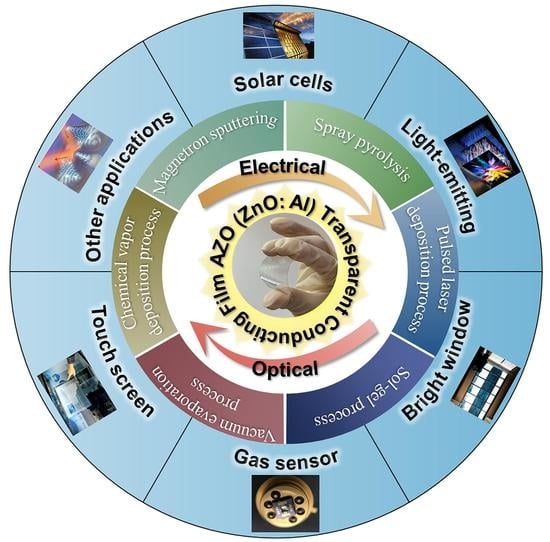
Progress in the Synthesis and Application of Transparent Conducting Film of AZO (ZnO:Al)
Abstract
:1. Introduction
2. Structure and Properties of AZO Thin Films
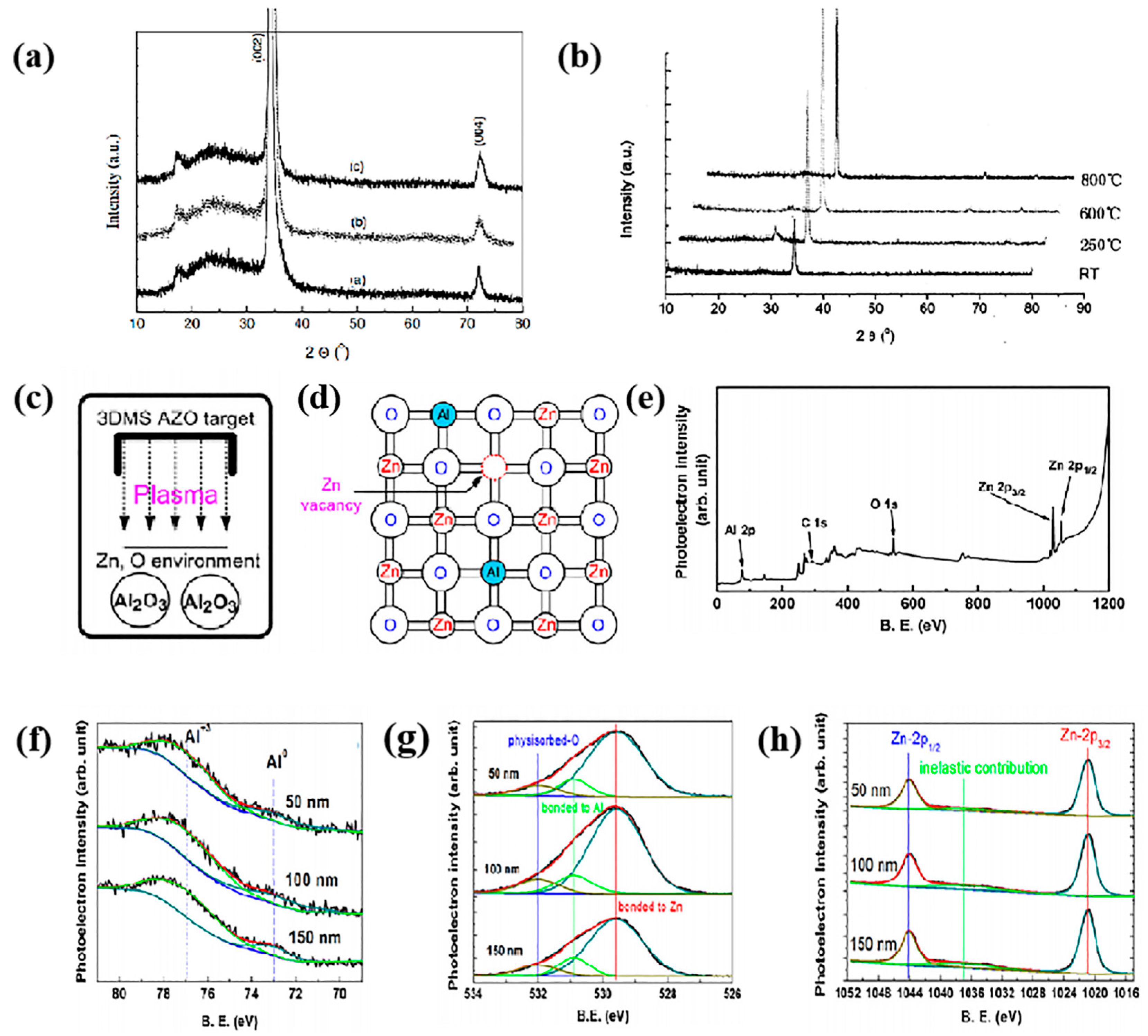
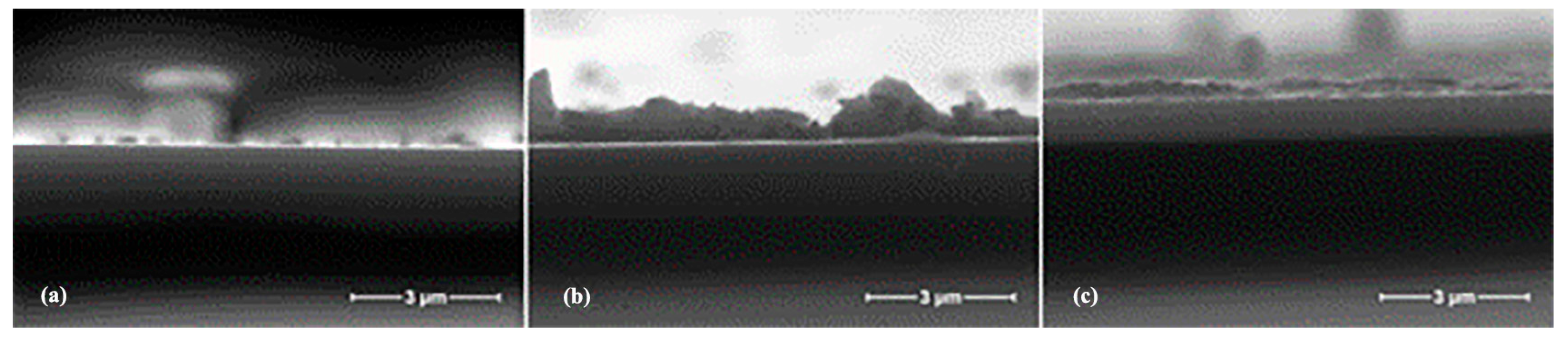
2.1. Structure of AZO Thin Films
2.2. Electrical Properties of AZO Thin Films
2.3. Optical Properties of AZO Thin Films
3. Preparation of AZO Thin Films
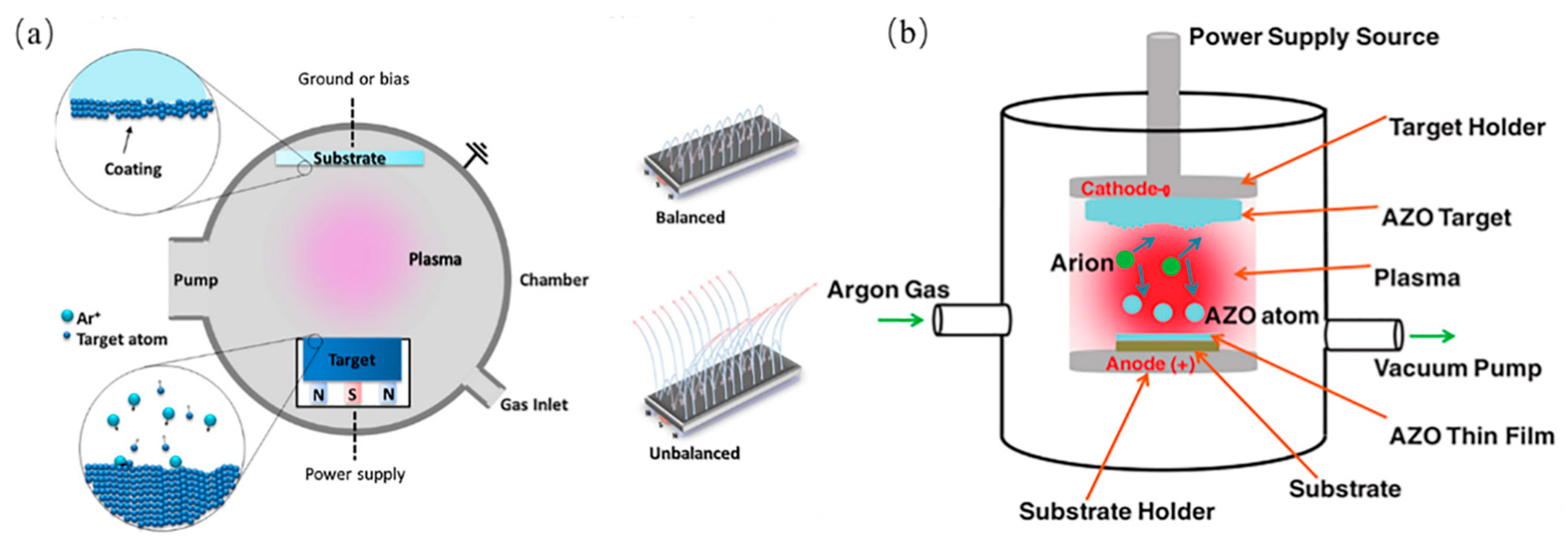
3.1. Magnetron Sputtering
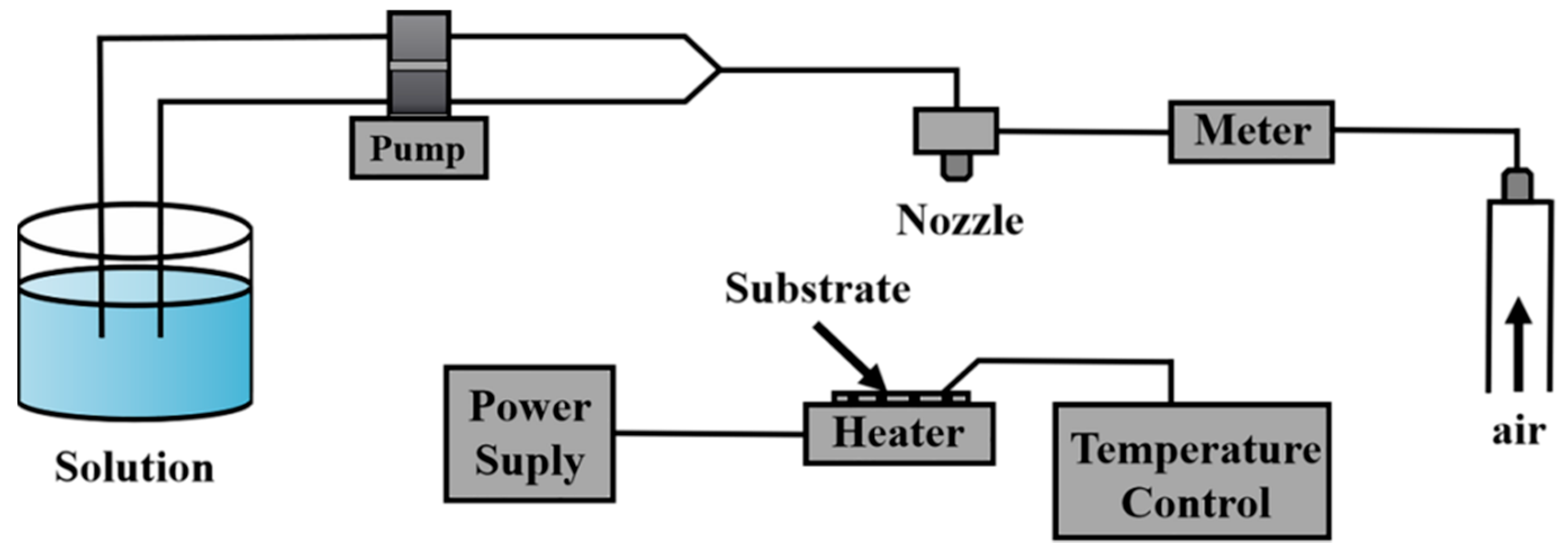
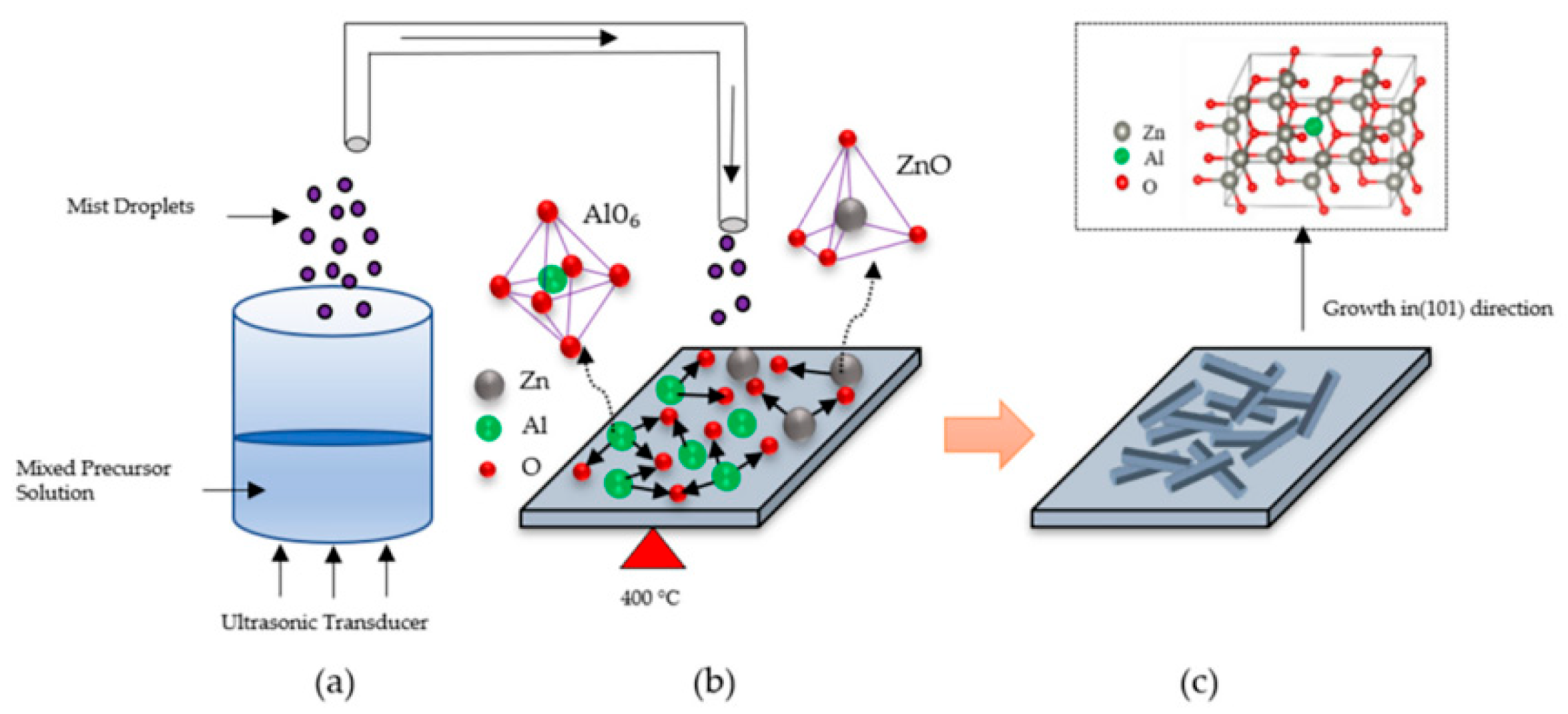
3.2. Spray Pyrolysis
3.3. Pulsed Laser Deposition Process
3.4. Sol–Gel Process
3.5. Vacuum Evaporation
3.6. Atomic Layer Deposition
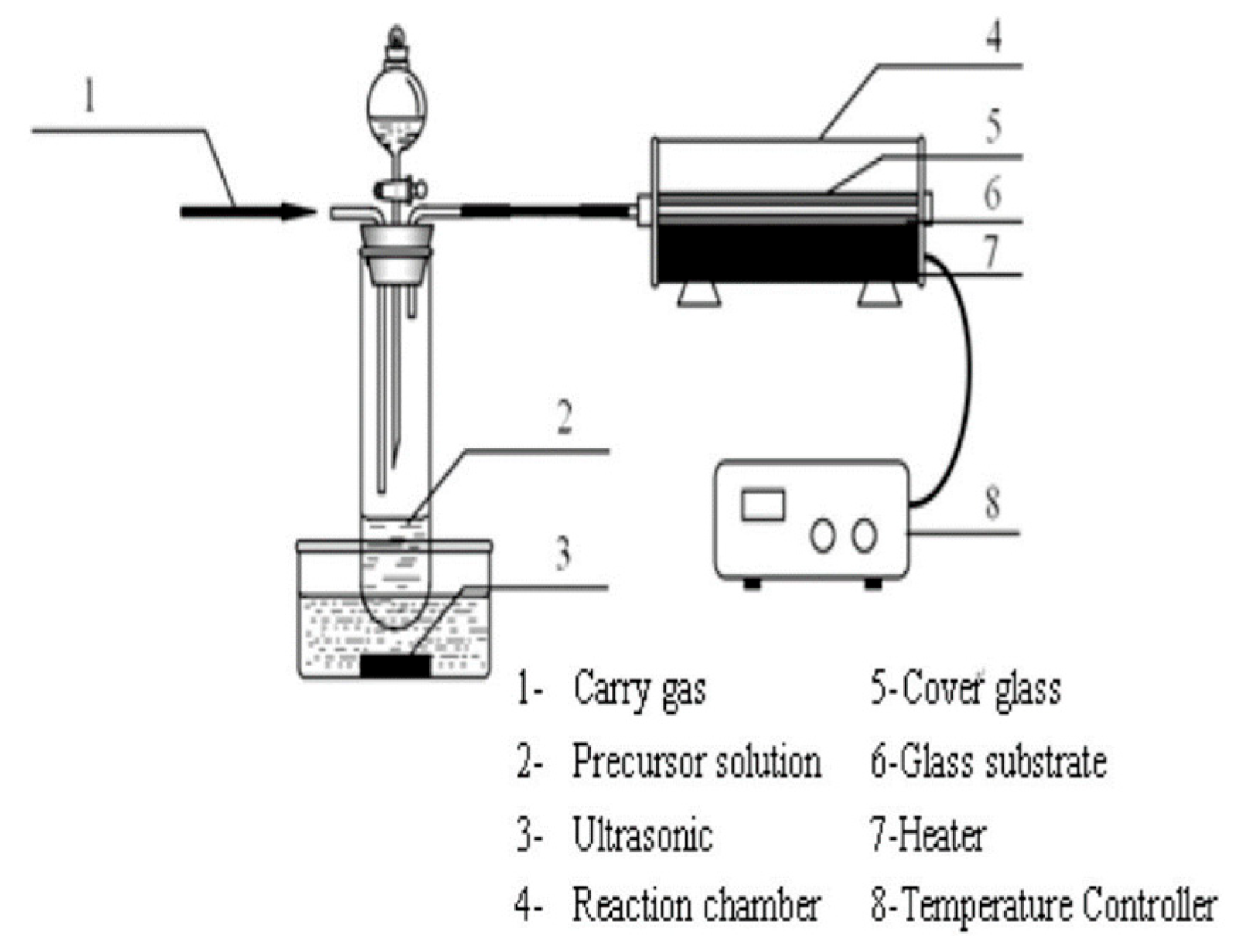
3.7. Chemical Vapor Deposition Process
4. Applications of AZO Thin Films
4.1. Application of AZO Transparent Conductive Films in Solar Cells
4.2. Application of AZO Transparent Conductive Films in Light-Emitting Diodes (LEDs)
4.3. Application of AZO Transparent Conductive Film in Bright Windows
4.4. Application of AZO Transparent Conductive Film in Gas Sensor
4.5. Application of AZO Transparent Conductive Film in Touch Screens
4.6. Other Applications of AZO Films
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Preeti; Kumar, S. Extraction and analysis of TCO coated glass from waste amorphous silicon thin film solar module. Sol. Energy Mater. Sol. Cells 2023, 253, 112227. [Google Scholar] [CrossRef]
- Kwok, C.K.G.; Liu, C.; Yu, K.M. Amorphous CdO-In2O3 alloy thin films with high conductivity and transparency synthesized by sol-gel method. J. Alloys Compd. 2022, 893, 162341. [Google Scholar] [CrossRef]
- Charoenphon, S.; Tubtimtae, A.; Watanabe, I.; Jungthawan, S.; T-Thienprasert, J.; Boonchun, A.; Reunchan, P. The role of native point defects and donor impurities in the electrical properties of ZnSb2O4: A hybrid density-functional study. Phys. Chem. Chem. Phys. 2023, 25, 19116–19125. [Google Scholar] [PubMed]
- Zhao, X.; Shen, H.; Zhou, C.; Lin, S.; Li, X.; Zhao, X.; Deng, X.; Li, J.; Lin, H. Preparation of aluminum doped zinc oxide films with low resistivity and outstanding transparency by a sol–gel method for potential applications in perovskite solar cell. Thin Solid Films 2016, 605, 208. [Google Scholar]
- Lee, Y.-C.; Chen, Y.-C.; Lin, H.-T.; Xu, L.; Lee, M.-T.; Juang, J.-Y. Maskless deposition of patterned transparent conductive oxides via laser-assisted atmospheric pressure plasma jet. J. Alloys Compd. 2023, 931, 167607. [Google Scholar] [CrossRef]
- Nunes, P.; Fortunato, E.; Martins, R. Influence of the post-treatment on the properties of ZnO thin films. Thin Solid Films 2001, 383, 277. [Google Scholar] [CrossRef]
- Nitta, A.; Uwatoko, Y.; Nagata, T.; Yoshimura, Y. Improvement of electrical characteristics of flexible AZO/Ag/Cu/AZO transparent conductive films by Cu deposition. J. Vac. Sci. Technol. B 2023, 41, 022205. [Google Scholar] [CrossRef]
