Abstract
Multicomponent equimolar perovskite oxides (ME-POs) have recently emerged as a highly promising class of materials with unique synergistic effects, making them well-suited for applications in such areas as photovoltaics and micro- and nanoelectronics. High-entropy perovskite oxide thin film in the (Gd0.2Nd0.2La0.2Sm0.2Y0.2)CoO3 (RECO, where RE = Gd0.2Nd0.2La0.2Sm0.2Y0.2, C = Co, and O = O3) system was synthesized via pulsed laser deposition. The crystalline growth in an amorphous fused quartz substrate and single-phase composition of the synthesized film was confirmed by X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). Surface conductivity and activation energy were determined using a novel technique implementing atomic force microscopy (AFM) in combination with current mapping. The optoelectronic properties of the deposited RECO thin film were characterized using UV/VIS spectroscopy. The energy gap and nature of optical transitions were calculated using the Inverse Logarithmic Derivative (ILD) and four-point resistance method, suggesting direct allowed transitions with altered dispersions. The narrow energy gap of RECO, along with its relatively high absorption properties in the visible spectrum, positions it as a promising candidate for further exploration in the domains of low-energy infrared optics and electrocatalysis.
1. Introduction
The demand for cutting-edge technology requires the development of new materials, which meet the ever-increasing requirements for conductivity, thermal stability, durability, and environmental resilience. In response to this need, high-entropy materials (HEMs) have garnered widespread attention, particularly the pioneering class of high-entropy alloys (HEAs) known for their remarkable versatility [1]. In 2015, expanding upon the concept of HEAs, Rost et al. [2] introduced a novel approach to ionic compounds by populating cation positions with several elements in equiatomic amounts. This breakthrough led to the emergence of a new class of materials called high-entropy oxides (HEOs), which encompass later, more complex structures, including spinel- [3] and perovskite-type oxides [4]. HEOs have gained significant attention from the research community due to their exceptional properties, attributed in part to the cocktail effect [5,6]. The thermodynamic stability of these materials is achieved by minimizing the Gibbs free energy. In the context of HEOs, phase stabilization relates to the Gibbs free energy Equation (1),
where ∆G represents the change in Gibbs free energy, ∆H is the change of enthalpy, T is the absolute temperature, and ∆S is the change of entropy of the system [7]. The calculation of ∆S, which plays a crucial role in stabilizing the single-phase structure in high-entropy materials, is based on Equation (2),
where R is the universal gas constant and xi is the molar concentration of each element in the mixture [7,8]. For instance, the entropy of HEOs consisting of five metal ions in the cationic sublattice is equal to 1.61 R [9].
Since the discovery of HEOs, the majority of research efforts have been directed toward bulk HEO systems, with relatively less attention given to thin film developments. The first reports on high-entropy oxide films (HEOFs) appeared in 2016, when Rost et al., [10] successfully prepared the entropy-stabilized oxide film using pulsed laser deposition (PLD), thus paving the way for further exploration [11]. Notably, perovskite thin films have garnered special attention due to their tunable physical and chemical properties, as evidenced by the recent studies on thermoelectric behavior, quantum effects, and structural and optical controlling [12,13,14,15]. The combination of various effects in a single material through 2D nanostructuring, exploiting the properties of the ABO3 family of perovskites and harnessing the synergistic effects of high configuration entropy has attracted significant interest. This approach promises the occurrence of pioneering physical phenomena, such as colossal magnetoresistance and photo- and electrocatalytic properties, alongside the simultaneous presence of emerging phases [16,17,18,19,20]. Consequently, these led to a growing focus on the fabrication of the high-entropy perovskite thin films used in a wide spectrum of fields, including nanoelectronics [21,22], electrocatalysis [23,24], and photovoltaics [25,26]. Moreover, the high-entropy effect resulting from the general configurational disorder of HEMs can effectively modulate the structural disorder, enabling access to unexplored quantum phase spaces and facilitating fundamental studies on the electronic band structure of those multicomponent materials [27,28,29].
The reliable production of uniform thin films on the planar crystalline and amorphous substrates via pulsed laser deposition is crucial for the development of cutting-edge applications, including artificial intelligence, memory, and logic devices [30,31,32,33,34,35,36,37]. One of the principal challenges encountered in thin film deposition processes for complex multicomponent oxide systems is the inherent risk of losing precise control over the stoichiometry of the film. Deviations from the desired composition can lead to the formation of secondary phases and hinder the attainment of a single-phase structure, which is particularly critical in multicomponent perovskite-type oxide systems [38]. Furthermore, the formation of defects, such as oxygen vacancies, during the film growth process can negatively impact film properties and stability [39]. To address these issues, the optimization of the deposition conditions, such as substrate temperature and oxygen pressure, becomes crucial in minimizing defect formation and enhancing film quality [40]. Motivated by the aforementioned potential, we have undertaken the challenge of nanostructuring the high-entropy perovskite-type oxide system that was the subject of our previous work [41].
This study aimed to deposit a thin film from the RECO system (Gd0.2Nd0.2La0.2Sm0.2Y0.2; C = Co; O = O3) onto a SiO2 substrate using pulsed laser deposition in order to investigate the feasibility of producing uniform films of a high-entropy multicomponent single-phase material. Our focus was primarily on the comprehensive characterization of the structural, chemical, electrical, and optical properties of the RECO thin film, intending to identify potential applications for this material.
