Combined Sulfur and Peroxide Vulcanization of Filled and Unfilled EPDM-Based Rubber Compounds
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Preparation and Curing of Rubber Compounds
2.2.2. Determination of Curing Characteristics
2.2.3. Determination of Cross-Link Density
2.2.4. Investigation of Physical–Mechanical Characteristics
2.2.5. Determination of Dynamical–Mechanical Properties
3. Results and Discussion
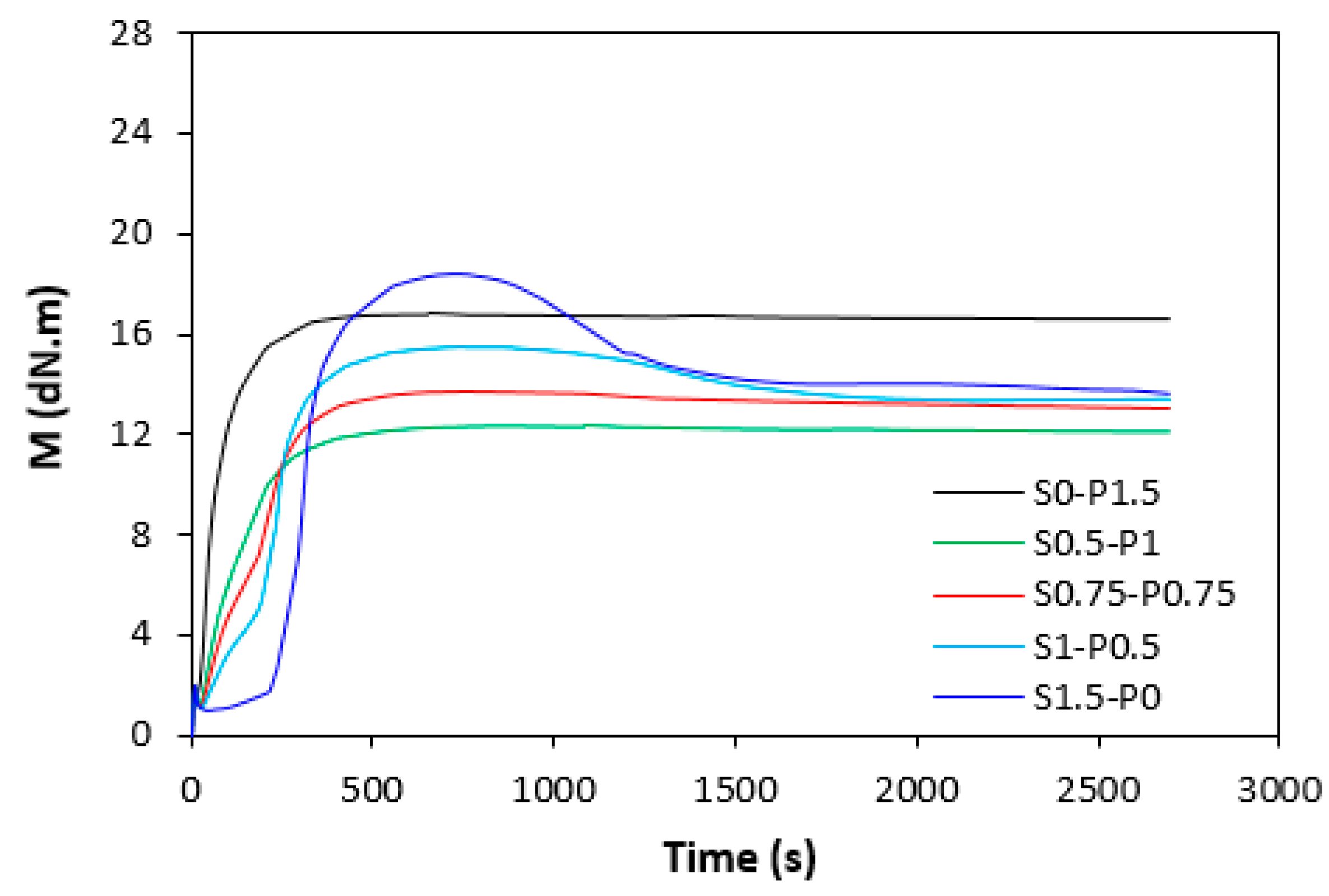
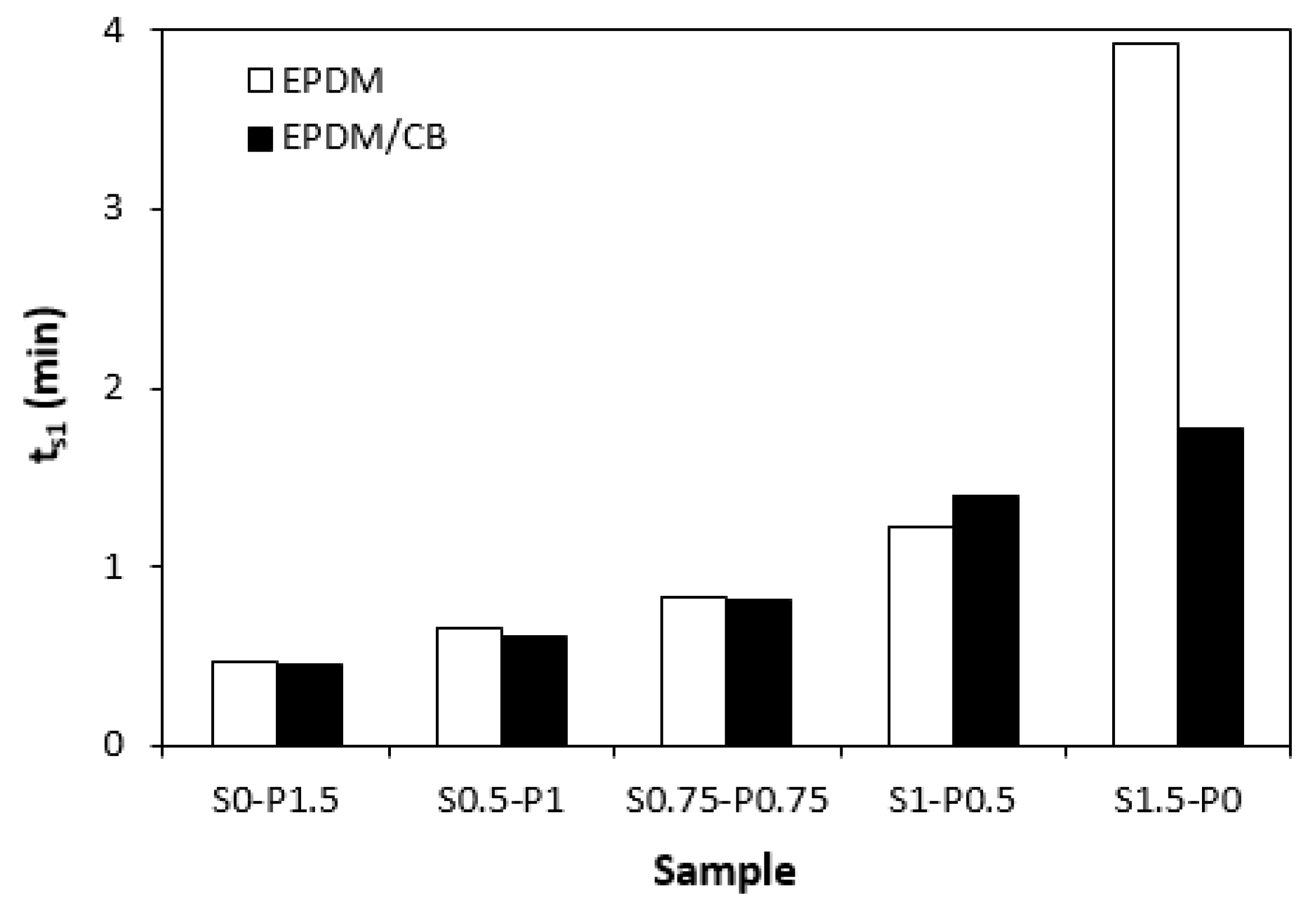
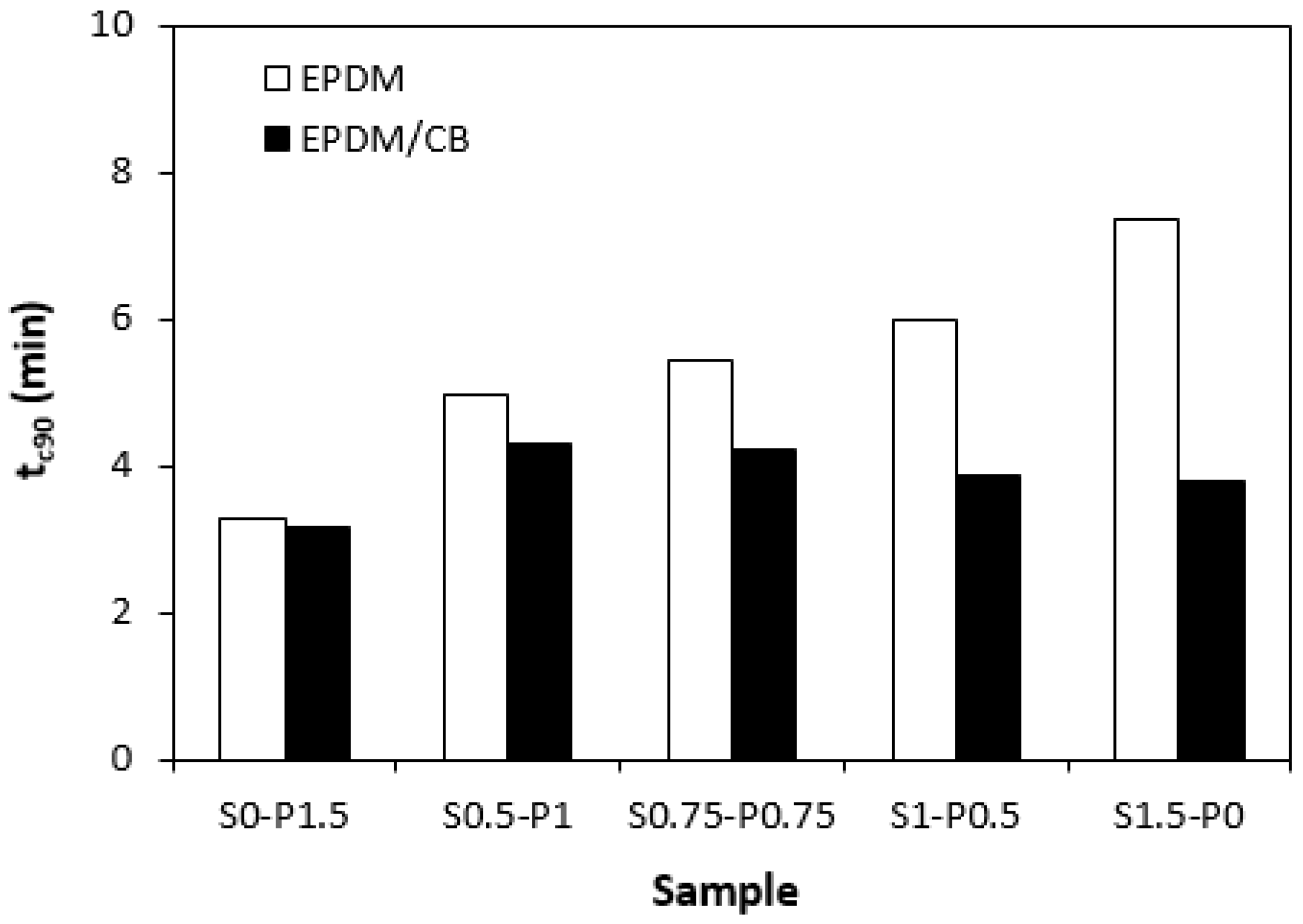
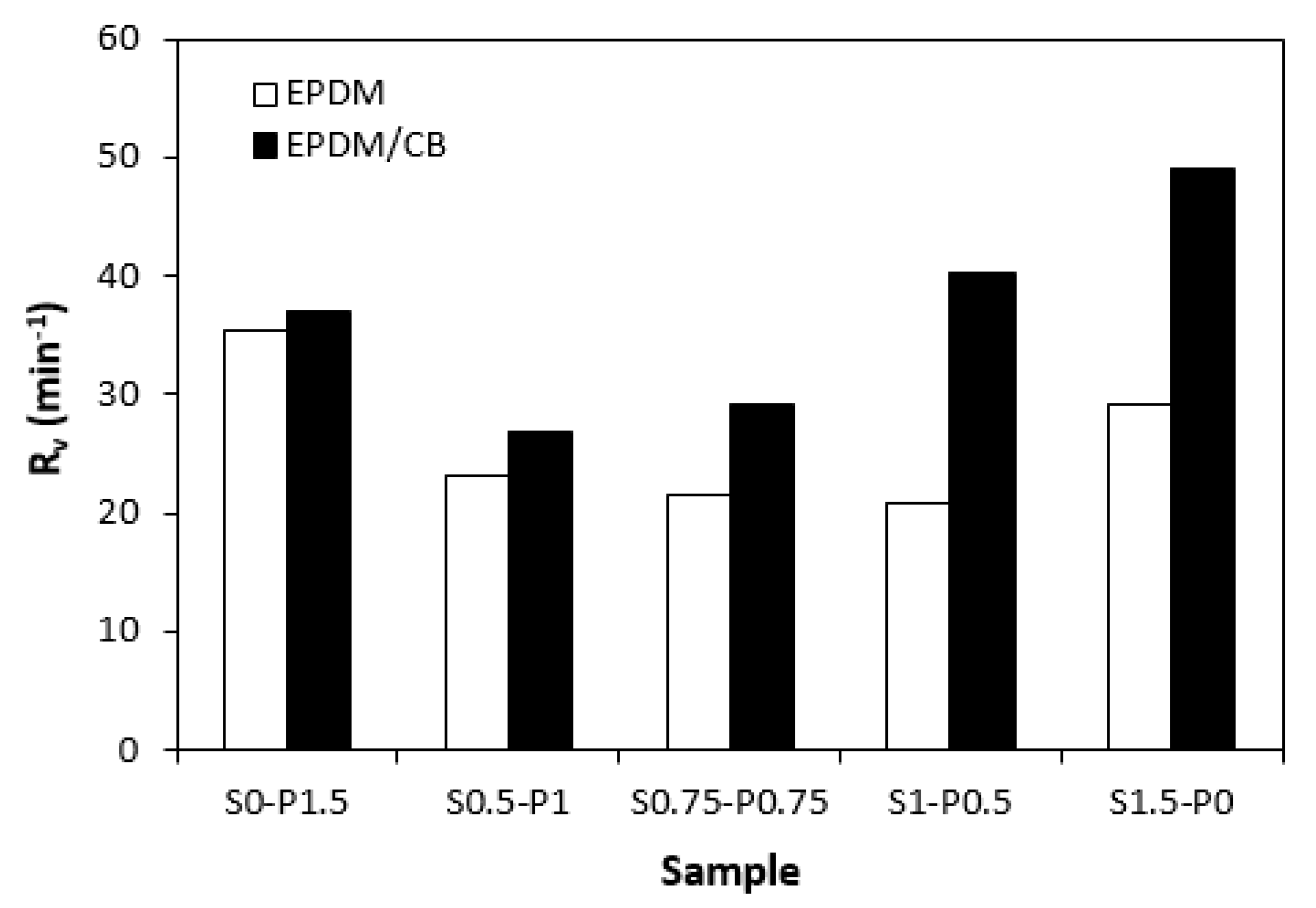
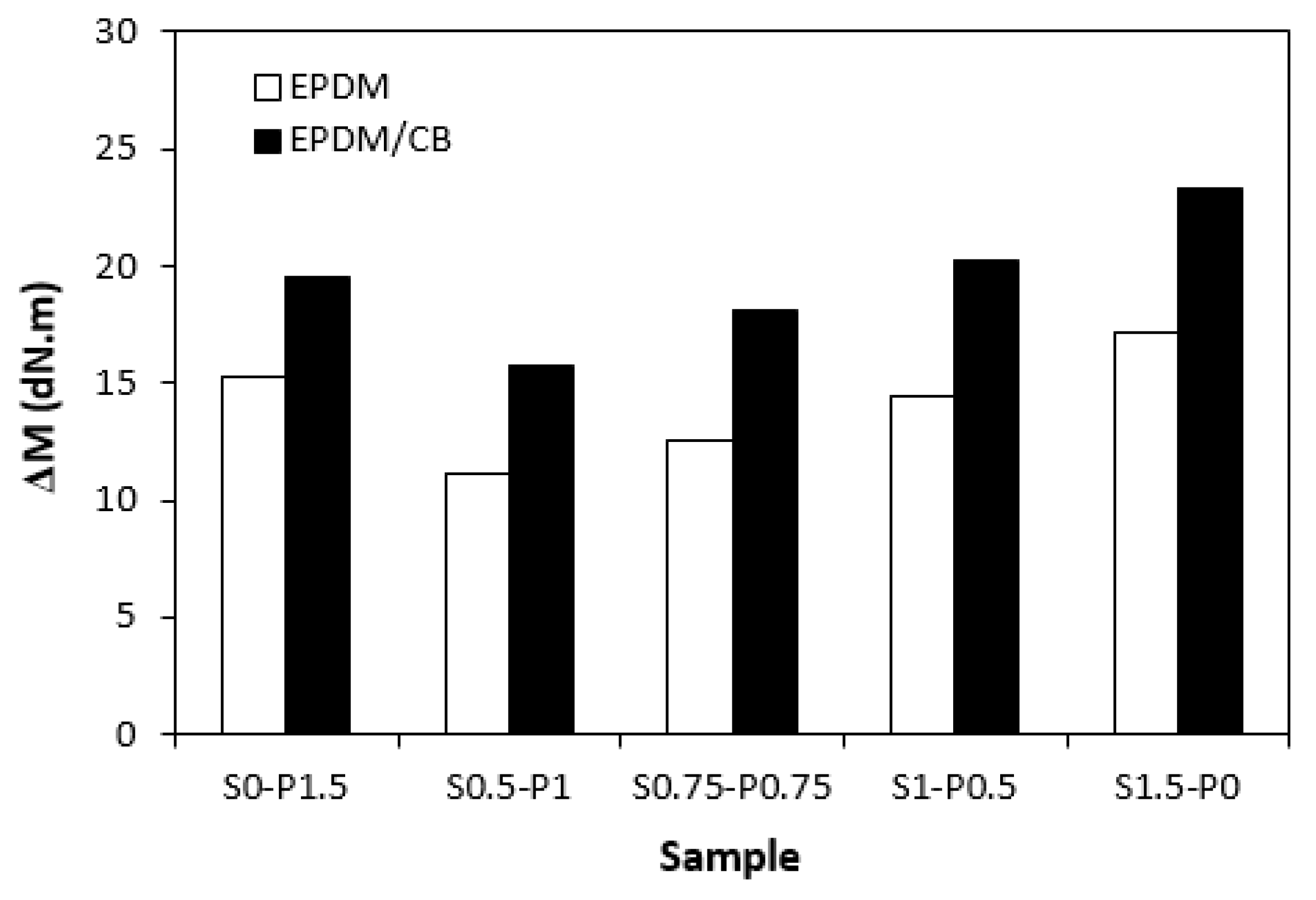
3.1. Curing Process
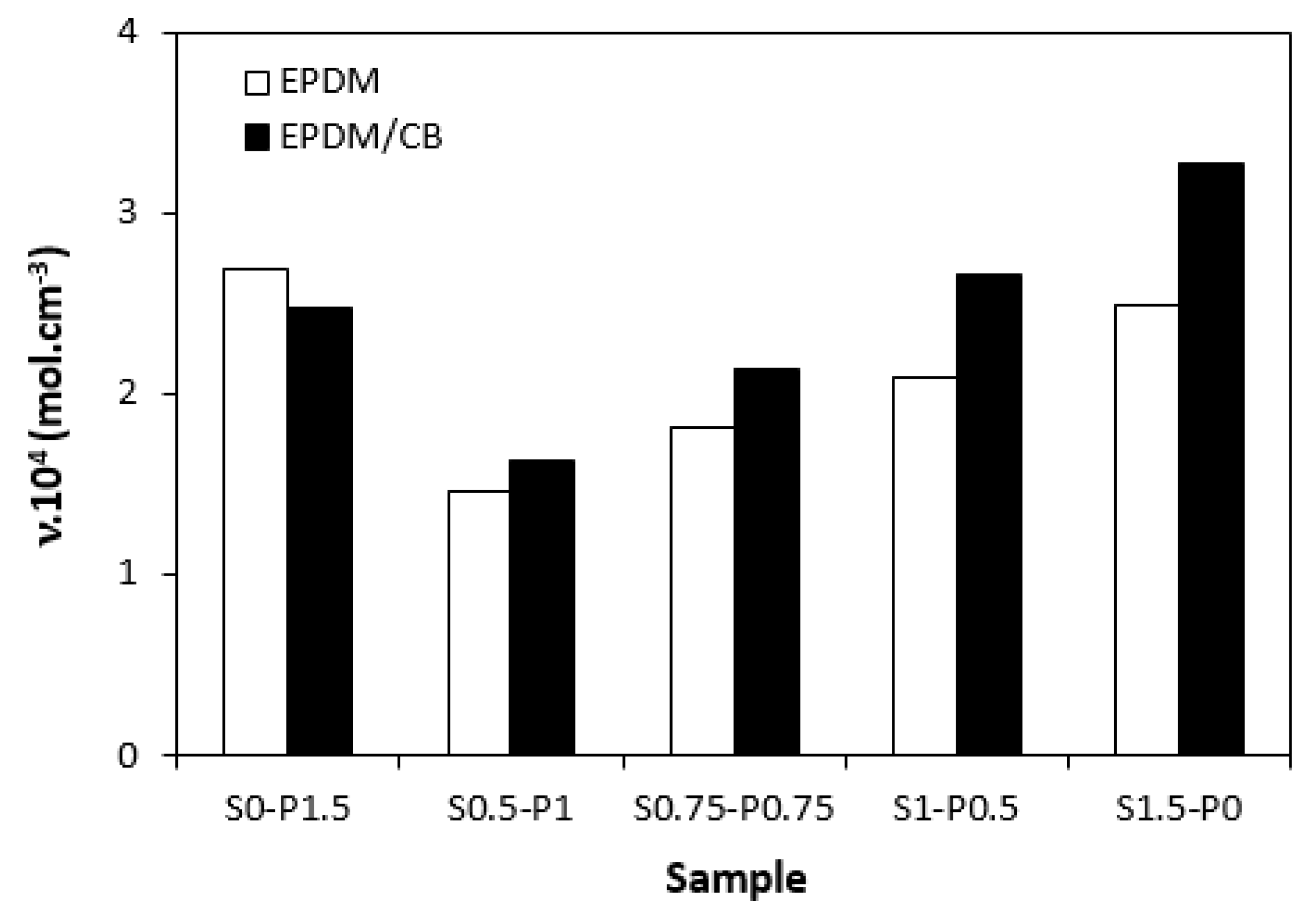
3.2. Cross-Link Density
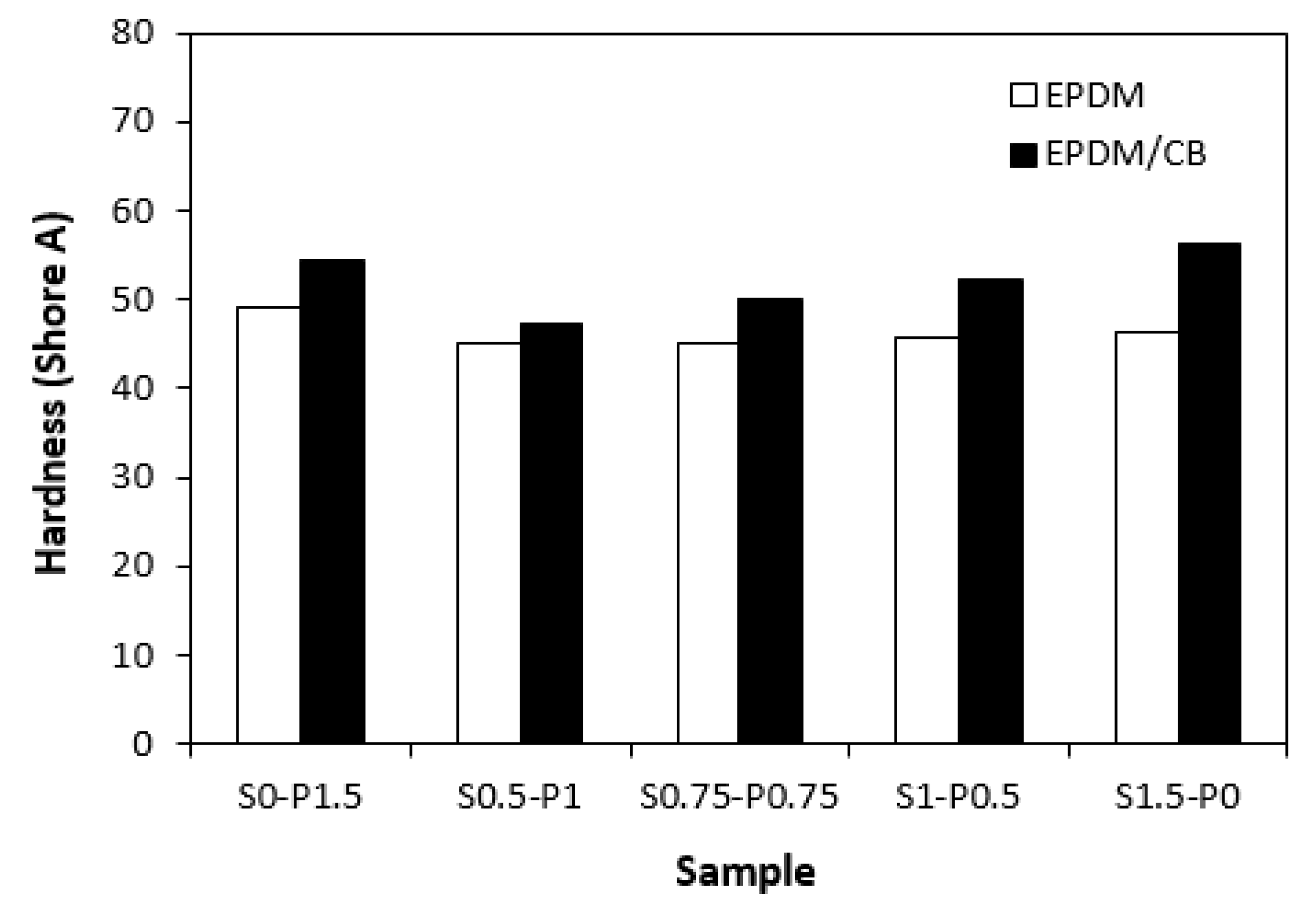
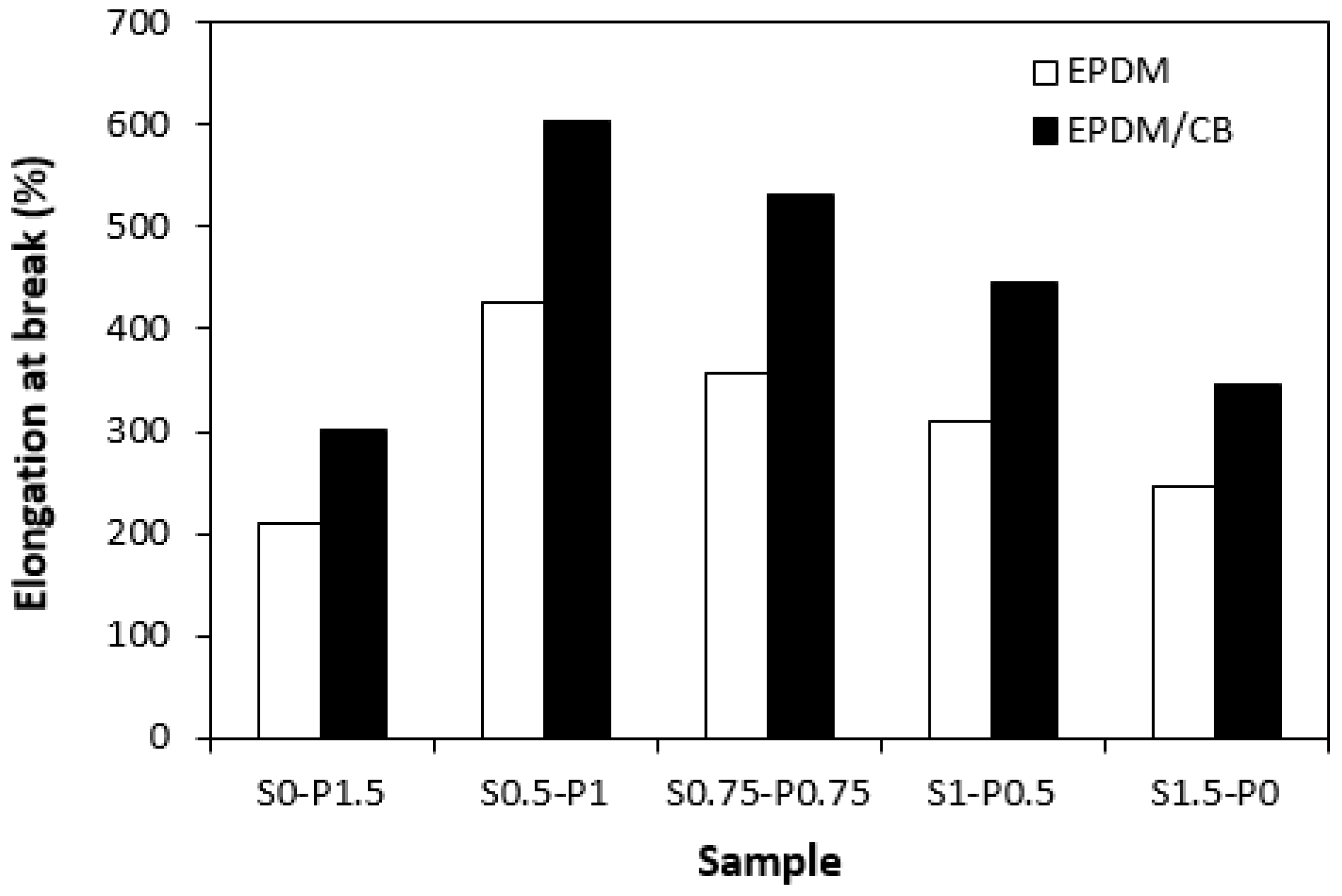
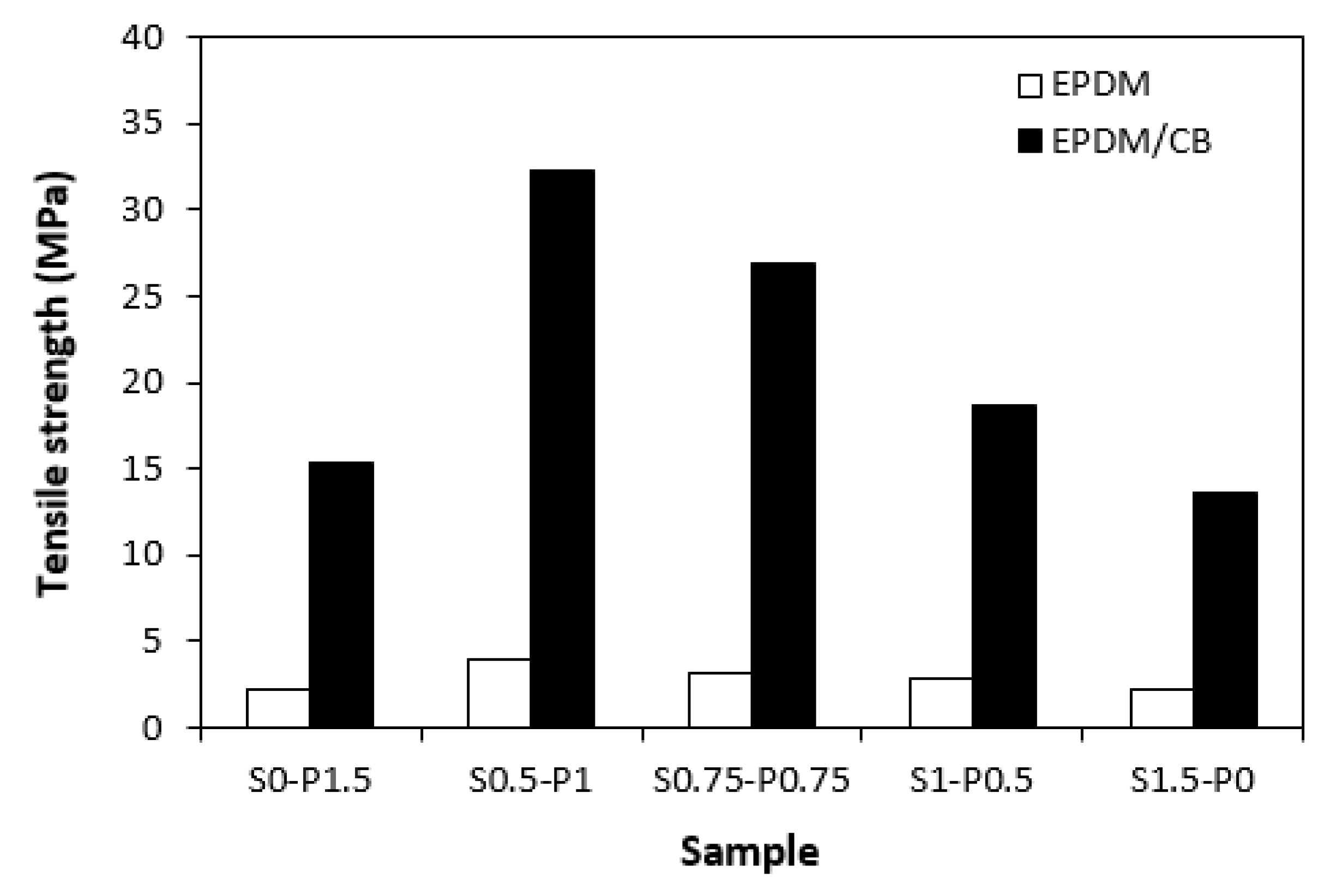
3.3. Physical–Mechanical Properties
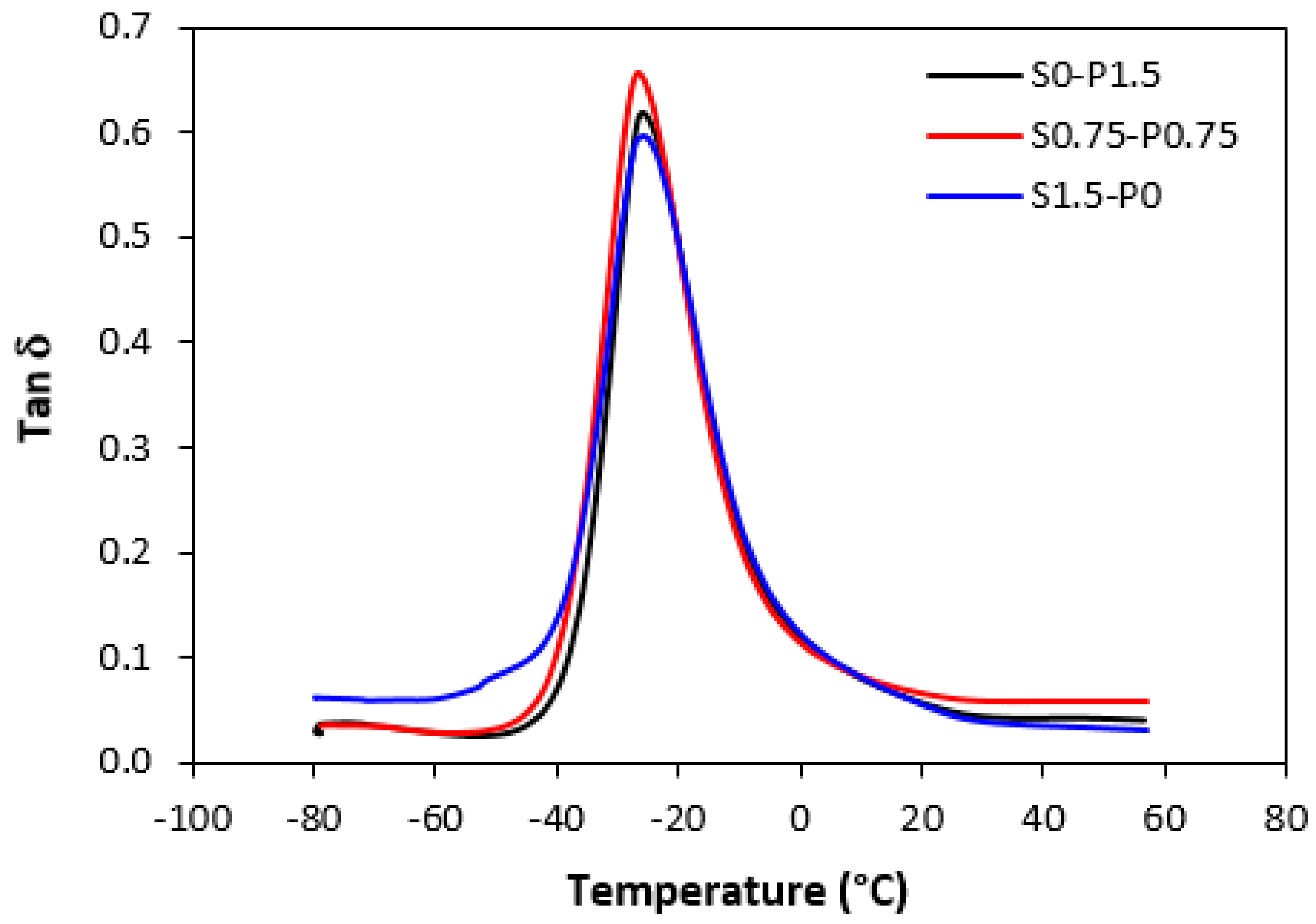
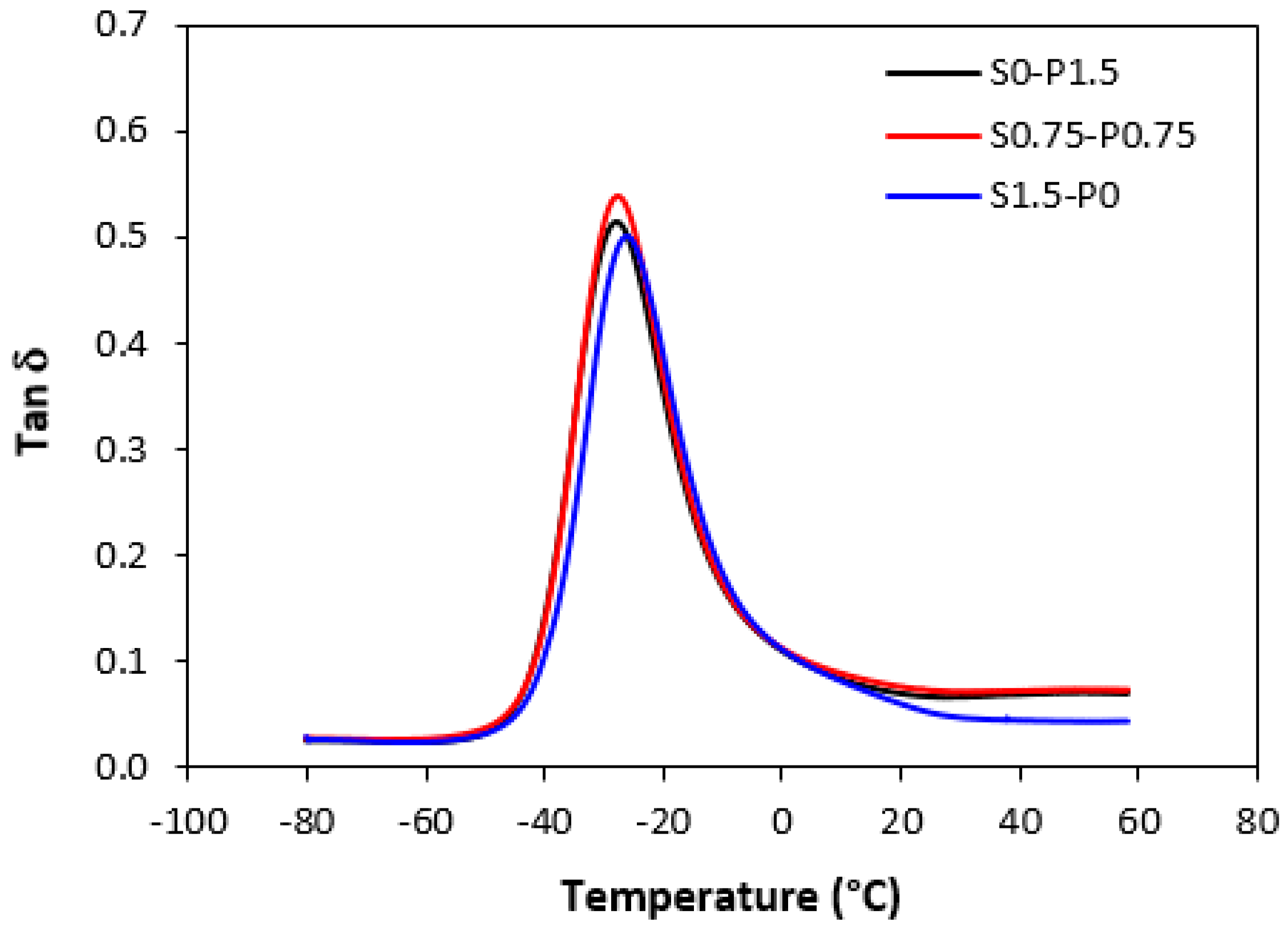
3.4. Dynamical–Mechanical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akiba, M.; Hashim, A.S. Vulcanization and crosslinking in elastomers. Prog. Polym. Sci. 1997, 22, 475–521. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Sulphur and peroxide vulcanisation of rubber compounds—Overview. Chem. Pap. 2016, 70, 1533–1555. [Google Scholar] [CrossRef]
- Lian, Q.; Li, Y.; Li, K.; Cheng, J.; Zhang, J. Insights into the vulcanization mechanism through a simple and facile approach to the sulfur cleavage behavior. Macromolecules 2017, 50, 803–810. [Google Scholar] [CrossRef]
- Dondi, D.; Buttafava, A.; Zeffiro, A.; Palamini, C.; Lostritto, A.; Giannini, L.; Faucitano, A. The mechanisms of the sulphur-only and catalytic vulcanization of polybutadiene: An EPR and DFT study. Eur. Polym. J. 2015, 62, 222–235. [Google Scholar] [CrossRef]
