Abstract
Ni/CaO, a low-cost dual-functional material (DFM), has been widely studied for integrated CO2 capture and hydrogenation. The core of this dual-functional material should possess both good CO2 capture–conversion performance and structural stability. Here, we synthesized Ni/CaO DFMs modified with alkali metals (Na, K, and Li) through a combination of precipitation and combustion methods. It was found that Na-modified Ni/CaO (Na-Ni/CaO) DFM offered stable CO2 capture–conversion activity over 20 cycles, with a high CO2 capture capacity of 10.8 mmol/g and a high CO2 conversion rate of 60.5% at the same temperature of 650 °C. The enhanced CO2 capture capacity was attributed to the improved surface basicity of Na-Ni/CaO. In addition, the incorporation of Na into DFMs had a favorable effect on the formation of double salts, which shorten the CO2 capture and release process and promoted DFM stability by hindering their aggregation and the sintering of DFMs.
1. Introduction
The excessive emissions of anthropogenic CO2 into the atmosphere are recognized as a primary driver of global warming and climate change [1,2,3]. To combat this issue, collaborative efforts from researchers and governments are required to curtail CO2 emissions. Carbon capture and storage (CCS) have been considered the primary strategy to achieve emission reduction targets, but its high cost and significant energy consumption in CO2 purification, compression, and transportation limit its widespread application [4,5,6,7]. An alternative approach, known as CO2 capture and utilization (CCU), aims to convert the captured CO2 into value-added fuels and chemicals, offering a promising solution to the CO2 challenge [8,9,10]. Integrated CO2 capture and utilization (ICCU) technology, which combines capture and conversion in a single column under the same temperature, has garnered increasing interest due to its potential to reduce capital and operating costs while saving energy [11,12,13].
Generally, the successful implementation of ICCU relies on the development of dual-functional materials (DFMs) capable of serving as both CO2 adsorbents and catalytic components for CO2 conversion. DFMs cyclically capture CO2 from combustion flue gas with a CO2 concentration of 4–14 vol%, subsequently converting the adsorbed CO2 into valuable products, such as synthetic fuels or other chemicals, while regenerating the adsorbent. CO2 adsorbents are typically based on alkaline or alkaline–earth compounds, as they react with acidic CO2 to form corresponding carbonates. Among these materials, CaO-based composites are extensively studied in ICCU processes, due to their low cost, high availability (e.g., limestone), and excellent theoretical CO2 capacity (17.8 mmol/gCaO) [14,15]. On the other hand, catalysts such as Pt [16], Ni [17], or Fe [18] are widely used to catalyze the C1 chemistry reactions, including methanation, reverse water–gas shift (RWGS), and dry reforming of methane (DRM) in ICCU processes for CO2 conversion. As a result, Ni/CaO DFMs are extensively employed in ICCU technology due to their optimal cost-to-activity ratio [19,20,21,22,23,24,25,26,27]. However, the development of Ni/CaO DFMs with both high CO2 capture capacity and efficient catalytic activity remains a significant challenge since most CO2 conversion reactions are conducted outside at a high temperature, but CaO does not maintain its stability during carbonation–calcination cycles at high temperatures, given the relatively low Tammann temperature of CaCO3 (533 °C). Numerous efforts have been dedicated to resolving this sintering issue. In previous studies, we successfully applied a three-dimensional Ni/CaO network composed of mesopores and macropores in a stable high-temperature ICCU process thanks to its unique porosity structure [28,29]. Recently, the doping approach of DFMs with alkali metals, such as Li, Na, K, and Cs, has been considered a promising strategy to promote the ICCU process. Previous research has developed Ca adsorbents and investigated the promoting effect of alkali metals. Ahmed et al. conducted a study on a series of double salts, which included K- and Na-promoted Ca adsorbents, specifically designed for CO2 capture at 650 °C. The results of their research showed that the K−Ca double salt displayed an exceptional CO2 adsorption capacity of 10.7 mmol/g [30]. The K−Ca double-salt materials were also utilized in a combined CO2 capture–utilization process aimed at producing syngas [31]. In this process, the CO2 sorption capacity was measured at 0.95 mmol/g, with a CO2 conversion rate of 65%. However, there are limited studies available on the subject of integrated CO2 capture and hydrogenation utilizing alkali-metal-doped Ni/CaO catalysts.
Herein, we successfully synthesized and utilized modified Ni/CaO dual-functional materials for integrated CO2 capture (Equation (1)) and reverse water–gas shift (RWGS) reaction (Equation (2)). The RWGS reaction is a highly valuable CO2 conversion process that provides a promising opportunity to align the high temperature required for CO2 capture with the generation of CO, which can be further utilized as a raw material in various chemical processes to produce valuable chemicals [32]. To achieve this, we employed a facile precipitation–combustion method to synthesize Ni nanoparticles modified with alkali metals (Na, K, and Li) on CaO (M-Ni/CaO), creating effective DFMs for selective CO production through ICCU. Thorough investigations of the physical and chemical properties of the modified Ni/CaO DFMs were conducted using techniques such as XRD, N2 adsorption–desorption, SEM, EDX, TEM, CO2-TPD, and TG. The introduction of different doping elements (Na, K, and Li) into the Ni/CaO DFMs had a profound impact on CO2 capture and CO formation. Additionally, we assessed the cycle stability of the Na-doped Ni/CaO DFMs by performing 20 cycles of integrated CO2 capture and hydrogenation. The evaluation results are promising and are expected to provide valuable guidance for the development of highly effective DFMs and the establishment of an efficient pathway to enhance ICCU technology.
2. Experimental Section
2.1. Preparation of Ni/CaO and M/CaO (M = Li, Na, and K) DFMs
All reagents were procured from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and utilized without any additional purification steps. All gases were procured from Shanghai Youjiali Liquid Helium Co., Ltd. (Shanghai, China) and had a nominal purity of at least 99.99%. For Li-doped Ni/CaO DFM, 0.70 g of nickel nitrate hexahydrate (Ni(NO3)2·6H2O), 22.14 g of calcium nitrate tetrahydrate (Ca(NO3)2·4H2O), 0.28 g of lithium carbonate (Li2CO3), and 21.01 g of citric acid monohydrate (C6H8O7·H2O) were dissolved in 50 mL of an aqueous solution with a magnetic stirrer at 40 °C under sufficient agitation. Then, 1.0 mol/L of an ammonium carbonate ((NH4)2CO3) solution was added dropwise using a peristaltic pump until the pH value of the mixed solution reached 8.0, where the nickel and calcium were precipitated in the form of carbonates species. The suspension was further evaporated in the superfluous water at 80 °C. The obtained solid was dried at 100 °C overnight and then calcined at 200 °C for 2 h and 700 °C for 5 h, with a heating rate of 2 °C/min. The precursor particles consisted of citric acid complexes and carbonated species of nickel and calcium, which underwent decomposition during the combustion stage at a high calcination temperature. The Na- and K-doped Ni/CaO DFMs were prepared using the same process as described above, and the mass fractions of Ni, the added carbonates, and CaO in each DFM were kept at 2.5 wt%, 5 wt%, and 92.5 wt%, respectively. The obtained sample was denoted as M-Ni/CaO, where M refers to the added alkali metal element (Li, Na, and K). For comparison, the Ni/CaO DFM was also prepared and used as the reference sample.