2. Materials and Methods
Target and thin film deposition: The selection of the RECO (RE = Gd0.2Nd0.2La0.2Sm0.2Y0.2; C = Co; O = O3) for this study was based on the successful synthesis of this system, as detailed in our previous work [41]. The selection of this specific configuration was primarily driven by the requirement of matching the oxidation state and coordination number with the prototype structure of LaCoO3 in the Pnma space group. Additionally, the similarity in ionic radii of cations on a specific site (A or B) was quantitatively examined using the tolerance factor introduced by Goldschmidt for perovskite-type oxides [42] and the parameter δ proposed by Zheng for high-entropy materials [43], yielding values of 0.932 [−] and 0.344 %, respectively. The thin film has been deposited on a fused quartz (SiO2) substrate employing the pulsed laser deposition (PLD) technique. The KrF 248 nm excimer laser (Compex Pro 110) with a pulse of 10 ns and energy density of 1.5 J cm−2 has been used for the ablation-deposition processes. The distance between the rotating substrate holder and the RECO target was set to 5 cm. All other parameters, i.e., substrate temperature of 750 °C, deposition rate of 10 Hz and 5000 pulses, and oxygen background pressure of 40 mTorr, have resulted in a thin film with a thickness of approximately 100 nm. The parameters were optimized based on work on the deposition of a thin film of perovskite with a similar structure, namely LaCoO3, as described in reference [44], and tuned by further testing of the RECO target material in the PLD process.
AFM: Topography and conductive parameters of the obtained thin film were studied with atomic force microscopy (AFM). Images were obtained with a Dimension Icon XR (Bruker, Santa Barbara, CA, USA) working in the PeakForce Tapping (PFT) mode in the air using Platinum-Iridium (Pt/Ir) front-side-coated, electrically conductive tips with a nominal spring constant of 3 N·m−1 and nominal radius of curvature of 25 nm. PeakForce TUNA mode was used for conductivity imaging with a bias voltage equal to 10 V and for collecting I–U curves from the random spots on (Gd0.2Nd0.2La0.2Sm0.2Y0.2)CoO3 surfaces. I–U plots for all samples were collected in the same conditions using ramp mode with a setpoint value corresponding to the maximum force of 1 nN and the voltage applied between the tip and the sample surface in the range −5 V to 5 V. The plots were recorded in at least 25 random points for each sample. Topography and conductive images with the corresponding I−U plots were obtained at room temperature (298 K) and elevated temperatures (323 K, 348 K, and 373 K). All elevated temperatures measurements were done on the High Temperature Heater element from Bruker Co. (Santa Barbara, CA, USA) added to the AFM stage and cooled by the flow of chilled water driven by the Masterflex L/S peristaltic pump (Cole Parmer, Vernon Hills, IL, USA). The temperature values were set by the Thermal Application Controller (Bruker) driven by the software (Nanoscope 9.7) and controlled by an external thermometer connected directly to the sample surface. All measurements were done in the environmental chamber providing thermal and acoustic insulation.
XRD: X-ray diffraction studies were made through an X’Pert MPD diffractometer equipped with CuKα radiation. The substrate and target prepared according to the procedure described in our previous study [41] were measured in Bragg-Brentano and grazing incident X-ray diffraction (GIXD) geometry at an incidence angle of 1°. Thin film thickness and interface roughness were obtained via X-ray reflectivity (XRR) measurements.
XPS: X-ray photoelectron spectroscopy analyses were carried out in a PHI Versa ProbeII Scanning XPS system using monochromatic Al Kα (1486.6 eV) X-rays focused to a 100 µm spot and scanned over the area of 400 µm × 400 µm. The photoelectron take-off angle was 45° and the pass energy in the analyzer was set to 46.95 eV to obtain high energy resolution spectra for the Co 2p, O 1s, Sm 3d, Nd 3d, Gd 4d, La 3d, Y 3d, and Bi 4f regions. A dual beam charge compensation with 7 eV Ar+ ions and 1 eV electrons was used to maintain a constant sample surface potential regardless of the sample conductivity. All XPS spectra were charge referenced to the unfunctionalized, saturated carbon (C−C) C1s peak at 285.0 eV. The operating pressure in the analytical chamber was less than 3·10−9 mbar. Deconvolution of spectra was carried out using PHI MultiPak software (v.9.9.0.8). Spectrum background was subtracted using the Shirley method. The Lorentzian-Gaussian function was employed for fitting, with the Gaussian part accounting for at least 80% of the curve shape.
Conductivity measurements: Resistivity of RECO on SiO2 was measured using the standard four-point resistance method (FPRM) with a Keithley model 2400 source meter unit. To determine the activation energy of the obtained material, the temperature range of 340–450 K at intervals of 10 K was applied. Measurements were made in an argon atmosphere to ensure high stability and to prevent external contamination of the sample.
UV/VIS measurements: Optical measurements of RECO/quartz film were made with a 1 nm step using the PerkinElmer UV–Vis Lambda 750 spectrophotometer.
3. Results and Discussion
Figure 1 shows the grazing incident X-ray diffraction (GIXD) profile for a thin RECO film (black line) that was deposited onto a SiO2 (fused quartz) substrate surface. The red profile corresponds to a measurement taken from an uncovered substrate, revealing its amorphous structure. The relatively high Full Width at Half Maximum (FWHM) of the RECO reflections is attributed to the limited thickness of the film. Additionally, the X-ray reflectivity profile of the RECO layer on quartz is presented in the inset of Figure 1. The density, layer thickness, and RMS roughness were determined through an optimal match between the experimental curve (Figure 1: red, measurement) and the calculated XRR profile (Figure 1: green, fit). The values for density, layer thickness, and roughness are presented in Table 1.