- Milani, G.; Milani, F. Fast and reliable meta-data model for the mechanistic analysis of NR vulcanized with sulphur. Polym. Test 2014, 33, 64–78. [Google Scholar] [CrossRef]
- Shahrampour, H.; Motavalizadehkakhky, A. The Effects of sulfur curing systems (insoluble-rhombic) on physical and thermal properties of the matrix polymeric of styrene butadiene rubber. Pet Chem. 2017, 57, 700–704. [Google Scholar] [CrossRef]
- Gao, Y.; Xue, Y.; ZG, L.; Wang, Z.; Chen, Q.; Shi, N.; Sun, F. Self-accelerating decomposition temperature and quantitative structure-property relationship of organic peroxides. Process. Saf. Environ. Prot. 2015, 94, 322–328. [Google Scholar] [CrossRef]
- Radosavljević, J.; Nikolić, L. The effect of organic peroxides on the curing behavior of EPDM isolation medium voltage cables. Adv. Technol. 2018, 71, 56–63. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Peroxide vulcanization of natural rubber. Part I: Effect of temperature and peroxide concentration. J. Polym. Eng. 2014, 34, 617–624. [Google Scholar] [CrossRef]
- Rodríguez Garraza, A.L.; Mansilla, M.A.; Depaoli, E.L.; Macchi, C.; Cerveny, S.; Marzocca, A.J.; Somoza, A. Comparative study of thermal, mechanical and structural properties of polybutadiene rubber isomers vulcanized using peroxide. Polym. Test 2016, 52, 117–123. [Google Scholar] [CrossRef]
- Wang, H.; Zhuang, T.; Shi, X.; Duin, M.V.; Zhao, S. Peroxide cross-linking of EPDM using moving die rheometer measurements. II: Effects of the process oils. Rubber Chem. Technol. 2018, 91, 561–576. [Google Scholar] [CrossRef]