- Chang, J.S.; Strunk, J.; Chong, M.N.; Poh, P.E.; Ocon, J.D. Multi-dimensional zinc oxide (ZnO) nanoarchitectures as efficient photocatalysts: What is the fundamental factor that determines photoactivity in ZnO? J. Hazard. Mater. 2020, 381, 120958. [Google Scholar]
- Cruz, D.M.; Mostafavi, E.; Vernet-Crua, A.; Barabadi, H.; Shah, V.; Cholula-Díaz, J.L.; Guisbiers, G.; Webster, T.J. Green nanotechnology-based zinc oxide (ZnO) nanomaterials for biomedical applications: A review. J. Phys. Mater. 2020, 3, 034005. [Google Scholar]
- Lanje, A.S.; Sharma, S.J.; Ningthoujam, R.S.; Ahn, J.-S.; Pode, R.B. Low temperature dielectric studies of zinc oxide (ZnO) nanoparticles prepared by precipitation method. Adv. Powder Technol. 2013, 24, 331. [Google Scholar] [CrossRef]
- Murdoch, G.B.; Hinds, S.; Sargent, E.H.; Tsang, S.W.; Mordoukhovski, L.; Lu, Z.H. Aluminum doped zinc oxide for organic photovoltaics. Appl. Phys. Lett. 2009, 94, 213301. [Google Scholar] [CrossRef]
- Chi, C.-Y.; Chen, H.-I.; Chen, W.-C.; Chang, C.-H.; Liu, W.-C. Formaldehyde sensing characteristics of an aluminum-doped zinc oxide (AZO) thin-film-based sensor. Sens. Actuators B Chem. 2018, 255, 3017. [Google Scholar] [CrossRef]
- Fang, G.J.; Li, D.J.; Yao, B.-L. Effect of vacuum annealing on the properties of transparent conductive AZO thin films prepared by dc magnetron sputtering. Phys. Status Solidi A 2002, 193, 139. [Google Scholar] [CrossRef]
- Fang, Z.B.; Yan, Z.J.; Tan, Y.S.; Liu, X.Q.; Wang, Y.Y. Influence of post-annealing treatment on the structure properties of ZnO films. Appl. Surf. Sci. 2005, 241, 303. [Google Scholar]
- Wen, L.; Sahu, B.B.; Kim, H.R.; Han, J.G. Study on the electrical, optical, structural, and morphological properties of highly transparent and conductive AZO thin films prepared near room temperature. Appl. Surf. Sci. 2019, 473, 649. [Google Scholar] [CrossRef]
- Prepelita, P.; Craciun, V.; Garoi, F.; Staicu, A. Effect of annealing treatment on the structural and optical properties of AZO samples. Appl. Surf. Sci. 2015, 352, 23. [Google Scholar]
- Meng, R.; Zhang, S.; Guo, H.; Sun, Y.; Guo, J.; Ao, J.; Liu, W.; Liu, F.; Zhang, Y. Al-doped ZnO thin films with excellent optoelectronic properties prepared using H2-assisted reactive magnetron sputtering at low temperatures for potential application in photovoltaic devices. J. Mater. Sci. Mater. Electron. 2022, 33, 10267. [Google Scholar] [CrossRef]
- Yang, T.; Song, S.; Li, Y.; Xin, Y.; Du, G.; Lv, M.; Han, S. The enhanced conductivity and stability of AZO thin films with a TiO2 buffer layer. Phys. B Condens. Matter 2012, 407, 4518. [Google Scholar] [CrossRef]
- Oh, B.-Y.; Jeong, M.-C.; Moon, T.-H.; Lee, W.; Myoung, J.-M.; Hwang, J.-Y.; Seo, D.-S. Transparent conductive Al-doped ZnO films for liquid crystal displays. J. Appl. Phys. 2006, 99, 124505. [Google Scholar]
- Gong, S.C.; Choi, Y.-J.; Kim, H.; Park, C.-S.; Park, H.-H.; Jang, J.G.; Chang, H.J.; Yeom, G.Y. Aluminum-doped zinc oxide formed by atomic layer deposition for use as anodes in organic light emitting diodes. J. Vac. Sci. Technol. A 2013, 31, 01A101. [Google Scholar]