2.2. Characterization of Ni/CaO and M-Ni/CaO (M = Li, Na, and K) DFMs
The crystalline structures of the DFMs were investigated using a Bruker D8 Advance diffractometer to perform X-ray diffraction (XRD) with Cu Kα radiation (λ = 0.15418 nm). The patterns were collected in the 2θ range of 10° to 90°with a scanning speed of 8°/min. The actual masses of Ni and alkali metal elements (Li, Na, and K) in the DFMs were measured via inductively coupled plasma−atomic emission spectrometry (ICP−AES) conducted on a PerkinElmer emission spectrometer. The specific surface area and the pore size distribution were determined via N2 adsorption–desorption at −196 °C on a Micromeritics ASAP 2020 Sorptometer (Micromeritics Instrument Ltd., Shanghai, China. The DFMs were degassed for 6 h at 200 °C prior to each measurement. The surface morphologies and elemental distribution of the DFMs were performed using an FEI Nova Nano SEM 450 (FEI Company, Hillsboro, OR, USA) scanning electron microscope (SEM) coupled with an EDAX energy-dispersive X-ray spectrometer. All samples were sputtered with a thin layer of Pt via low-vacuum sputter coating before imaging via SEM. The transmission electron microscope (TEM) images of the DFMs were obtained on a JEOL JEM-2010F field-emission scanning electron microscope operating at a working voltage of 200 kV. CO2 temperature-programmed desorption (CO2-TPD) of the DFMs was determined using an automatic PCA-1200 (Beijing builder Co., Ltd., Beijing, China) chemisorption analyzer. Prior to each measurement, 0.2 g of DFMs was used for pretreatment with Ar at 700 °C for 1 h and then cooled to room temperature. Then, chemical adsorption was conducted using a 10 vol% CO2/Ar for 1 h at 80 °C, followed by desorption under pure Ar at temperatures ranging from room temperature to 900 °C.
2.3. CO2 Capture and Capture–Release of Ni/CaO and M-Ni/CaO (M = Li, Na, and K) DFMs
CO2 capture and capture–release experiments of the DFMs were conducted using an SDT650 (TA Instruments, New Castle, DE, USA) thermogravimetric analyzer. For the CO2 capture experiment, approximately 8 mg of each DFM was heated to a test temperature (550, 600, 650, or 700 °C) at a heating rate of 10 °C/min in pure Ar at 100 mL/min for 0.5 h to eliminate impurities. Subsequently, a 10 vol% CO2/Ar at 100 mL/min was introduced for CO2 capture for 2 h. For the CO2 capture–release test, about 8 mg of each DFM was heated up to 650 °C in pure Ar for 0.5 h. After 0.5 h of CO2 capture in 10 vol% CO2/Ar at 100 mL/min, pure Ar was introduced for CO2 release for 1.5 h. Additionally, 10 cycles of CO2 capture–release tests were performed to evaluate the cyclic stability of the DFMs.
2.4. Integrated CO2 Capture and Hydrogenation of Ni/CaO and M-Ni/CaO (M = Li, Na, and K) DFMs
The integrated CO2 capture and hydrogenation were conducted in a fixed-bed column with a vertical quartz reactor. A thermocouple was put in the middle of the reactor to detect the actual bed temperature. The reactants CO2 and H2, as well as the dilution gases of Ar, were precisely controlled with mass flow controllers (MFCs) and thoroughly mixed before being introduced into the reactor. Briefly, 1 g of the pelletized sample was pre-reduced in a 10 vol% H2/Ar (100 mL/min) at 650 °C for 3 h. Then, CO2 capture was conducted in a 10 vol% CO2/Ar (100 mL/min) for 1 h. Subsequently, the DFMs were hydrogenated in a 10 vol% H2/Ar (100 mL/min) after the reactor was purged with Ar for 10 min. The outlet gas was analyzed online using a GC9800 gas chromatograph. In addition, 20 cycles of combined CO2 capture and hydrogenation were performed to investigate the cyclic stability of Na-Ni/CaO.
The concentrations of the reactants (CO2 and H2) and the product (CO) in the outlet gases were calculated based on Equation (3). In addition, there was no CH4 detected in the outlet gases. The conversion of CO2 (CCO2) was calculated by following Equation (4).
An SDT650 (TA Instruments, New Castle, DE, USA) thermogravimetric analyzer was used to analyze the capacities of the captured and released CO2 of DFMs. The DFMs before and after the CO2 capture stage or the hydrogenation stage were heated to 1000 °C in pure Ar. The CO2 capture (Nac), release capacity (Ndc), carbon balance, and CO yield (YCO) of DFMs were calculated based on the following equations:
where m0 and m1 represent the masses of the DFMs before and after the CO2 capture stage, m2 and m3 represent the masses of the DFMs before and after the hydrogenation stage, and MCO2 represents the molecular weight of CO2.
3. Results and Discussion
3.1. Characterization of the Ni/CaO and M-Ni/CaO (M = Li, Na, and K) DFMs
Figure 1a includes the XRD patterns of the calcined Ni/CaO modified with alkali metals (Li-Ni/CaO, Na-Ni/CaO, and K-Ni/CaO) and Ni/CaO. Intense and narrow diffraction peaks at around 32.2°, 37.3°, 53.9°, 64.2°, and 67.4° could be observed for all the prepared materials, which are characteristic of highly crystalline CaO with a cubic structure [33]. The weak peaks around 18.1° and 34.1° are characteristic of Ca(OH)2, which is related to the existence of moisture. Furthermore, small diffraction peaks at 43.3° and 62.8° are ascribed to the characteristic peak of NiO. The addition of Na and K did not give rise to new crystalline phases, indicating that alkali metals were present in an amorphous and/or highly dispersed state, and the crystalline structure of the CaO remained unchanged, but a slight decrease in the intensity of the characteristic diffraction peaks of NiO was observed for Na-Ni/CaO and K-Ni/CaO compared with Ni/CaO, which is attributed to the stabilization of metal particles caused by the doping of alkali metals [34,35]. However, a new peak with higher crystallinity attributed to Li0.3Ni1.7O2 was detected for Li-Ni/CaO [36].
