Sustained Delivery of the Antiviral Protein Griffithsin and Its Adhesion to a Biological Surface by a Silk Fibroin Scaffold
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Grft
2.2. Production of HIV-1 Pseudovirus
2.3. Preparation of Silk Fibroin Solution
2.4. Production of Silk-Based Discs
2.5. Morphology of Silk-Based Discs
2.6. Adhesion Tests
2.7. Measurement of Sustained Release of Grft from the SF Discs
2.8. HIV Inhibition Assays
2.9. Biocompatibility Assays
2.10. Degradation Evaluation
2.11. Statistical and Mathematical Analysis
3. Results
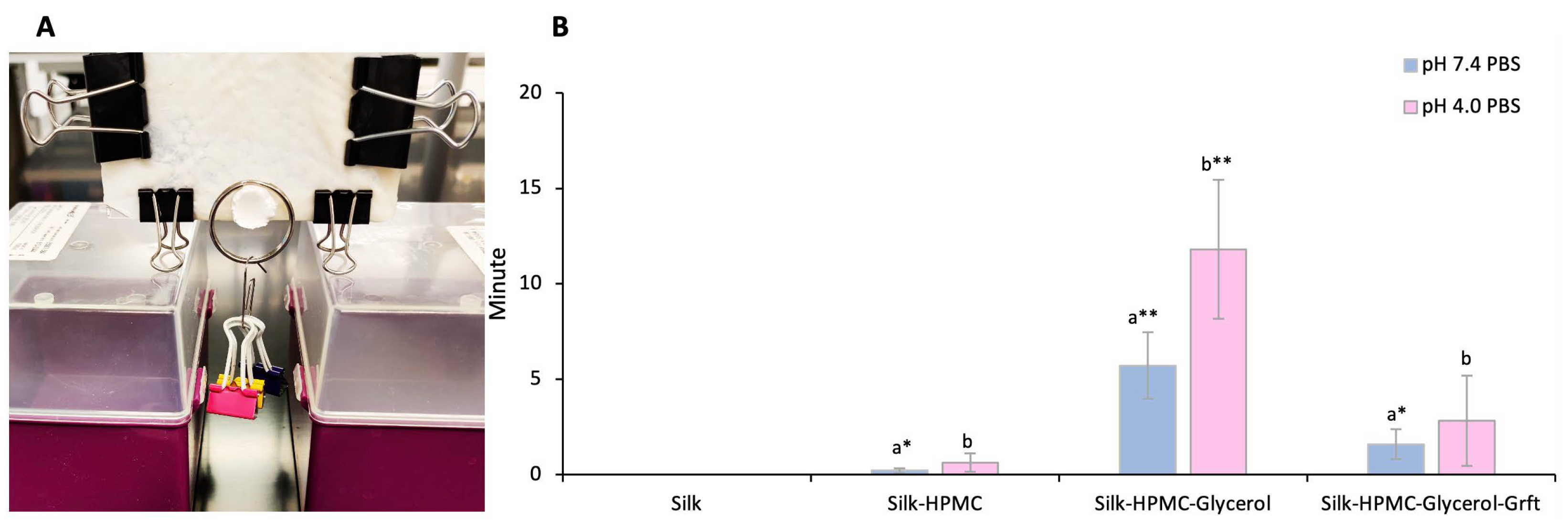
3.1. HPMC Increases the Adhesion of the Silk Discs to Sample Tissue
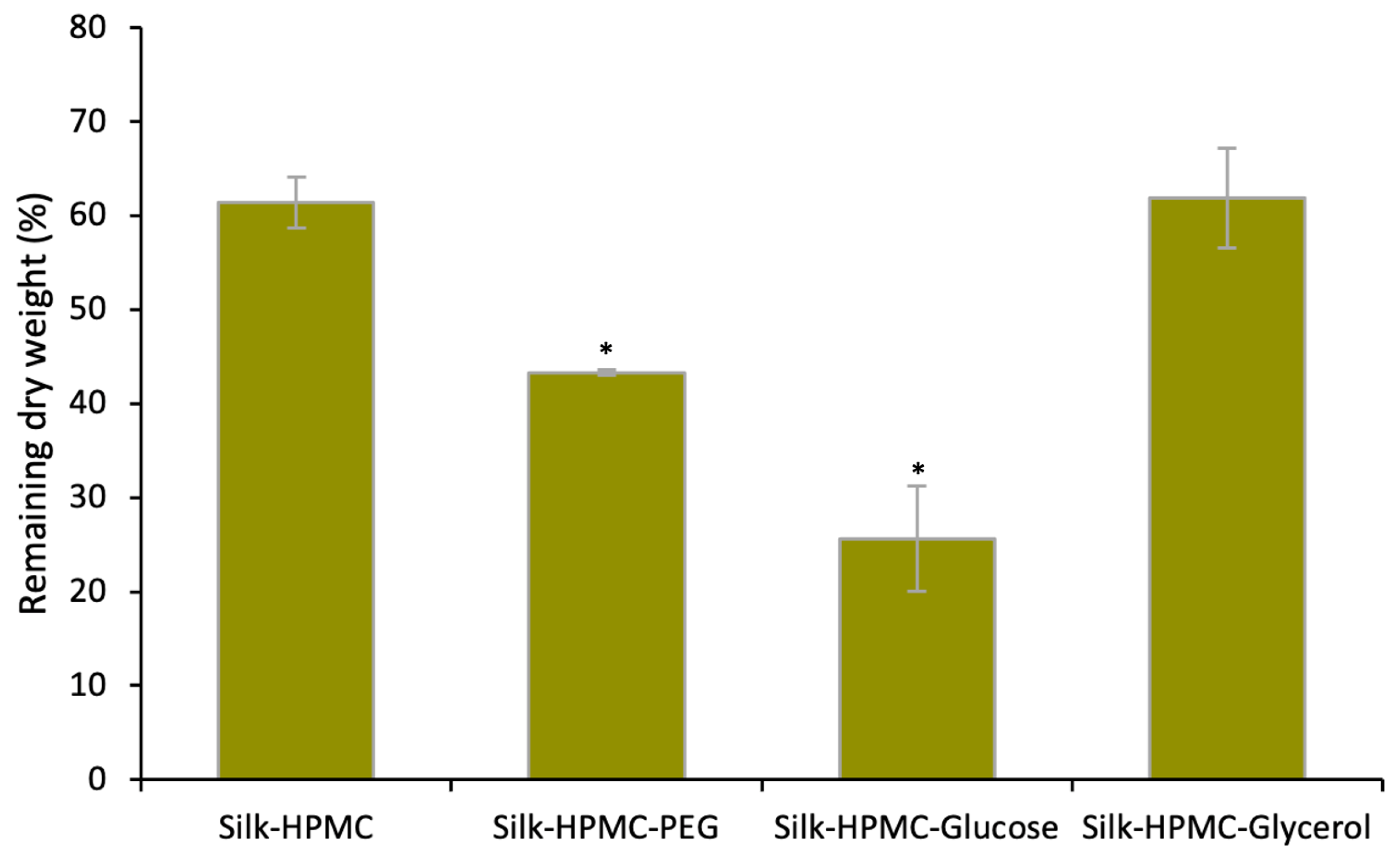
3.2. Glycerol Decreases the Disintegration of Silk–HPMC Discs
3.3. The Morphology and FTIR Spectra of the Silk Discs
3.4. Adhesion of the Four Silk-Based Discs
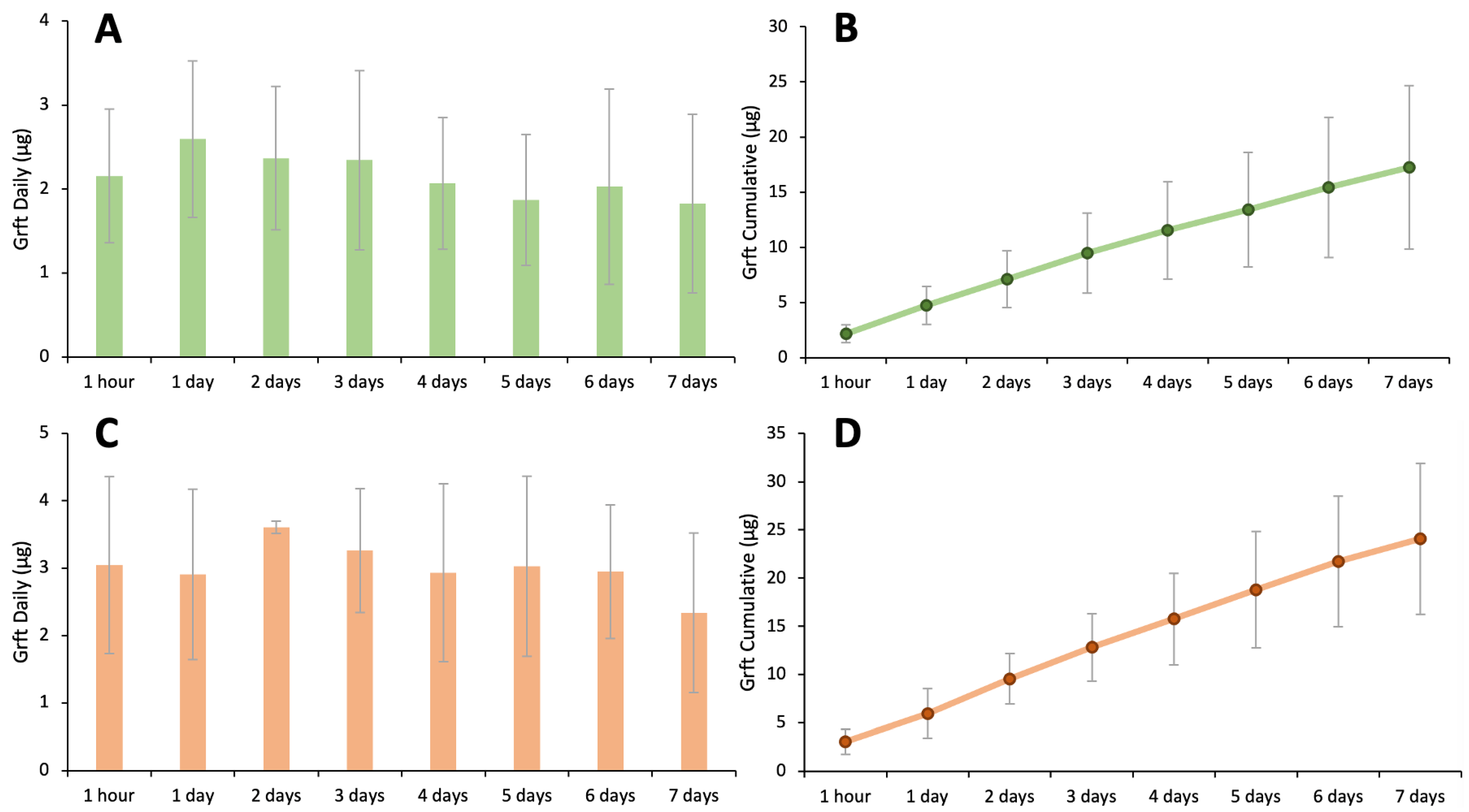
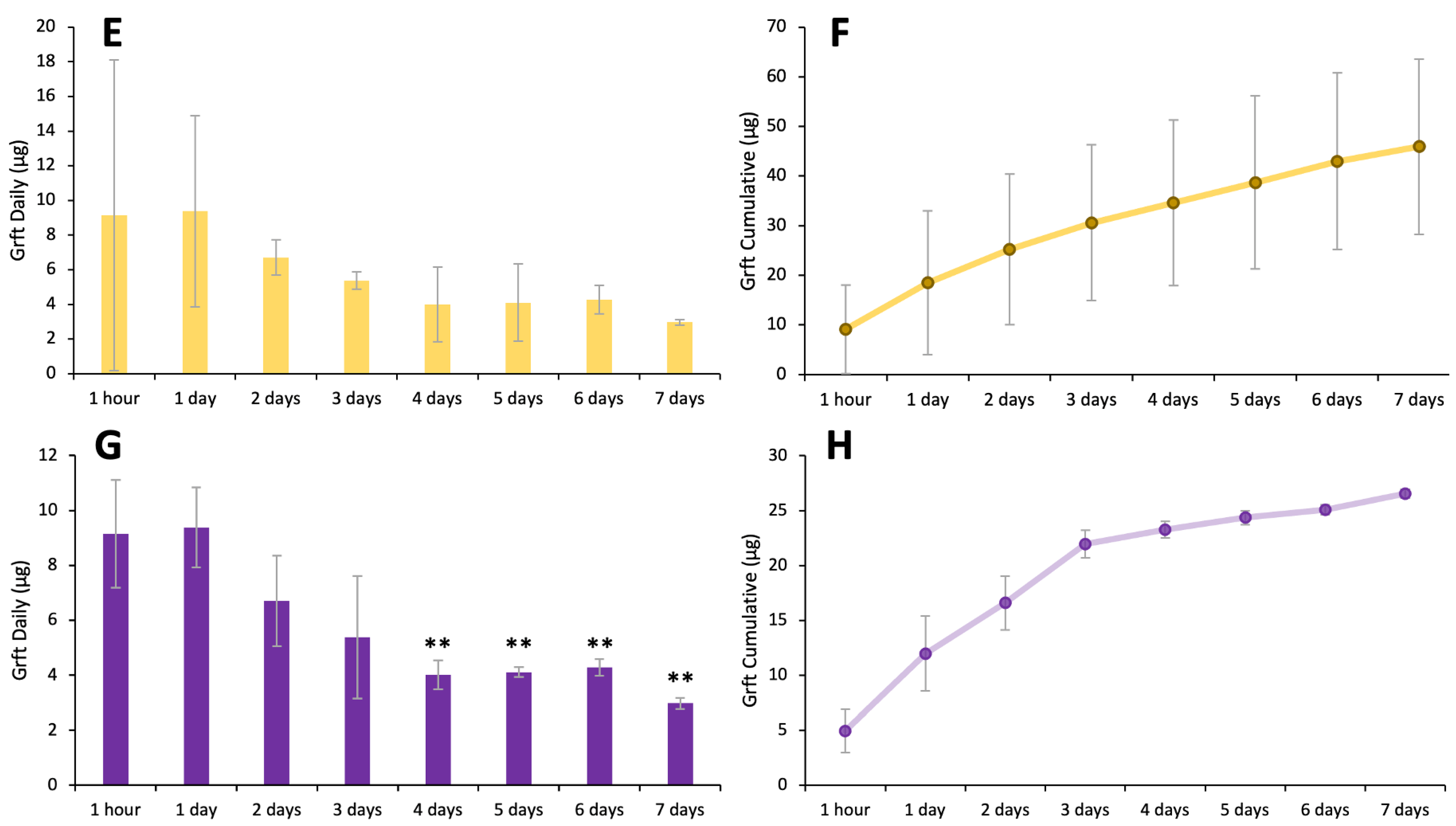
3.5. Silk–HPMC–Glycerol–Grft Discs Show Sustained Release of Griffithsin