- Shen, H.; Zhang, H.; Lu, L.; Jiang, F.; Yang, C. Preparation and properties of AZO thin films on different substrates. Prog. Nat. Sci. Mater. Int. 2010, 20, 44. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wu, M.Z.; Wang, Y.Y.; Yu, Y.M.; Wu, X.M.; Zhuge, L.J. Influence of thickness and annealing temperature on the electrical, optical and structural properties of AZO thin films. Vacuum 2013, 89, 127. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Yu, Y.; Zhu, Y.; Ge, M. Preparation and luminescence properties of rare-earth doped fiber with spectral blue-shift: SrAl2O4: Eu2+, Dy3+ phosphors/triarylsulfonium hexafluoroantimonate based on polypropylene substrate. J. Rare Earths 2017, 35, 530. [Google Scholar]
- Singh, R.; Mukherjee, S.K. RF sputtered AZO thin films: A potential TCO for various opto-electronic applications. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, W.; Xiao, Y.; Yang, P. Manipulating the optoelectronic characteristic of AZO films by magnetron sputtering power. Vacuum 2023, 210, 111849. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Chen, J.; Fang, L.; Wang, L. Structural and optoelectronic properties of AZO thin films prepared by RF magnetron sputtering at room temperature. Trans. Nonferrous Met. Soc. China 2016, 26, 1655. [Google Scholar]
- Gondoni, P.; Ghidelli, M.; Di Fonzo, F.; Russo, V.; Bruno, P.; Martí-Rujas, J.; Bottani, C.E.; Bassi, A.L.; Casari, C.S. Structural and functional properties of Al: ZnO thin films grown by Pulsed Laser Deposition at room temperature. Thin Solid Films 2012, 520, 4707. [Google Scholar]
- Tanaka, H.; Ihara, K.; Miyata, T.; Sato, H.; Minami, T. Low resistivity polycrystalline ZnO: Al thin films prepared by pulsed laser deposition. J. Vac. Sci. Technol. A 2004, 22, 1757. [Google Scholar]
- Znaidi, L.; Touam, T.; Vrel, D.; Souded, N.; Yahia, S.B.; Brinza, O.; Fischer, A.; Boudrioua, A. AZO thin films by sol-gel process for integrated optics. Coatings 2013, 3, 126. [Google Scholar] [CrossRef] [Green Version]
- Ng, Z.-N.; Chan, K.-Y.; Tohsophon, T. Effects of annealing temperature on ZnO and AZO films prepared by sol–gel technique. Appl. Surf. Sci. 2012, 258, 9604. [Google Scholar]
- Cui, J.; Sun, S.; Lan, M.; Liu, S.; Piao, Y.; Li, G.; Wang, Q. Effect of low-dimensional carbon composite on the thermoelectric properties of vacuum evaporated ZnO: Al films. Thin Solid Films 2023, 766, 139641. [Google Scholar]
- Li, C.; Wai, H.S. Influence of Seeds Layer on the Control of Growth and Crystallinity of AZO Films Deposited by Mist Vapor Deposition Applying for Photocatalytic Activity. Top. Catal. 2023, 66, 523–532. [Google Scholar] [CrossRef]
- Kim, W.-H.; Maeng, W.J.; Kim, M.-K.; Kim, H. Low pressure chemical vapor deposition of aluminum-doped zinc oxide for transparent conducting electrodes. J. Electrochem. Soc. 2011, 158, D495. [Google Scholar] [CrossRef]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159. [Google Scholar]