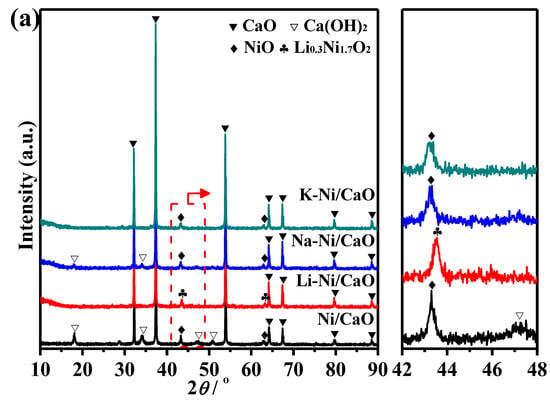
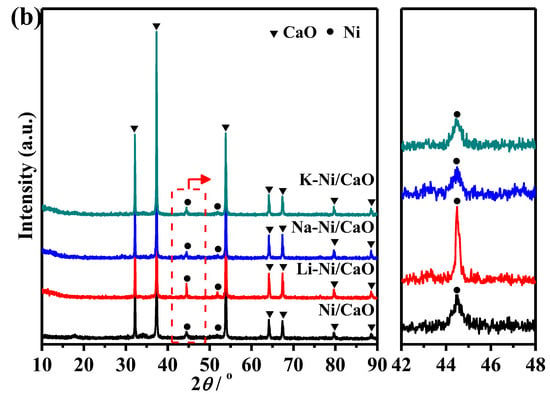
Figure 1.
XRD patterns of (a) calcined and (b) reduced Ni/CaO and M-Ni/CaO (M = Li, Na, and K) DFMs.
The crystal phase of the different samples after subjecting them to the reduction pretreatment at 650 °C in the 10 vol% H2/Ar mixture for 3 h was also assessed in the XRD patterns (Figure 1b). All samples maintained the intense and narrow diffraction peaks belonging to CaO, indicating the high stability of this oxide. As expected, no characteristic diffraction peaks were discernible for Ca(OH)2, Li0.3Ni1.7O2, and NiO phases. Diffraction peaks at 44.5° and 51.8° were ascribed to metallic Ni, which originated from the reduction of NiO and/or Li0.3Ni1.7O2. The average crystallite sizes of Ni in the reduced materials were estimated using the Scherrer equation, and the results are shown in Table 1. Na-Ni/CaO and K-Ni/CaO presented relatively smaller Ni crystallites, indicating that the addition of Na and K was conducive to the dispersion of Ni. The small size of Ni particles is considered crucial for achieving high catalytic activity in CO2 hydrogenation [37,38]. Therefore, as Li-Ni/CaO had larger Ni crystallites than Ni/CaO, it revealed the most severe Li0.3Ni1.7O2 aggregation.

Table 1.
Physicochemical properties of the reduced Ni/CaO and M-Ni/CaO (M = Li, Na, and K) DFMs.
Regarding textural properties, Figure 2 shows the N2 adsorption–desorption isotherms and pore size distributions for the reduced DFMs. All of them exhibited a type-IV isotherm and an H3-shaped hysteresis loop, which are characteristic of slit-shaped pores formed by the decomposition of the citric acid complex and carbonated species. As shown in Table 1, the addition of alkali metals to Ni/CaO resulted in a slight decrease in the specific surface areas to 12.6 and 12.2 m2/g for Na-Ni/CaO and K-Ni/CaO, respectively, whereas these DFMs maintained comparable pore volumes as well as average pore sizes. As for Li-Ni/CaO, the specific surface area, pore volume, and average pore size dramatically decreased to 5.9 m2/g, 0.04 cm3/g, and 19.8 nm, respectively, due to the pore structure blockage caused by the large Li0.3Ni1.7O2 particles. The larger specific surface area of DFMs is believed to correlate with high CO2 capture capacity [39]. In addition, Figure 2 shows that Na-Ni/CaO and K-Ni/CaO maintained bimodal pore size distributions with mesopores in the range of 2–5 nm and macropores in the range of 20–100 nm, which was similar to the pore structure of three-dimensional networks consisting of meso- and macropores with excellent elasticity and stability for Ni/CaO, as previously reported [30,31]. Meso- and macropores are responsible for CO2 uptake and diffusion control, respectively [40].
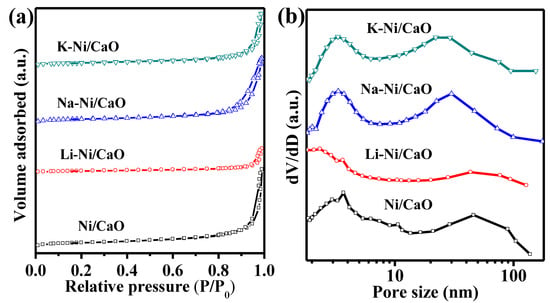
Figure 2.
(a) N2 adsorption–desorption isotherms and (b) pore size distributions of the reduced Ni/CaO (☐), Li-Ni/CaO (○), Na-Ni/CaO (△) and K-Ni/CaO (▽) DFMs.
To investigate the effect of alkali metal doping on the morphology and particle size of the reduced DFMs, SEM/EDX and TEM were conducted. As shown in Figure 3a–d, the Ni/CaO DFMs prepared via a facile precipitation–combustion method exhibited a three-dimensional network with porous morphology, which was composed of CaO particles ranging in size from 50 to 100 nm. The porous morphology was maintained very well after doping with alkali metals, including Na and K. However, it was noticed that the porous structure of Li-Ni/CaO partly collapsed, which agreed with the obvious decrease in specific surface areas. Additionally, SEM-EDX analysis was conducted to explore the distribution mappings of Na, Ni, and Ca elements in Na-Ni/CaO (Figure 3e,f). As could be observed, Na (blue color), Ni (red color), and Ca (yellow color) elements coexisted with a homogeneous distribution in all the analyzed areas, indicating their good dispersion. From the TEM image of Ni/CaO (Figure 4a), some dark crystal particles were observed with a mean size of ca. 15 nm distributed on the surface, where the lattice distance of 0.203 nm could be clearly seen and represented the lattice plane (111) of the metallic Ni phase [41]. After doping with alkali metals, the average sizes of Ni nanoparticles on the Li-, Na-, and K-Ni/CaO DFMs varied as 21.8 ± 3.8 nm, 12.2 ± 2.0 nm, and 13.3 ± 2.3 nm (Figure 4b–d), respectively, which was consistent with the XRD results (Table 1), suggesting that the introduction of Na or K into Ni/CaO DFMs certainly help to uniform and stabilize Ni particles to achieve the expected catalytic activity.