- Cong, C.; Liu, Q.; Li, J.; Meng, X.; Zhou, Q. The effect of peroxide crosslinking on the synergistic crosslink of double bond and nitrile group of nitrile rubber in H2S environment. Polym. Test 2019, 76, 298–304. [Google Scholar] [CrossRef]
- Kruželák, J.; Kvasničáková, A.; Hložeková, K.; Hudec, I. Influence of dicumyl peroxide and Type I and II co-agents on cross-linking and physical–mechanical properties of rubber compounds based on NBR. Plast Rubber Compos. 2020, 49, 307–320. [Google Scholar] [CrossRef]
- Laing, B.; De Keyzer, J.; Seveno, D.; Van Bael, A. Effect of co-agents on adhesion between peroxide cured ethylene–propylene–diene monomer and thermoplastics in two-component injection molding. J. Appl. Polym. Sci. 2020, 137, 48414. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Peroxide vulcanization of natural rubber. Part II: Effect of peroxides and co-agents. J. Polym. Eng. 2015, 35, 21–29. [Google Scholar] [CrossRef]
- Lee, Y.S.; Ha, K. Effects of co-agent type and content on curing characteristics and mechanical properties of HNBR composite. Elastomers. Compos. 2020, 55, 95–102. [Google Scholar]
- Rim, K.; Park, J. Organic co-agents for maintaining mechanical properties of rubber elastomers at high processing temperatures. Korean, J. Chem. Eng. 2023. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Vulcanization of rubber compounds with peroxide curing systems. Rubber Chem. Technol. 2017, 90, 60–88. [Google Scholar] [CrossRef]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Synergistic mechanisms underlie the peroxide and coagent improvement of natural-rubber-toughened poly(3-hydroxybutyrate-co-3-hydroxyvalerate) mechanical performance. Polymers. 2019, 11, 565. [Google Scholar] [CrossRef]
