Antibacterial and Biocompatible Polyethylene Composites with Hybrid Clay Nanofillers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Synthesis
2.2. Methods for Characterization
2.3. Antibacterial Tests
2.4. In Vitro Biocompatibility
3. Results and Discussion
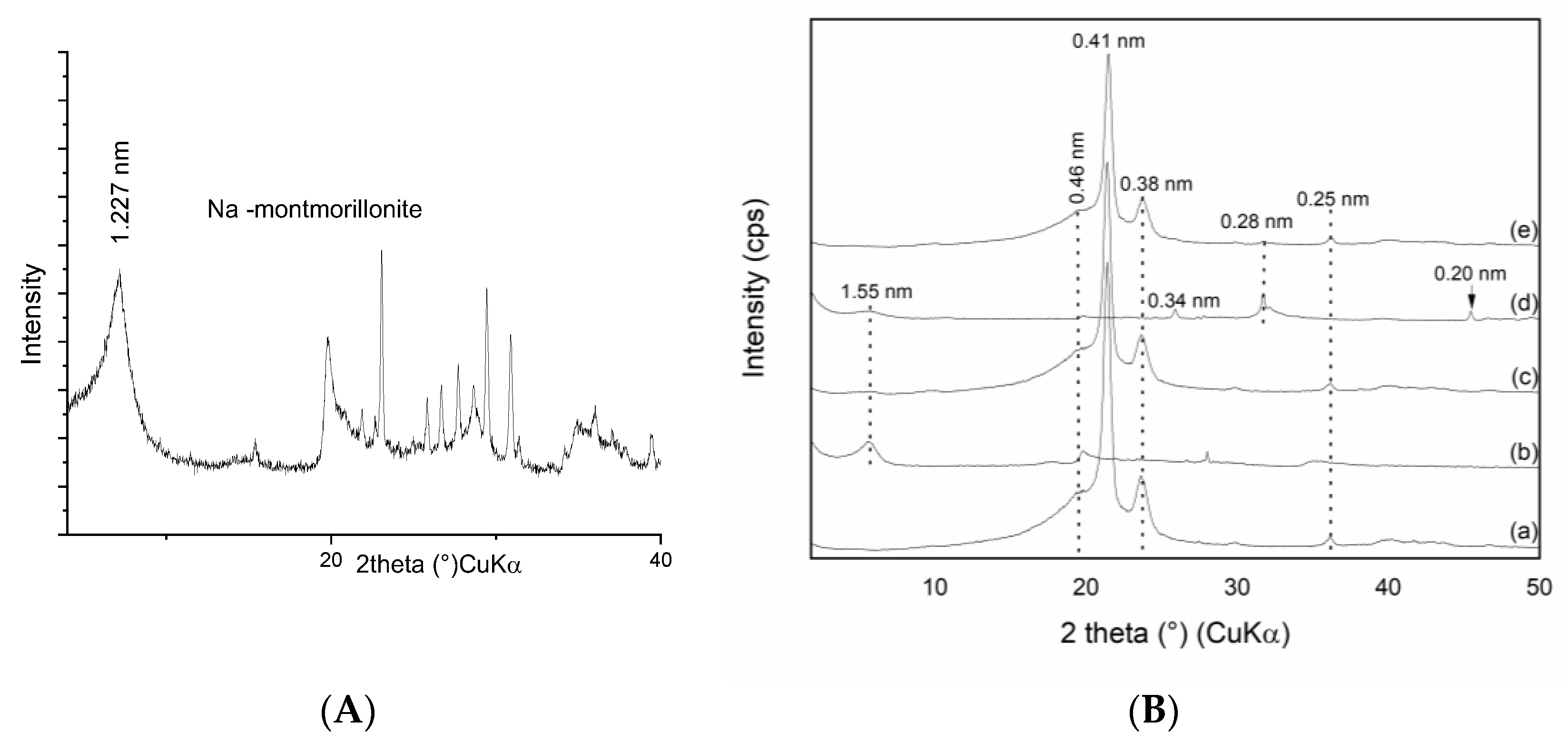
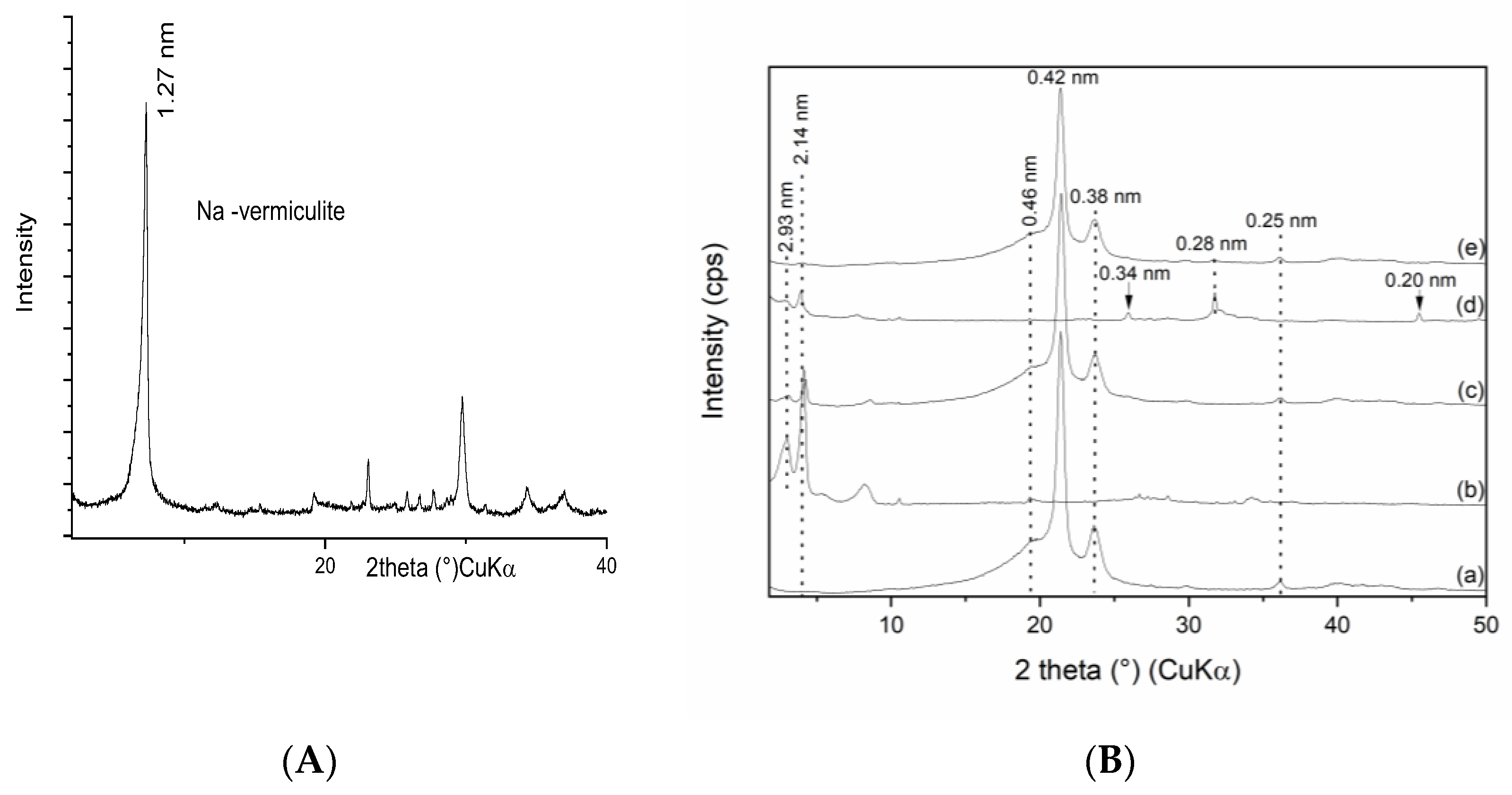
3.1. X-ray Diffraction Phase Analysis
3.2. Differential Scanning Calorimetry Structure Study
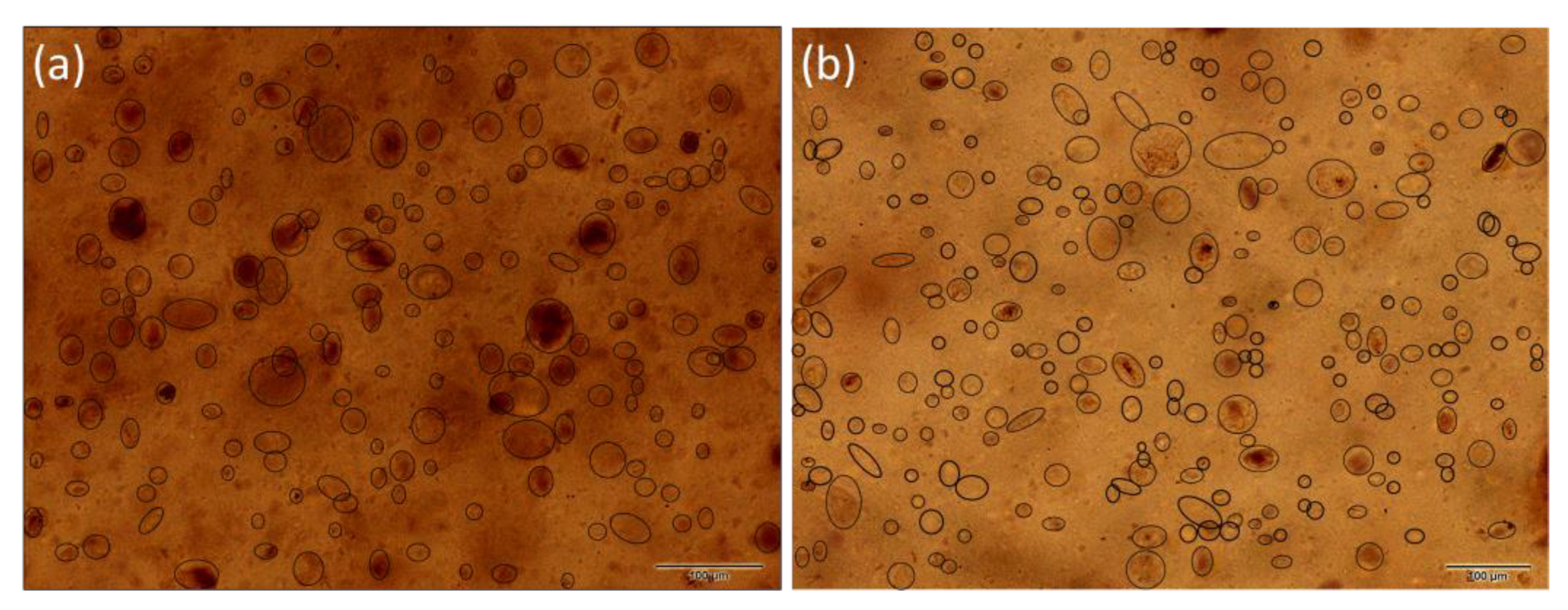
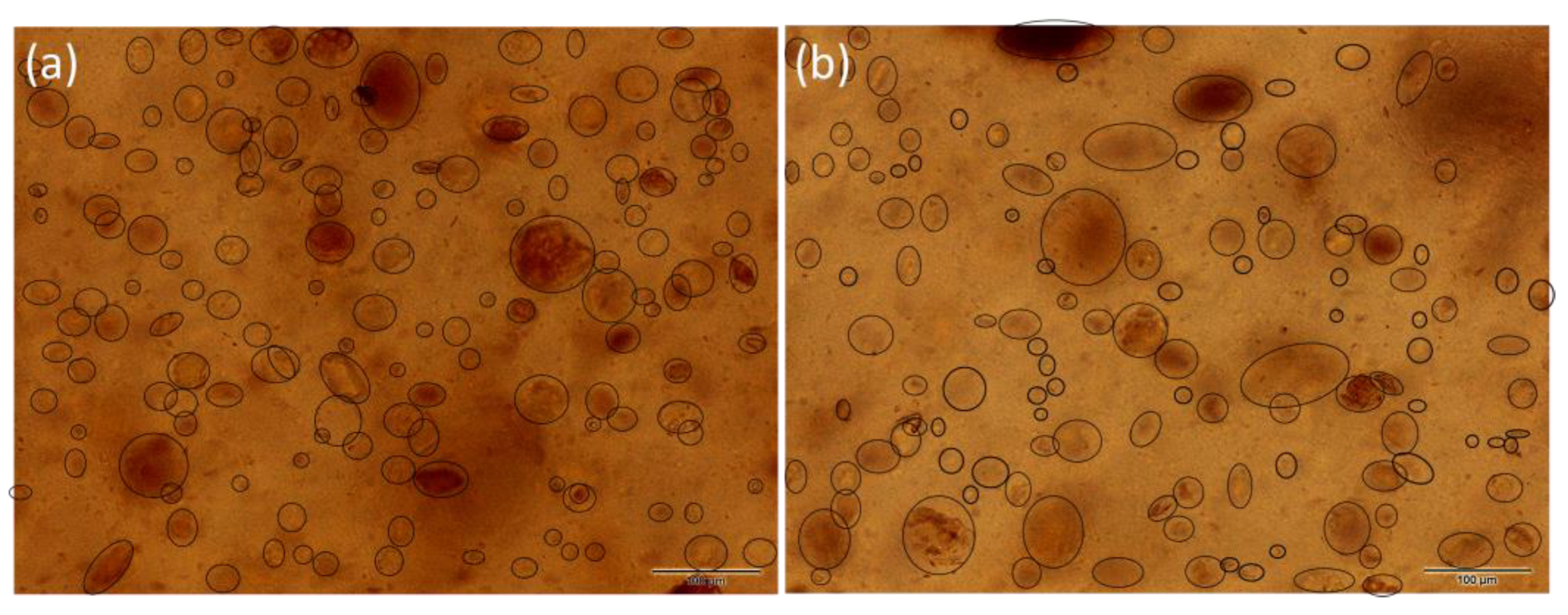
3.3. Light Microscopy Imagining and Particle Size Distribution
3.4. Antibacterial Test
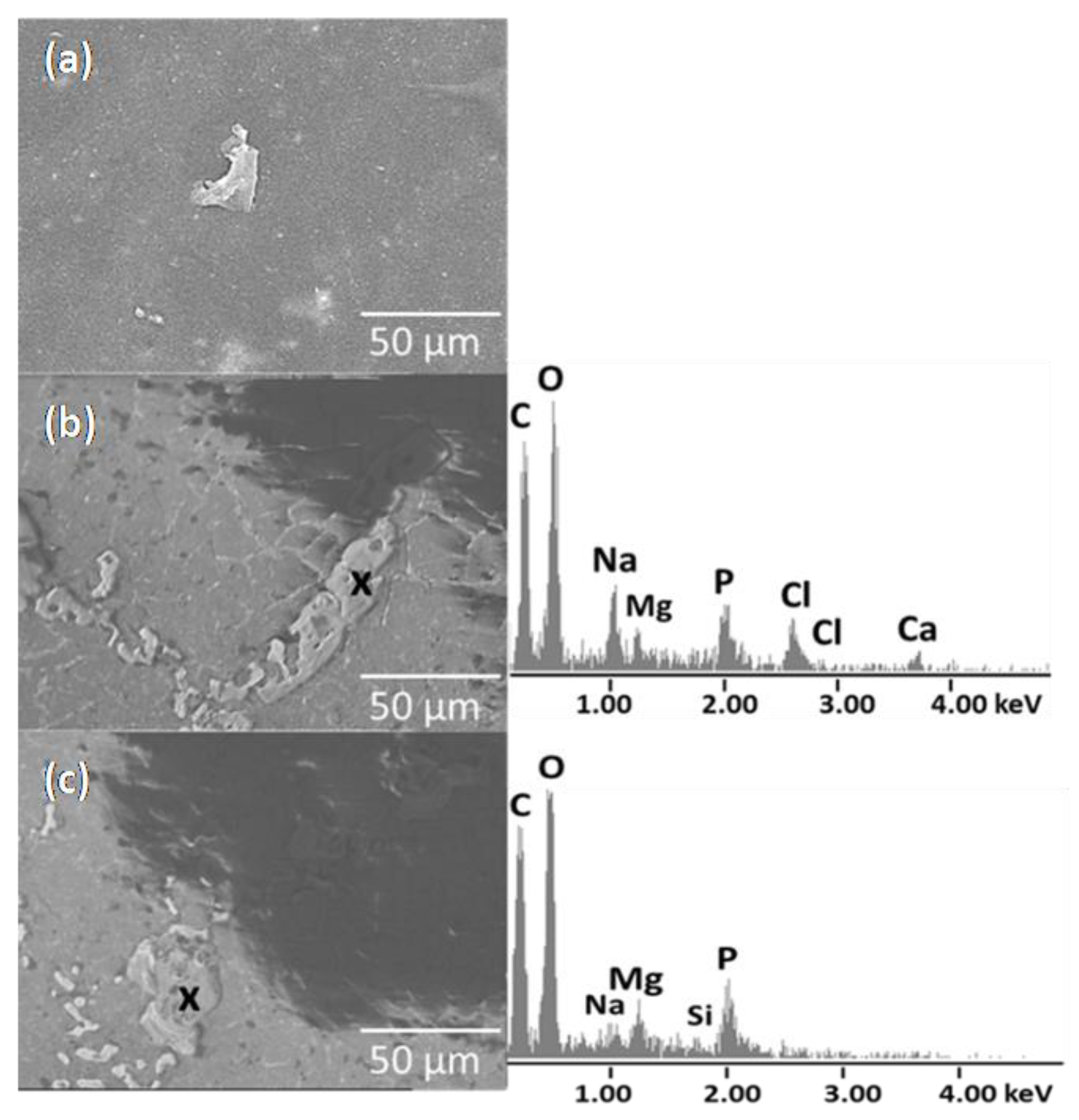
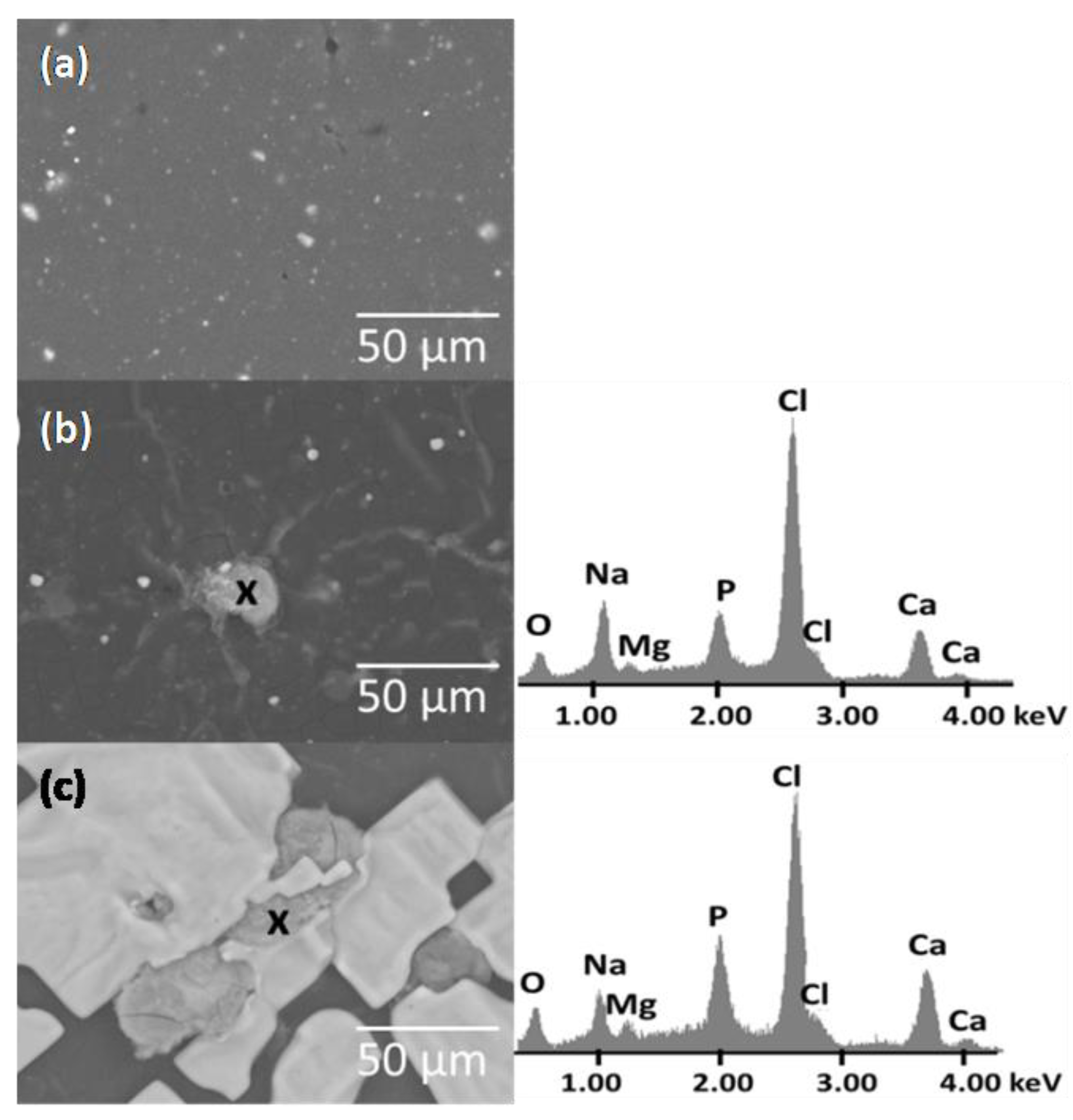
3.5. In Vitro Biocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruiz-Hitzky, E.; Van Meerbeek, A. Clay mineral–and organoclay–polymer nanocomposite. Dev. Clay Sci. 2006, 1, 583–621. [Google Scholar]
- Katti, K.S.; Katti, D.R.; Dash, R. Synthesis and characterization of a novel chitosan/montmorillonite/hydroxyapatite nanocomposite for bone tissue engineering. Biomed. Mater. 2008, 3, 034122. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite based materials for bone tissue engineering: A brief and comprehensive introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Ambre, A.; Katti, K.S.; Katti, D.R. In situ mineralized hydroxyapatite on amino acid modified nanoclays as novel bone biomaterials. Mater. Sci. Eng. C 2011, 31, 1017–1029. [Google Scholar] [CrossRef]
- Duan, S.; Wu, R.; Xiong, Y.H.; Ren, H.M.; Lei, C.; Zhao, Y.Q.; Zhang, X.Y.; Xu, F.J. Multifunctional antimicrobial materials: From rational design to biomedical applications. Prog. Mater. Sci. 2022, 125, 100887. [Google Scholar] [CrossRef]
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Eng. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef]
- Kolpen, M.; Kragh, K.N.; Enciso, J.B.; Faurholt-Jepsen, D.; Lindegaard, B.; Egelund, G.B.; Jensen, A.V.; Ravn, P.; Mabuza Mathiensen, I.H.; Gheorge, A.G.; et al. Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax 2022, 77, 1015–1022. [Google Scholar] [CrossRef]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med. 2002, 113, 5–13. [Google Scholar] [CrossRef]
- Matl, F.D.; Obermeier, A.; Repmann, S.; Friess, W.; Stemberger, A.; Kuehn, K.D. New anti-infective coatings of medical implants. Antimicrob. Agents Chemother. 2008, 52, 1957–1963. [Google Scholar] [CrossRef]
- Das-Gupta, D.K. Polyethylene: Structure, morphology, molecular motion and dielectric behavior. IEEE Elect. Insul. Mag. 1994, 10, 5–15. [Google Scholar] [CrossRef]
- Androsch, R.; Di Lorenzo, M.L.; Schick, C.; Wunderlich, B. Mesophases in polyethylene, polypropylene, and poly (1-butene). Polymer 2010, 51, 4639–4662. [Google Scholar] [CrossRef]
- Holešová, S.; Samlíková, M.; Ritz, M.; Pazdziora, E. Antibacterial polyethylene/clay nanocomposites using chlorhexidine as organic modifier. Mater. Today Proc. 2015, 2, 246–252. [Google Scholar] [CrossRef]
- Hundáková, M.; Tokarský, J.; Valášková, M.; Slobodian, P.; Pazdziora, E.; Kimmer, D. Structure and antibacterial properties of polyethylene/organo-vermiculite composites. Solid State Sci. 2015, 48, 197–204. [Google Scholar] [CrossRef]
- Martynková, G.S.; Valášková, M. Antimicrobial nanocomposites based on natural modified materials: A review of carbons and clays. J. Nanosci. Nanotechnol. 2014, 14, 673–693. [Google Scholar] [CrossRef]