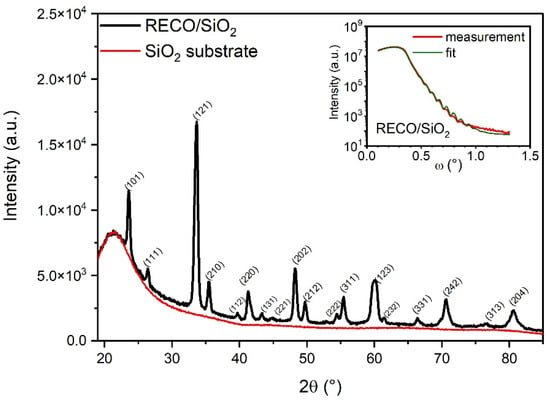
Figure 1.
X-ray diffraction pattern of RECO thin film deposited onto a fused quartz (SiO2) substrate. Inset shows a plot with fitting and experimental X-ray reflectivity profile for RECO thin film layer on a quartz substrate.

Table 1.
XRR results calculated for RECO thin film on a quartz substrate.
The XRD pattern obtained for the RECO thin film presents a good fit with the orthorhombic group of the Pnma symmetry (as indexed in ICSD 01-086-1208), which is consistent with our previous work on bulk RECO material [41]. The presence of distinct peaks in the XRD profile coming from different crystallographic planes with a pattern with the space group Pnma supports the conclusion of disoriented crystallite growth on the amorphous SiO2 substrate. This observation equivalently indicates the absence of epitaxial growth, which can be attributed to the lack of a regular lattice structure, and the inability to induce a preferred crystallographic orientation on the RECO thin film [45]. The interplanar spacing calculated from the position of the peaks is smaller than 0.2% compared to the RECO bulk powder sample. Grain sizes were determined by applying the Debye Scherrer formula to the Full Width at Half Maximum (FWHM) values of the peaks [46]. The calculated grain sizes for these peaks range from 13 to 20 nm. These results suggest that the structure and properties of the RECO thin film may differ from those of the bulk material.
X-ray photoelectron spectroscopy (XPS) has been used to investigate the surface chemistry of the deposited RECO thin film. The O 1s spectrum depicted in Figure 2a shows two peaks—the first one at 528.5 eV originating from lattice oxygen in metal oxides and the second one at 531.1 eV, which comes from either defective oxygen or organic contamination [47]. The XPS Co 2p3/2 region spectra (Figure 2b) are similar and show multiplet splitting structure characteristics for first-row transition metal species containing unpaired electrons. From the inspection of line positions (first peak centered at 779.5 eV) and the very low intensity of shake-up satellites, it can be stated that cobalt is in Co3+ and Co2+ oxidation states such as in Co3O4 [47,48,49]. The XPS Sm 3d5/2 spectrum is shown in Figure 2c. The Sm 3d5/2 core level peak maximum was found at 1081.5 eV, indicating the literature value characteristic for samarium oxidation state Sm3+ [50]. The XPS Gd 4d spectrum is depicted in Figure 2d. From inspection of the fitted line positions (main peak at 140.5 eV) and the spectrum shape, it can be stated that gadolinium exists predominantly in the Gd3+ oxidation state, as observed in gadolinium oxide (Gd2O3) [51]. The Nd 3d5/2 spectra are shown in Figure 2e. The observed overlap between the peaks of neodymium (Nd) and the Auger oxygen (O) KLL lines was taken into consideration during the fitting process. Despite this overlap, the Auger lines were successfully extracted from the data. Nd 3d5/2 lines were found at about 980 eV, indicating the metallic state of the neodymium [52]. The La 3d5/2 spectra presented in Figure 2f were fitted with two components. In the case of lanthanum (La), only one chemical state is observed. However, due to the phenomenon of multiplet splitting, each state manifests as two distinct lines. The assigned chemical state for lanthanum (La) was determined to be La2O3 based on the observed lines at the binding energies of 832 and 837 eV [50,51]. Deconvolution of spectra collected for Y 3d regions results in lines centered at positions typical for Yttrium in the Y3+ oxidation state, similar to that found in YSZ (Figure 2g) [50]. The presence of bismuth has been detected in the spectrum of yttrium, and from the position of the line of Bi 4f7/2, it can be determined that bismuth is in the oxidation state of Bi3+ [50,51]. The bismuth traces identified on the surface can be attributed to the contamination from the pulsed laser deposition chamber, which was instinctively used to prepare BiFeO3 layers before RECO/SiO2 film deposition.
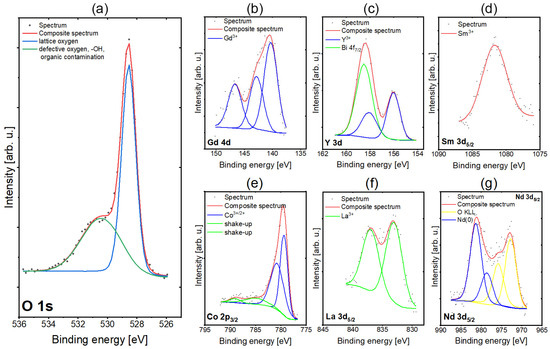
Figure 2.
