Mono- and Co-Doped Mn-Doped CsPbCl3 Perovskites with Enhanced Doping Efficiency and Photoluminescent Performance
Abstract
:1. Introduction
2. Calculation Methods
3. Results and Discussions
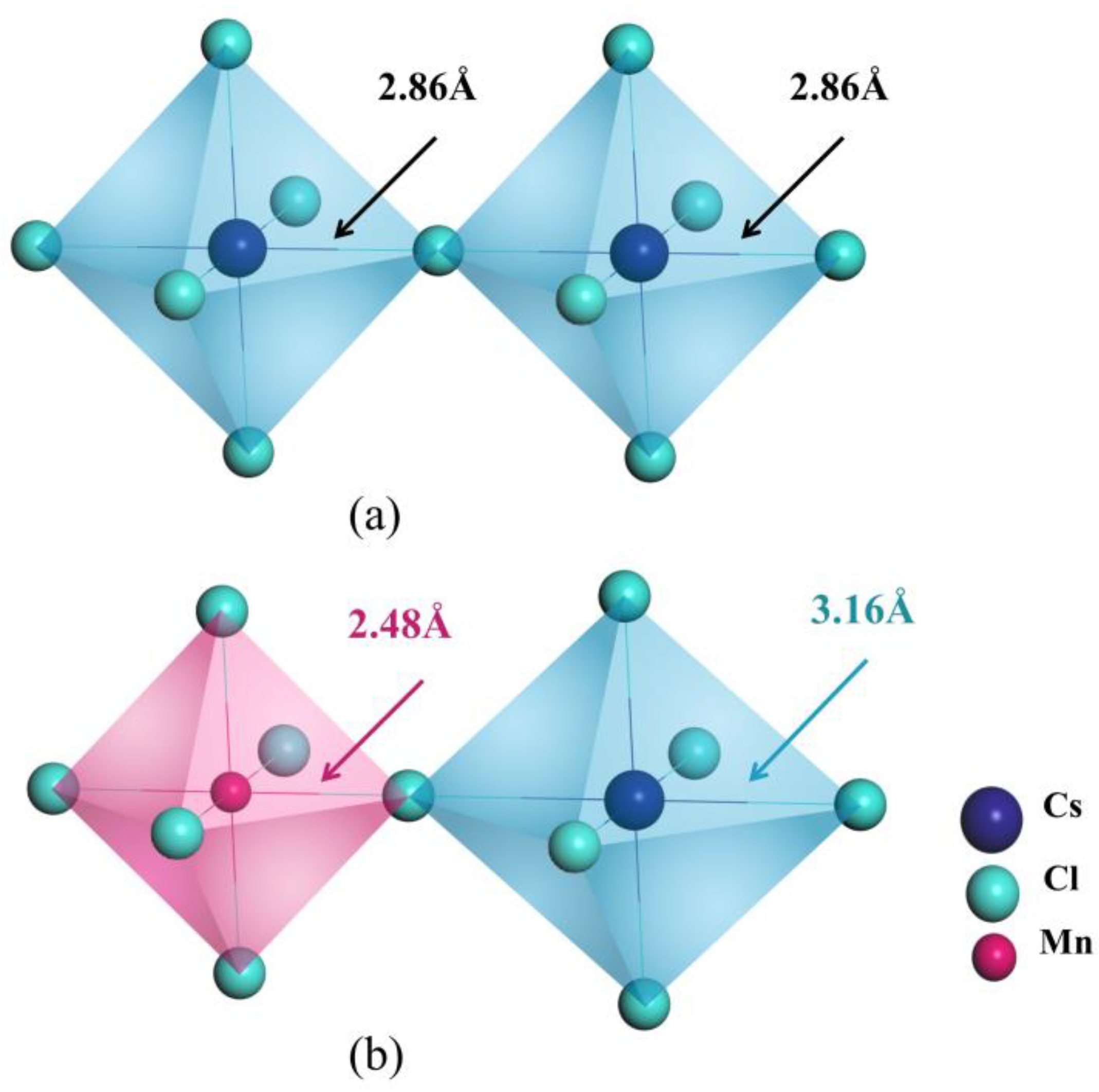
3.1. Geometric Properties
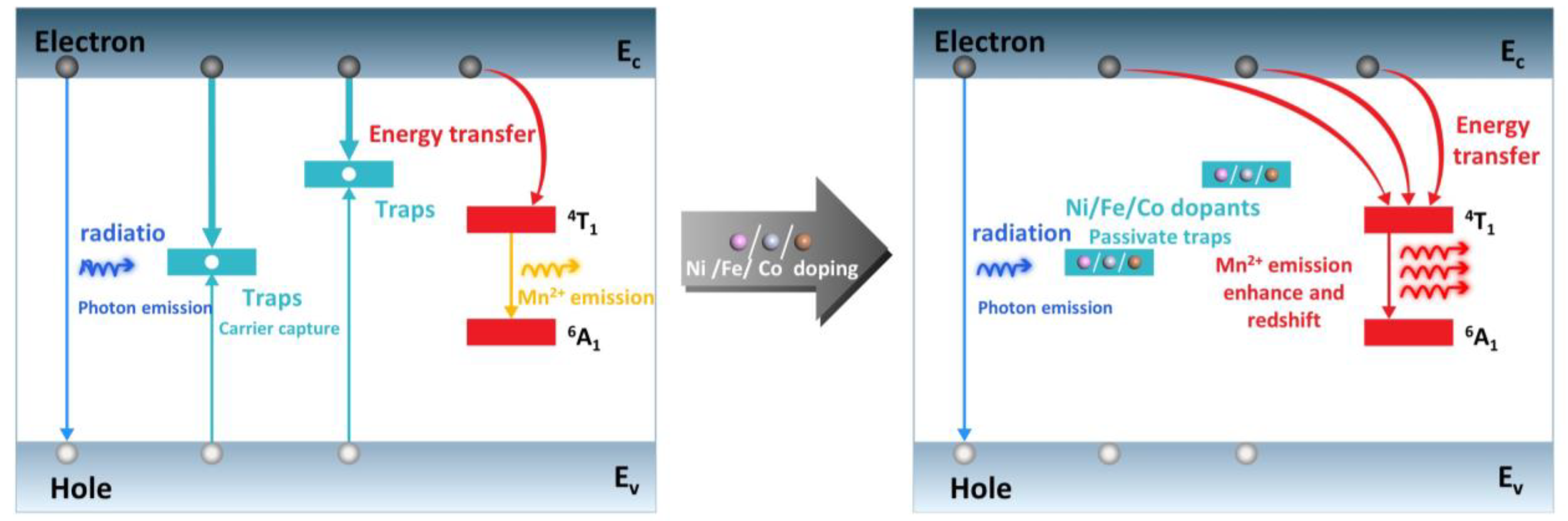
3.2. Electronic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.-L.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. Research Progress in Perovskite Solar Cell Materials and Devices. J. Xiamen Univ. 2015, 54, 619–629. [Google Scholar]
- Koscher, B.A.; Swabeck, J.K.; Bronstein, N.D.; Alivisatos, A.P. Essentially trap-free CsPbBr3 colloidal nanocrystals by postsynthetic thiocyanate surface treatment. J. Am. Chem. Soc. 2018, 139, 6566–6569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, X.; Zhang, T.; Wang, X.; Kan, M.; Shi, J.; Zhao, Y. The Role of Dimethylammonium Iodide in CsPbI3 Perovskite Fabrication: Additive or Dopant? Angew. Chem. Int. Ed. 2019, 58, 16691–16696. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, J.; Cui, J.-R.; Guo, X.-Y. Research Progress of Perovskite Solar Cells. J. Inorg. Mater. 2015, 30, 1131–1138. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Ji, X.; Liang, J.; Yu, Q. Progress toward Applications of Perovskite Solar Cells. Energy Fuels 2020, 34, 6624–6633. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, X.; Feng, X. Recent progress of perovskite solar cells. Electron. Compon. Mater. 2014, 33, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Shi, J.; Chen, J.; Tan, Z.; Lei, H. Lead-Free Halide Double Perovskite for High-Performance Photodetectors: Progress and Perspective. Materials 2023, 16, 4490. [Google Scholar] [CrossRef]
- Samsonova, A.Y.; Yudin, V.I.; Shurukhina, A.V.; Kapitonov, Y.V. Excitonic Enhancement and Excited Excitonic States in CsPbBr3 Halide Perovskite Single Crystals. Materials 2023, 16, 185. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Li, J.; McKechnie, S. Interactions between molecules and perovskites in halide perovskite solar cells. Sol. Energy Mater. Sol. Cells 2018, 175, 1–19. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, C.; Jiang, X.-F.; Liu, M.; Xia, R.; Shi, T.; Chen, D.; Xue, Q.; Zhao, Y.-J.; Su, S.; et al. High-Performance Color-Tunable Perovskite Light Emitting Devices through Structural Modulation from Bulk to Layered Film. Adv. Mater. 2017, 29, 1603157. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)(2)PbI3-based perovskite solar cells. Nat. Energy 2017, 2, 16177. [Google Scholar] [CrossRef]
- Liu, T.; Chen, K.; Hu, Q.; Zhu, R.; Gong, Q. Inverted Perovskite Solar Cells: Progresses and Perspectives. Adv. Energy Mater. 2016, 6, 1600457. [Google Scholar] [CrossRef]
- Song, Y.; Bi, W.; Wang, A.; Liu, X.; Kang, Y.; Dong, Q. Efficient lateral-structure perovskite single crystal solar cells with high operational stability. Nat. Commun. 2020, 11, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Sun, W.; Li, Y.; Ye, S.; Rao, H.; Gu, F.; Liu, Z.; Bian, Z.; Huang, C. Simplification of device structures for low-cost, high-efficiency perovskite solar cells. J. Mater. Chem. A 2017, 5, 4756–4773. [Google Scholar] [CrossRef]
- Zuo, C.; Bolink, H.J.; Han, H.; Huang, J.; Cahen, D.; Ding, L. Advances in Perovskite Solar Cells. Adv. Sci. 2016, 3, 1500324. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Kershaw, S.V.; Li, T.; Wang, C.; Zhang, X.; Wang, W.; Li, D.; Wang, Y.; Lu, M.; et al. Zn-Alloyed CsPbI3 Nanocrystals for Highly Efficient Perovskite Light-Emitting Devices. Nano Lett. 2019, 19, 1552–1559. [Google Scholar] [CrossRef]
- Cao, Z.; Li, J.; Wang, L.; Xing, K.; Yuan, X.; Zhao, J.; Gao, X.; Li, H. Enhancing luminescence of intrinsic and Mn doped CsPbCl3 perovskite nanocrystals through Co2+ doping. Mater. Res. Bull. 2020, 121, 110608. [Google Scholar] [CrossRef]
- Ji, S.; Yuan, X.; Cao, S.; Ji, W.; Zhang, H.; Wang, Y.; Li, H.; Zhao, J.; Zou, B. Near-Unity Red Mn2+ Photoluminescence Quantum Yield of Doped CsPbCl3 Nanocrystals with Cd Incorporation. J. Phys. Chem. Lett. 2020, 11, 2142–2149. [Google Scholar] [CrossRef]