- Valaskova, M.; Martynková, G.S. (Eds.) . Clay Minerals in Nature—Their Characterization, Modification and Application; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Šupová, M.; Martynková, G.S.; Barabaszová, K. Effect of nanofillers dispersion in polymer matrices: A review. Sci. Adv. Mater. 2011, 3, 1–25. [Google Scholar] [CrossRef]
- Christenson, E.M.; Anseth, K.S.; van den Beucken, J.J.P.; Chan, C.K.; Ercan, B.; Jansen, J.A.; Laurencin, C.T.; Li, W.-J.; Murugan, R.; Nair, L.S.; et al. Nanobiomaterial applications in orthopedics. J. Orthop. Res. 2007, 25, 11–22. [Google Scholar] [CrossRef]
- Jaworski, J.W.; Cho, S.; Kim, Y.; Jung, J.H.; Jeon, H.S.; Min, B.K.; Kwon, K.Y. Hydroxyapatite supported cobalt catalysts for hydrogen generation. J. Colloid Interface Sci. 2013, 394, 401–408. [Google Scholar] [CrossRef]
- Pazourková, L.; Kupková, J.; Hundáková, M.; Seidlerová, J.; Martynková, G.S. Sorption of Cd2+ on clay mineral/hydroxyapatite nanocomposites. J. Nanosci. Nanotechnol. 2016, 16, 7788–7791. [Google Scholar] [CrossRef]