High-resolution individual XPS spectra of RECO thin films for binding energies and chemical states and peak fitting for (a) oxygen, (b) cobalt, (c) samarium, (d) gadolinium, (e) neodymium, (f) lanthanum and (g) yttrium.
Atomic concentrations of each element from the RECO system derived from XPS analysis, as presented in Table 2, allow for the determination of the stoichiometry of the analyzed samples. It is observed that the stoichiometry can be expressed as (Y0.29La0.22Nd0.18Sm0.19Gd0.27)CoO3.43, which is determined to exhibit a high degree of agreement with the theoretical formulation for a multicomponent RECO structure. This result provides strong evidence of the successful synthesis of the RECO material with the desired composition.

Table 2.
Surface composition (at-%) determined by XPS after pre-sputtering the surface with a 2 kV Ar+ ion beam.
The conductivity of the RECO on a nanoscale amorphous quartz layer was investigated using atomic force microscopy operating in the PF-TUNA mode, allowing simultaneous gathering of the sample surface topography images along with its conductivity parameters, including current distribution image and current-voltage (I-U) plots. Conductivity measurements in the studied system are based on the electrical contact between the AFM probe and the conductive surface of the path [53,54]. It should be noted that the nature of the contact and the resulting conductivity parameters can be influenced by variations in surface roughness, resulting in different contact areas between the AFM tip and the sample surface. The AFM topography and current images were measured at room temperature (298 K) and the elevated temperatures of 323, 348, and 373 K. The graphical data in Figure 3a,b clearly demonstrate a significant increase in conductivity observed at the surface as the temperature applied to the thin film increases. The analysis of the cross-section profile presented in Figure 3c enabled the determination of the average surface roughness of the sample, yielding a value of 2.1 ± 0.4 nm, and it exhibited no significant changes with variations in temperature or electric current, indicating its thermal and electrical stability. Furthermore, the relatively small roughness of the thin film makes it non-susceptible to the common effect of measuring higher current at the contact areas of the measuring tip and its sides with the surface of the sample. This characteristic ensures that the mapped conductivity distribution remains relatively homogeneous and is unaffected by the surface non-uniformity effects, providing a balanced representation of the current distribution image. The corresponding I−U plots (Figure 3d) confirm the observed behavior, as the current values reach up to 1 nA for potentials above 4 V. The obtained current-voltage plots were used to calculate the activation energy (Ea), which was found to be 1.23 ± 0.12 eV using the Arrhenius plot from Figure 3e.
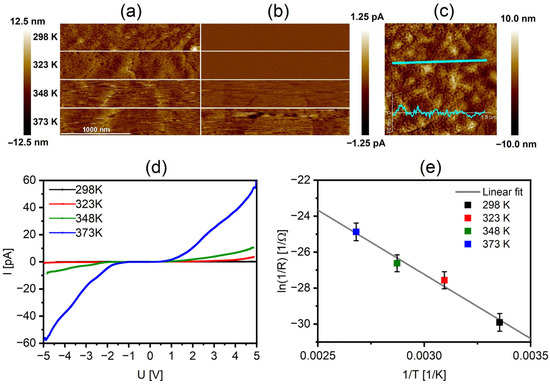
Figure 3.
AFM images of the RECO on SiO2 thin film recorded at 298, 323, 348, and 373 K: (a) topography, (b) current distribution image, (c) cross-section profile (d) I−U plots in the range between–5 and 5 V, and (e) two-point probe method plots of ln(1/R) as a function of the reciprocal of the temperature (1/T) using the Arrhenius equation.
The UV-VIS spectroscopic measurements were performed on the deposited RECO layer on the quartz substrate, and the measured absorbance of the thin layer was used to determine the energy gap of the semiconductor and the nature of its optical transitions. In this study, we implemented the Inverse Logarithmic Derivative (ILD) method to analyze the absorbance data for the RECO film deposited on the quartz substrate, according to the calculation method described in the reference [55]. The resulting graph (Figure 4a) exhibits three distinct regions, each corresponding to different optical transitions. The first is characterized by strong absorption, indicating the direct allowed transition of electrons from the valence band to the conduction band [56]. The energy gaps and the corresponding parameters m were then calculated from the graph, and the results are presented in Table 3. Analysis reveals that the RECO layer exhibits direct allowed transitions with a modified dispersion constant only for parameter m = 0.77 and the corresponding energy gap (Ea) of 0.88 eV. Additionally, the presence of two other absorption peaks suggests the involvement of other types of transitions, including indirect allowed and direct/indirect forbidden transitions [57,58]. The observed phenomena could potentially arise from the nanometer thickness of the RECO semiconducting film and the refractive index of the quartz substrate and have direct implications for the design and optimization of the optoelectronic devices, both of which may potentially rely on the RECO thin film [59,60]. Further investigation of the underlying factors contributing to the modified dispersion constant or effective dimensionality could lead to a deeper understanding of the unique properties of the RECO thin film.
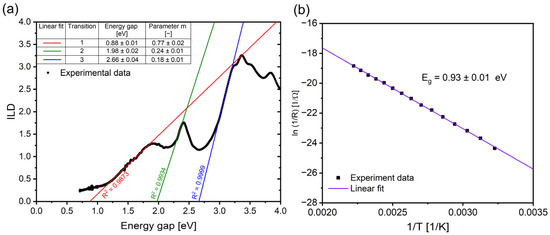
Figure 4.