- Lyu, J.; Dong, B.; Pan, G.; Sun, L.; Bai, X.; Hu, S.; Shen, B.; Zhou, B.; Wang, L.; Xu, W.; et al. Ni2+ and Pr3+ Co-doped CsPbCl3 perovskite quantum dots with efficient infrared emission at 1300 nm. Nanoscale 2021, 13, 16598–16607. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, S.; Li, J.; Li, H.; Yuan, X.; Zhao, J. Improved ultraviolet radiation stability of Mn2+-doped CsPbCl3 nanocrystals via B-site Sn doping. Crystengcomm 2019, 21, 6238–6245. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, X.; Han, L.; Li, J.; Zhang, X.; Hua, J.; Wang, J.; Zhao, J.; Li, H. A-Site FA(+) Doping-Enhanced Photoluminescence Efficiency and Photostability of Mn-Doped Perovskite Nanocrystals. J. Phys. Chem. C 2022, 126, 3582–3590. [Google Scholar] [CrossRef]
- Yuce, H.; Mandal, M.; Yalcinkaya, Y.; Andrienko, D.; Demir, M.M. Improvement of Photophysical Properties of CsPbBr3 and Mn2+:CsPb(Br,Cl)(3) Perovskite Nanocrystals by Sr2+ Doping for White Light-Emitting Diodes. J. Phys. Chem. C 2022, 126, 11277–11284. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Liu, G.; Ma, Y.; Gong, L.; Guan, R.; Cui, X.; Yan, J.; Zhao, J.; Yang, J. Pressure-Engineered Optical and Charge Transport Properties of Mn2+/Cu2+ Codoped CsPbCl3 Perovskite Nanocrystals via Structural Progression. ACS Appl. Mater. Interfaces 2020, 12, 48225–48236. [Google Scholar] [CrossRef]
- Bhat, A.A.; Khandy, S.A.; Islam, I.; Tomar, R. Optical, electrochemical and photocatalytic properties of cobalt doped CsPbCl3 nanostructures: A one-pot synthesis approach. Sci. Rep. 2021, 11, 16473. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. Zn(II)-Doped Cesium Lead Halide Perovskite Nanocrystals with High Quantum Yield and Wide Color Tunability for Color-Conversion Light-Emitting Displays. ACS Appl. Nano Mater. 2020, 3, 7621–7632. [Google Scholar] [CrossRef]
- Roy, B.; Mahato, S.; Bose, S.; Ghorai, A.; Srivastava, S.K.; Das, N.C.; Ray, S.K. Cu-Doping Induced Phase Transformation in CsPbI3 Nanocrystals with Enhanced Structural Stability and Photoluminescence Quantum Yield. Chem. Mater. 2023, 35, 1601–1609. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Kim, J.; Jain, A.; Quintero-Bermudez, R.; Tan, H.R.; Long, G.K.; Tan, F.R.; Johnston, A.; Zhao, Y.C.; Voznyy, O.; et al. Suppression of atomic vacancies via incorporation of isovalent small ions to increase the stability of halide perovskite solar cells in ambient air. Nat. Energy 2018, 3, 648–654. [Google Scholar] [CrossRef]