- Ferraz, C.C.; de Almeida Gomes, B.P.; Zaia, A.A.; Teixeira, F.B.; de Souza-Filho, F.J. In vitro assessment of the antimicrobial action and the mechanical ability of chlorhexidine gel as an endodontic irrigant. J. Endod. 2001, 27, 452–455. [Google Scholar] [CrossRef]
- Daud, N.M.; Al-Ashwal, R.H.; Kadir, M.R.; Saidin, S. Polydopamine-assisted chlorhexidine immobilization on medical grade stainless steel 316L: Apatite formation and in vitro osteoblastic evaluation. Ann. Anat.-Anat. Anz. 2018, 220, 29–37. [Google Scholar] [CrossRef]
- Kotnala, S.; Bhushan, B.; Nayak, A. Hydroxyapatite Polymer Nano-composites And Their Role in Biomedical Applications. Trends Biomater. Artif. Organs. 2022, 1, 36. [Google Scholar]
- Ghosh, S.; Ghosh, S.; Atta, A.K.; Pramanik, N. A succinct overview of hydroxyapatite based nanocomposite biomaterials: Fabrications, physicochemical properties and some relevant biomedical applications. J. Bionanosci. 2018, 12, 143–158. [Google Scholar] [CrossRef]
- Dawson, J.I.; Oreffo, R.O. Clay: New opportunities for tissue regeneration and biomaterial design. Adv. Mater. 2013, 25, 4069–4086. [Google Scholar] [CrossRef]
