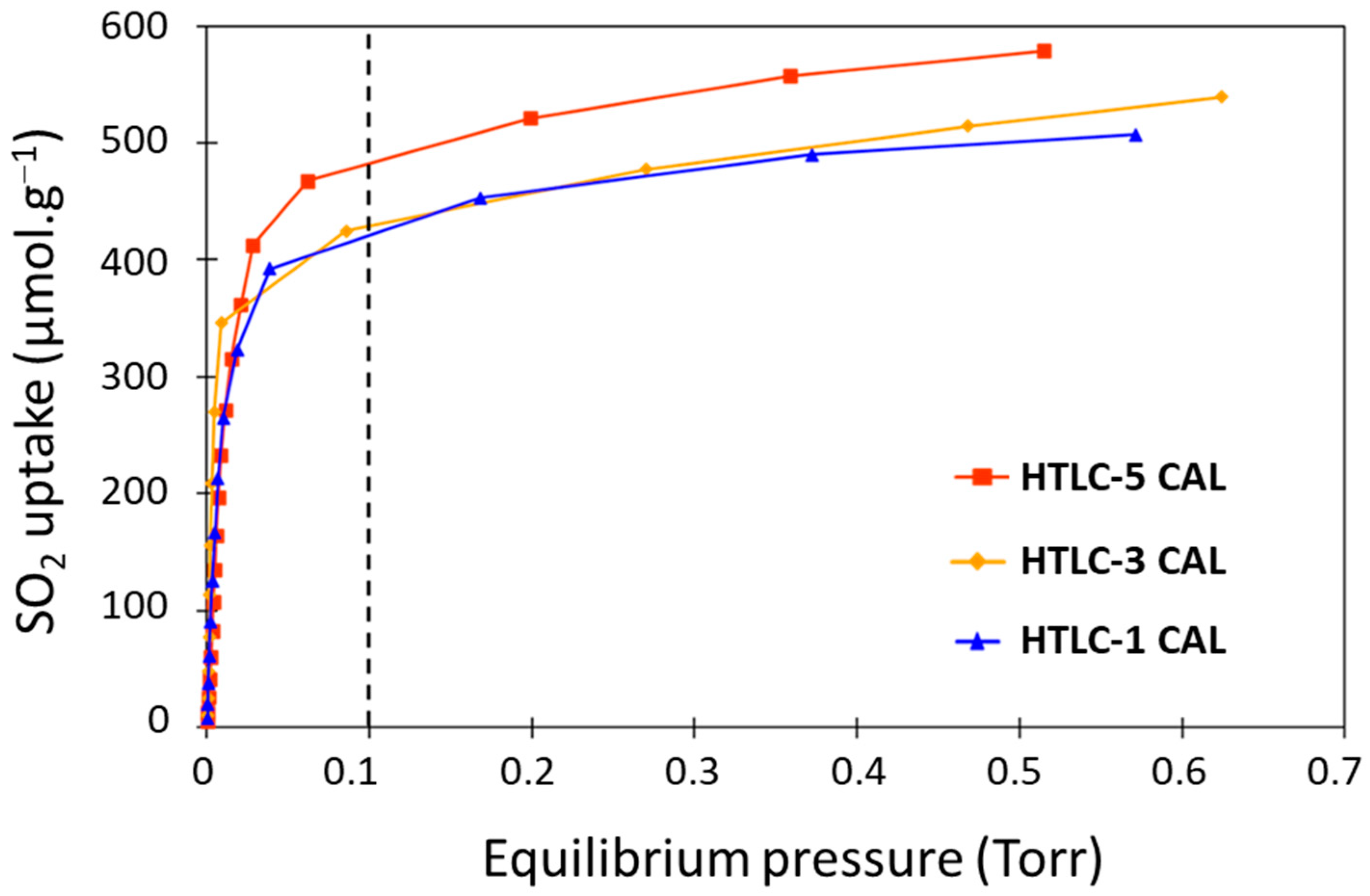



| Sample | Al Source | Mg/Al Ratio | NaOH Amount (mL) |
|---|---|---|---|
| HTCL-1 | AlCl3.6H2O | 3.02 | 28 |
| HTCL-3 | Al(C5H7O2)3 | 2.99 | 22 |
| HTCL-5 | AlCl3.6H2O | 3.00 | 16 |
| Sample | Weight Loss 25–200 °C (%) | Weight Loss 200–600 °C (%) | Total Weight Loss (%) | Comments | Ref. |
|---|---|---|---|---|---|
| HTCL-1 | 17.02 | 26.04 | 43.06 | - | This article |
| HTCL-3 | 13.03 | 31.32 | 44.36 | - | This article |
| HTCL-5 | 16.44 | 25.46 | 41.90 | - | This article |
| LDH1 | 12.7 | 29.3 | 42.00 | Commercial MgAl LDH | [50] |
| LDH2 | 15.2 | 26.7 | 41.90 | Commercial MgAl LDH | [50] |
| LDH_amm | 15 | 38.83 | 53.83 | Coprecipitation with ammonia | [51] |
| LDH_70 | 15 | 29 | 44.00 | Coprecipitation at low supersaturation, then pretreatment at 70 °C | [52] |
| LDH_140 | 10 | 30 | 40.00 | Coprecipitation at low supersaturation, then pretreatment at 140 °C | [52] |
| Mg/Al_LDH | 24 | 23 | 47.00 | Coprecipitation at low supersaturation | [53] |
| Sample | Mg/Al Molar Ratio | Tpretreatment (°C) | Tadsorption (°C) | SSA (m2·g−1) | CO2 Adsorption Capacity (mmol·g−1) | Ref. |
|---|---|---|---|---|---|---|
| HTLC-1 CAL | 3 | 300 | 200 | 138 | 0.386 | This work |
| HTLC-3 CAL | 3 | 300 | 200 | 180 | 0.527 | This work |
| HTLC-5 CAL | 3 | 300 | 200 | 139 | 0.417 | This work |