- Twigg, C.; Dees, M.; van Duin, M. Hot-air ageing characteristics of peroxide-cured EPDM rubber with 5-vinyl-2-norbornene as third monomer. Kautsch. Gummi. Kunstst. 2010, 63, 436–445. [Google Scholar]
- Milani, G.; Milani, F. EPDM accelerated sulfur vulcanization: A kinetic model based on a genetic algorithm. J. Math. Chem. 2011, 49, 1357–1383. [Google Scholar] [CrossRef]
- Butuc, S.G.; van Leerdam, K.; Rossenar, B.; Swart, J.; Geurts, F.; Bossinga-Geurts, B.; Verwer, P.; Talma, A.; Blume, A. Elucidation of the role of ZnO in sulfur cure in novel EPDM-CTS blends. Polym. Test. 2023, 117, 107843. [Google Scholar] [CrossRef]
- Kraus, G. Swelling of filler-reinforced vulcanizates. J. Appl. Polym. Sci. 1963, 7, 861–871. [Google Scholar] [CrossRef]
- Nikoleva, S.; Mihaylov, M.; Dishovsky, N. Mixed peroxide/sulfur vulcanization of ethylene-propylene terpolymer based composites. Curing characteristics, curing kinetics and mechanical properties. J. Chem. Technol. Metall. 2022, 57, 881–894. [Google Scholar]
- Raffaele, E.; Dusserre, G.; del Confetto, S.; Eberling-Fux, N.; Descamps, C.; Cutard, T. Effect of dicumyl peroxide concentration on the polymerization kinetics of a polysilazane system. Polym. Eng. Sci. 2018, 58, 859–869. [Google Scholar]
- Ghosh, J.; Ghorai, S.; Jalan, A.K.; Roy, M.; De, D. Manifestation of accelerator type and vulcanization system on the properties of silica-reinforced SBR/devulcanize SBR blend vulcanizates. Adv. Polym. Technol. 2018, 37, 2636–2650. [Google Scholar] [CrossRef]