- Bi, C.H.; Wang, S.X.; Li, Q.; Kershaw, S.V.; Tian, J.J.; Rogach, A.L. Thermally Stable Copper(II)-Doped Cesium Lead Halide Perovskite Quantum Dots with Strong Blue Emission. J. Phys. Chem. Lett. 2019, 10, 943–952. [Google Scholar] [CrossRef]
- Liu, W.; Lin, Q.; Li, H.; Wu, K.; Robel, I.; Pietryga, J.M.; Klimov, V.I. Mn2+-Doped Lead Halide Perovskite Nanocrystals with Dual-Color Emission Controlled by Halide Content. J. Am. Chem. Soc. 2016, 138, 14954–14961. [Google Scholar] [CrossRef]
- Parobek, D.; Dong, Y.T.; Qiao, T.; Son, D.H. Direct Hot-Injection Synthesis of Mn-Doped CsPbBr3 Nanocrystals. Chem. Mater. 2018, 30, 2939–2944. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, C.; Zhao, Z.Y.; Zhang, W.C.; Li, K.; Ye, Y.; Zhu, C.F.; Meng, X.G. Enhanced luminescence of Mn doped CsPbCl3 and CsPb(Cl/Br)(3) perovskite nanocrystals stabilized in glasses. J. Alloys Compd. 2020, 827, 154349. [Google Scholar] [CrossRef]
- Wu, W.; Cong, W.-Y.; Guan, C.; Sun, H.; Yin, R.; Yu, G.; Lu, Y.-B. Investigation of the Mn dopant-enhanced photoluminescence performance of lead-free Cs2AgInCl6 double perovskite crystals. Phys. Chem. Chem. Phys. 2020, 22, 1815–1819. [Google Scholar] [CrossRef]
- Locardi, F.; Cirignano, M.; Baranov, D.; Dang, Z.Y.; Prato, M.; Drago, F.; Ferretti, M.; Pinchetti, V.; Fanciulli, M.; Brovelli, S.; et al. Colloidal Synthesis of Double Perovskite Cs2AgInCl6 and Mn-Doped Cs2AgInCl6 Nanocrystals. J. Am. Chem. Soc. 2018, 140, 12989–12995. [Google Scholar] [CrossRef] [PubMed]
- Dalpian, G.M.; Chelikowsky, J.R. Self-purification in semiconductor nanocrystals. Phys. Rev. Lett. 2006, 96, 226802. [Google Scholar] [CrossRef] [PubMed]
- Guerra, R.; Ossicini, S. Preferential Positioning of Dopants and Co-Dopants in Embedded and Freestanding Si Nanocrystals. J. Am. Chem. Soc. 2014, 136, 4404–4409. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Li, D.; Xu, J.; Li, W.; Chen, K. Size-dependent phosphorus doping effect in nanocrystalline-Si-based multilayers. Appl. Surf. Sci. 2018, 461, 66–71. [Google Scholar] [CrossRef]