3.6. The Griffithsin Released from the Silk–HPMC–Glycerol–Grft Discs Shows Inhibitory Activity against HIV In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- HIV. Available online: https://www.who.int/data/gho/data/themes/hiv-aids (accessed on 10 April 2023).
- Current Trends Update on Acquired Immune Deficiency Syndrome (AIDS)—United States. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00001163.htm (accessed on 10 April 2023).
- Unexplained Immunodeficiency and Opportunistic Infections in Infants—New York, New Jersey, California. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00001208.htm (accessed on 10 April 2023).
- HIV.Gov. Who Is at Risk for HIV and AIDS? Available online: https://www.hiv.gov/hiv-basics/overview/about-hiv-and-aids/who-is-at-risk-for-hiv/ (accessed on 11 April 2023).
- KFF. The Global HIV/AIDS Epidemic. Available online: https://www.kff.org/global-health-policy/fact-sheet/the-global-hivaids-epidemic/ (accessed on 12 April 2023).
- Nájera, R.; Herrera, M.I.; de Andrés, R. Human Immunodeficiency Virus and Related Retroviruses. West. J. Med. 1987, 147, 702. [Google Scholar]
- Hoorelbeke, B.; Van Montfort, T.; Xue, J.; Liwang, P.J.; Tanaka, H.; Igarashi, Y.; Van Damme, E.J.M.; Sanders, R.W.; Balzarini, J. HIV-1 Envelope Trimer Has Similar Binding Characteristics for Carbohydrate-Binding Agents as Monomeric Gp120. FEBS Lett. 2013, 587, 860–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moebius, U.; Clayton, L.K.; Abraham, S.; Harrison, S.C.; Reinherz, E.L. The Human Immunodeficiency Virus Gp120 Binding Site on CD4: Delineation by Quantitative Equilibrium and Kinetic Binding Studies of Mutants in Conjunction with a High-Resolution CD4 Atomic Structure. J. Exp. Med. 1992, 176, 507–517. [Google Scholar] [CrossRef]
- IAVI. The HIV Challenge. Available online: https://www.iavi.org/news-resources/the-hiv-challenge/the-challenge-of-developing-an-hiv-vaccine (accessed on 11 April 2023).