- Charoeythornkhajhornchai, P.; Samthong, C.; Somwangthanaroj, A. Influence of sulfenamide accelerators on cure kinetics and properties of natural rubber foam. J. Appl. Polym. Sci. 2017, 134, 44822. [Google Scholar] [CrossRef]
- Boonkerd, K.; Limphirat, W. Investigation of crosslink structure of natural rubber during vulcanization using X-ray absorption near edge spectroscopy. J. Met. Mater. Miner. 2020, 30, 119–123. [Google Scholar] [CrossRef]
- Hayeemasae, N.; Masa, A. Relationship between stress relaxation behavior and thermal stability of natural rubber vulcanizates. Polímeros 2020, 30, e2020016. [Google Scholar] [CrossRef]
- Azevedo, M.; Monks, A.M.; Kerschbaumer, R.C.; Schlögl, S.; Holzer, C. Peroxide-based crosslinking of solid silicone rubber, part I: Insights into the influence of dicumyl peroxide concentration on the curing kinetics and thermodynamics determined by a rheological approach. Polymers 2022, 14, 4404. [Google Scholar] [CrossRef]
- Przybysz, M.; Hejna, A.; Haponiuk, J.; Formela, K. Structural and thermo-mechanical properties of poly(ε-caprolactone) modified by various peroxide initiators. Polymers 2019, 11, 1101. [Google Scholar] [CrossRef] [PubMed]
- Orza, R.A.; Magusin, P.C.M.M.; Litvinov, V.M.; Van Duin, M.; Michels, M.A.J. Mechanism for peroxide cross-Linking of EPDM rubber from MAS 13C NMR spectroscopy. Macromolecules 2009, 42, 8914–8924. [Google Scholar] [CrossRef]
- Saleesung, T.; Reichert, D.; Saalwächter, K.; Sirisinha, C. Correlation of crosslink densities using solid state NMR and conventional techniques in peroxide-crosslinked EPDM rubber. Polymer 2015, 56, 309–317. [Google Scholar] [CrossRef]