- George, S.M.; Nayak, C.; Singh, I.; Balani, K. Multifunctional hydroxyapatite composites for orthopedic applications: A review. ACS Biomater. Sci. Eng. 2022, 8, 3162–3186. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Tashmetov, M.Y.; Ismatov, N.B.; Allayarov, S.R. X-ray Diffraction Study of the Structure of Gamma-Irradiated Low-Density Polyethylene. High Energy Chem. 2022, 56, 175–179. [Google Scholar] [CrossRef]
- Tanniru, M.; Yuan, Q.; Misra, R.D.K. On significant retention of impact strength in clay–reinforced high-density polyethylene (HDPE) nanocomposites. Polymer 2006, 47, 2133–2146. [Google Scholar] [CrossRef]
- Holešová, S.; Valášková, M.; Plevová, E.; Pazdziora, E.; Matějová, K. Preparation of novel organovermiculites with antibacterial activity using chlorhexidine diacetate. J. Colloid Interface Sci. 2010, 342, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Holešová, S.; Samlíková, M.; Pazdziora, E.; Valášková, M. Antibacterial activity of organomontmorillonites and organovermiculites prepared using chlorhexidine diacetate. Appl. Clay Sci. 2013, 83, 17–23. [Google Scholar] [CrossRef]
- Hotta, S.; Paul, D. Nanocomposites formed from linear low density polyethylene and organoclays. Polymer 2004, 45, 7639–7654. [Google Scholar] [CrossRef]