Figure 3.
SEM images of (a) Ni/CaO, (b) Li-Ni/CaO, (c) Na-Ni/CaO, (d) K-Ni/CaO DFMs, and (e) EDX and (f) elemental mappings of Na-Ni/CaO DFM.
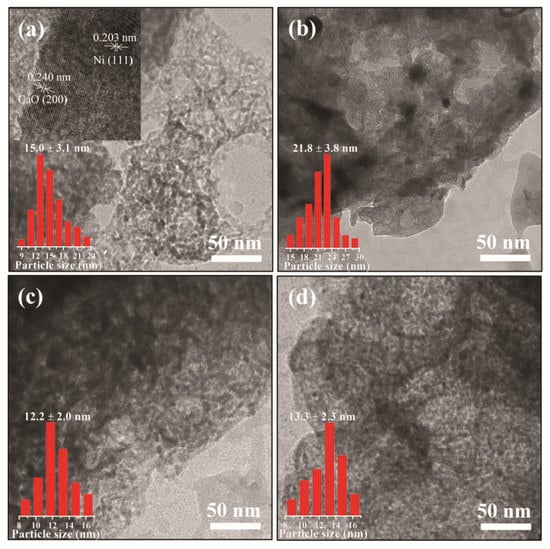
Figure 4.
TEM images of (a) Ni/CaO, (b) Li-Ni/CaO, (c) Na-Ni/CaO, and (d) K-Ni/CaO DFMs.
The effect of alkali metal doping on CO2 capture and activation was evaluated in CO2-TPD experiments on Ni/CaO and M-Ni/CaO DFMs, which were pre-reduced in situ under H2 at 650 °C before CO2 adsorption at 80 °C. As shown in Figure 5, three types of CO2 desorption peaks were detected for Ni/CaO: (1) the minor peak below 200 °C represents the desorption of the physisorbed CO2 (classified as weak basic sites); (2) the peaks located in the temperature range of 250–550 °C typically originate from the chemisorbed CO2 on the outer surface, including the Ca(OH)2 phase (classified as medium basic sites); (3) the peaks located at temperature between 550 °C and 800 °C are attributed to CO2 adsorbed at the bulk part of CaO particles (classified as strong basic sites) [19,42]. In comparison to Ni/CaO, the TPD profiles of Na-Ni/CaO and K-Ni/CaO exhibited similar types of basic sites but with a higher desorption temperature and an increase in peak intensity. As for the Li-Ni/CaO DFM, the low intensity of the desorption peaks suggested a relatively low CO2 capture capacity. More specific information about the different basic site distributions was obtained from the integration of the corresponding desorption peaks. As presented in Table S1, the total basicity of the DFMs follows a descending order of Na-Ni/CaO (987.1 μmol/g) > K-Ni/CaO (879.5 μmol/g) > Ni/CaO (766.7 μmol/g) > Li-Ni/CaO (572.7 μmol/g). Particularly, the number of medium and strong basic sites, which were the main adsorption sites for CO2, were significantly increased with Na or K doping, indicating that the deposition of Na or K onto CaO enhanced its affinity for CO2 and endowed it with excellent CO2 capture capability.

Figure 5.
CO2-TPD profiles of the reduced Ni/CaO and M-Ni/CaO (M = Li, Na, and K) DFMs.
3.2. CO2 Capture and Capture–Release of Ni/CaO and M-Ni/CaO (M = Li, Na, and K) DFMs
The temperature of CO2 capture in the DFMs was tested using the TGA technique under a simulated gas of 10 vol% CO2/Ar over a wide temperature range of 50 °C to 1000 °C (Figure 6a). It could be observed that the weight of the as-prepared DFMs remarkably increased in the temperature range of 550–700 °C, in which CO2 chemisorption could be activated to allow diffusion processes throughout the bulk of the materials. The carbonated CaO and alkali-metal-modified CaO were completely regenerated, with a sharp decrease in weight occurring at 740–800 °C, which corresponded to the decomposition of CaCO3. This suggested that all DFMs exhibited good CO2 capture capacities at temperatures between 550 °C and 700 °C. Furthermore, at 550, 600, 650, and 700 °C, the CO2 isothermal capture capacities and adsorption rates of all DFMs increased with temperature and reached the maximum level at 650 °C (Figure S1 and Table S2).
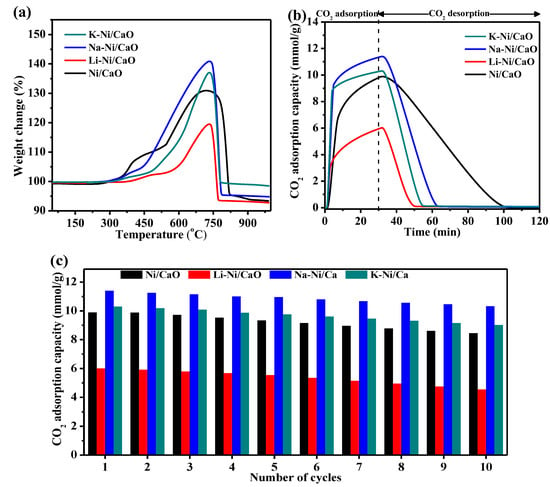
Figure 6.
(a) The changes in the weights of Ni/CaO and M-Ni/CaO (M = Li, Na, and K) DFMs over the temperature range of 50 °C to 1000 °C; CO2 capture–release profiles (b) and cyclic CO2 capture–release stability (c) of Ni/CaO and M-Ni/CaO (M = Li, Na, and K) DFMs at 650 °C.