(a) the Inverse Logarithmic Derivative (ILD) of αhv as a function of photon energy hv with fitted straight lines for RECO/SiO2 and measured energy gap values and the transition parameter m. (b) calculated energy gap and plot of ln(1/R) as a function of the reciprocal of temperature (1/T) obtained using the Arrhenius equation derived from the four-point resistance method (FPRM).

Table 3.
Energy gap from different methods.
Figure 4b depicts a plot of the natural logarithm of the reciprocal of resistance (ln(1/R)) as a function of the reciprocal of temperature (1/T) derived from the four-point resistance method (FPRM). The energy gap was determined from the Arrhenius plot using the slope of the ln(R) versus (1/T) line, which was calculated using the Arrhenius equation [61]:
where E represents the energy gap, k is the Boltzmann constant, R0 and R1 are the resistances at the initial and final temperatures, and Δ(1/T) is the change in the reciprocal of temperature. The energy gap values obtained for the RECO thin film system exhibit relatively consistent results across different measurement methods, differing by no more than 30%. In particular, the energy gaps obtained from the AFM U−I, FPRM, and UV/VIS methods were found to be 1.23, 0.93, and 0.88 eV, respectively. These findings indicate that RECO thin film may be a promising candidate for optoelectronic and electrocatalyst devices requiring narrow energy gap values. Examples of such applications include oxygen evolution catalysis [62], low-energy light-emitting diodes [63,64], or near-infrared photodetectors [65]. The combination of a narrow energy gap with relatively good visible light adsorption may allow the RECO thin films to become a promising component to consider for solar cell application [66]. Furthermore, the observed increase in conductivity with rising temperatures indicates that the energy carriers play an increasing role in the optical transition of the RECO thin film [67]. These observations highlight the importance of understanding the modified dispersion constant or effective dimension of the material in affecting both the electrical and optical properties of potential RECO-based electronic devices. Further investigation of these aspects is essential to gain deeper insights and facilitate the development of the potential optimized RECO-based electronic devices.
4. Conclusions
In this study, the RECO thin film was successfully deposited on the amorphous SiO2 substrate. Structural and chemical analysis revealed that the thin film with an orthorhombically distorted (Pnma) crystal lattice is consistent with the bulk material. The film was found to be composed of Y, La, Nd, Sm, Gd, Co, and O elements with stoichiometry consistent with the theoretical composition. Additionally, the presence of a Co element in both Co2+ and Co3+ oxidation states has been confirmed, while the rare-earth elements were found to be in their trivalent states. Electronic and optical measurements revealed activation energy values of 1.23 eV (AFM U-I), 0.93 eV (FPRM), and 0.88 (UV/VIS), respectively. The measured values categorize the RECO thin film as a narrow gap semiconductor material and indicate that it exhibits distinct electronic and optical properties compared to its bulk form, thus enabling the RECO material to be susceptible to tuning the energy gap value by nanostructuring or introducing local strain in thin film fabrication. Furthermore, the notable narrow energy gap of RECO, coupled with its relatively good absorption in the visible spectrum, makes it a compelling candidate for further investigation for applications in low-energy infrared optical devices, electrocatalysis of the oxygen evolution reaction, and efficient light energy storage in solar cells. Further studies are needed to explore the potential applications of the RECO thin film and to optimize the deposition process to improve and tune its structural and optoelectronic properties. Nevertheless, the successful deposition and characterization of the RECO thin film provide a basis for future research on the properties and potential applications of this material.
Author Contributions
Conceptualization, P.A.K. and A.Ż.; data curation, P.A.K., W.S., A.Ż., M.S., M.M., J.P. and J.K.; formal analysis, P.A.K. and A.Ż.; writing—original draft preparation, P.A.K., W.S., A.Ż., M.S., M.M., J.P. and J.K., writing—review and editing, P.A.K., W.S., A.Ż., M.S., M.M., J.P. and J.K.; visualization, P.A.K. and W.S.; validation, A.Ż., W.W.K. and M.D., supervision, A.Ż. and W.W.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Acknowledgments
We would like to thank Witold Reczyński of the AGH University of Science and Technology for his comments and final revision of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-Entropy Alloy: Challenges and Prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.-P. Entropy-Stabilized Oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and Microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 High Entropy Oxide Characterized by Spinel Structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Djenadic, R.; Sarkar, A.; Clemens, O.; Loho, C.; Botros, M.; Chakravadhanula, V.S.K.; Kübel, C.; Bhattacharya, S.S.; Gandhi, A.S.; Hahn, H. Multicomponent Equiatomic Rare Earth Oxides. Mater. Res. Lett. 2017, 5, 102–109. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-Entropy Ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Zhang, R.-Z.; Reece, M.J. Review of High Entropy Ceramics: Design, Synthesis, Structure and Properties. J. Mater. Chem. A 2019, 7, 22148–22162. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Bała, P.; Górecki, K.; Bednarczyk, W.; Wątroba, M.; Lech, S.; Kawałko, J. Effect of High-Temperature Exposure on the Microstructure and Mechanical Properties of the Al5Ti5Co35Ni35Fe20 High-Entropy Alloy. J. Mater. Res. Technol. 2020, 9, 551–559. [Google Scholar] [CrossRef]
- High-Entropy Alloys—2nd Edition. Available online: https://www.elsevier.com/books/high-entropy-alloys/murty/978-0-12-816067-1 (accessed on 13 May 2023).
