Comparative Synthesis of Silver Nanoparticles: Evaluation of Chemical Reduction Procedures, AFM and DLS Size Analysis
Abstract
1. Introduction
2. Materials and Procedures
2.1. Materials
2.2. Chemical Synthesis of Silver Nanoparticles
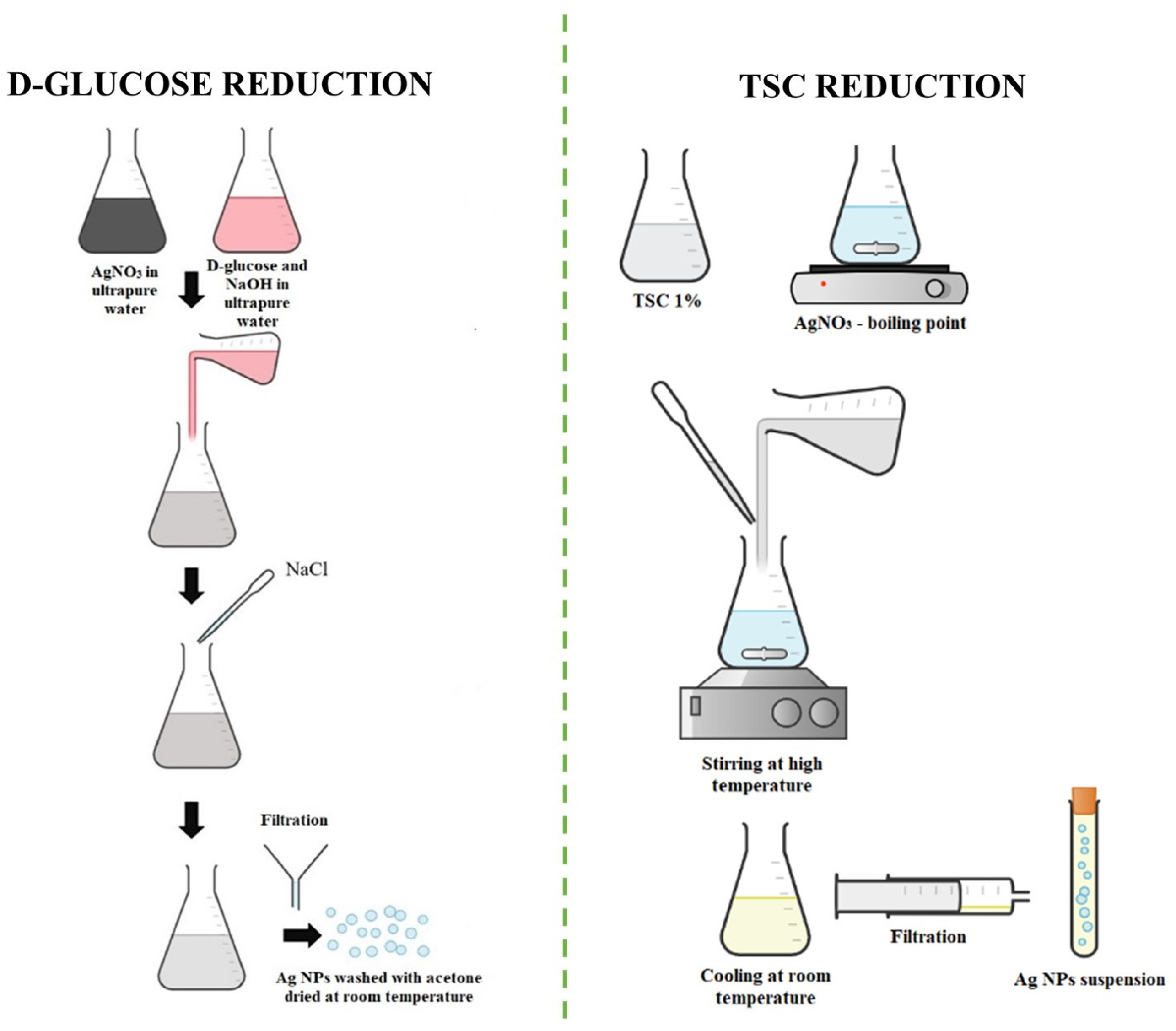
2.2.1. D-glucose Reduction (AgNP-R1)
2.2.2. TSC Reduction (AgNP-R2)
2.2.3. Argentum+77 Pure Life Product (Ag-77)
2.3. Characterization Techniques
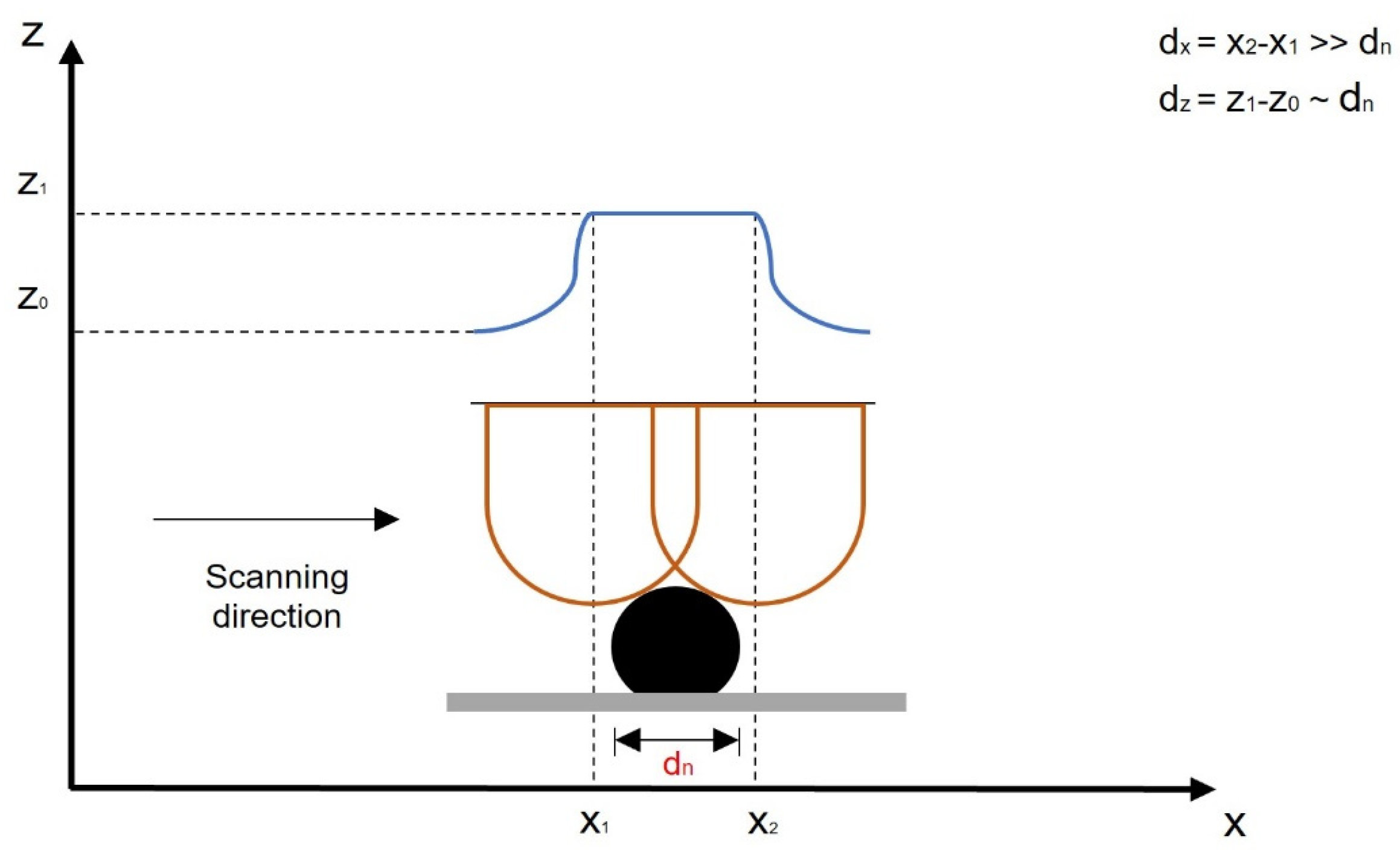
2.3.1. AFM
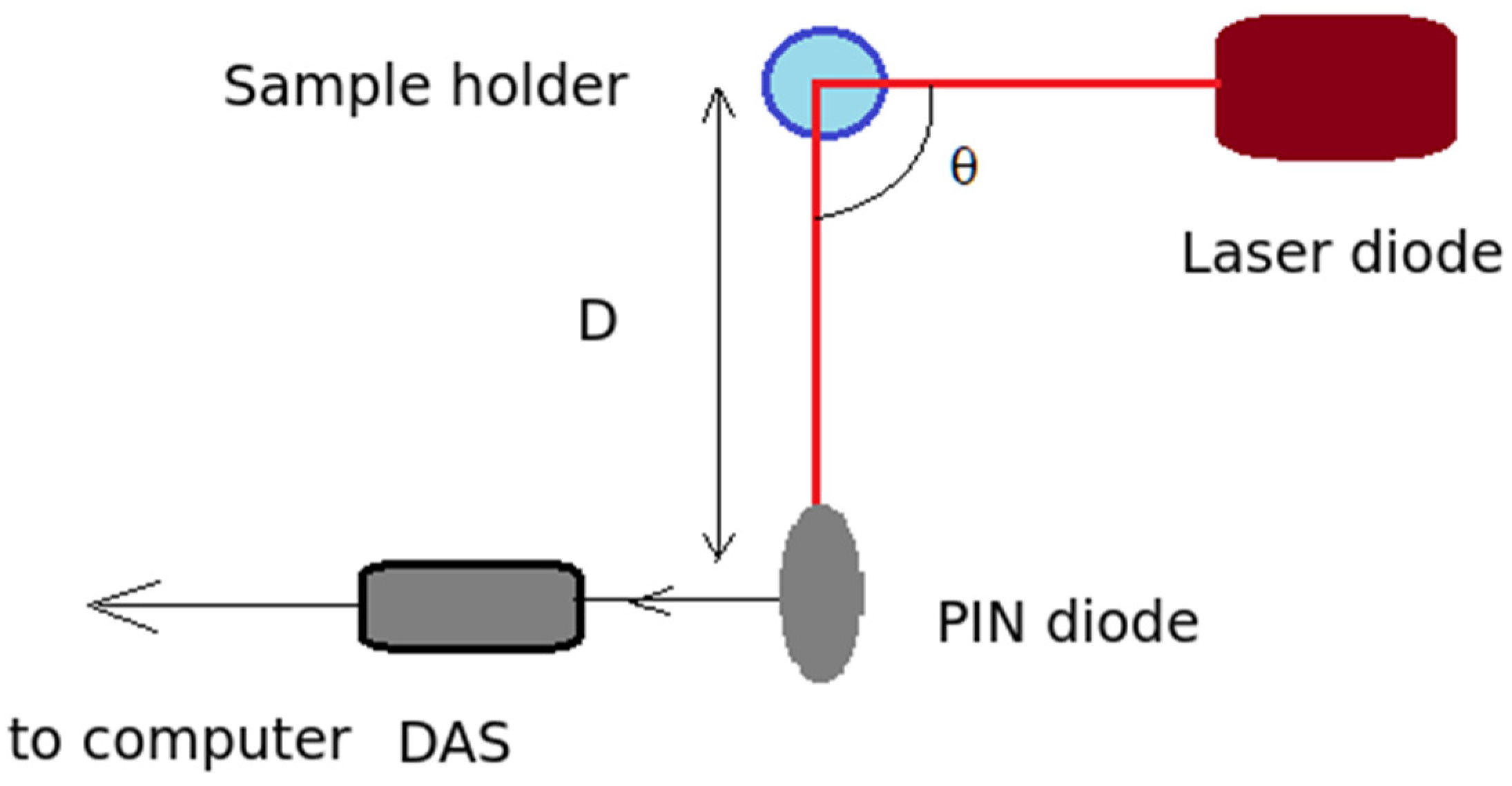
2.3.2. A Brief Overview of the Dynamic Light Scattering (DLS) Data Processing Procedure
- Error Calculation for DLS Diameters
2.3.3. UV-VIS
2.3.4. FT-IR Spectroscopy
3. Results and Discussions
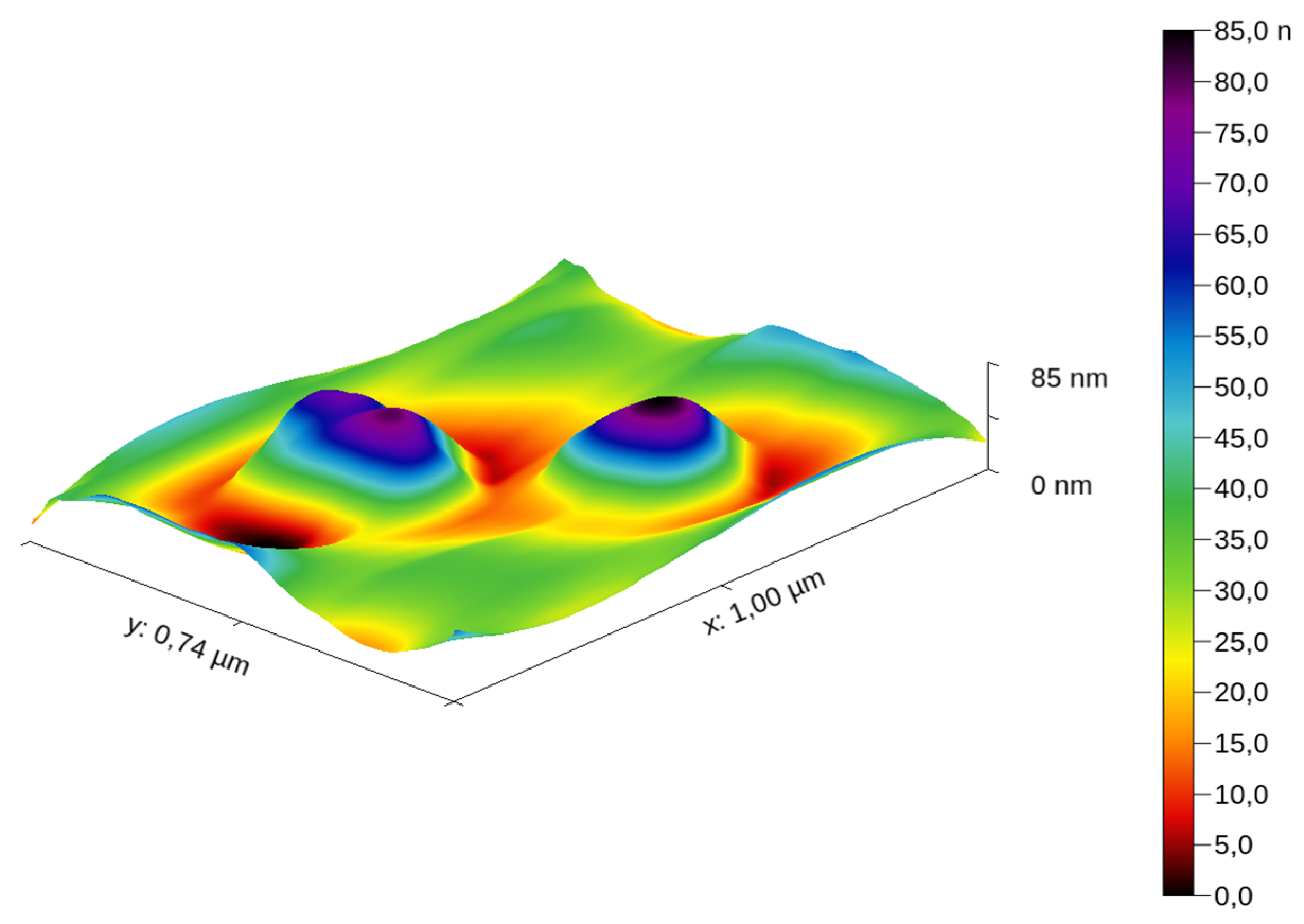
3.1. AFM Results
3.2. DLS Particle Sizing Results
3.3. UV-VIS Results
3.4. FT-IR Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heiligtag, F.J.; Niederberger, M. The fascinating world of nanoparticle research. Mater. Today 2013, 16, 262–271. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Bhushan, B. Introduction to Nanotechnology. In Springer Handbook of Nanotechnology; Bhushan, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 2, pp. 1–13. [Google Scholar]
- Varanda, L.C.; Souza, C.G.; Moraes, D.A.; Neves, H.R.; Souza, J.B.; Silva, M.F.; Bini, R.A.; Albers, R.F.; Silva, T.L.; Beck, W. Size and shape-controlled nanomaterials based on modified polyol and thermal decomposition approaches. A brief review. An. Acad. Bras. Ciências 2019, 91, e20181180. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Sahani, S.; Sharma, Y.C. Advancements in applications of nanotechnology in global food industry. Food Chem. 2020, 342, 128318. [Google Scholar] [CrossRef]
- Periasamy, S.; Jegadeesan, U.; Sundaramoorthi, K.; Rajeswari, T.; Tokala, V.N.B.; Bhattacharya, S.; Muthusamy, S.; Sankoh, M.; Nellore, M.K. Comparative Analysis of Synthesis and Characterization of Silver Nanoparticles Extracted Using Leaf, Flower, and Bark of Hibiscus rosasinensis and Examine Its Antimicrobicidal Activity. J. Nanomater. 2022, 2022, 8123854. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Begum, K.M.S. Chitosan-cellulose hydrogel conjugated with L-histidine and Zinc oxide nanoparticles for sustained drug delivery: Kinetics and in-vitro biological studies. Carbohydr. Polym. 2020, 236, 116101. [Google Scholar] [CrossRef]
- Novaes, J.; Silva, E.A.d.; Bernardo, P.M.F.; Yapuchura, E.R. Preparation and characterization of Chitosan/Collagen blends containing silver nanoparticles. Polímeros 2020, 30, e2020015. [Google Scholar] [CrossRef]