Therefore, the isothermal CO2 capture–release properties of all DFMs at 650 °C were investigated. As shown in Figure 6b, the capture of CO2 on the DFMs followed two different processes. The CO2 capture capacity exhibited a rapid increase during the initial stage (within 10 min), which was the chemical-reaction-controlled stage. Subsequently, it slowly increased in the latter stage (within 10–30 min), which was the product-layer-diffusion-controlled stage. Compared with Ni/CaO, the Na- or K-modified DFMs showed a higher CO2 capacity both in the reaction-controlled and diffusion-controlled processes, contributing to a higher overall CO2 capture capacity. The CO2 capture capacities were ranked in the following order: Na-Ni/CaO (11.4 mmol/g) > K-Ni/CaO (10.3 mmol/g) > Ni/CaO (9.9 mmol/g) > Li-Ni/CaO (6.0 mmol/g). In addition, the alkali-metal-modified DFMs showed a shorter time span of CO2 release than Ni/CaO, indicating the fast regeneration of the sorbents, which was essential to maintain its structural stability and mitigated CaO sintering [43,44]. The significantly enhanced desorption kinetics of a double-salt adsorbent containing K− and Na−CaO compared with that of bare CaO has previously been reported in several papers [30]. The XRD pattern of Na-Ni/CaO with 15 wt% Na doping after the capture of CO2 showed the characteristic peaks of the Na2Ca(CO3)2 phase (Figure S2), which confirmed the formation of double carbonates. The cycle stability of all DFMs was evaluated with 10 cycles of CO2 capture and release at 650 °C (Figure 6c). Among them, Na-Ni/CaO showed the best cycle stability, retaining over 90% capacity after 10 cycles with only a slight decrease from 11.4 to 10.3 mmol/g, which was better than Ni/CaO (from 9.9 to 8.4 mmol/g). All these confirmed that not only CO2 capture capacities but also the cycle stability of the modified DFMs were improved through the doping of Na and K.
3.3. Integrated CO2 Capture and Hydrogenation of Ni/CaO and M-Ni/CaO (M = Li, Na, and K) DFMs
The integrated CO2 capture and hydrogenation performance of all the DFMs obtained from the fixed bed are illustrated in Figure 7. The whole process includes a CO2 capture stage and a hydrogenation stage. In the CO2 capture stage, a 10 vol% CO2/Ar was introduced into the bed and was maintained for 1 h until the DFMs reached adsorption saturation. Based on the TG profiles of the DFMs after the CO2 capture stage (Figure S3), the CO2 capture capacities of all DFMs followed an ascending order: Li-Ni/CaO (7.0 mmol/g) < Ni/CaO (11.1 mmol/g) < K-Ni/CaO (11.6 mmol/g) < Na-Ni/CaO (12.0 mmol/g). This trend was consistent with the capture capacities illustrated in Figure S1 and the ranking of the number of medium and strong basic sites presented in Table S1. Following the CO2 capture stage, pure Ar was introduced for 10 min to remove the excess CO2 in the bed, and then it was switched to 10 vol% H2/Ar to initiate the hydrogenation stage. As shown in Table 2, the Ni/CaO DFM achieved a CO2 conversion rate and a CO yield of 60.3% and 6.3 mmol/g, respectively, with the hydrogenation stage lasting for 75 min until the complete decomposition of CaCO3. It was found that the incorporation of secondary dopants into the NiO–CaO catalysts exerted a significant influence on the overall composition of the product gas, particularly with regard to the water–gas shift reaction [45]. The incorporation of Na and K in the Ni/CaO DFM resulted in higher CO2 conversions and CO yields for the doped DFMs compared with Ni/CaO, indicating that the addition of Na and K could promote the hydrogenation of the calcium carbonate species (CaCO3 and released CO2) towards the production of CO by RWGS. In comparison, the hydrogenation stages of the Na- and K-modified Ni/CaO DFMs were completed in only 45 min, owing to their high CO2 conversion and rapid CO2 release rates. Among all the DFMs, the Na-Ni/CaO DFM demonstrated the highest CO2 conversion rate and CO yield of 62.0% and 7.1 mmol/g, respectively, which is attributed to its abundant basic sites and uniform dispersion of fine Ni particles. The preparation method, reaction types, testing conditions, CO2 capture capacities, and product yields in the integrated CO2 capture and hydrogenation using different Ni/CaO-based DFMs are listed in Table S3. The results demonstrated that the Na/Ni–CaO in this work exhibited significantly higher CO2 capture capacity and better product yield than most Ni/CaO-based DFMs. Due to the larger Ni particle size and fewer basic sites caused by the poor pore structure, Li-Ni/CaO exhibited the lowest CO2 capture capacity (7.0 mmol/g), CO2 conversion rate (35.8%), and CO yield (2.3 mol/g) among all the DFMs. However, Li-Ni/CaO also underwent fast hydrogenation, i.e., within 55 min, possibly due to its low CO2 capture capacity.
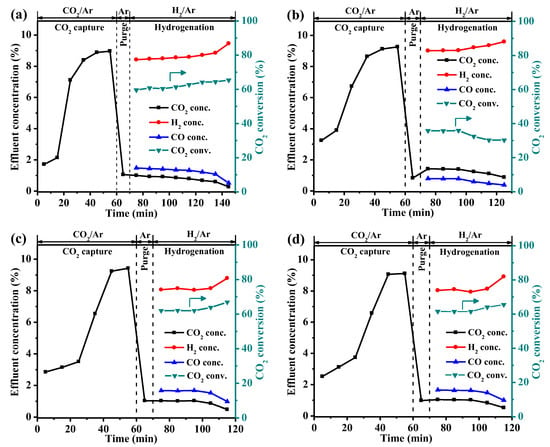
Figure 7.
Integrated CO2 capture and hydrogenation of (a) Ni/CaO a, (b) Li-Ni/CaO, (c) Na-Ni/CaO, and (d) K-Ni/CaO DFM. Temperature: 650 °C; GHSV: 6000 mL·g−1·h−1. a Reprinted with permission from ref [28]. Copyright 2023 Elsevier.

Table 2.
The integrated CO2 capture and hydrogenation performance of Ni/CaO and M-Ni/CaO (M = Li, Na, and K) DFMs at 650 °C.
The carbon balance of the DFM was defined as the ratio between the total yields of CO2 and CO in the hydrogenation stage and the CO2 capture capacity in the capture stage. It was calculated that the carbon balances of all the DFMs were calculated to be higher than 90%, indicating that nearly all of the captured CO2 in the capture stage was subsequently released as CO and CO2 during the hydrogenation stage.