| HTLC_Low | 2 | 500 | 450 | 63 | 0.270 | [61] |
| HTLC_High | 2 | 500 | 450 | 154 | 0.260 | [61] |
| LDO200 | 3 | 400 | 200 | 167 | 0.486 | [62] |
| Htlc-200 | 3 | 200 | 200 | 66 | 0.604 | [63] |
| Htlc-400 | 3 | 400 | 200 | 184 | 0.896 | [63] |
| Htlc | 3 | - | 300 | 74 | 0.5 | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaillot, D.; Folliard, V.; Miehé-Brendlé, J.; Auroux, A.; Dzene, L.; Bennici, S. Basic Properties of MgAl-Mixed Oxides in CO2 Adsorption at High Temperature. Materials 2023, 16, 5698. https://doi.org/10.3390/ma16165698
Chaillot D, Folliard V, Miehé-Brendlé J, Auroux A, Dzene L, Bennici S. Basic Properties of MgAl-Mixed Oxides in CO2 Adsorption at High Temperature. Materials. 2023; 16(16):5698. https://doi.org/10.3390/ma16165698
Chicago/Turabian StyleChaillot, Dylan, Vincent Folliard, Jocelyne Miehé-Brendlé, Aline Auroux, Liva Dzene, and Simona Bennici. 2023. "Basic Properties of MgAl-Mixed Oxides in CO2 Adsorption at High Temperature" Materials 16, no. 16: 5698. https://doi.org/10.3390/ma16165698
APA StyleChaillot, D., Folliard, V., Miehé-Brendlé, J., Auroux, A., Dzene, L., & Bennici, S. (2023). Basic Properties of MgAl-Mixed Oxides in CO2 Adsorption at High Temperature. Materials, 16(16), 5698. https://doi.org/10.3390/ma16165698




_CHAINOK.jpg)