- Johnson & Johnson. Janssen and Global Partners to Discontinue Phase 3 Mosaico HIV Vaccine Clinical Trial. Available online: https://www.jnj.com/janssen-and-global-partners-to-discontinue-phase-3-mosaico-hiv-vaccine-clinical-trial (accessed on 11 April 2023).
- Kufel, W.D. Antibody-Based Strategies in HIV Therapy. Int. J. Antimicrob. Agents 2020, 56, 106186. [Google Scholar] [CrossRef] [PubMed]
- NIH. Protease Inhibitor-Based Regimens. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/protease-inhibitor-based-regimens (accessed on 18 June 2023).
- The Only H.I.V. Vaccine in Advanced Trials Has Failed. What Now?—The New York Times. Available online: https://www.nytimes.com/2023/01/18/health/hiv-vaccine-janssen.html (accessed on 11 April 2023).
- HIV.Gov. FDA Approves First Drug for Reducing the Risk of Sexually Acquired HIV Infection. Available online: https://www.hiv.gov/blog/fda-approves-first-drug-for-reducing-the-risk-of-sexually-acquired-hiv-infection/ (accessed on 14 April 2023).
- PrEP Effectiveness | PrEP | HIV Basics | HIV/AIDS | CDC. Available online: https://www.cdc.gov/hiv/basics/prep/prep-effectiveness.html (accessed on 10 April 2023).
- Unaids. Putting HIV Prevention among Adolescent Girls and Young Women on the Fast-Track and Engaging Men and Boys. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_HIV_prevention_among_adolescent_girls_and_young_women.pdf (accessed on 19 July 2023).
- Krakower, D.S.; Jain, S.; Mayer, K.H. Antiretrovirals for Primary HIV Prevention: The Current Status of Pre- and Post-Exposure Prophylaxis. Curr. HIV/AIDS Rep. 2015, 12, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HIV.Gov. Microbicides. Available online: https://www.hiv.gov/hiv-basics/hiv-prevention/potential-future-options/microbicides/ (accessed on 9 May 2023).
- Shattock, R.J.; Rosenberg, Z. Microbicides: Topical Prevention against HIV. Cold Spring Harb. Perspect. Med. 2012, 2, a007385. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-Based Biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Surgical Specialties, Inc. Suture Nonabsorbable—Silk 510(k) FDA Premarket Notification K930826. Available online: https://fda.report/PMN/K930826 (accessed on 18 June 2023).
- Jewell, M.; Daunch, W.; Bengtson, B.; Mortarino, E. The Development of SERI® Surgical Scaffold, an Engineered Biological Scaffold. Ann. N. Y. Acad. Sci. 2015, 1358, 44–55. [Google Scholar] [CrossRef]
- Silk Fabrics in the Management of Atopic Dermatitis. Available online: https://www.skintherapyletter.com/atopic-dermatitis/silk-fabrics/ (accessed on 19 June 2023).
- Ricci, G.; Patrizi, A.; Bellini, F.; Medri, M. Use of Textiles in Atopic Dermatitis: Care of Atopic Dermatitis. Curr. Probl. Dermatol. 2006, 33, 127–143. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.S.; Kim, D.K.; Park, C.H.; Lee, H.R. Clinical Outcomes of Silk Patch in Acute Tympanic Membrane Perforation. Clin. Exp. Otorhinolaryngol. 2015, 8, 117. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J.; et al. Silk Fibroin Biomaterial Shows Safe and Effective Wound Healing in Animal Models and a Randomized Controlled Clinical Trial. Adv. Healthc. Mater. 2017, 6, 1700121. [Google Scholar] [CrossRef]
- Yucel, T.; Lovett, M.L.; Kaplan, D.L. Silk-Based Biomaterials for Sustained Drug Delivery. J. Control. Release 2014, 190, 381–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, B.; Justin Raj, S. Therapeutic Applications and Properties of Silk Proteins from Bombyx Mori. Front. Life Sci. 2013, 6, 55–60. [Google Scholar] [CrossRef]
- Wang, Y.; Rudym, D.D.; Walsh, A.; Abrahamsen, L.; Kim, H.J.; Kim, H.S.; Kirker-Head, C.; Kaplan, D.L. In Vivo Degradation of Three-Dimensional Silk Fibroin Scaffolds. Biomaterials 2008, 29, 3415. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Hajra, S.; Jeong, H.; Panigrahi, B.K.; Pakawanit, P.; Dubal, D.; Hong, S.; Kim, H.J. Biocompatible CaTiO3-PVDF Composite-Based Piezoelectric Nanogenerator for Exercise Evaluation and Energy Harvesting. Nano Energy 2022, 102, 107682. [Google Scholar] [CrossRef]
- Bosio, V.E.; Rybner, C.; Kaplan, D. Concentric-Mineralized Hybrid Silk-Based Scaffolds for Bone Tissue Engineering in Vitro Models. J. Mater. Chem. B 2023. online ahead of print. [Google Scholar] [CrossRef]
- Bosio, V.E.; Brown, J.; Rodriguez, M.J.; Kaplan, D.L. Biodegradable Porous Silk Microtubes for Tissue Vascularization. J. Mater. Chem. B 2017, 5, 1227–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosio, V.E.; Islan, G.A.; Martínez, Y.N.; Durán, N.; Castro, G.R. Nanodevices for the Immobilization of Therapeutic Enzymes. Crit. Rev. Biotechnol. 2015, 36, 447–464. [Google Scholar] [CrossRef]