- Huang, F.; Gao, Y.; Zhang, Y.; Cheng, T.; Ou, H.; Yang, L.; Liu, J.; Shi, L.; Liu, J. Silver-decorated polymeric micelles combined with curcumin for enhanced antibacterial activity. ACS Appl. Mater. Interfaces 2017, 9, 16880–16889. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications—A review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Jouyban, A.; Rahimpour, E. Optical sensors based on silver nanoparticles for determination of pharmaceuticals: An overview of advances in the last decade. Talanta 2020, 217, 121071. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, R.; Negi, B. Conventional and green methods of synthesis of silver nanoparticles and their antimicrobial properties. Curr. Res. Green Sustain. Chem. 2021, 4, 100205. [Google Scholar] [CrossRef]
- Ameen, F.; Abdullah, M.M.; Al-Homaidan, A.A.; Al-Lohedan, H.A.; Al-Ghanayem, A.A.; Almansob, A. Fabrication of silver nanoparticles employing the cyanobacterium Spirulina platensis and its bactericidal effect against opportunistic nosocomial pathogens of the respiratory tract. J. Mol. Struct. 2020, 1217, 128392. [Google Scholar] [CrossRef]
- Helmlinger, J.; Sengstock, C.; Groß-Heitfeld, C.; Mayer, C.; Schildhauer, T.; Köller, M.; Epple, M. Silver nanoparticles with different size and shape: Equal cytotoxicity, but different antibacterial effects. RSC Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef]
- Nicolae-Maranciuc, A.; Chicea, D.; Chicea, L.M. Ag nanoparticles for biomedical applications—Synthesis and characterization—A review. Int. J. Mol. Sci. 2022, 23, 5778. [Google Scholar] [CrossRef]
- Begum, I.; Ameen, F.; Soomro, Z.; Shamim, S.; AlNadhari, S.; Almansob, A.; Al-Sabri, A.; Arif, A. Facile fabrication of malonic acid capped silver nanoparticles and their antibacterial activity. J. King Saud. Univ. Sci. 2021, 33, 101231. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Morsi, M.M.; Hussien, N.A.; Alyamani, A.A.; Al Sufyani, N.M. Comparative Analysis of Nanosilver Particles Synthesized by Different Approaches and Their Antimicrobial Efficacy. J. Nanomater. 2021, 2021, 2204776. [Google Scholar] [CrossRef]
- Ahmad, N.; Bhatnagar, S.; Ali, S.S.; Dutta, R. Phytofabrication of bioinduced silver nanoparticles for biomedical applications. Int. J. Nanomed. 2015, 10, 7019. [Google Scholar] [CrossRef]
- Amiri, M.; Mahmoudi-Moghaddam, H. Green synthesis of ZnO/ZnCo2O4 and its application for electrochemical determination of bisphenol A. Microchem. J. 2021, 160, 105663. [Google Scholar] [CrossRef]
- Razavi, R.; Amiri, M.; Alshamsi, H.A.; Eslaminejad, T.; Salavati-Niasari, M. Green synthesis of Ag nanoparticles in oil-in-water nano-emulsion and evaluation of their antibacterial and cytotoxic properties as well as molecular docking. Arab. J. Chem. 2021, 14, 103323. [Google Scholar] [CrossRef]