- Pazourková, L.; Reli, M.; Hundáková, M.; Pazdziora, E.; Predoi, D.; Simha Martynková, G.; Lafdi, K. Study of the structure and antimicrobial activity of Ca-deficient ceramics on chlorhexidine nanoclay substrate. Materials 2019, 12, 2996. [Google Scholar] [CrossRef] [PubMed]
- Pazourková, L.; Peikertová, P.; Hundáková, M.; Martynková, G.S. Preparation of calcium deficient hydroxyapatite on the montmorillonite substrate: Structure and morphology. Mater. Today Proc. 2021, 37, 35–41. [Google Scholar] [CrossRef]


| Sample | Tm/(°C) | Tc/(°C) | ΔHm/(J/g) | Xc/% |
|---|---|---|---|---|
| PE | 108.83 | 93.4 | 111.9 | 38 |
| PE/MCA | 108.03 | 94.58 | 103.84 | 36.53 |
| PE/MCAH | 108.73 | 93.79 | 107.71 | 37.90 |
| PE/VCA | 108.74 | 93.78 | 89.95 | 31.65 |
| PE/VCAH | 108.44 | 93.82 | 101.27 | 35.63 |
| Sample | Median (μm) | Mode (μm) | Distribution Type |
|---|---|---|---|
| MCA | 11.10 | 12.31; 262.37 | bimodal |
| MCAH | 12.95 | 0.339; 12.35; 101.46 | trimodal |
| VCA | 15.75 | 12.41 | monomodal |
| VCAH | 13.51 | 12.30; 200 | bimodal |
| Sample | 24 h | 48 h | 72 h | 96 h |