- Mu, X.; He, W.; Rivera, V.A.M.; De Alba, R.A.D.; Newman, D.J.; Zhang, Y.S. Small Tissue Chips with Big Opportunities for Space Medicine. Life Sci. Space Res. 2022, 35, 150–157. [Google Scholar] [CrossRef]
- Mu, X.; Gerhard-Herman, M.D.; Zhang, Y.S. Building Blood Vessel Chips with Enhanced Physiological Relevance. Adv. Mater. Technol. 2023, 8, 2201778. [Google Scholar] [CrossRef]
- Liu, J.; Xie, X.; Wang, T.; Chen, H.; Fu, Y.; Cheng, X.; Wu, J.; Li, G.; Liu, C.; Liimatainen, H.; et al. Promotion of Wound Healing Using Nanoporous Silk Fibroin Sponges. ACS Appl. Mater. Interfaces 2023, 15, 12696–12707. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J.; Zhao, Z.; Liu, Y.; Tam, W.C.; Zheng, Z.; Wang, X.; Li, Y.; Liu, Z.; Li, Y.; et al. A Negative-Response Strain Sensor towards Wearable Microclimate Changes for Body Area Sensing Networks. Chem. Eng. J. 2023, 459, 141628. [Google Scholar] [CrossRef]
- Zhang, L.; Herrera, C.; Coburn, J.; Olejniczak, N.; Ziprin, P.; Kaplan, D.L.; Liwang, P.J. Stabilization and Sustained Release of HIV Inhibitors by Encapsulation in Silk Fibroin Disks. ACS Biomater. Sci. Eng. 2017, 3, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, B.; Morgan, J.L.; Herrera, C.; Harrington, K.; Perez-Ramirez, B.; Liwang, P.J.; Kaplan, D.L. Sustained Release Silk Fibroin Discs: Antibody and Protein Delivery for HIV Prevention Corresponding Authors: HHS Public Access. J. Control. Release 2019, 301, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Florczak, A.; Grzechowiak, I.; Deptuch, T.; Kucharczyk, K.; Kaminska, A.; Dams-Kozlowska, H. Silk Particles as Carriers of Therapeutic Molecules for Cancer Treatment. Materials 2020, 13, 4946. [Google Scholar] [CrossRef]
- Yavuz, B.; Chambre, L.; Harrington, K.; Kluge, J.; Valenti, L.; Kaplan, D.L. Silk Fibroin Microneedle Patches for the Sustained Release of Levonorgestrel. ACS Appl. Bio Mater. 2020, 3, 5375–5382. [Google Scholar] [CrossRef]
- Fischer, K.; Nguyen, K.; LiWang, P.J. Griffithsin Retains Anti-HIV-1 Potency with Changes in Gp120 Glycosylation and Complements Broadly Neutralizing Antibodies PGT121 and PGT126. Antimicrob. Agents Chemother. 2020, 64, e01084-19. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Gao, Y.; Hoorelbeke, B.; Kagiampakis, I.; Zhao, B.; Demeler, B.; Balzarini, J.; LiWang, P.J. The Role of Individual Carbohydrate-Binding Sites in the Function of the Potent Anti-HIV Lectin Griffithsin. Mol. Pharm. 2012, 9, 2613–2625. [Google Scholar] [CrossRef]
- Xue, J.; Hoorelbeke, B.; Kagiampakis, I.; Demeler, B.; Balzarini, J.; LiWang, P.J. The Griffithsin Dimer Is Required for High-Potency Inhibition of HIV-1: Evidence for Manipulation of the Structure of Gp120 as Part of the Griffithsin Dimer Mechanism. Antimicrob. Agents Chemother. 2013, 57, 3979–3989. [Google Scholar] [CrossRef] [Green Version]
- O’Keefe, B.R.; Giomarelli, B.; Barnard, D.L.; Shenoy, S.R.; Chan, P.K.S.; McMahon, J.B.; Palmer, K.E.; Barnett, B.W.; Meyerholz, D.K.; Wohlford-Lenane, C.L.; et al. Broad-Spectrum In Vitro Activity and In Vivo Efficacy of the Antiviral Protein Griffithsin against Emerging Viruses of the Family Coronaviridae. J. Virol. 2010, 84, 2511. [Google Scholar] [CrossRef] [Green Version]
- Alsaidi, S.; Cornejal, N.; Mahoney, O.; Melo, C.; Verma, N.; Bonnaire, T.; Chang, T.; O’keefe, B.R.; Sailer, J.; Zydowsky, T.M.; et al. Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model. Mar. Drugs 2021, 19, 418. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, P.; Albecka, A.; Belouzard, S.; Vercauteren, K.; Verhoye, L.; Wychowski, C.; Leroux-Roels, G.; Palmer, K.E.; Dubuisson, J. Griffithsin Has Antiviral Activity against Hepatitis C Virus. Antimicrob. Agents Chemother. 2011, 55, 5159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derby, N.; Lal, M.; Aravantinou, M.; Kizima, L.; Barnable, P.; Rodriguez, A.; Lai, M.; Wesenberg, A.; Ugaonkar, S.; Levendosky, K.; et al. Griffithsin Carrageenan Fast Dissolving Inserts Prevent SHIV HSV-2 and HPV Infections in Vivo. Nat. Commun. 2018, 9, 3881. [Google Scholar] [CrossRef] [Green Version]
- Ishag, H.Z.A.; Li, C.; Huang, L.; Sun, M.x.; Wang, F.; Ni, B.; Malik, T.; Chen, P.y; Mao, X. Griffithsin Inhibits Japanese Encephalitis Virus Infection in Vitro and in Vivo. Arch. Virol. 2013, 158, 349. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, N.; Cai, Y.; Zhang, H.; Li, J.; Liu, J.; Ye, C.; Wang, Y.; Dang, Y.; Li, W.; et al. An Algal Lectin Griffithsin Inhibits Hantaan Virus Infection in Vitro and in Vivo. Front. Cell Infect. Microbiol. 2022, 12, 1813. [Google Scholar] [CrossRef] [PubMed]