- Lokhande, A.C.; Babar, P.T.; Karade, V.C.; Jang, J.S.; Lokhande, V.C.; Lee, D.J.; Kim, I.C.; Patole, S.P.; Qattan, I.A.; Lokhande, C.D.; et al. A viable green route to produce Ag nanoparticles for antibacterial and electrochemical supercapacitor applications. Mater. Today Chem. 2019, 14, 100181. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Kobylinska, N.; Shakhovsky, A.; Khainakova, O.; Klymchuk, D.; Avdeeva, L.; Ratushnyak, Y.; Duplij, V.; Matvieieva, N. ‘Hairy’root extracts as source for ‘green’ synthesis of silver nanoparticles and medical applications. RSC Adv. 2020, 10, 39434–39446. [Google Scholar] [CrossRef]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 18. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Sajjad, W.; Wu, F.; Bibi, N.; Shah, K.; Yali, Z.; Wang, W. Green synthesis of silver nanoparticles and their shortcomings, animal blood a potential source for silver nanoparticles: A review. J. Hazard. Mater. Adv. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Islam, M.A.; Jacob, M.V.; Antunes, E. A critical review on silver nanoparticles: From synthesis and applications to its mitigation through low-cost adsorption by biochar. J. Environ. Manag. 2021, 281, 111918. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Volpe, A.; Clemente, M.; Picca, R.A.; Ancona, A.; Lugarà, P.M.; Palazzo, G.; Cioffi, N. The Pros and Cons of the Use of Laser Ablation Synthesis for the Production of Silver Nano-Antimicrobials. Antibiotics 2018, 7, 67. [Google Scholar] [CrossRef]
- López-Lorente, A.I.; Picca, R.A.; Izquierdo, J.; Kranz, C.; Mizaikoff, B.; Di Franco, C.; Cárdenas, S.; Cioffi, N.; Palazzo, G.; Valentini, A. Ion beam sputtering deposition of silver nanoparticles and TiOx/ZnO nanocomposites for use in surface enhanced vibrational spectroscopy (SERS and SEIRAS). Microchim. Acta 2018, 185, 153. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385. [Google Scholar]
- Banne, S.V.; Patil, M.; Kulkarni, R.; Patil, S. Synthesis and characterization of silver nano particles for EDM applications. Mater. Today Proc. 2017, 4, 12054–12060. [Google Scholar] [CrossRef]
- Vu, X.H.; Duong, T.T.T.; Pham, T.T.H.; Trinh, D.K.; Nguyen, X.H.; Dang, V.-S. Synthesis and study of silver nanoparticles for antibacterial activity against Escherichia coli and Staphylococcus aureus. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025019. [Google Scholar] [CrossRef]
- Diantoro, M.; Fitrianingsih, R.; Mufti, N.; Fuad, A. Synthesis of silver nanoparticles by chemical reduction at various fraction of MSA and their structure characterization. In Proceedings of the 4th International Conference on Mathematics and Natural Sciences: Science for Health, Food and Sustainable Energy, Bandung, Indonesia, 8–9 November 2012. [Google Scholar]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthès, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of nanoparticles in tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Rajoriya, P.; Barcelos, M.C.; Ferreira, D.C.; Misra, P.; Molina, G.; Pelissari, F.M.; Shukla, P.K.; Ramteke, P.W. Green silver nanoparticles: Recent trends and technological developments. J. Polym. Environ. 2021, 29, 2711–2737. [Google Scholar] [CrossRef]