- Norris, D.J.; Efros, A.L.; Erwin, S.C. Doped Nanocrystals. Science 2008, 319, 1776–1779. [Google Scholar] [CrossRef]
- Chang, P.; Zhai, Y.; Wu, N.; Zhang, H.; Zhu, Q.-Q.; Wang, L. Fluorescence properties of potassium ions doped CsPbCl3: Mn perovskite quantum dots. Chin. J. Liq. Cryst. Disp. 2021, 36, 1352–1361. [Google Scholar] [CrossRef]
- Zhang, R.; Yuan, Y.X.; Zhang, J.F.; Liu, H.Y.; Chen, G.; Li, K.; Hong, M.Q.; Zuo, W.B.; Wang, C.N.; Yang, W.; et al. Improving the Mn2+ emission and stability of CsPb(Cl/Br)(3) nanocrystals by Ni2+ doping in ambient air. J. Mater. Sci. 2021, 56, 7494–7507. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Heyd, J.; Peralta, J.E.; Scuseria, G.E.; Martin, R.L. Energy band gaps and lattice parameters evaluated with the Heyd-Scuseria-Ernzerhof screened hybrid functional. J. Chem. Phys. 2005, 123, 174101. [Google Scholar] [CrossRef] [PubMed]
- Blochl, P.E.; Forst, C.J.; Schimpl, J. Projector augmented wave method: Ab initio molecular dynamics with full wave functions. Bull. Mater. Sci. 2003, 26, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Setyawan, W.; Curtarolo, S. High-throughput electronic band structure calculations: Challenges and tools. Comput. Mater. Sci. 2010, 49, 299–312. [Google Scholar] [CrossRef] [Green Version]
- Maintz, S.; Deringer, V.L.; Tchougreeff, A.L.; Dronskowski, R. LOBSTER: A Tool to Extract Chemical Bonding from Plane-Wave Based DFT. J. Comput. Chem. 2016, 37, 1030–1035. [Google Scholar] [CrossRef] [Green Version]
- Erwin, S.C.; Zu, L.; Haftel, M.I.; Efros, A.L.; Kennedy, T.A.; Norris, D.J. Doping semiconductor nanocrystals. Nature 2005, 436, 91–94. [Google Scholar] [CrossRef]






| Doping Strategies | Defect Type | Ef (eV) | Doping Strategies | Defect Type | Ef (eV) |
|---|---|---|---|---|---|
| Intrinsic Defects | VCl | 3.15 | Mono-Doping | MnPb | 3.96 |
| VCs | 3.96 | MnPbVCl1 | 6.83 | ||
| VPb | 3.96 | MnPbVCl2 | 6.86 | ||
| Mono-Doping | NiPbVCl1 | 4.78 | MnPbVCl3 | 6.85 | |