- Velasco, S.C.; Cavaleiro, A.; Carvalho, S. Functional properties of ceramic-Ag nanocomposite coatings produced by magnetron sputtering. Prog. Mater. Sci. 2016, 84, 158. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Heo, S.; Park, K.; Song, M.H.; Kim, J.-Y.; Kyung, G.; Ruoff, R.S.; Park, J.-U.; Bien, F. Research on flexible display at Ulsan National Institute of Science and Technology. Npj Flex. Electron. 2017, 1, 9. [Google Scholar]
- Rana, V.S.; Rajput, J.K.; Pathak, T.K.; Pal, P.K.; Purohit, L.P. Impact of RF Sputtering Power on AZO Thin Films for Flexible Electro-Optical Applications. Cryst. Res. Technol. 2021, 56, 2000144. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, P.; Shi, S.; Zhang, M.; He, G.; Lv, J.; Sun, Z. Deposition and characterization of AZO thin films on flexible glass substrates using DC magnetron sputtering technique. Ceram. Int. 2017, 43, 4536. [Google Scholar]
- Shi, Q.; Zhou, K.; Dai, M.; Hou, H.; Lin, S.; Wei, C.; Hu, F. Room temperature preparation of high performance AZO films by MF sputtering. Ceram. Int. 2013, 39, 1135. [Google Scholar] [CrossRef]
- Shiravand, M.; Ghobadi, N.; Hatam, E.G. The investigation of structural, surface topography, and optical behaviors of Al-doped ZnO thin films with annealing temperature deposited by RF magnetron sputtering. J. Mater. Sci. Mater. Electron. 2023, 34, 713. [Google Scholar]
- Yang, W.; Liu, Z.; Peng, D.-L.; Zhang, F.; Huang, H.; Xie, Y.; Wu, Z. Room-temperature deposition of transparent conducting Al-doped ZnO films by RF magnetron sputtering method. Appl. Surf. Sci. 2009, 255, 5669. [Google Scholar] [CrossRef]
- Guillén, C.; Herrero, J. Optical, electrical and structural characteristics of Al: ZnO thin films with various thicknesses deposited by DC sputtering at room temperature and annealed in air or vacuum. Vacuum 2010, 84, 924. [Google Scholar]
- Kaid, M.A.; Ashour, A. Preparation of ZnO-doped Al films by spray pyrolysis technique. Appl. Surf. Sci. 2007, 253, 3029. [Google Scholar] [CrossRef]
- Pandey, R.; Yuldashev, S.; Nguyen, H.D.; Jeon, H.C.; Kang, T.W. Fabrication of aluminium doped zinc oxide (AZO) transparent conductive oxide by ultrasonic spray pyrolysis. Curr. Appl. Phys. 2012, 12, S56. [Google Scholar] [CrossRef]
- Alyamani, A.; Tataroğlu, A.; El Mir, L.; Al-Ghamdi, A.A.; Dahman, H.; Farooq, W.A.; Yakuphanoğlu, F. Photoresponse and photocapacitor properties of Au/AZO/p-Si/Al diode with AZO film prepared by pulsed laser deposition (PLD) method. Appl. Phys. A 2016, 122, 297. [Google Scholar] [CrossRef]
- Sibiński, M.; Znajdek, K.; Walczak, S.; Słoma, M.; Górski, M.; Cenian, A. Comparison of ZnO: Al, ITO and carbon nanotube transparent conductive layers in flexible solar cells applications. Mater. Sci. Eng. B 2012, 177, 1292. [Google Scholar] [CrossRef]
- Anyanwu, V.O.; Moodley, M.K. PLD of transparent and conductive AZO thin films. Ceram. Int. 2023, 49, 5311. [Google Scholar] [CrossRef]
- Kim, H.; Gilmore, C.M.; Horwitz, J.S.; Piqué, A.; Murata, H.; Kushto, G.P.; Schlaf, R.; Kafafi, Z.H.; Chrisey, D.B. Transparent conducting aluminum-doped zinc oxide thin films for organic light-emitting devices. Appl. Phys. Lett. 2000, 76, 259. [Google Scholar] [CrossRef]