- Hamida, R.S.; Abdelmeguid, N.E.; Ali, M.A.; Bin-Meferij, M.M.; Khalil, M.I. Synthesis of silver nanoparticles using a novel cyanobacteria Desertifilum sp. extract: Their antibacterial and cytotoxicity effects. Int. J. Nanomed. 2019, 2020, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Quiroz, C.; Acevedo, N.; Zapata-Giraldo, J.; Botero, L.E.; Quintero, J.; Zárate-Triviño, D.; Saldarriaga, J.; Pérez, V.Z. Optimization of silver nanoparticle synthesis by chemical reduction and evaluation of its antimicrobial and toxic activity. Biomater. Res. 2019, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Shameli, K.; Ahmad, M.B.; Zargar, M.; Yunus, W.M.Z.W.; Ibrahim, N.A.; Shabanzadeh, P.; Moghaddam, M.G. Synthesis and characterization of silver/montmorillonite/chitosan bionanocomposites by chemical reduction method and their antibacterial activity. Int. J. Nanomed. 2011, 6, 271–284. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Arellano-Jimenez, M.J.; Lopez, F.D.; Lopez-Ribot, J.L. Protocol optimization for a fast, simple and economical chemical reduction synthesis of antimicrobial silver nanoparticles in non-specialized facilities. BMC Res. Notes 2019, 12, 773. [Google Scholar] [CrossRef]
- Halder, S.; Ahmed, A.N.; Gafur, M.A.; Seong, G.; Hossain, M.Z. Size-Controlled Facile Synthesis of Silver Nanoparticles by Chemical Reduction Method and Analysis of Their Antibacterial Performance. ChemistrySelect 2021, 6, 9714–9720. [Google Scholar] [CrossRef]
- Marinescu, L.; Ficai, D.; Ficai, A.; Oprea, O.; Nicoara, A.I.; Vasile, B.S.; Boanta, L.; Marin, A.; Andronescu, E.; Holban, A.-M. Comparative Antimicrobial Activity of Silver Nanoparticles Obtained by Wet Chemical Reduction and Solvothermal Methods. Int. J. Mol. Sci. 2022, 23, 5982. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. Rsc Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Quate, C.; Gerber, C.; Binnig, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef]
- Binnig, G.; Gerber, C.; Stoll, E.; Albrecht, T.; Quate, C. Atomic resolution with atomic force microscope. Europhys. Lett. 1987, 3, 1281. [Google Scholar] [CrossRef]
- Gerber, C.; Lang, H.P. How the doors to the nanoworld were opened. Nat. Nanotechnol. 2006, 1, 3–5. [Google Scholar] [CrossRef]
- Hansma, H.; Kim, K.; Laney, D.; Garcia, R.; Argaman, M.; Allen, M.; Parsons, S. Properties of biomolecules measured from atomic force microscope images: A review. J. Struct. Biol. 1997, 119, 99–108. [Google Scholar] [CrossRef]