- Rost, C.M. Entropy-Stabilized Oxides: Explorations of a Novel Class of Multicomponent Materials. Ph.D. thesis, North Carolina State University, Raleigh, NC, USA, 2016. [Google Scholar]
- Zhang, Y.; Zhang, Z.; Wang, X.; Yao, W.; Liang, X. Structure and Properties of High-Entropy Amorphous Thin Films: A Review. JOM 2022, 74, 794–807. [Google Scholar] [CrossRef]
- Zheng, Y.; Zou, M.; Zhang, W.; Yi, D.; Lan, J.; Nan, C.-W.; Lin, Y.-H. Electrical and Thermal Transport Behaviours of High-Entropy Perovskite Thermoelectric Oxides. J. Adv. Ceram. 2021, 10, 377–384. [Google Scholar] [CrossRef]
- Ferro, S.M.; Wobben, M.; Ehrler, B. Rare-Earth Quantum Cutting in Metal Halide Perovskites—A Review. Mater. Horiz. 2021, 8, 1072–1083. [Google Scholar] [CrossRef]
- Dhole, S.; Chen, A.; Nie, W.; Park, B.; Jia, Q. Strain Engineering: A Pathway for Tunable Functionalities of Perovskite Metal Oxide Films. Nanomaterials 2022, 12, 835. [Google Scholar] [CrossRef] [PubMed]
- Corey, Z.J.; Lu, P.; Zhang, G.; Sharma, Y.; Rutherford, B.X.; Dhole, S.; Roy, P.; Wang, Z.; Wu, Y.; Wang, H.; et al. Structural and Optical Properties of High Entropy (La,Lu,Y,Gd,Ce)AlO3 Perovskite Thin Films. Adv. Sci. 2022, 9, 2202671. [Google Scholar] [CrossRef]
- Uehara, M.; Mori, S.; Chen, C.-L.; Cheong, S.-W. Percolative Phase Separation Underlies Colossal Magnetoresistance in Mixed-Valent Manganites. Nature 1999, 399, 560–563. [Google Scholar] [CrossRef]
- Dagotto, E.; Hotta, T.; Moreo, A. Colossal Magnetoresistant Materials: The Key Role of Phase Separation. Phys. Rep. 2001, 344, 1–153. [Google Scholar] [CrossRef]
- Bresolin, B.-M.; Park, Y.; Bahnemann, D.W. Recent Progresses on Metal Halide Perovskite-Based Material as Potential Photocatalyst. Catalysts 2020, 10, 709. [Google Scholar] [CrossRef]
- Tabish, A.; Varghese, A.M.; Wahab, M.A.; Karanikolos, G.N. Perovskites in the Energy Grid and CO2 Conversion: Current Context and Future Directions. Catalysts 2020, 10, 95. [Google Scholar] [CrossRef]
- Górecki, K.; Bała, P.; Bednarczyk, W.; Kawałko, J. Cryogenic Behaviour of the Al5Ti5Co35Ni35Fe20 Multi-Principal Component Alloy. Mater. Sci. Eng. A 2019, 745, 346–352. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Li, H.-B.; Hattori, A.N.; Tanaka, H. Comprehensive Determination of Proton Diffusion in Protonated NdNiO3 Thin Film by a Combination of Electrochemical Impedance Spectroscopy and Optical Observation. Appl. Phys. Express 2023, 16, 035501. [Google Scholar] [CrossRef]
- Qi, H.; Chen, L.; Deng, S.; Chen, J. High-Entropy Ferroelectric Materials. Nat. Rev. Mater. 2023, 8, 355–356. [Google Scholar] [CrossRef]
- Kante, M.V.; Weber, M.L.; Ni, S.; van den Bosch, I.C.G.; van der Minne, E.; Heymann, L.; Falling, L.J.; Gauquelin, N.; Tsvetanova, M.; Cunha, D.M.; et al. A High-Entropy Oxide as High-Activity Electrocatalyst for Water Oxidation. ACS Nano 2023, 17, 5329–5339. [Google Scholar] [CrossRef] [PubMed]
- Esquius, J.R.; Liu, L. High Entropy Materials as Emerging Electrocatalysts for Hydrogen Production through Low-Temperature Water Electrolysis. Mater. Futures 2023, 2, 022102. [Google Scholar] [CrossRef]
- Tu, J.; Ding, J.; Xi, G.; Li, H.; Yang, Q.; Tian, J.; Zhang, L. Controllable Chemical Composition in Double-Perovskite Bi0.5Sm0.5FeO3 Epitaxial Thin Films for Ferroelectric, Photovoltaic, and Ferromagnetic Properties. Chem. Eng. J. 2023, 453, 139726. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, Z.; Song, Y.; Yang, G.; Qian, W.; Yang, M.; Zhu, Y.; Ran, R.; Wang, W.; Zhou, W.; et al. High-Entropy Perovskite Oxide: A New Opportunity for Developing Highly Active and Durable Air Electrode for Reversible Protonic Ceramic Electrochemical Cells. Nano-Micro Lett. 2022, 14, 217. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Zhivulin, V.E.; Trofimov, E.A.; Gudkova, S.A.; Punda, A.Y.; Valiulina, A.N.; Gavrilyak, M.; Zaitseva, O.V.; Taskaev, S.V.; Khandaker, M.U.; et al. A-Site Cation Size Effect on Structure and Magnetic Properties of Sm(Eu,Gd)Cr0.2Mn0.2Fe0.2Co0.2Ni0.2O3 High-Entropy Solid Solutions. Nanomaterials 2022, 12, 36. [Google Scholar] [CrossRef]