- Kek, R.; Ong, G.L.; Yap, S.L.; Lim, L.K.; Koh, S.F.; Nee, C.H.; Tou, T.Y.; Yap, S.S. Growth of Al-doped ZnO nanostructures in low pressure background gas by pulsed laser deposition. Mater. Sci. Semicond. Process. 2022, 145, 106636. [Google Scholar] [CrossRef]
- Darwish, A.M.; Muhammad, A.; Sarkisov, S.S.; Mele, P.; Saini, S.; Liu, J.; Shiomi, J. Thermoelectric properties of Al-doped ZnO composite films with polymer nanoparticles prepared by pulsed laser deposition. Compos. Part B Eng. 2019, 167, 406. [Google Scholar] [CrossRef]
- Aurobind, S.V.; Amirthalingam, K.P.; Gomathi, H. Sol-gel based surface modification of electrodes for electro analysis. Adv. Colloid Interface Sci. 2006, 121, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R.; Scalarone, D.; Magnacca, G. Sol-gel chemistry, templating and spin-coating deposition: A combined approach to control in a simple way the porosity of inorganic thin films/coatings. Microporous Mesoporous Mater. 2017, 248, 18. [Google Scholar]
- Tan, W.K.; Muto, H.; Kawamura, G.; Lockman, Z.; Matsuda, A. Nanomaterial fabrication through the modification of sol–gel derived coatings. Nanomaterials 2021, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Rahman, M.; Farhad, S.F.U.; Podder, J. Structural, optical and photocatalysis properties of sol–gel deposited Al-doped ZnO thin films. Surf. Interfaces 2019, 16, 120–126. [Google Scholar] [CrossRef]
- Khan, M.I.; Neha, T.R.; Billah, M.M. UV-irradiated sol-gel spin coated AZO thin films: Enhanced optoelectronic properties. Heliyon 2022, 8, e08743. [Google Scholar]
- Ghosh, S.; Chakraborty, J. Obviating the post annealing step for production of Al-doped zinc oxide films using sol–gel method: Use of rapid cooling and gas blanketing. Thin Solid Films 2016, 605, 173. [Google Scholar]
- Wang, J.; Cao, X.; Liu, S.; Guo, Y.; Wang, Z.; Li, X.; Ren, Y.; Xia, Z.; Wang, H.; Liu, C.; et al. Preparation, structural and sintering properties of AZO nanoparticles by sol-gel combustion method. Ceram. Int. 2020, 46, 17659. [Google Scholar] [CrossRef]
- Abegunde, O.O.; Akinlabi, E.T.; Oladijo, O.P.; Akinlabi, S.; Ude, A.U.; Abegunde, O.O.; Akinlabi, E.T.; Oladijo, O.P.; Akinlabi, S.; Ude, A.U. Overview of thin film deposition techniques. AIMS Mater. Sci. 2019, 6, 174. [Google Scholar] [CrossRef]
- Miyata, T.; Minamino, Y.; Ida, S.; Minami, T. Highly transparent and conductive ZnO: Al thin films prepared by vacuum arc plasma evaporation. J. Vac. Sci. Technol. A 2004, 22, 1711–1715. [Google Scholar] [CrossRef]
- Mattox, D.M. Physical vapor deposition (PVD) processes. Met. Finish. 2002, 100, 394–408. [Google Scholar] [CrossRef]
- Vyas, S.; Singh, S.; Chakrabarti, P. Tailoring Energy Bandgap of Al Doped ZnO Thin Films Grown by Vacuum Thermal Evaporation Method. J. Nanosci. Nanotechnol. 2015, 15, 9636–9642. [Google Scholar] [CrossRef]
- Abdulmunem, O.M.; Ali, M.J.M.; Hassan, E.S. Optical and structural characterization of aluminium doped zinc oxide thin films prepared by thermal evaporation system. Opt. Mater. 2020, 109, 110374. [Google Scholar] [CrossRef]