- Dufrêne, Y.F.; Ando, T.; Garcia, R.; Alsteens, D.; Martinez-Martin, D.; Engel, A.; Gerber, C.; Müller, D.J. Imaging modes of atomic force microscopy for application in molecular and cell biology. Nat. Nanotechnol. 2017, 12, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics; John Wiley & Sons: New York, NY, USA, 1977; p. 376. [Google Scholar]
- Washington, C. Particle Size Analysis in Pharmaceutics and Other Industries: Theory and Practice, 1st ed.; CRC Press: London, UK, 1992; p. 250. [Google Scholar]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Chu, B. Laser Light Scattering: Basic Principles and Practice, 2nd ed.; Academic Press: Cambridge, MA, USA, 1991; p. 354. [Google Scholar]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Maiti, S.; Krishnan, D.; Barman, G.; Ghosh, S.K.; Laha, J.K. Antimicrobial activities of silver nanoparticles synthesized from Lycopersicon esculentum extract. J. Anal. Sci. Technol. 2014, 5, 40. [Google Scholar] [CrossRef]
- Binnig, G.; Rohrer, H.; Gerber, C.; Weibel, E. Surface studies by scanning tunneling microscopy. Phys. Rev. Lett. 1982, 49, 57. [Google Scholar] [CrossRef]
- Giessibl, F.J. Advances in atomic force microscopy. Rev. Mod. Phys. 2003, 75, 949–983. [Google Scholar] [CrossRef]
- Gomès, S.; Assy, A.; Chapuis, P.O. Scanning thermal microscopy: A review. Phys. Status Solidi A 2015, 212, 477–494. [Google Scholar] [CrossRef]
- Chicea, D. Nanoparticles and nanoparticle aggregates sizing by DLS and AFM. J. Optoelectron. Adv. Mater. 2010, 4, 1310–1315. [Google Scholar]
- Chicea, D.; Neamtu, B.; Chicea, R.; Chicea, L. The application of AFM for biological sample imaging. Dig. J. Nanomater. Biostructures 2010, 5, 1015–1022. [Google Scholar]
- Chicea, D.; Indrea, E.; Cretu, C. Assesing Fe3O4 nanoparticle size by DLS, XRD and AFM. J. Optoelectron. Adv. Mater. 2012, 14, 460–466. [Google Scholar]
- Chicea, D. A study of nanoparticle aggregation by coherent light scattering. Curr. Nanosci. 2012, 8, 259–265. [Google Scholar] [CrossRef]
- Chicea, D.; Leca, C.; Olaru, S.; Chicea, L.M. An advanced sensor for particles in gases using dynamic light scattering in air as solvent. Sensors 2021, 21, 5115. [Google Scholar] [CrossRef]
- Langowski, J.; Bryan, R. Maximum entropy analysis of photon correlation spectroscopy data using a Bayesian estimate for the regularization parameter. Macromolecules 1991, 24, 6346–6348. [Google Scholar] [CrossRef]
- Jaynes, E. Information Theory and Statistical Mechanics. Phys. Rev. 1957, 106, 620–630. [Google Scholar] [CrossRef]
- Provencher, S. A constrained regularization method for inverting data represented by linear algebraic or integral equations. Comput. Phys. Commun. 1982, 9, 213–227. [Google Scholar] [CrossRef]
- Provencher, S. An eigenfunction expansion method for the analysis of exponential decay curves. J. Chem. Phys. 1976, 64, 2772–2777. [Google Scholar] [CrossRef]