- Crakes, K.R.; Herrera, C.; Morgan, J.L.; Olstad, K.; Hessell, A.J.; Ziprin, P.; LiWang, P.J.; Dandekar, S. Efficacy of Silk Fibroin Biomaterial Vehicle for in Vivo Mucosal Delivery of Griffithsin and Protection against HIV and SHIV Infection Ex Vivo. J. Int. AIDS Soc. 2020, 23, e25628. [Google Scholar] [CrossRef]
- Zhao, B.; Mankowski, M.K.; Snyder, B.A.; Ptak, R.G.; LiWang, P.J. Highly Potent Chimeric Inhibitors Targeting Two Steps of HIV Cell Entry. J. Biol. Chem. 2011, 286, 28370. [Google Scholar] [CrossRef] [Green Version]
- Kagiampakis, I.; Gharibi, A.; Mankowski, M.K.; Snyder, B.A.; Ptak, R.G.; Alatas, K.; LiWang, P.J. Potent Strategy To Inhibit HIV-1 by Binding Both Gp120 and Gp41. Antimicrob. Agents Chemother. 2011, 55, 264. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Gao, F.; Mascola, J.R.; Stamatatos, L.; Polonis, V.R.; Koutsoukos, M.; Voss, G.; Goepfert, P.; Gilbert, P.; Greene, K.M.; et al. Human Immunodeficiency Virus Type 1 Env Clones from Acute and Early Subtype B Infections for Standardized Assessments of Vaccine-Elicited Neutralizing Antibodies. J. Virol. 2005, 79, 10108–10125. [Google Scholar] [CrossRef] [Green Version]
- Naldini, L.; Blomer, U.; Gage, F.H.; Trono, D.; Verma, I.M. Efficient Transfer, Integration, and Sustained Long-Term Expression of the Transgene in Adult Rat Brains Injected with a Lentiviral Vector. Proc. Natl. Acad. Sci. USA 1996, 93, 11382–11388. [Google Scholar] [CrossRef]
- Harrison, T.; Graham, F.; Williams, J. Host-Range Mutants of Adenovirus Type 5 Defective for Growth in HeLa Cells. Virology 1977, 77, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Graham, F.L.; Smiley, J.; Russell, W.C.; Nairn, R. Characteristics of a Human Cell Line Transformed by DNA from Human Adenovirus Type 5. J. Gen. Virol. 1977, 36, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Platt, E.J.; Wehrly, K.; Kuhmann, S.E.; Chesebro, B.; Kabat, D. Effects of CCR5 and CD4 Cell Surface Concentrations on Infections by Macrophagetropic Isolates of Human Immunodeficiency Virus Type 1. J. Virol. 1998, 72, 2855–2864. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials Fabrication from Bombyx Mori Silk Fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The Immunology of the Porcine Skin and Its Value as a Model for Human Skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Khiao In, M.; Richardson, K.C.; Loewa, A.; Hedtrich, S.; Kaessmeyer, S.; Plendl, J. Histological and Functional Comparisons of Four Anatomical Regions of Porcine Skin with Human Abdominal Skin. Anat. Histol. Embryol. 2019, 48, 207–217. [Google Scholar] [CrossRef]
- Zhou, X.; Ding, S.; Wang, D.; Chen, L.; Feng, K.; Huang, T.; Li, Z.; Cai, Y. Identification of Cell Markers and Their Expression Patterns in Skin Based on Single-Cell RNA-Sequencing Profiles. Life 2022, 12, 550. [Google Scholar] [CrossRef]
- Lucas, A.M.; Stettenheim, P.R.; Project, A.A. Avian Anatomy Integument Part I; USGPO: Washington, DC, USA, 1972.
- Panico, A.; Paladini, F.; Pollini, M. Development of Regenerative and Flexible Fibroin-Based Wound Dressings. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.E.; Davidowski, S.K.; Xu, D.; Cebe, P.; Onofrei, D.; Holland, G.P.; Kaplan, D.L. Thermal and Structural Properties of Silk Biomaterials Plasticized by Glycerol. Biomacromolecules 2016, 17, 3911–3921. [Google Scholar] [CrossRef]
- Lawrence, B.D.; Omenetto, F.; Chui, K.; Kaplan, D.L. Processing Methods to Control Silk Fibroin Film Biomaterial Features. J. Mater. Sci. 2008, 43, 6967–6985. [Google Scholar] [CrossRef]
- FDA. FDA Approves First Injectable Treatment for HIV Pre-Exposure Prevention. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-injectable-treatment-hiv-pre-exposure-prevention (accessed on 23 June 2023).
- FDA. Vaginal Microbicides:Development for the Prevention of HIV Infection PDF. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/vaginal-microbicidesdevelopment-prevention-hiv-infection-pdf (accessed on 20 June 2023).
- Department of Epidemiology. Study Suggests Dapivirine Vaginal Ring Is Safe to Use as HIV Prevention during Breastfeeding. Available online: https://epi.washington.edu/news/study-suggests-dapivirine-vaginal-ring-is-safe-to-use-as-hiv-prevention-during-breastfeeding/ (accessed on 23 June 2023).