- Van Toan, N.; Tuoi, T.T.K.; Inomata, N.; Toda, M.; Ono, T. Aluminum doped zinc oxide deposited by atomic layer deposition and its applications to micro/nano devices. Sci. Rep. 2021, 11, 1204. [Google Scholar]
- Saini, S.; Mele, P.; Oyake, T.; Shiomi, J.; Niemelä, J.-P.; Karppinen, M.; Miyazaki, K.; Li, C.; Kawaharamura, T.; Ichinose, A.; et al. Porosity-tuned thermal conductivity in thermoelectric Al-doped ZnO thin films grown by mist-chemical vapor deposition. Thin Solid Films 2019, 685, 180–185. [Google Scholar] [CrossRef]
- Wai, H.S.; Li, C. Effect of aluminum doping ratios on the properties of aluminum-doped zinc oxide films deposited by mist chemical vapor deposition method applying for photocatalysis. Nanomaterials 2022, 12, 195. [Google Scholar] [PubMed]
- Liu, L.; Wang, L.; Qin, X.; Cui, L.; Shao, G. Effects of intermittent atomization on the properties of Al-doped ZnO thin films deposited by aerosol-assisted chemical vapor deposition. Thin Solid Films 2016, 605, 163–168. [Google Scholar]
- Hussain, S.T.; Bakar, S.A.; Saima, B.; Muhammad, B. Low temperature deposition of silver sulfide thin films by AACVD for gas sensor application. Appl. Surf. Sci. 2012, 258, 9610–9616. [Google Scholar] [CrossRef]
- Potter, D.B.; Parkin, I.P.; Carmalt, C.J. The effect of solvent on Al-doped ZnO thin films deposited via aerosol assisted CVD. RSC Adv. 2018, 8, 33164–33173. [Google Scholar]
- Groenen, R.; Löffler, J.; Sommeling, P.M.; Linden, J.L.; Hamers, E.A.G.; Schropp, R.E.I.; van de Sanden, M.C.M. Surface textured ZnO films for thin film solar cell applications by expanding thermal plasma CVD. Thin Solid Films 2001, 392, 226–230. [Google Scholar] [CrossRef]
- Badgujar, A.C.; Yadav, B.S.; Jha, G.K.; Dhage, S.R. Room temperature sputtered aluminum-doped ZnO thin film transparent electrode for application in solar cells and for low-band-gap optoelectronic devices. ACS Omega 2022, 7, 14203–14210. [Google Scholar]
- Lin, G.-S.; Li, C.-Y.; Huang, K.-C.; Houng, M.-P. Using chemical wet-etching methods of textured AZO films on a-Si: H solar cells for efficient light trapping. Mater. Chem. Phys. 2015, 160, 264–270. [Google Scholar]
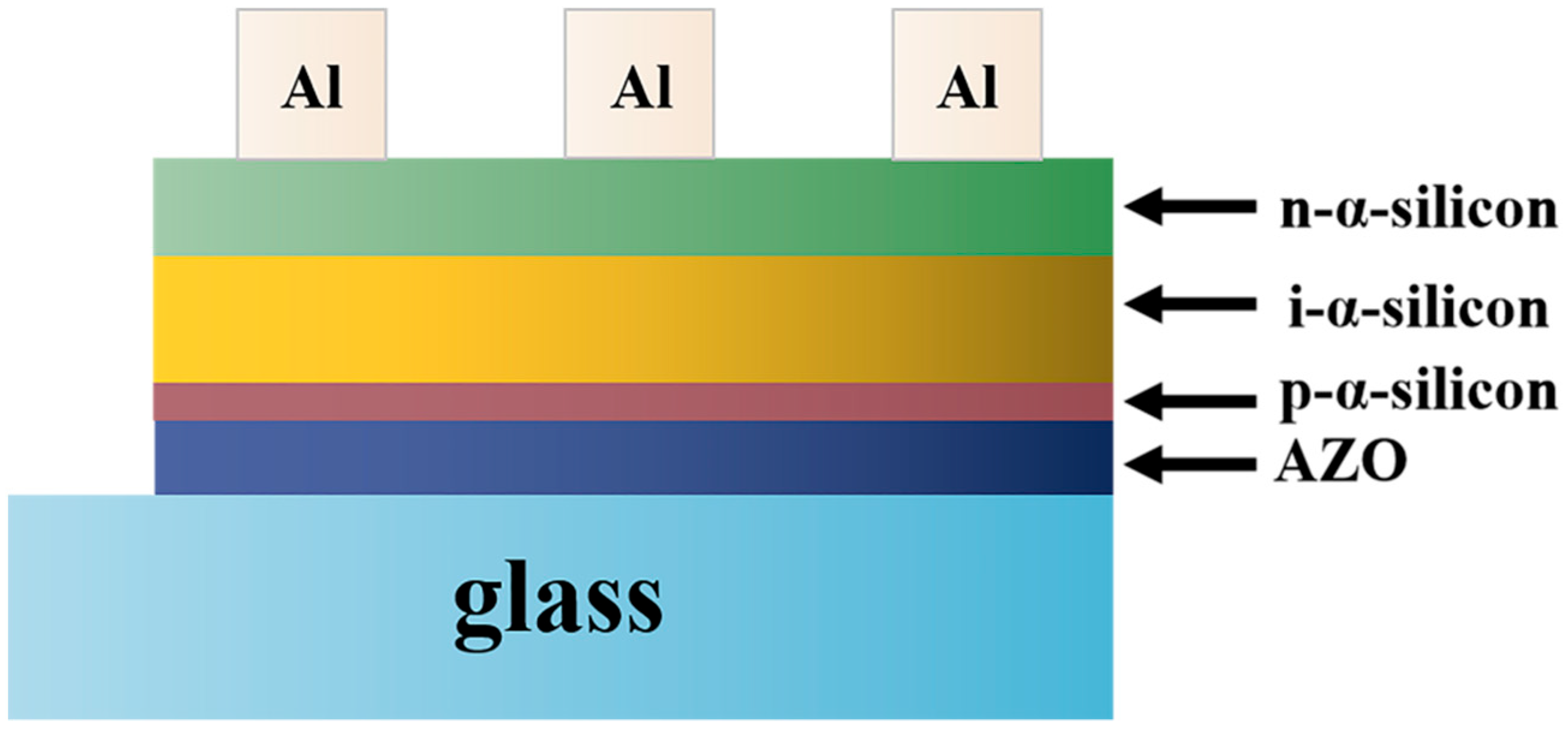
- Huang, C.-C.; Wang, F.-H.; Yang, C.-F. Effects of deposition temperature and hydrogen flow rate on the properties of the Al-doped ZnO thin films and amorphous silicon thin-film solar cells. Appl. Phys. A 2013, 112, 877–883. [Google Scholar]
- Chen, J.; Huang, Q.; Lei, W. Dual-Facets Emissive Quantum-Dot Light-Emitting Diode Based on AZO Electrode. Materials 2022, 15, 740. [Google Scholar] [PubMed]
- Wang, F.; Wang, Z.; Zhu, X.; Bai, Y.; Yang, Y.; Hu, S.; Liu, Y.; You, B.; Wang, J.; Li, Y.; et al. Highly efficient and super stable full-color quantum dots light-emitting diodes with solution-processed all-inorganic charge transport layers. Small 2021, 17, 2007363. [Google Scholar]
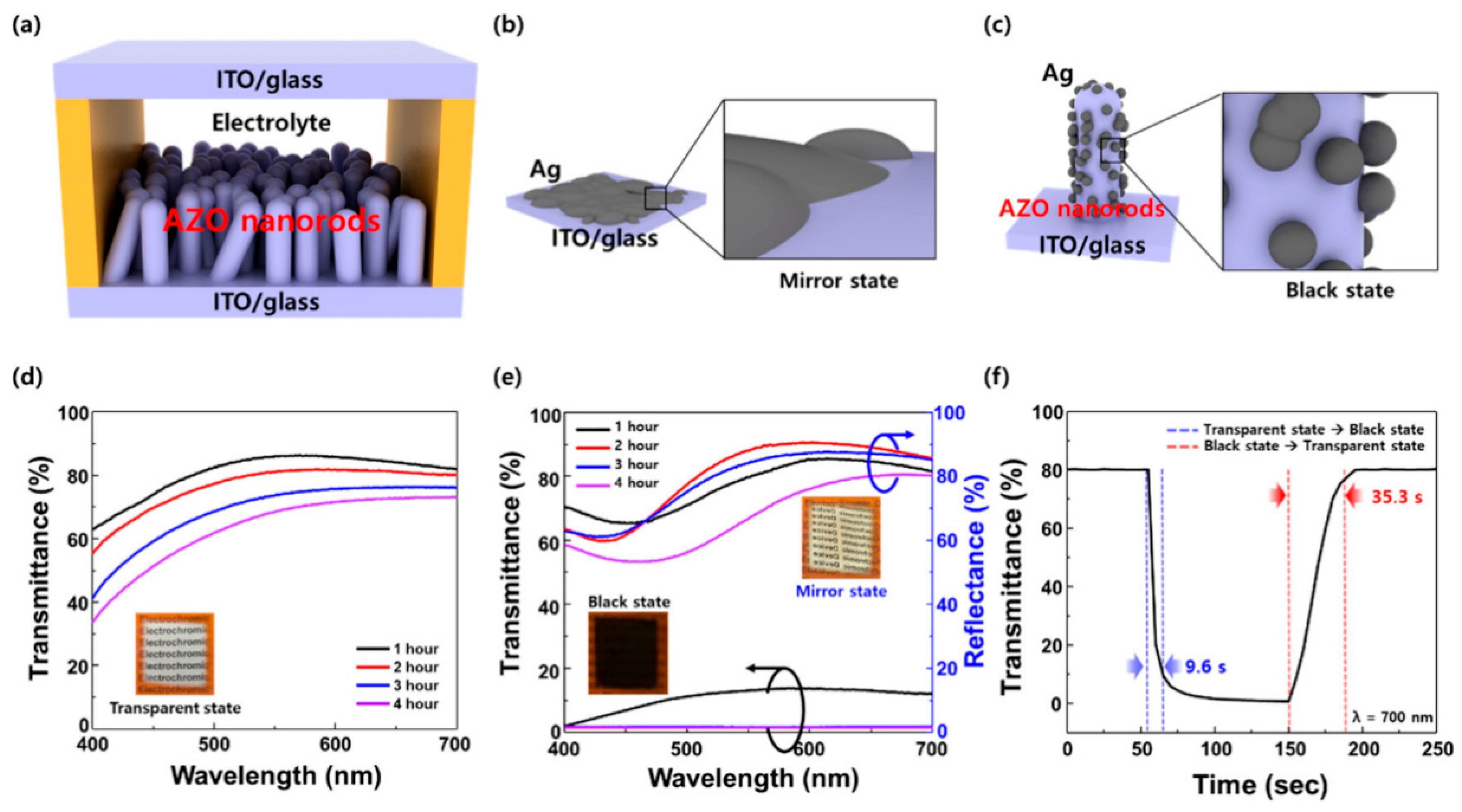
- Song, J.; Park, J.Y.; Kim, K.; Lee, J.-L. High-vacuum annealed Al-doped ZnO nanorods for fast-switching electrochromic windows with high-optical contrast and long-term stability. ACS Appl. Nano Mater. 2022, 5, 3946–3956. [Google Scholar]