| NiPbVCl2 | 5.16 | Co-Doping | MnNi1 | 2.56 | |
| NiPbVCl3 | 5.07 | MnNi2 | 2.84 | ||
| CoPbVCl1 | 5.63 | MnNi3 | 3.34 | ||
| CoPbVCl2 | 5.93 | MnCo1 | 3.17 | ||
| CoPbVCl3 | 5.85 | MnCo2 | 3.36 | ||
| FePbVCl1 | 6.06 | MnCo3 | 3.58 | ||
| FePbVCl2 | 6.28 | MnFe1 | 3.51 | ||
| FePbVCl3 | 6.19 | MnFe2 | 3.53 | ||
| MnFe3 | 3.69 |
| Ion | Cs+ | Cl− | Pb2+ | Mn2+ | Fe2+ | Co2+ | Ni2+ |
|---|---|---|---|---|---|---|---|
| CN | 12 | 6 | 6 | 6 | 6 | 6 | 6 |
| CR(pm) | 202 | 182 | 133 | 81 | 75 | 79 | 77 |
| Systems | Bonds | Bond Lengths | -IpCOHP |
|---|---|---|---|
| CsPbCl3 | Pb-Cl | 2.86 | 1.78 |
| Ni@CsPbCl3 | Ni-Cl | 2.52 | 3.52 |
| Co@CsPbCl3 | Co-Cl | 2.46 | 3.93 |
| Fe@CsPbCl3 | Fe-Cl | 2.45 | 4.07 |
| Mn@CsPbCl3 | Mn-Cl | 2.48 | 4.08 |
| MnNi1@CsPbCl3 | Mn-Cl | 2.30 | 4.32 |
| Ni-Cl | 3.42 | 4.43 | |
| MnCo1@CsPbCl3 | Mn-Cl | 2.32 | 4.15 |
| Co-Cl | 3.40 | 4.82 | |
| MnFe1@CsPbCl3 | Mn-Cl | 2.35 | 4.08 |
| Fe-Cl | 3.44 | 3.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Zhao, Y.; Liu, F.; Yan, Y.; Ma, Y.; Bao, H.; Wu, Z.; Cong, W.-Y.; Lu, Y.-B. Mono- and Co-Doped Mn-Doped CsPbCl3 Perovskites with Enhanced Doping Efficiency and Photoluminescent Performance. Materials 2023, 16, 5545. https://doi.org/10.3390/ma16165545
Jiang H, Zhao Y, Liu F, Yan Y, Ma Y, Bao H, Wu Z, Cong W-Y, Lu Y-B. Mono- and Co-Doped Mn-Doped CsPbCl3 Perovskites with Enhanced Doping Efficiency and Photoluminescent Performance. Materials. 2023; 16(16):5545. https://doi.org/10.3390/ma16165545
Chicago/Turabian StyleJiang, Hao, Yiting Zhao, Fangchao Liu, Yongqi Yan, Yinuo Ma, Hexin Bao, Zhongchen Wu, Wei-Yan Cong, and Ying-Bo Lu. 2023. "Mono- and Co-Doped Mn-Doped CsPbCl3 Perovskites with Enhanced Doping Efficiency and Photoluminescent Performance" Materials 16, no. 16: 5545. https://doi.org/10.3390/ma16165545
APA StyleJiang, H., Zhao, Y., Liu, F., Yan, Y., Ma, Y., Bao, H., Wu, Z., Cong, W.-Y., & Lu, Y.-B. (2023). Mono- and Co-Doped Mn-Doped CsPbCl3 Perovskites with Enhanced Doping Efficiency and Photoluminescent Performance. Materials, 16(16), 5545. https://doi.org/10.3390/ma16165545