- Baeten, J.M.; Palanee-Phillips, T.; Brown, E.R.; Schwartz, K.; Soto-Torres, L.E.; Govender, V.; Mgodi, N.M.; Matovu Kiweewa, F.; Nair, G.; Mhlanga, F.; et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N. Engl. J. Med. 2016, 375, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Karim, Q.A.; Karim, S.S.A.; Frohlich, J.A.; Grobler, A.C.; Baxter, C.; Mansoor, L.E.; Kharsany, A.B.M.; Sibeko, S.; Mlisana, K.P.; Omar, Z.; et al. Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women. Science 2010, 329, 1168–1174. [Google Scholar] [CrossRef] [Green Version]
- Marrazzo, J.M.; Ramjee, G.; Richardson, B.A.; Gomez, K.; Mgodi, N.; Nair, G.; Palanee, T.; Nakabiito, C.; van der Straten, A.; Noguchi, L.; et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. N. Engl. J. Med. 2015, 372, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delany-Moretlwe, S.; Lombard, C.; Baron, D.; Bekker, L.G.; Nkala, B.; Ahmed, K.; Sebe, M.; Brumskine, W.; Nchabeleng, M.; Palanee-Philips, T.; et al. Tenofovir 1% Vaginal Gel for Prevention of HIV-1 Infection in Women in South Africa (FACTS-001): A Phase 3, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Infect. Dis. 2018, 18, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Mayo, A.J.; Browne, E.N.; Montgomery, E.T.; Torjesen, K.; Palanee-Phillips, T.; Jeenarain, N.; Seyama, L.; Woeber, K.; Harkoo, I.; Reddy, K.; et al. Acceptability of the Dapivirine Vaginal Ring for HIV-1 Prevention and Association with Adherence in a Phase III Trial. AIDS Behav. 2021, 25, 2430–2440. [Google Scholar] [CrossRef]
- Naidoo, K.; Montgomery Elizabeth, T.; Katz, A.W.; Morgan, G.; Krishnaveni, R.; Lydia, S.T.; Sarita, N.; Leila, M.E. Efficacy and Action of the Dapivirine Vaginal Ring as Understood by Women Participating in an Open Label Extension Study. AIDS Behav. 2023, 27, 75–81. [Google Scholar] [CrossRef]
- UNAIDS. Full Report—In Danger: UNAIDS Global AIDS Update 2022. Available online: https://www.unaids.org/en/resources/documents/2022/in-danger-global-aids-update (accessed on 12 April 2023).
- Crakes, K.R.; Santos Rocha, C.; Grishina, I.; Hirao, L.A.; Napoli, E.; Gaulke, C.A.; Fenton, A.; Datta, S.; Arredondo, J.; Marco, M.L.; et al. PPARα-Targeted Mitochondrial Bioenergetics Mediate Repair of Intestinal Barriers at the Host-Microbe Intersection during SIV Infection. Proc. Natl. Acad. Sci. USA 2019, 16, 24819–24829. [Google Scholar] [CrossRef] [Green Version]
- Lagakos, S.W.; Gable, A.R. Methodological Challenges in Biomedical HIV Prevention Trials; National Academies Press: Washington, DC, USA, 2008; pp. 1–258. [Google Scholar] [CrossRef]
- Saag, M.S. Preventing HIV in Women—Still Trying to Find Their VOICE. N. Engl. J. Med. 2015, 372, 564–566. [Google Scholar] [CrossRef] [Green Version]
- Coburn, J.M.; Na, E.; Kaplan, D.L. Modulation of Vincristine and Doxorubicin Binding and Release from Silk Films. J. Control. Release 2015, 220, 229–238. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.Gov. Griffithsin-Based Rectal Microbicide for PREvention of Viral ENTry (PREVENT)—Full Text View. Available online: https://clinicaltrials.gov/ct2/show/NCT04032717 (accessed on 4 June 2023).
- Lusvarghi, S.; Bewley, C.A. Griffithsin: An Antiviral Lectin with Outstanding Therapeutic Potential. Viruses 2016, 8, 296. [Google Scholar] [CrossRef] [Green Version]
- Kouokam, J.C.; Huskens, D.; Schols, D.; Johannemann, A.; Riedell, S.K.; Walter, W.; Walker, J.M.; Matoba, N.; O’Keefe, B.R.; Palmer, K.E. Investigation of Griffithsin’s Interactions with Human Cells Confirms Its Outstanding Safety and Efficacy Profile as a Microbicide Candidate. PLoS ONE 2011, 6, e22635. [Google Scholar] [CrossRef] [Green Version]
- Tyo, K.M.; Lasnik, A.B.; Zhang, L.; Mahmoud, M.; Jenson, A.B.; Fuqua, J.L.; Palmer, K.E.; Steinbach-Rankins, J.M. Sustained-Release Griffithsin Nanoparticle-Fiber Composites against HIV-1 and HSV-2 Infections. J. Control. Release 2020, 321, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Barton, C.; Kouokam, J.C.; Lasnik, A.B.; Foreman, O.; Cambon, A.; Brock, G.; Montefiori, D.C.; Vojdani, F.; McCormick, A.A.; O’Keefe, B.R.; et al. Activity of and Effect of Subcutaneous Treatment with the Broad-Spectrum Antiviral Lectin Griffithsin in Two Laboratory Rodent Models. Antimicrob. Agents Chemother. 2014, 58, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyo, K.M.; Lasnik, A.B.; Zhang, L.; Jenson, A.B.; Fuqua, J.L.; Palmer, K.E.; Steinbach-Rankins, J.M. Rapid-Release Griffithsin Fibers for Dual Prevention of HSV-2 and HIV-1 Infections. Antimicrob. Agents Chemother. 2020, 64, 10–128. [Google Scholar] [CrossRef]
- Minooei, F.; Fried, J.R.; Fuqua, J.L.; Palmer, K.E.; Steinbach-Rankins, J.M. In Vitro Study on Synergistic Interactions Between Free and Encapsulated Q-Griffithsin and Antiretrovirals Against HIV-1 Infection. Int. J. Nanomed. 2021, 16, 1189–1206. [Google Scholar] [CrossRef] [PubMed]
- Teleshova, N.; Keller, M.J.; Romero, J.A.F.; Friedland, B.A.; Creasy, G.W.; Plagianos, M.G.; Ray, L.; Barnable, P.; Kizima, L.; Rodriguez, A.; et al. Results of a Phase 1, Randomized, Placebo-Controlled First-in-Human Trial of Griffithsin Formulated in a Carrageenan Vaginal Gel. PLoS ONE 2022, 17, e0261775. [Google Scholar] [CrossRef]
- Franzén Boger, M.; Benhach, N.; Hasselrot, T.; Brand, R.M.; Rohan, L.C.; Wang, L.; McGowan, I.; Edick, S.; Ho, K.; Meyn, L.; et al. A Topical Rectal Douche Product Containing Q-Griffithsin Does Not Disrupt the Epithelial Border or Alter CD4+ Cell Distribution in the Human Rectal Mucosa. Sci. Rep. 2023, 13, 7547. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, W.; Gu, C.; Cai, X.; Qu, D.; Lu, L.; Xie, Y.; Jiang, S. Griffithsin with A Broad-Spectrum Antiviral Activity by Binding Glycans in Viral Glycoprotein Exhibits Strong Synergistic Effect in Combination with A Pan-Coronavirus Fusion Inhibitor Targeting SARS-CoV-2 Spike S2 Subunit. Virol. Sin. 2020, 35, 857. [Google Scholar] [CrossRef] [PubMed]
- Ahan, R.E.; Hanifehnezhad, A.; Kehribar, E.; Oguzoglu, T.C.; Földes, K.; Özçelik, C.E.; Filazi, N.; Öztop, S.; Palaz, F.; Önder, S.; et al. A Highly Potent SARS-CoV-2 Blocking Lectin Protein. ACS Infect. Dis. 2022, 8, 1253–1264. [Google Scholar] [CrossRef]
- Phase 1 Clinical Trials of a Q-Griffithsin Nasal Spray for SARS-CoV-2 Prophylaxis—CROI Conference. Available online: https://www.croiconference.org/abstract/phase-1-clinical-trials-of-a-q-griffithsin-nasal-spray-for-sars-cov-2-prophylaxis/ (accessed on 8 June 2023).