- Sanger, A.; Kang, S.B.; Jeong, M.H.; Kim, C.U.; Baik, J.M.; Choi, K.J. All-transparent NO2 gas sensors based on freestanding Al-doped ZnO nanofibers. ACS Appl. Electron. Mater. 2019, 1, 1261–1268. [Google Scholar]
- Patil, V.L.; Dalavi, D.S.; Dhavale, S.B.; Tarwal, N.L.; Vanalakar, S.A.; Kalekar, A.S.; Kim, J.H.; Patil, P.S. NO2 gas sensing properties of chemically grown Al doped ZnO nanorods. Sens. Actuators Phys. 2022, 340, 113546. [Google Scholar]
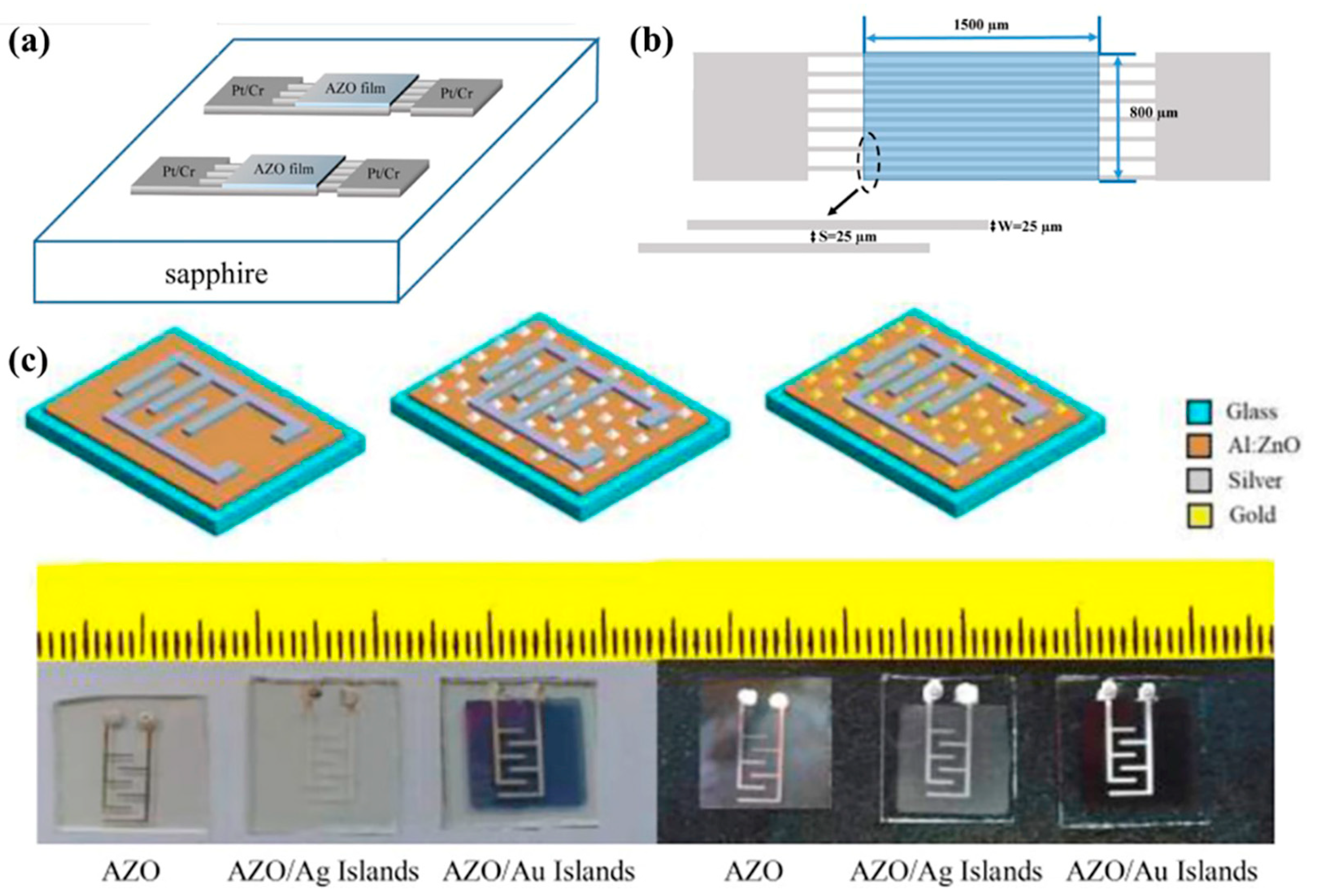
- Farajollahi, H.; Bafghi, Z.G.; Mohammadi, E.; Manavizadeh, N.; Salehi, A. Sensitivity enhancement of AZO-based ethanol sensor decorated by Au nano-islands. Curr. Appl. Phys. 2020, 20, 917. [Google Scholar]
- Lee, D.H.; Ryu, H.; Kwon, S.J.; Cho, E.-S. Process Optimization of Aluminum-Doped Zinc Oxide Films by In-Line Pulsed-DC Sputtering and Its Application to Resistive Touch Panels. Sci. Adv. Mater. 2020, 12, 1607. [Google Scholar]
- Su, Y.C.; Chiou, C.C.; Marinova, V.; Lin, S.H.; Bozhinov, N.; Blagoev, B.; Babeva, T.; Hsu, K.Y.; Dimitrov, D.Z. Atomic layer deposition prepared Al-doped ZnO for liquid crystal displays applications. Opt. Quantum Electron. 2018, 50, 205. [Google Scholar]
- Minami, T.; Miyata, T.; Ohtani, Y. Optimization of aluminum-doped ZnO thin-film deposition by magnetron sputtering for liquid crystal display applications. Phys. Status Solidi A 2007, 204, 3145–3151. [Google Scholar]
- Jeong, H.-C.; Kim, D.H.; Lee, D.W.; Oh, J.Y.; Lee, J.H.; Won, J.; Jang, J.I.; Eom, S.J.; Seo, D.-S. Solution-driven imprinting lithography of sol–gel ZnO thin films for liquid crystal display. Langmuir 2022, 38, 2561–2568. [Google Scholar]
- Szyszka, B.; Dewald, W.; Gurram, S.K.; Pflug, A.; Schulz, C.; Siemers, M.; Sittinger, V.; Ulrich, S. Recent developments in the field of transparent conductive oxide films for spectral selective coatings, electronics and photovoltaics. Curr. Appl. Phys. 2012, 12, S2–S11. [Google Scholar]
- Singh, A.V.; Kumar, M.; Mehra, R.M.; Wakahara, A.; Yoshida, A. Al-doped zinc oxide (ZnO:Al) thin films by pulsed laser ablation. J. Indian Inst. Sci. 2001, 81, 527. [Google Scholar]
- Tönbül, B.; Can, H.A.; Öztürk, T.; Akyıldız, H. Solution processed aluminum-doped ZnO thin films for transparent heater applications. Mater. Sci. Semicond. Process. 2021, 127, 105735. [Google Scholar]
- Jayathilake, D.S.Y.; Sagu, J.S.; Wijayantha, K.G.U. Transparent heater based on Al, Ga co-doped ZnO thin films. Mater. Lett. 2019, 237, 249–252. [Google Scholar]
- Mao, Z.; Yu, X.; Zhang, L.; Zhong, Y.; Xu, H. Novel infrared stealth property of cotton fabrics coated with nano ZnO:(Al, La) particles. Vacuum 2014, 104, 111–115. [Google Scholar]
- Wang, W.; Zhang, L.; Liu, Y.; Xu, H.; Zhong, Y.; Mao, Z. Synthesis and infrared stealth property of ordered mesoporous carbon–aluminum-doped zinc oxide composites. Ind. Eng. Chem. Res. 2013, 52, 15066–15074. [Google Scholar]
- Miao, D.; Jiang, S.; Shang, S.; Chen, Z. Effect of heat treatment on infrared reflection property of Al-doped ZnO films. Sol. Energy Mater. Sol. Cells 2014, 127, 163. [Google Scholar]