- Goko, T.; Arguello, C.J.; Hamann, A.; Wolf, T.; Lee, M.; Reznik, D.; Maisuradze, A.; Khasanov, R.; Morenzoni, E.; Uemura, Y.J. Restoration of Quantum Critical Behavior by Disorder in Pressure-Tuned (Mn,Fe)Si. npj Quantum Mater. 2017, 2, 44. [Google Scholar] [CrossRef]
- Sales, B.C.; Jin, K.; Bei, H.; Stocks, G.M.; Samolyuk, G.D.; May, A.F.; McGuire, M.A. Quantum Critical Behavior in a Concentrated Ternary Solid Solution. Sci. Rep. 2016, 6, 26179. [Google Scholar] [CrossRef]
- Chen, Q.; Han, T.; Zeng, J.; He, Z.; Liu, Y.; Sun, J.; Tang, M.; Zhang, Z.; Gao, P.; Liu, G. Perovskite-Based Memristor with 50-Fold Switchable Photosensitivity for In-Sensor Computing Neural Network. Nanomaterials 2022, 12, 2217. [Google Scholar] [CrossRef]
- Zeng, F.; Guo, Y.; Hu, W.; Tan, Y.; Zhang, X.; Feng, J.; Tang, X. Opportunity of the Lead-Free All-Inorganic Cs3Cu2I5 Perovskite Film for Memristor and Neuromorphic Computing Applications. ACS Appl. Mater. Interfaces 2020, 12, 23094–23101. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Han, H.; Park, C. Nanostructured Perovskites for Retina-Inspired Structurally Tunable Synapse. In Proceedings of the International Conference on Perovskite Memristors and Electronics 2021 (ICPME2021), Online, 13–14 December 2021. [Google Scholar] [CrossRef]
- Huang, X.; Guo, Y.; Liu, Y. Perovskite Photodetectors and Their Application in Artificial Photonic Synapses. Chem. Commun. 2021, 57, 11429–11442. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, X.; Liu, Q.; Jiang, Y.; Zhong, W.; Luo, F. An Artificial Synapse Based on CsPbI3 Thin Film. Micromachines 2022, 13, 284. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Han, J.S.; Hong, K.; Kim, S.Y.; Jang, H.W. Organic–Inorganic Hybrid Halide Perovskites for Memories, Transistors, and Artificial Synapses. Adv. Mater. 2018, 30, 1704002. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.K.; Ojha, S.K.; Kumar, S.; Saha, A.; Mandal, P.; Freeland, J.W.; Middey, S. Epitaxial Stabilization of Ultra Thin Films of High Entropy Perovskite. Appl. Phys. Lett. 2020, 116, 071601. [Google Scholar] [CrossRef]
- Son, Y.; Zhu, W.; Trolier-McKinstry, S.E. Electrocaloric Effect of Perovskite High Entropy Oxide Films. Adv. Electron. Mater. 2022, 8, 2200352. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, T.; Gild, J.; Zhou, N.; Nie, J.; Qin, M.; Harrington, T.; Vecchio, K.; Luo, J. A New Class of High-Entropy Perovskite Oxides. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Kotsonis, G.N.; Rost, C.M.; Harris, D.T.; Maria, J.-P. Epitaxial Entropy-Stabilized Oxides: Growth of Chemically Diverse Phases via Kinetic Bombardment. MRS Commun. 2018, 8, 1371–1377. [Google Scholar] [CrossRef]
- Schlom, D.G.; Chen, L.-Q.; Pan, X.; Schmehl, A.; Zurbuchen, M.A. A Thin Film Approach to Engineering Functionality into Oxides. J. Am. Ceram. Soc. 2008, 91, 2429–2454. [Google Scholar] [CrossRef]
- Krawczyk, P.A.; Jurczyszyn, M.; Pawlak, J.; Salamon, W.; Baran, P.; Kmita, A.; Gondek, Ł.; Sikora, M.; Kapusta, C.; Strączek, T.; et al. High-Entropy Perovskites as Multifunctional Metal Oxide Semiconductors: Synthesis and Characterization of (Gd0.2Nd0.2La0.2Sm0.2Y0.2)CoO3. ACS Appl. Electron. Mater. 2020, 2, 3211–3220. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-Solution Phase Formation Rules for Multi-Component Alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Jędrusik, M.; Cieniek, Ł.; Kopia, A.; Turquat, C.; Leroux, C. Structural Characterization of LaCoO3 Thin Films Grown by Pulsed Laser Deposition. Arch. Metall. Mater. 2020, 65, 793–797. [Google Scholar] [CrossRef]
- Schwarcz, D.; Burov, S. The Effect of Disordered Substrate on Crystallization in 2D. J. Phys. Condens. Matter 2019, 31, 445401. [Google Scholar] [CrossRef] [PubMed]
- Salaheldeen, M.; Garcia, A.; Corte-Leon, P.; Ipatov, M.; Zhukova, V.; Zhukov, A. Unveiling the Effect of Annealing on Magnetic Properties of Nanocrystalline Half-Metallic Heusler Co2FeSi Alloy Glass-Coated Microwires. J. Mater. Res. Technol. 2022, 20, 4161–4172. [Google Scholar] [CrossRef]
- Biesinger, M.; Payne, B.; Grosvenor, A.; Lau, L.; Gerson, A.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Okamoto, Y.; Adachi, T.; Maezawa, A.; Imanaka, T. Effect of ZnO Addition on Cobalt–Alumina Interaction Species. Bull. Chem. Soc. Jpn. 1991, 64, 236–242. [Google Scholar] [CrossRef]