- Gao, X.; Dai, Q.; Yao, L.; Dong, H.; Li, Q.; Cao, X. A Medical Adhesive Used in a Wet Environment by Blending Tannic Acid and Silk Fibroin. Biomater. Sci. 2020, 8, 2694–2701. [Google Scholar] [CrossRef]
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm (accessed on 7 June 2023).
- Li, Y.; Liao, M.; Zhou, J. Catechol-Cation Adhesion on Silica Surfaces: Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2017, 19, 29222–29231. [Google Scholar] [CrossRef] [PubMed]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine. Evid. Based Complement. Alternat Med. 2015, 2015, 593902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abruzzo, A.; Vitali, B.; Lombardi, F.; Guerrini, L.; Cinque, B.; Parolin, C.; Bigucci, F.; Cerchiara, T.; Arbizzani, C.; Gallucci, M.C.; et al. Mucoadhesive Buccal Films for Local Delivery of Lactobacillus Brevis. Pharmaceutics 2020, 12, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Fang, Y.; Xu, L.; Ahmed, A. High-Throughput Fabrication of Silk Fibroin/Hydroxypropyl Methylcellulose (SF/HPMC) Nanofibrous Scaffolds for Skin Tissue Engineering. Int. J. Biol. Macromol. 2021, 183, 1210–1221. [Google Scholar] [CrossRef]
- Umuhoza, D.; Yang, F.; Long, D.; Hao, Z.; Dai, J.; Zhao, A. Strategies for Tuning the Biodegradation of Silk Fibroin-Based Materials for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2020, 6, 1290–1310. [Google Scholar] [CrossRef]
- Bory, C.; Boulieu, R.; Souillet, G.; Chantin, C.; Guibaud, P.; Hershfield, M.S. Effect of Polyethylene Glycol-Modified Adenosine Deaminase (PEG-ADA) Therapy in Two ADA-Deficient Children: Measurement of Erythrocyte Deoxyadenosine Triphosphate as a Useful Tool. Adv. Exp. Med. Biol. 1991, 309A, 173–176. [Google Scholar] [CrossRef]
- Verbeken, G.; Verween, G.; De Vos, D.; Pascual, B.; De Corte, P.; Richters, C.; De Coninck, A.; Roseeuw, D.; Ectors, N.; Rose, T.; et al. Glycerol Treatment as Recovery Procedure for Cryopreserved Human Skin Allografts Positive for Bacteria and Fungi. Cell Tissue Bank 2012, 13, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Marshall, L.; Ghosh, M.M.; Boyce, S.G.; MacNeil, S.; Freedlander, E.; Kudesia, G. Effect of Glycerol on Intracellular Virus Survival: Implications for the Clinical Use of Glycerol-Preserved Cadaver Skin. Burns 1995, 21, 356–361. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, W.; Zhang, N.; Bains, A.; Martinez, A.; LiWang, P.J. Sustained Delivery of the Antiviral Protein Griffithsin and Its Adhesion to a Biological Surface by a Silk Fibroin Scaffold. Materials 2023, 16, 5547. https://doi.org/10.3390/ma16165547
Guan W, Zhang N, Bains A, Martinez A, LiWang PJ. Sustained Delivery of the Antiviral Protein Griffithsin and Its Adhesion to a Biological Surface by a Silk Fibroin Scaffold. Materials. 2023; 16(16):5547. https://doi.org/10.3390/ma16165547
Chicago/Turabian StyleGuan, Wenyan, Ning Zhang, Arjan Bains, Airam Martinez, and Patricia J. LiWang. 2023. "Sustained Delivery of the Antiviral Protein Griffithsin and Its Adhesion to a Biological Surface by a Silk Fibroin Scaffold" Materials 16, no. 16: 5547. https://doi.org/10.3390/ma16165547
APA StyleGuan, W., Zhang, N., Bains, A., Martinez, A., & LiWang, P. J. (2023). Sustained Delivery of the Antiviral Protein Griffithsin and Its Adhesion to a Biological Surface by a Silk Fibroin Scaffold. Materials, 16(16), 5547. https://doi.org/10.3390/ma16165547