- Atay, H.Y.; İçin, Ö. Manufacturing radar-absorbing composite materials by using magnetic Co-doped zinc oxide particles synthesized by Sol-Gel. J. Compos. Mater. 2020, 54, 4059. [Google Scholar]
- Ghosh, J.; Ghosh, R.; Giri, P.K. Tuning the visible photoluminescence in Al doped ZnO thin film and its application in label-free glucose detection. Sens. Actuators B Chem. 2018, 254, 681. [Google Scholar]
- García-Salinas, F.; Vázquez-Durán, A.; Yáñez-Limón, J.M. Comparative study of Al-doped ZnO films deposited by sol–gel and by sputtering using a sintered target from ZnO nanoparticles synthesized by sol–gel. Bol. Soc. Esp. Cerám. Vidr. 2023, 62, 134–144. [Google Scholar]
- Valerini, D.; Tammaro, L.; Vigliotta, G.; Picariello, E.; Banfi, F.; Cavaliere, E.; Ciambriello, L.; Gavioli, L. Ag functionalization of Al-doped ZnO nanostructured coatings on PLA substrate for antibacterial applications. Coatings 2020, 10, 1238. [Google Scholar]



| Method | Advantages | Disadvantages |
|---|---|---|
| Magnetron sputtering | Low substrate temperature, high deposition rate, good film adhesion, uniform film thickness, good controllability and stability, simple operation, and low deposition cost. | Low in target material utilization rate, plasma instability. |
| Spray pyrolysis | Easy to control microstructure, high film-forming rate, easy doping, large area film formation. | Only Newtonian fluids. |
| Pulsed laser deposition | Operating at lower temperatures, control coating density and coating purity, high deposition rate, works on complex shapes. | High equipment cost, slow sedimentary speed. |
| Sol–gel | Good uniformity of products, high purity, low process temperature, easy to prepare various new materials. | Harmful organic solvents, long production time, cracking easily. |
| Vacuum evaporation | Excellent corrosion resistance. | Need a decompression device, high viscosity. |
| Atomic layer deposition | Uniformity of sediment thickness. | The film grows slowly. |
| Chemical vapor deposition | Fast deposition speed, easy control of film composition, good adhesion, compactness, uniformity of the obtained film. | Reduce the product quality. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Yu, W.; Zhang, L.; Hao, X. Progress in the Synthesis and Application of Transparent Conducting Film of AZO (ZnO:Al). Materials 2023, 16, 5537. https://doi.org/10.3390/ma16165537
Zhang D, Yu W, Zhang L, Hao X. Progress in the Synthesis and Application of Transparent Conducting Film of AZO (ZnO:Al). Materials. 2023; 16(16):5537. https://doi.org/10.3390/ma16165537
Chicago/Turabian StyleZhang, Dingyi, Wenhe Yu, Lu Zhang, and Xiangyang Hao. 2023. "Progress in the Synthesis and Application of Transparent Conducting Film of AZO (ZnO:Al)" Materials 16, no. 16: 5537. https://doi.org/10.3390/ma16165537
APA StyleZhang, D., Yu, W., Zhang, L., & Hao, X. (2023). Progress in the Synthesis and Application of Transparent Conducting Film of AZO (ZnO:Al). Materials, 16(16), 5537. https://doi.org/10.3390/ma16165537