- Craig, I.; Thompson, A.; Thompson, W.J. Practical numerical algorithms why laplace transforms are difficult to invert numerically. Comput. Phys. 1994, 8, 648–653. [Google Scholar] [CrossRef]
- Davies, B.; Martin, B. Numerical inversion of the Laplace transform: A survey and comparison of methods. J. Comput. Phys. 1979, 33, 1–32. [Google Scholar] [CrossRef]
- Gurney, K. An Introduction to Neural Networks; UCL Press—Taylor & Francis Group: London, UK, 1997; p. 317. [Google Scholar]
- Haykin, S. Neural Networks and Learning Machines, 3rd ed.; Pearson Education: Upper Saddle River, NJ, USA, 2009; p. 906. [Google Scholar]
- Chicea, D. Using neural networks for dynamic light scattering time series processing. Meas. Sci. Technol. 2017, 28, 055206. [Google Scholar] [CrossRef]
- Chicea, D.; Rei, S.M. A fast artificial neural network approach for dynamic light scattering time series processing. Meas. Sci. Technol. 2018, 29, 105201. [Google Scholar] [CrossRef]
- Chicea, D. An artificial neural network assisted dynamic light scattering procedure for assessing living cells size in suspension. Sensors 2020, 20, 3425. [Google Scholar] [CrossRef]
- Clark, N.A.; Lunacek, J.H.; Benedek, G.B. A study of Brownian motion using light scattering. Am. J. Phys. 1970, 38, 575–585. [Google Scholar] [CrossRef]
- Dubin, S.B.; Lunacek, J.H.; Benedek, G.B. Observation of the spectrum of light scattered by solutions of biological macromolecules. Proc. Natl. Acad. Sci. USA 1967, 57, 1164–1171. [Google Scholar] [CrossRef]
- Goodman, J.W. Statistical Optics; Wiley: Hoboken, NJ, USA, 2000; p. 572. [Google Scholar]
- Hecht, E. Optics, 5th ed.; Pearson Education Limited: London, UK, 2017; p. 730. [Google Scholar]
- Knapp, J.Z.; Barber, T.A.; Lieberman, A. Liquid and Surface Borne Particle Measurement Handbook; Taylor & Francis: Monticello, NY, USA, 1996; p. 896. [Google Scholar]
- Khintchine, A. Korrelationstheorie der stationären stochastischen Prozesse. Math. Ann. 1933, 109, 604–615. [Google Scholar] [CrossRef]
- Wiener, N. Generalized harmonic analysis. Acta Math. 1930, 55, 117–258. [Google Scholar] [CrossRef]
- Leong, Y.S.; Ker, P.J.; Jamaludin, M.Z.; Nomanbhay, S.M.; Ismail, A.; Abdullah, F.; Looe, H.M.; Shukri, C.N.S.M. New near-infrared absorbance peak for inhibitor content detection in transformer insulating oil. Sens. Actuators B Chem. 2018, 266, 577–582. [Google Scholar] [CrossRef]
- Monteiro, D.; Gorup, L.; Silva, S.; Negri, M.; De Camargo, E.; Oliveira, R.; Barbosa, D.d.B.; Henriques, M. Silver colloidal nanoparticles: Antifungal effect against adhered cells and biofilms of Candida albicans and Candida glabrata. Biofouling 2011, 27, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.S.; Ker, P.J.; Jamaludin, M.Z.M.; Nomanbhay, S.; Ismail, A.; Abdullah, F.; Looe, H.M.; Lo, C.K. UV-Vis Spectroscopy: A New Approach for Assessing the Color Index of Transformer Insulating Oil. Sensors 2018, 18, 2175. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gaud, B.; Jaybhaye, S. Optimization of synthesis parameters of silver nanoparticles and its antimicrobial activity. Mater. Sci. Energy Technol. 2020, 3, 232–236. [Google Scholar] [CrossRef]