|---|---|---|---|---|
| PE/VCA | 115 | 65 | 46 | 19 |
| PE/VCAH | 246 | 224 | 254 | 157 |
| PE/MCA | 126 | 102 | 67 | 21 |
| PE/MCAH | 155 | 152 | 143 | 116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klecandová, L.; Nakonieczny, D.S.; Reli, M.; Simha Martynková, G. Antibacterial and Biocompatible Polyethylene Composites with Hybrid Clay Nanofillers. Materials 2023, 16, 5179. https://doi.org/10.3390/ma16145179
Klecandová L, Nakonieczny DS, Reli M, Simha Martynková G. Antibacterial and Biocompatible Polyethylene Composites with Hybrid Clay Nanofillers. Materials. 2023; 16(14):5179. https://doi.org/10.3390/ma16145179
Chicago/Turabian StyleKlecandová, Lenka, Damian S. Nakonieczny, Magda Reli, and Gražyna Simha Martynková. 2023. "Antibacterial and Biocompatible Polyethylene Composites with Hybrid Clay Nanofillers" Materials 16, no. 14: 5179. https://doi.org/10.3390/ma16145179
APA StyleKlecandová, L., Nakonieczny, D. S., Reli, M., & Simha Martynková, G. (2023). Antibacterial and Biocompatible Polyethylene Composites with Hybrid Clay Nanofillers. Materials, 16(14), 5179. https://doi.org/10.3390/ma16145179