- Kruželák, J.; Hakošová, S.; Kvasničáková, A.; Hudec, I. Dicumyl peroxide used as curing agent for different types of rubber matrices. Part I: Effect of temperature. Kautsch. Gummi. Kunstst. 2020, 10, 36–42. [Google Scholar]
- Strohmeier, L.; Balasooriya, W.; Schrittesser, B.; van Duin, M.; Schlögl, S. Hybrid in situ reinforcement of EPDM rubber compounds based on phenolic novolac resin and ionic coagent. Appl. Sci. 2022, 12, 2432. [Google Scholar] [CrossRef]
- Parathodika, A.R.; Raju, A.T.; Das, M.; Bhattacharya, A.B.; Neethirajan, J.; Naskar, K. Exploring hybrid vulcanization system in high-molecular weight EPDM rubber composites: A statistical approach. J. App. Polym. Sci. 2022, 139, e52721. [Google Scholar] [CrossRef]
- Parathodika, A.R.; Sreethu, T.K.; Maji, P.; Susoff, M.; Naskar, K. Influence of molecular and crosslink network structure on vulcanizate properties of EPDM elastomers. Express. Polym. Lett. 2023, 17, 722–737. [Google Scholar] [CrossRef]
- Çakır, N.Y.; Inan, Ö.; Ergün, M.; Kodal, M.; Özkoç, G. Unlocking the potential use of reactive POSS as a coagent for EPDM/PP-based TPV. Polymers 2023, 15, 2267. [Google Scholar] [CrossRef]
- Lin, Y.; Amornkitbamrung, L.; Mora, P.; Jubsilp, C.; Hemvichian, K.; Soottitantawat, A.; Ekgasit, S.; Rimdusit, S. Effects of coagent functionalities on properties of ultrafine fully vulcanized powdered natural rubber prepared as toughening filler in rigid PVC. Polymers 2021, 13, 289. [Google Scholar] [CrossRef]
- Shimizu, T.; Inagaki, S. Development of a novel cross-linking agent with excellent resistance to high-temperature vapour. Seal Technol. 2017, 5, 7–11. [Google Scholar] [CrossRef]
- Sun, Y.; Ch, F.; Zhao, Y.; Jia, L. Peroxide-cured isobutylene-isoprene rubber composite: Methacrylate coagent and enhanced mechanical properties by in situ formed methacrylate domains. Ind. Eng. Chem. Res. 2021, 60, 2728–2735. [Google Scholar] [CrossRef]