- Tamilselvan, S.; Soniya, R.M.; Vasantharaja, R.; Kannan, M.; Supriya, S.; Dass Batvari, B.P.; Ramesh, T.; Govindaraju, K. Silver nanoparticles based spectroscopic sensing of eight metal ions in aqueous solutions. Environ. Res. 2022, 212, 113585. [Google Scholar] [CrossRef]
- Santos, C.A.d.; Seckler, M.M.; Ingle, A.P.; Rai, M. Comparative antibacterial activity of silver nanoparticles synthesised by biological and chemical routes with pluronic F68 as a stabilising agent. IET Nanobiotechnol. 2016, 10, 200–205. [Google Scholar] [CrossRef]
- Fu, L.-M.; Hsu, J.-H.; Shih, M.-K.; Hsieh, C.-W.; Ju, W.-J.; Chen, Y.-W.; Lee, B.-H.; Hou, C.-Y. Process Optimization of Silver Nanoparticle Synthesis and Its Application in Mercury Detection. Micromachines 2021, 12, 1123. [Google Scholar] [CrossRef]
- Eid, M.M. Characterization of Nanoparticles by FTIR and FTIR-Microscopy. In Handbook of Consumer Nanoproducts; Shadpour, M., Chaudhery, M.H., Eds.; Springer: Singapore, 2022; Volume 1, pp. 645–673. [Google Scholar]
- Biswal, A.K.; Misra, P.K. Biosynthesis and characterization of silver nanoparticles for prospective application in food packaging and biomedical fields. Mater. Chem. Phys. 2020, 250, 123014. [Google Scholar] [CrossRef]
- Thiruvengadam, V.; Bansod, A.V. Characterization of silver nanoparticles synthesized using chemical method and its antibacterial property. Biointerface Res. Appl. Chem. 2020, 10, 7257–7264. [Google Scholar] [CrossRef]
- Chicea, D. Coherent light scattering on nanofluids: Computer simulation results. Appl. Opt. 2008, 47, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.; Mayers, B.; Xia, Y. Shape-Controlled Synthesis of Metal Nanostructures: The Case of Silver. Chem. Eur. J. 2005, 11, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Abkhalimov, E.V.; Ershov, V.A.; Ershov, B.G. “Pure” silver hydrosol: Nanoparticles and stabilizing carbonate ions. J. Nanopart. Res. 2019, 21, 93. [Google Scholar] [CrossRef]
- Awad, M.A.; Eid, A.M.; Elsheikh, T.M.; Al-Faifi, Z.E.; Saad, N.; Sultan, M.H.; Selim, S.; Al-Khalaf, A.A.; Fouda, A. Mycosynthesis, characterization, and mosquitocidal activity of silver nanoparticles fabricated by Aspergillus niger strain. J. Fungi 2022, 8, 396. [Google Scholar] [CrossRef]