3.4. The Cyclic Integrated CO2 Capture and Hydrogenation of Na-Ni/CaO DFMs
The cycle stability of the Na-Ni/CaO DFM was evaluated during 20 cycles of integrated CO2 capture and hydrogenation. The change in CO2, H2, and CO concentrations over time are depicted in Figure 8. The Na-Ni/CaO DFM exhibited similar trends in the concentrations of CO2, H2, and CO with increasing cycle time, indicating a relatively stable conversion rate of CO2. The spent Na-Ni/CaO achieved a CO2 conversion rate of 60.5% after 20 cycles. As shown in Figure S4, it was determined that the spent Na-Ni/CaO retained a high CO2 capture capacity of 10.8 mmol/g, indicating a mere 10% loss in capture capacity as compared to its initial performance. Figure 9 presents the SEM and TEM images of the spent Na-Ni/CaO after 20 cycles of the integrated process. The images show a slight aggregation of CaO particles and the disappearance of some mesopores, while the three-dimensional network’s porous structure and macropores still remained stable (Figure 9a). Additionally, the porous structure of the spent Na-Ni/CaO was also analyzed using the N2 adsorption–desorption isotherms and pore size distribution (Figure S5), which exhibited a slightly lower specific surface area and pore volume than those of fresh Na-Ni/CaO. In addition, the TEM image shows that the spent Na-Ni/CaO also maintained a uniform dispersion of Ni particles, with an average size of 14.5 nm after 20 cycles (Figure 9b). Furthermore, it is noteworthy that neither SEM nor TEM images reveal the existence of carbon deposition, indicating that the decline in CO2 conversion could not be attributed to this factor. Therefore, we concluded that the great cycle stability of Na-Ni/CaO might be ascribed to the variation in Na species (Figure S2). One explanation is that Na2O forms Na2Ca(CO3)2, which is conducive to CO2 capture and release and greatly shortens the cycle time of the whole integrated process. Another possibility is that Na2Ca(CO3)2 can be reduced to Na2O, which acts as a high-temperature refractory oxide to retard the sintering of CaO particles during the hydrogenation stage.
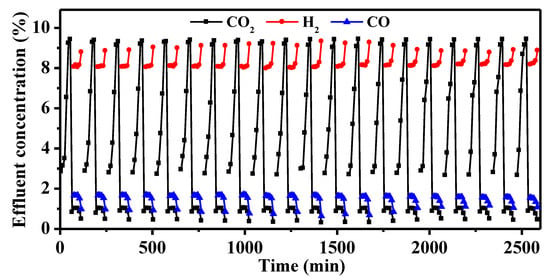
Figure 8.
Twenty cycles of integrated CO2 capture and hydrogenation of the Na-Ni/CaO DFM. Temperature: 650 °C; GHSV: 6000 mL·g−1·h−1.
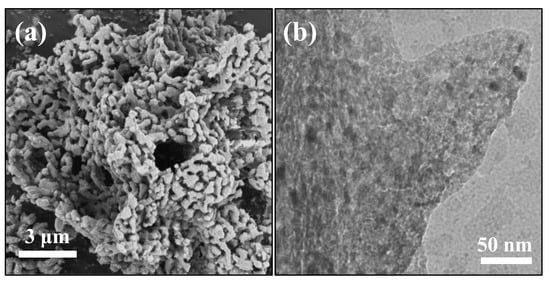
Figure 9.
(a) SEM and (b) TEM images of the spent Na-Ni/CaO DFM after 20 cycles of integrated CO2 capture and hydrogenation.
4. Conclusions
In this study, we successfully synthesized and utilized Ni/CaO DFMs modified with alkaline metals (Li, Na, and K) for producing CO through integrated CO2 capture and hydrogenation. The incorporation of Na and K into the DFMs significantly increased the surface basicity, resulting in an enhanced CO2 capture capacity. Additionally, Na and K improved the dispersion of Ni particles, leading to smaller particle sizes in the modified DFMs, which in turn contributed to higher catalytic activity. The performance evaluation of the DFMs regarding CO2 capture and release revealed that Na- and K-doped Ni/CaO DFMs demonstrated not only high CO2 capture capacities but also faster kinetics for both capture and release, surpassing those of Ni/CaO alone. However, the negative effects of Li on the integrated CO2 capture and hydrogenation were observed in the Li-Ni/CaO catalyst, which was due to the low surface basicity, pore structure blockage, and large Ni particle sizes. Among all the tested DFMs, Na-Ni/CaO exhibited the highest CO2 capture capacity of 12.0 mmol/g and CO2 conversion rate of 62.0% with a CO production yield of 7.1 mmol/g in the integrated CO2 capture and hydrogenation process at 650 °C. Moreover, the formation of double salts, particularly Na2Ca(CO3)2, played a crucial role in enhancing the CO2 capture and release rate of the DFMs, contributing to their stability by preventing the aggregation and sintering of CaO. The recyclability and surface morphology of the spent material clearly indicated that Na-Ni/CaO had high stability even after undergoing 20 cycles.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma16155430/s1, Table S1: Weak, medium, strong and total basicity of the reduced Ni/CaO and M-Ni/CaO (M = Li, Na, K) DFMs. Figure S1: CO2 capture profiles in the temperature range of 550 to 700 °C for 2 h. (a) Ni/CaO, (b) Li-Ni/CaO, (c) Na-Ni/CaO, and (d) K-Ni/CaO; Table S2: The CO2 capture capacities of the M-Ni/CaO (M = Li, Na, and K) DFMs at the temperature range of 550–700 °C for 2 h; Figure S2: XRD pattern of Na-Ni/CaO with 15% Na doping after (a) reduction, (b) CO2 capture stage, and (c) integrated CO2 capture and hydrogenation; Figure S3: TG profiles of all DFMs (a) after the CO2 capture stage and (b) the hydrogenation stage; Figure S4: TG profile of the spent Na-Ni/CaO after adsorption in CO2/Ar for 1 h; Figure S5: N2 adsorption–desorption isotherms and pore size distribution (insert part) of Na-Ni/CaO DFM after 20 cycles of integrated CO2 capture and hydrogenation. Table S3. The combined CO2 capture and hydrogenation performance over our proposed Na-Ni/CaO DFM and recently documented Ni/CaO based DFMs.