- Heideman, G.; Datta, R.N.; Noordermeer, J.W.M.; van Baarle, B. Influence of zinc oxide during different stages of sulfur vulcanization. Elucidated by model compound studies. J. Appl. Polym. Sci. 2005, 95, 1388–1404. [Google Scholar] [CrossRef]
- van Duin, M. Chemistry of EPDM cross-linking. Kautsch. Gummi. Kunstst. 2002, 55, 150–156. [Google Scholar]
- Ravindran, A.; Kamaraj, M.; Vasanthmurali, N.; Meghavarshini, V.; Balachandran, M. Nanosilica reinforced EPDM silicone rubber blends: Experimental and theoretical evaluation of mechanical and solvent sorption properties. Mater. Today Proc. 2021, 46, 4381–4386. [Google Scholar] [CrossRef]
- Bandzierz, K.; Reuvekamp, L.; Dryzek, J.; Dierkes, W.; Blume, A.; Bielinski, D. Influence of network structure on glass transition temperature of elastomers. Materials 2016, 9, 607. [Google Scholar] [CrossRef]



| S0–P1.5 | S0.5–P1 | S0.75–P0.75 | S1–P0.5 | S1.5–P0 | |
|---|---|---|---|---|---|
| EPDM | 100 | 100 | 100 | 100 | 100 |
| ZnO | 0 | 1 | 2 | 3 | 4 |
| Stearic acid | 0 | 0.5 | 1 | 1.5 | 2 |
| Sulfur | 0 | 0.5 | 0.75 | 1 | 1.5 |
| CBS | 0 | 1 | 2 | 3 | 4 |
| DCP | 1.5 | 1 | 0.75 | 0.5 | 0 |
| TMPTMA | 4 | 3 | 2 | 1 | 0 |
| S0–P1.5 | S0.5–P1 | S0.75–P0.75 | S1–P0.5 | S1.5–P0 | |
|---|---|---|---|---|---|
| EPDM | 100 | 100 | 100 | 100 | 100 |
| CB | 25 | 25 | 25 | 25 | 25 |
| ZnO | 0 | 1 | 2 | 3 | 4 |
| Stearic acid | 0 | 0.5 | 1 | 1.5 | 2 |
| Sulfur | 0 | 0.5 | 0.75 | 1 | 1.5 |
| CBS | 0 | 1 | 2 | 3 | 4 |
| DCP | 1.5 | 1 | 0.75 | 0.5 | 0 |
| TMPTMA | 4 | 3 | 2 | 1 | 0 |