- Gul, A.; Fozia; Shaheen, A.; Ahmad, I.; Khattak, B.; Ahmad, M.; Ullah, R.; Bari, A.; Ali, S.S.; Alobaid, A. Green Synthesis, Characterization, Enzyme Inhibition, Antimicrobial Potential, and Cytotoxic Activity of Plant Mediated Silver Nanoparticle Using Ricinus communis Leaf and Root Extracts. Biomolecules 2021, 11, 206. [Google Scholar] [CrossRef]
- Dayakar, T.; Rao, K.V.; Park, J.; Sadasivuni, K.K.; Rao, K.R. Non-enzymatic biosensing of glucose based on silver nanoparticles synthesized from Ocimum tenuiflorum leaf extract and silver nitrate. Mater. Chem. Phys. 2018, 216, 502–507. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Antibacterial effect of silver nanoparticles is stronger if the production host and the targeted pathogen are closely related. Biomedicines 2022, 10, 628. [Google Scholar] [CrossRef]
- Setyaningrum, D.; Riyanto, S.; Rohman, A. Analysis of corn and soybean oils in red fruit oil using FTIR spectroscopy in combination with partial least square. Int. Food Res. J. 2013, 20, 1977–1981. [Google Scholar]
- Sowden, J.C.; Schaffer, R. The Reaction of D-Glucose, D-Mannose and D-Fructose in 0.035 N Sodium Hydroxide at 35°. J. Am. Chem. Soc. 1952, 74, 499–504. [Google Scholar] [CrossRef]
- Nagasawa, T.; Sato, K.; Shimada, Y.; Kasumi, T. Efficient conversion of D-glucose to D-fructose in the presence of organogermanium compounds. J. Appl. Glycosci. 2016, 63, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef] [PubMed]


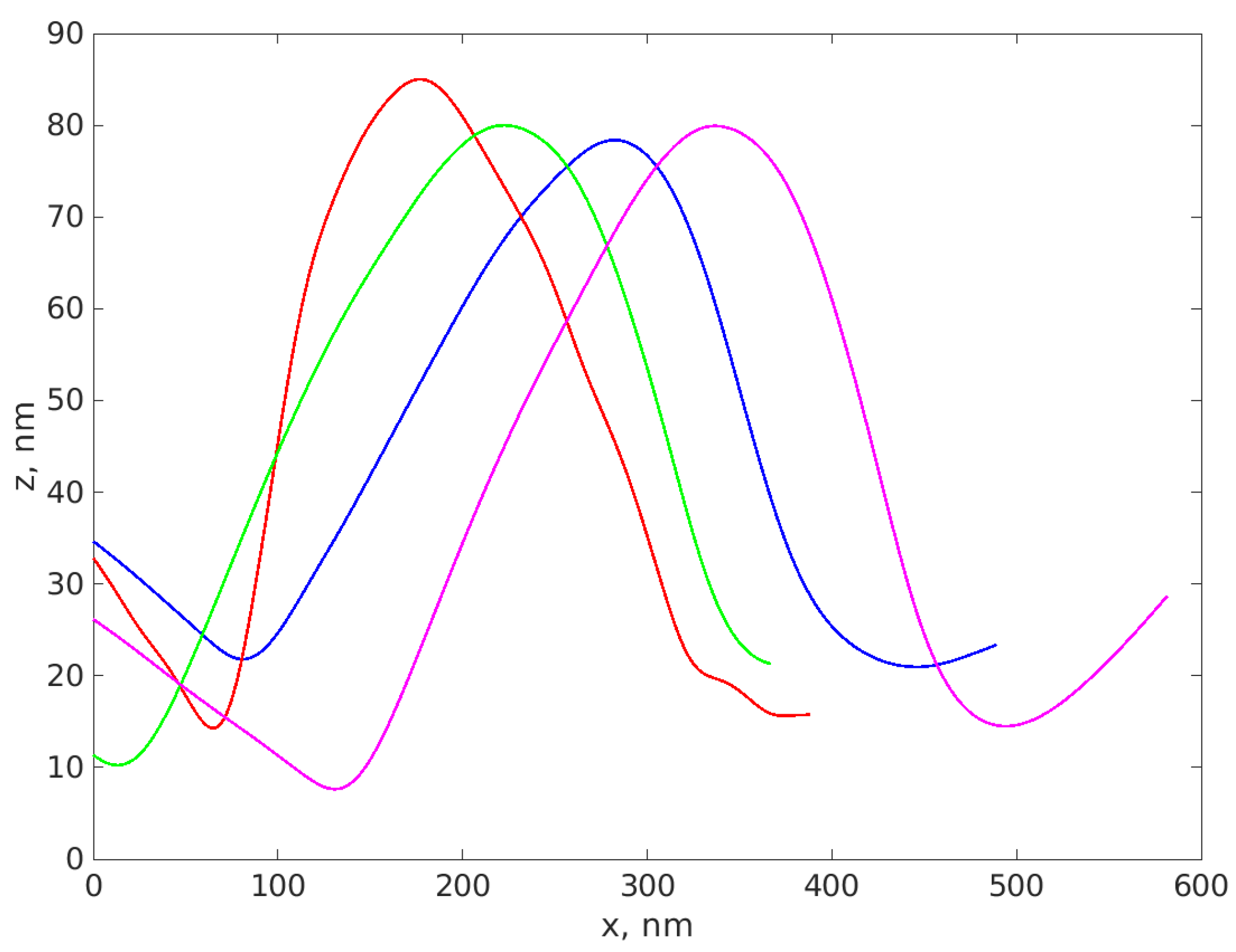
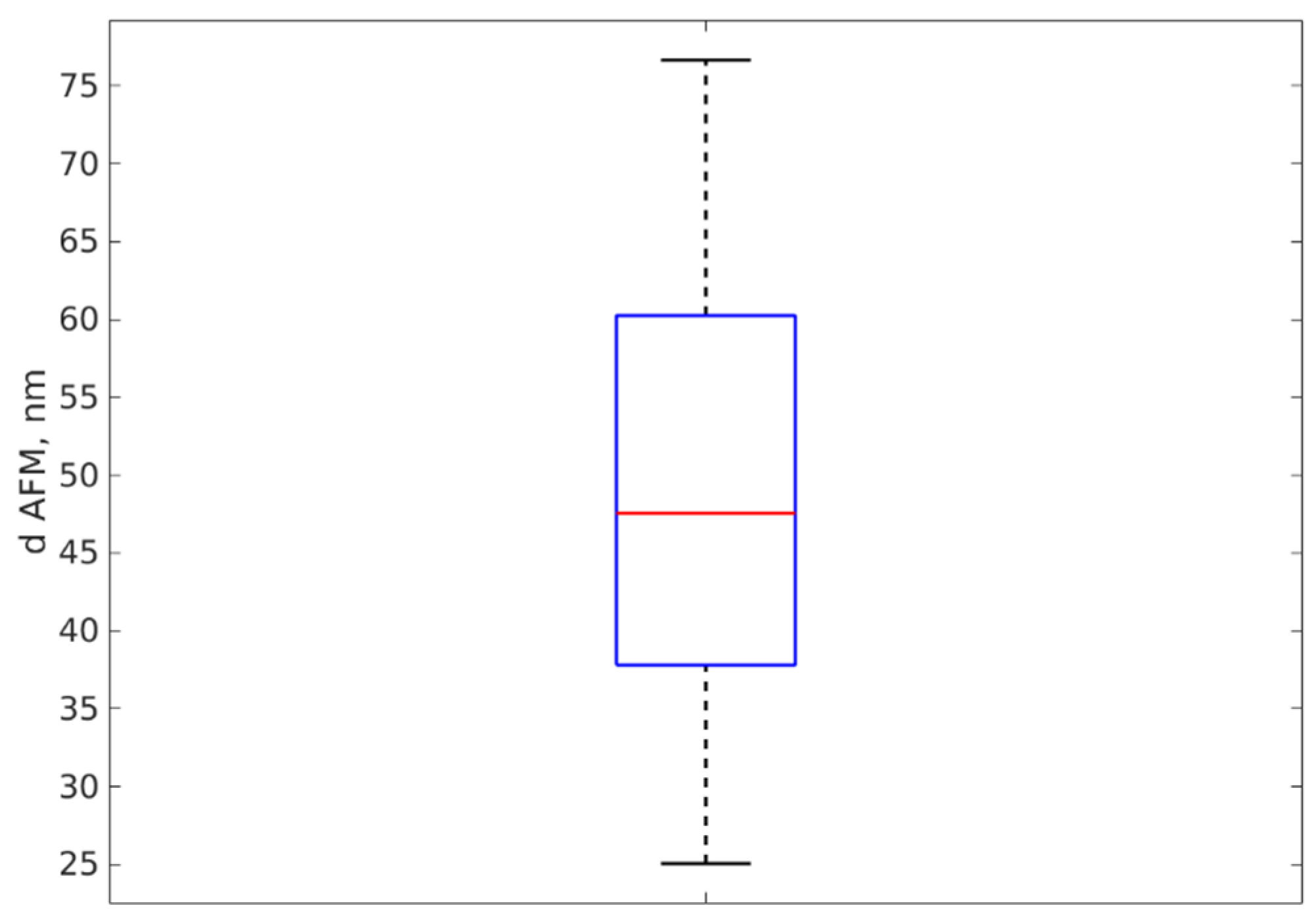
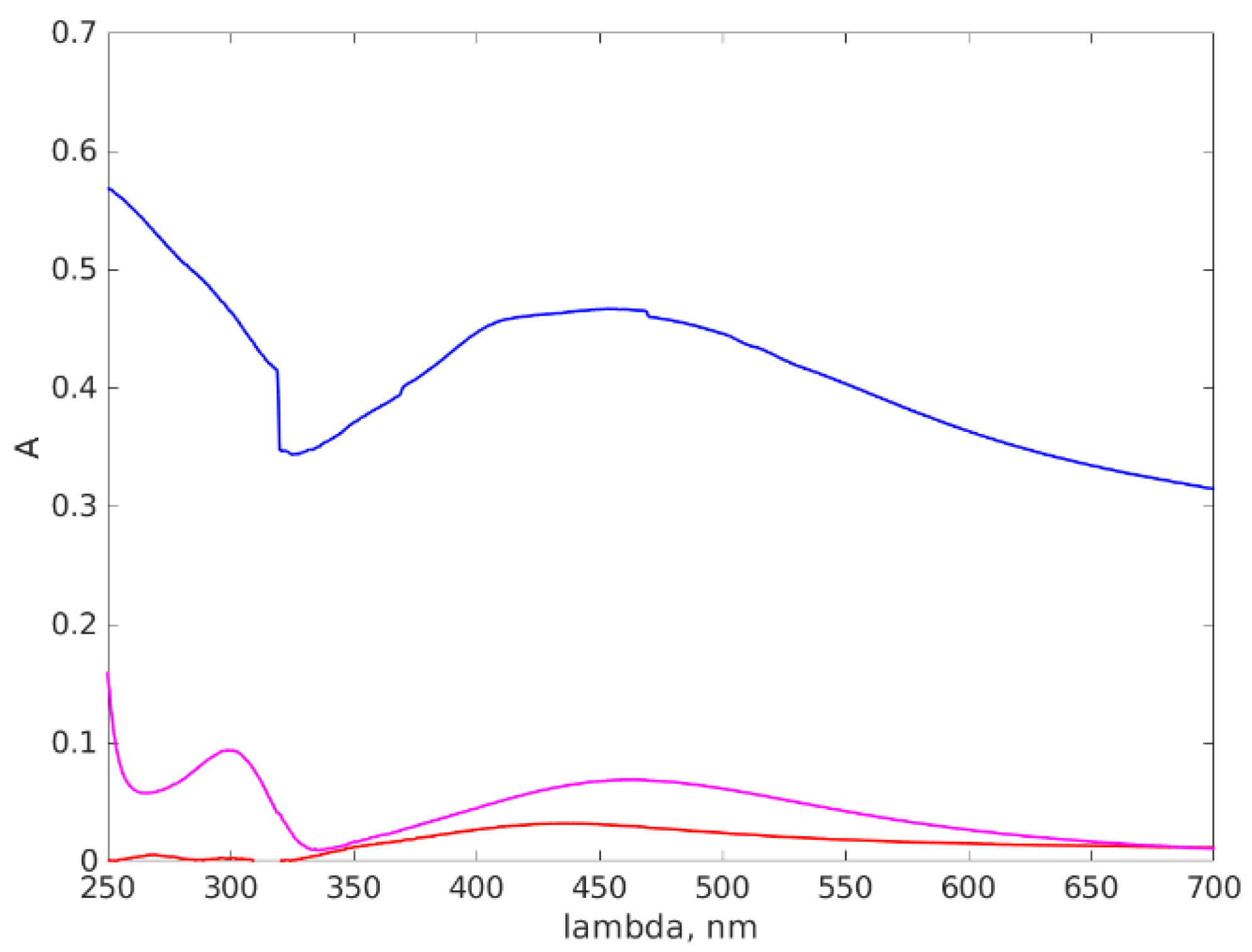
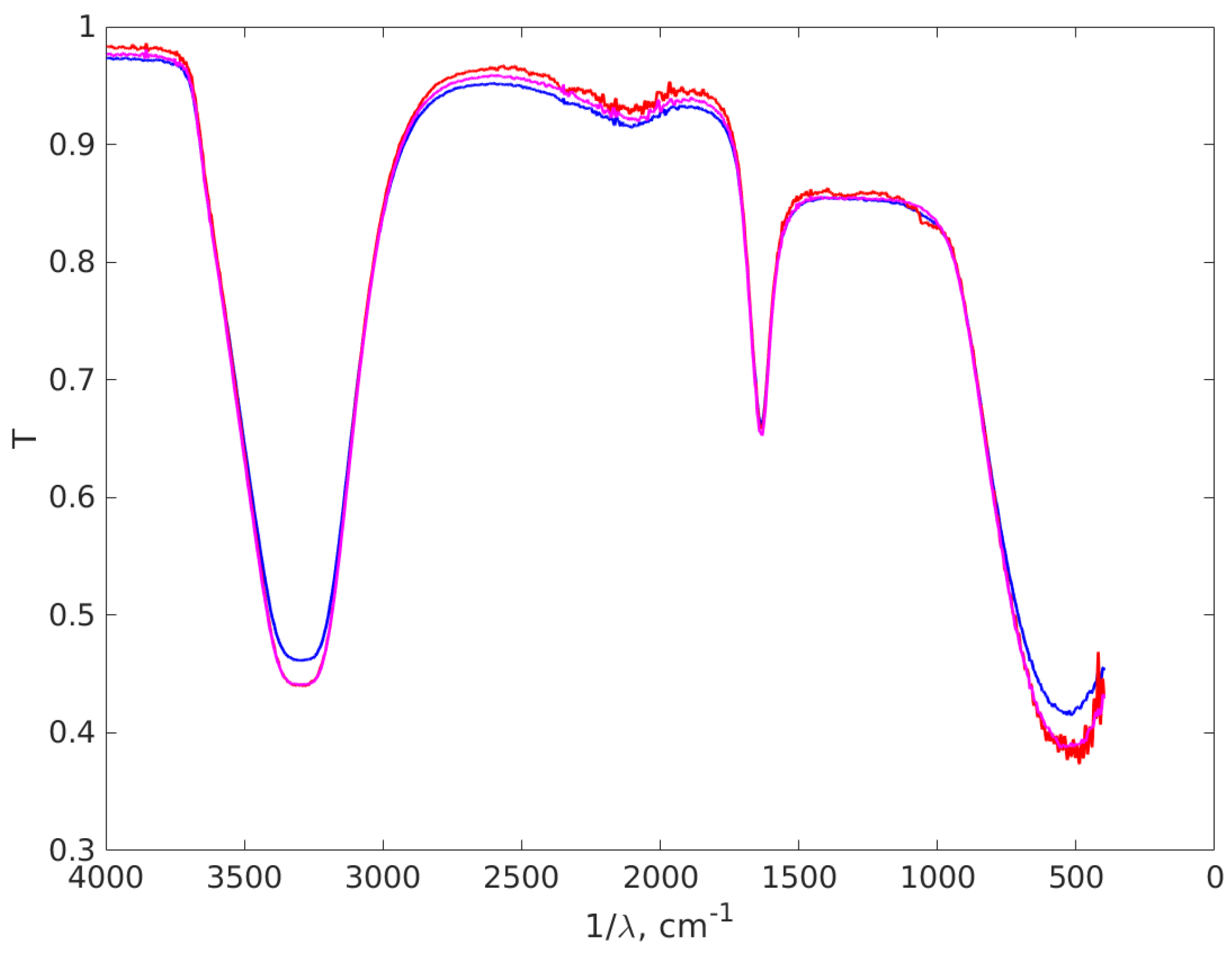
| No. | Sample | d DLS, nm | Δd DLS, nm | d AFM, nm |
| 1 | AgNP-R1 | 1140 | 107 | 973 |
| 2 | AgNP-R2 | 58 | 6 | 49 |
| 3 | Ag-77 | 431 | 41 | 387 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chicea, D.; Nicolae-Maranciuc, A.; Doroshkevich, A.S.; Chicea, L.M.; Ozkendir, O.M. Comparative Synthesis of Silver Nanoparticles: Evaluation of Chemical Reduction Procedures, AFM and DLS Size Analysis. Materials 2023, 16, 5244. https://doi.org/10.3390/ma16155244
Chicea D, Nicolae-Maranciuc A, Doroshkevich AS, Chicea LM, Ozkendir OM. Comparative Synthesis of Silver Nanoparticles: Evaluation of Chemical Reduction Procedures, AFM and DLS Size Analysis. Materials. 2023; 16(15):5244. https://doi.org/10.3390/ma16155244
Chicago/Turabian StyleChicea, Dan, Alexandra Nicolae-Maranciuc, Aleksandr S. Doroshkevich, Liana Maria Chicea, and Osman Murat Ozkendir. 2023. "Comparative Synthesis of Silver Nanoparticles: Evaluation of Chemical Reduction Procedures, AFM and DLS Size Analysis" Materials 16, no. 15: 5244. https://doi.org/10.3390/ma16155244
APA StyleChicea, D., Nicolae-Maranciuc, A., Doroshkevich, A. S., Chicea, L. M., & Ozkendir, O. M. (2023). Comparative Synthesis of Silver Nanoparticles: Evaluation of Chemical Reduction Procedures, AFM and DLS Size Analysis. Materials, 16(15), 5244. https://doi.org/10.3390/ma16155244