Author Contributions
Conceptualization, Y.H., Q.X. and X.W.; investigation, Y.H. and Y.S.; resources, Q.X., X.W. and X.L.; data curation, Y.H.; writing—original draft preparation, Y.H. and Y.S.; writing—review and editing, X.W., H.C. and X.Z.; funding acquisition, Q.X. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 51574163); the Science and Technology Commission of Shanghai Municipality (Grant No. 21DZ1208900); and the Shanghai Engineering Research Center of Green Remanufacture of Metal Parts (No. 19DZ2252900).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, Y.-S.; Zhang, X.; Liu, J.-W.; Lee, Y.; Li, X.-S. Natural gas hydrate resources and hydrate technologies: A review and analysis of the associated energy and global warming challenges. Energy Environ. Sci. 2021, 14, 5611–5668. [Google Scholar] [CrossRef]
- Cao, Z.; Myers, R.J.; Lupton, R.C.; Duan, H.; Sacchi, R.; Zhou, N.; Miller, T.R.; Cullen, J.M.; Ge, Q.; Liu, G. The sponge effect and carbon emission mitigation potentials of the global cement cycle. Nat. Commun. 2020, 11, 3777. [Google Scholar] [CrossRef]
- Jeong-Potter, C.; Abdallah, M.; Sanderson, C.; Goldman, M.; Gupta, R.; Farrauto, R. Dual function materials (Ru+ Na2O/Al2O3) for direct air capture of CO2 and in situ catalytic methanation: The impact of realistic ambient conditions. Appl. Catal. B 2022, 307, 120990. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon capture and utilization update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef]
- Abanades, J.C.; Rubin, E.S.; Mazzotti, M.; Herzog, H.J. On the climate change mitigation potential of CO2 conversion to fuels. Energy Environ. Sci. 2017, 10, 2491–2499. [Google Scholar] [CrossRef]
- Hu, L.; Urakawa, A. Continuous CO2 capture and reduction in one process: CO2 methanation over unpromoted and promoted Ni/ZrO2. J. CO2 Util. 2018, 25, 323–329. [Google Scholar] [CrossRef]
- Lv, Z.; Qin, C.; Chen, S.; Hanak, D.P.; Wu, C. Efficient-and-stable CH4 reforming with integrated CO2 capture and utilization using Li4SiO4 sorbent. Sep. Purif. Technol. 2021, 277, 119476. [Google Scholar] [CrossRef]
- Fernandez, J.R.; Garcia, S.; Sanz-Perez, E.S. CO2 capture and utilization editorial. Ind. Eng. Chem. Res. 2020, 59, 6767–6772. [Google Scholar] [CrossRef]
- Fu, L.; Ren, Z.; Si, W.; Ma, Q.; Huang, W.; Liao, K.; Huang, Z.; Wang, Y.; Li, J.; Xu, P. Research progress on CO2 capture and utilization technology. J. CO2 Util. 2022, 66, 102260. [Google Scholar] [CrossRef]
- Lim, J.; Kim, J. Designing and integrating NOx, SO2 and CO2 capture and utilization process using desalination wastewater. Fuel 2022, 327, 124986. [Google Scholar] [CrossRef]
- Duyar, M.S.; Trevino, M.A.A.; Farrauto, R.J. Dual function materials for CO2 capture and conversion using renewable H2. Appl. Catal. B 2015, 168, 370–376. [Google Scholar] [CrossRef]
- Duyar, M.S.; Wang, S.; Arellano-Trevino, M.A.; Farrauto, R.J. CO2 utilization with a novel dual function material (DFM) for capture and catalytic conversion to synthetic natural gas: An update. J. CO2 Util. 2016, 15, 65–71. [Google Scholar] [CrossRef]
- Merkouri, L.P.; Reina, T.R.; Duyar, M.S. Closing the carbon cycle with dual function materials. Energy Fuels 2021, 35, 19859–19880. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Karami, D.; Mahinpey, N. Solution combustion synthesis of zirconia-stabilized calcium oxide sorbents for CO2 capture. Fuel 2020, 269, 117432. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Peng, Y.; Yang, Y.; Sun, J.; Chen, H.; Zhou, Z.; Xu, M. One-step synthesis of highly efficient CaO-based CO2 sorbent pellets via gel-casting technique. Fuel Process. Technol. 2017, 160, 70–77. [Google Scholar] [CrossRef]
- Li, L.; Miyazaki, S.; Yasumura, S.; Ting, K.W.; Toyao, T.; Maeno, Z.; Shimizu, K.I. Continuous CO2 Capture and Selective Hydrogenation to CO over Na-Promoted Pt Nanoparticles on Al2O3. ACS Catal. 2022, 12, 2639–2650. [Google Scholar] [CrossRef]
- Hu, J.; Hongmanorom, P.; Galvita, V.V.; Li, Z.; Kawi, S. Bifunctional Ni-Ca based material for integrated CO2 capture and conversion via calcium-looping dry reforming. Appl. Catal. B 2021, 284, 119734. [Google Scholar] [CrossRef]
- Shao, B.; Hu, G.; Alkebsi, K.A.M.; Ye, G.; Lin, X.; Du, W.; Hu, J.; Wang, M.; Liu, H.; Qian, F. Heterojunction-redox catalysts of FexCoyMg10CaO for high-temperature CO2 capture and in situ conversion in the context of green manufacturing. Energy Environ. Sci. 2021, 14, 2291–2301. [Google Scholar] [CrossRef]
- Bermejo-López, A.; Pereda-Ayo, B.; González-Marcos, J.A.; González-Velasco, J.R. Ni loading effects on dual function materials for capture and in-situ conversion of CO2 to CH4 using CaO or Na2CO3. J. CO2 Util. 2019, 34, 576–587. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.; Zhao, J.; Shen, B.; Shi, J.; Huang, J.; Wu, C. Dual functional catalytic materials of Ni over Ce-modified CaO sorbents for integrated CO2 capture and conversion. Appl. Catal. B 2019, 244, 63–75. [Google Scholar] [CrossRef]
- Jo, S.B.; Woo, J.H.; Lee, J.H.; Kim, T.Y.; Kang, H.I.; Lee, S.C.; Kim, J.C. A novel integrated CO2 capture and direct methanation process using Ni/CaO catal-sorbents. Sustain. Energy Fuels 2020, 4, 4679–4687. [Google Scholar] [CrossRef]