| Sample | Tg (°C) | tan δ (−20 °C) | tan δ (0 °C) | tan δ (20 °C) | tan δ (50 °C) |
|---|---|---|---|---|---|
| S0–P1.5 | −26 | 0.50 | 0.12 | 0.06 | 0.04 |
| S0.75–P0.75 | −26.6 | 0.50 | 0.12 | 0.07 | 0.06 |
| S1.5–P0 | −25.8 | 0.50 | 0.12 | 0.06 | 0.03 |
| Sample | Tg (°C) | tan δ (−20 °C) | tan δ (0 °C) | tan δ (20 °C) | tan δ (50 °C) |
|---|---|---|---|---|---|
| S0–P1.5 | −27.9 | 0.35 | 0.11 | 0.07 | 0.07 |
| S0.75–P0.75 | −27.6 | 0.38 | 0.11 | 0.08 | 0.07 |
| S1.5–P0 | −26.3 | 0.39 | 0.11 | 0.06 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruželák, J.; Mikolajová, M.; Kvasničáková, A.; Džuganová, M.; Chodák, I.; Hronkovič, J.; Preťo, J.; Hudec, I. Combined Sulfur and Peroxide Vulcanization of Filled and Unfilled EPDM-Based Rubber Compounds. Materials 2023, 16, 5596. https://doi.org/10.3390/ma16165596
Kruželák J, Mikolajová M, Kvasničáková A, Džuganová M, Chodák I, Hronkovič J, Preťo J, Hudec I. Combined Sulfur and Peroxide Vulcanization of Filled and Unfilled EPDM-Based Rubber Compounds. Materials. 2023; 16(16):5596. https://doi.org/10.3390/ma16165596
Chicago/Turabian StyleKruželák, Ján, Mária Mikolajová, Andrea Kvasničáková, Michaela Džuganová, Ivan Chodák, Ján Hronkovič, Jozef Preťo, and Ivan Hudec. 2023. "Combined Sulfur and Peroxide Vulcanization of Filled and Unfilled EPDM-Based Rubber Compounds" Materials 16, no. 16: 5596. https://doi.org/10.3390/ma16165596
APA StyleKruželák, J., Mikolajová, M., Kvasničáková, A., Džuganová, M., Chodák, I., Hronkovič, J., Preťo, J., & Hudec, I. (2023). Combined Sulfur and Peroxide Vulcanization of Filled and Unfilled EPDM-Based Rubber Compounds. Materials, 16(16), 5596. https://doi.org/10.3390/ma16165596