- Dash, K.; Folkesson, B.; Larsson, R.; Mohapatra, M. An XPS Investigation on a Series of Schiff Base Dioxime Ligands and Cobalt Complexes. J. Electron Spectrosc. Relat. Phenom. 1989, 49, 343–357. [Google Scholar] [CrossRef]
- Wagner, A.; Naumkin, A.; Kraut-Vass, A.; Allison, J.; Powell, C.; Rumble, J. NIST X-Ray Photoelectron Spectroscopy Database 1, Version 2; Natl Std. Ref. Data Series (NIST NSRDS); National Institute of Standards and Technology: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Wandelt, K.; Brundle, C. The Interaction of Oxygen with Gadolinium: UPS and XPS Studies. Surf. Sci. 1985, 157, 162–182. [Google Scholar] [CrossRef]
- Uwamino, Y.; Ishizuka, T.; Yamatera, H. X-Ray Photoelectron Spectroscopy of Rare-Earth Compounds. J. Electron Spectrosc. Relat. Phenom. 1984, 34, 67–78. [Google Scholar] [CrossRef]
- Szuwarzyński, M.; Mazur, Ł.; Borkowski, M.; Maćkosz, K.; Giżyński, K.; Mazur, T. Enhanced Assembly of Ag Nanoparticles for Surface-Independent Fabrication of Conductive Patterns. ACS Appl. Nano Mater. 2022, 5, 12711–12719. [Google Scholar] [CrossRef]
- Wolski, K.; Szuwarzyński, M.; Zapotoczny, S. A Facile Route to Electronically Conductive Polyelectrolyte Brushes as Platforms of Molecular Wires. Chem. Sci. 2015, 6, 1754–1760. [Google Scholar] [CrossRef]
- Jarosiński, Ł.; Pawlak, J.; Al-Ani, S.K.J. Inverse Logarithmic Derivative Method for Determining the Energy Gap and the Type of Electron Transitions as an Alternative to the Tauc Method. Opt. Mater. 2019, 88, 667–673. [Google Scholar] [CrossRef]
- Cisneros, J.I. Optical Characterization of Dielectric and Semiconductor Thin Films by Use of Transmission Data. Appl. Opt. 1998, 37, 5262–5270. [Google Scholar] [CrossRef]
- Al-Ani, S.K.J.; Hogarth, C.A. The Optical Properties of Amorphous V2O5 and SiO Thin Films and of the Mixed Dielectric System SiO/V2O5. J. Mater. Sci. 1985, 20, 1185–1192. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Kats, M.A.; Capasso, F. Optical Absorbers Based on Strong Interference in Ultra-Thin Films. Laser Photonics Rev. 2016, 10, 735–749. [Google Scholar] [CrossRef]
- Babbe, F.; Sutter-Fella, C.M. Optical Absorption-Based In Situ Characterization of Halide Perovskites. Adv. Energy Mater. 2020, 10, 1903587. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Gustafson, W.J.; Murray, S.E.; Shoemaker, D.P.; Ertekin, E.; Krogstad, J.A.; Perry, N.H. Perovskite Na-Ion Conductors Developed from Analogous Li3xLa2/3-xTiO3 (LLTO): Chemo-Mechanical and Defect Engineering. J. Mater. Chem. A 2021, 9, 21241–21258. [Google Scholar] [CrossRef]
- Katzbaer, R.R.; dos Santos Vieira, F.M.; Dabo, I.; Mao, Z.; Schaak, R.E. Band Gap Narrowing in a High-Entropy Spinel Oxide Semiconductor for Enhanced Oxygen Evolution Catalysis. J. Am. Chem. Soc. 2023, 145, 6753–6761. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Li, Z.; Zhang, G.; Yang, G.-J. Lead-Free Perovskite [H3NC6H4NH3]CuBr4 with Both a Bandgap of 1.43 EV and Excellent Stability. J. Mater. Chem. A 2020, 8, 5484–5488. [Google Scholar] [CrossRef]
- Vashishtha, P.; Bishnoi, S.; Li, C.-H.A.; Jagadeeswararao, M.; Hooper, T.J.N.; Lohia, N.; Shivarudraiah, S.B.; Ansari, M.S.; Sharma, S.N.; Halpert, J.E. Recent Advancements in Near-Infrared Perovskite Light-Emitting Diodes. ACS Appl. Electron. Mater. 2020, 2, 3470–3490. [Google Scholar] [CrossRef]
- Ding, N.; Xu, W.; Zhou, D.; Pan, G.; Li, D.; Ji, Y.; Chen, X.; Yang, D.; Bai, X.; Ma, C.-G.; et al. Upconversion Ladder Enabled Super-Sensitive Narrowband near-Infrared Photodetectors Based on Rare Earth Doped Florine Perovskite Nanocrystals. Nano Energy 2020, 76, 105103. [Google Scholar] [CrossRef]
- Alarifi, I.M. Advanced Selection Materials in Solar Cell Efficiency and Their Properties—A Comprehensive Review. Mater. Today Proc. 2023, 81, 403–414. [Google Scholar] [CrossRef]
- Milot, R.L.; Eperon, G.E.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Temperature-Dependent Charge-Carrier Dynamics in CH3NH3PbI3 Perovskite Thin Films. Adv. Funct. Mater. 2015, 25, 6218–6227. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).