- Chai, K.H.; Leong, L.K.; Wong, D.S.H.; Tsai, D.H.; Sethupathi, S. Effect of CO2 adsorbents on the Ni-based dual-function materials for CO2 capturing and in situ methanation. J. Chin. Chem. Soc. 2020, 67, 998–1008. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; Xu, S.; Osman, A.I.; Stenning, G.; Han, J.; Sun, S.; Rooney, D.; Williams, P.T.; Wang, F.; et al. Understanding the interaction between active sites and sorbents during the integrated carbon capture and utilization process. Fuel 2021, 286, 119308. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Y.; Yu, J.; Liu, F.H.; Sun, J.; Wang, X.; Wang, T.; Zhao, C. Ni-CaO dual function materials prepared by different synthetic modes for integrated CO2 capture and conversion. Chem. Eng. J. 2022, 428, 132110. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, C.; Guan, S.; Xu, S.; Williams, P.T.; Wu, C. Ni/support-CaO bifunctional combined materials for integrated CO2 capture and reverse water-gas shift reaction: Influence of different supports. Sep. Purif. Technol. 2022, 298, 121604. [Google Scholar] [CrossRef]
- Hu, J.; Hongmanorom, P.; Chirawatkul, P.; Kawi, S. Efficient integration of CO2 capture and conversion over a Ni supported CeO2-modified CaO microsphere at moderate temperature. Chem. Eng. J. 2021, 426, 130864. [Google Scholar] [CrossRef]
- Hu, J.; Hongmanorom, P.; Chen, J.; Wei, W.; Chirawatkul, P.; Galvita, V.V.; Kawi, S. Tandem distributing Ni into CaO framework for isothermal integration of CO2 capture and conversion. Chem. Eng. J. 2023, 452, 139460. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Q.; Zou, X.; Wang, X.; Cheng, H.; Zou, X.; Lu, X. MxOy (M = Mg, Zr, La, Ce) modified Ni/CaO dual functional materials for integrated CO2 capture and hydrogenation. Int. J. Hydrog. Energy 2023, 48, 24871–24883. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Q.; Sheng, Y.; Wang, X.; Cheng, H.; Zou, X.; Lu, X. Catalytic Synthesis of CO by Combining CO2 Capture and Hydrogenation over Three-Dimensional Ni/CaO Networks. Energy Fuels 2021, 37, 7871–7880. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Thakkar, H.; Li, X.; Rownaghi, A.A.; Rezaei, F. Development of potassium-and sodium-promoted CaO adsorbents for CO2 capture at high temperatures. Ind. Eng. Chem. Res. 2017, 56, 8292–8300. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Rownaghi, A.A.; Rezaei, F. Integrated capture and utilization of CO2 for syngas production over dual-function materials. ACS Sustain. Chem. Eng. 2018, 6, 13551–13561. [Google Scholar] [CrossRef]
- Yang, L.; Pastor-Pérez, L.; Gu, S.; Sepúlveda-Escribano, A.; Reina, T.R. Highly efficient Ni/CeO2-Al2O3 catalysts for CO2 upgrading via reverse water-gas shift: Effect of selected transition metal promoters. Appl. Catal. B 2018, 232, 464–471. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, M.; Su, Z.; Wu, K.; Zhang, Y.; Long, D. Two-dimensional CaO/carbon heterostructures with unprecedented catalytic performance in room-temperature H2S oxidization. Appl. Catal. B 2021, 280, 119444. [Google Scholar] [CrossRef]
- Karimi, S.; Bibak, F.; Meshkani, F.; Rastegarpanah, A.; Deng, J.; Liu, Y.; Dai, H. Promotional roles of second metals in catalyzing methane decomposition over the Ni-based catalysts for hydrogen production: A critical review. Int. J. Hydrog. Energy 2021, 46, 20435–20480. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Shen, B.; Wu, C. Preparation, modification and development of Ni-based catalysts for catalytic reforming of tar produced from biomass gasification. Renew. Sustain. Energy Rev. 2018, 94, 1086–1109. [Google Scholar] [CrossRef]
- Miyazaki, T.; Michitani, K.; Ookawa, M.; Yamaguchi, T. On the behavior of the selective oxidation by LiNiO2: Oxidative coupling of methane. Res. Chem. Intermed. 2022, 28, 479–484. [Google Scholar] [CrossRef]
- Vogt, C.; Groeneveld, E.; Kamsma, G.; Nachtegaal, M.; Lu, L.; Kiely, C.J.; Berben, P.H.; Meirer, F.; Weckhuysen, B.M. Unravelling structure sensitivity in CO2 hydrogenation over nickel. Nat. Catal. 2018, 1, 127–134. [Google Scholar] [CrossRef]
- Wu, H.C.; Chang, Y.C.; Wu, J.H.; Lin, J.H.; Lin, I.K.; Chen, C.S. Methanation of CO2 and reverse water gas shift reactions on Ni/SiO2 catalysts: The influence of particle size on selectivity and reaction pathway. Catal. Sci. Technol. 2015, 5, 4154–4163. [Google Scholar] [CrossRef]
- Faria, A.C.; Trujillano, R.; Rives, V.; Miguel, C.V.; Rodrigues, A.E.; Madeira, L.M. Cyclic operation of CO2 capture and conversion into methane on Ni-hydrotalcite based dual function materials (DFMs). J. CO2 Util. 2023, 72, 102476. [Google Scholar] [CrossRef]
- Chen, C.; Huang, H.; Yu, Y.; Shi, J.; He, C.; Albilali, R.; Pan, H. Template-free synthesis of hierarchical porous carbon with controlled morphology for CO2 efficient capture. Chem. Eng. J. 2018, 353, 584–594. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.; Jia, X.; Xu, B.; Lin, H.; Yang, H.; Song, L.; Wang, X. Amorphous nickel-cobalt complexes hybridized with 1T-phase molybdenum disulfide via hydrazine-induced phase transformation for water splitting. Nat. Commun. 2017, 8, 15377. [Google Scholar] [CrossRef] [PubMed]
- Ashok, J.; Kathiraser, Y.; Ang, M.L.; Kawi, S. Bi-functional hydrotalcite-derived NiO–CaO–Al2O3 catalysts for steam reforming of biomass and/or tar model compound at low steam-to-carbon conditions. Appl. Catal. B 2015, 172, 116–128. [Google Scholar] [CrossRef]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.; Zhu, X.; Wang, J.; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Sivasangar, S.; Mastuli, M.S.; Islam, A.; Taufiq-Yap, Y.H. Screening of modified CaO-based catalysts with a series of dopants for the supercritical water gasification of empty palm fruit bunches to produce hydrogen. RSC Adv. 2015, 5, 36798–36808. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).