Zinc Evaporation from Brass Scraps in the Atmosphere of Inert Gas
Abstract
1. Introduction
2. Materials and Methods
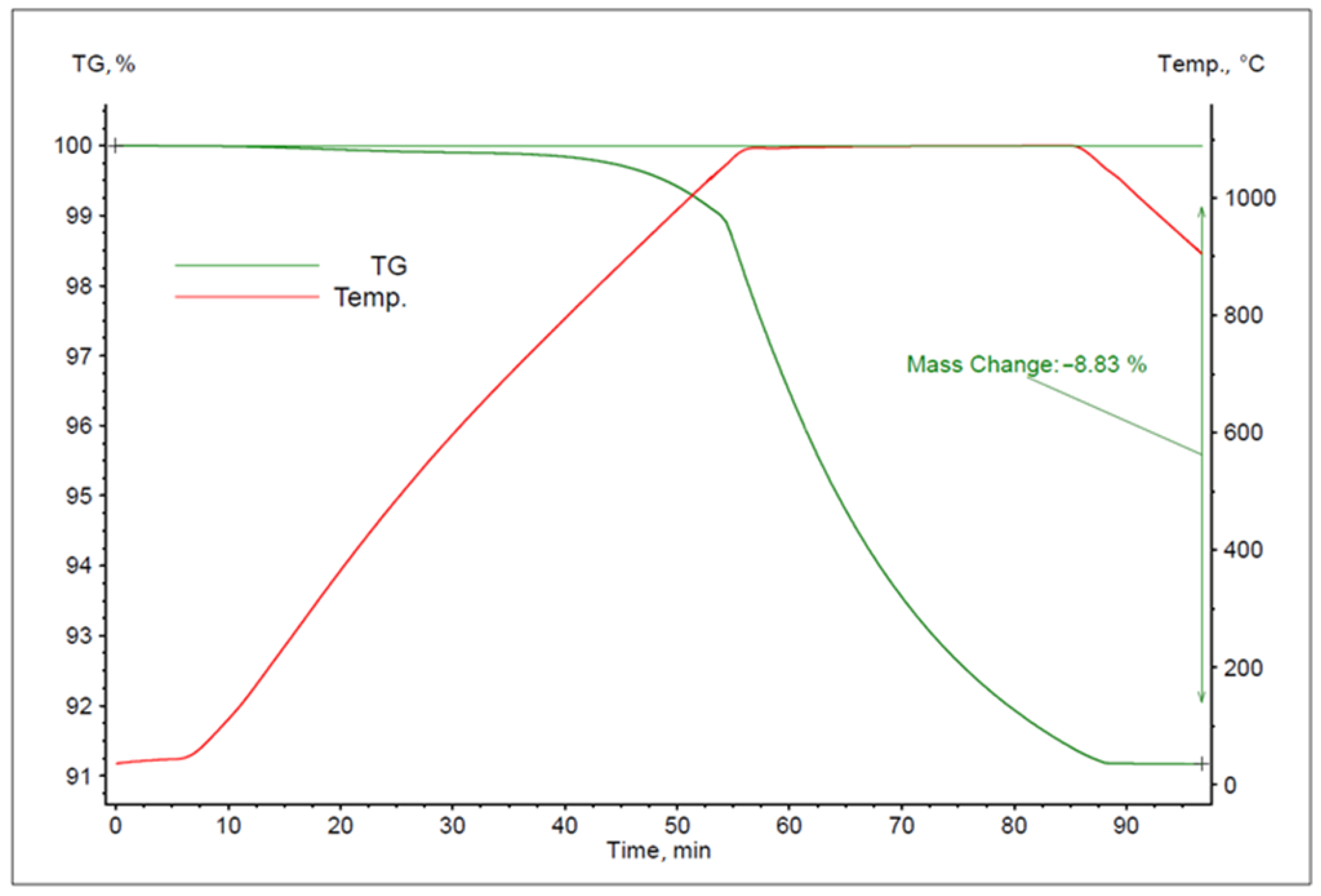
- Sample heating up to a desired temperature (1080, 1120, 1160, 1200, 1240 °C) at 20 °C/min;
- Isothermal holding of the sample for 30 min at the set temperature;
- Sample cooling to 900 °C.
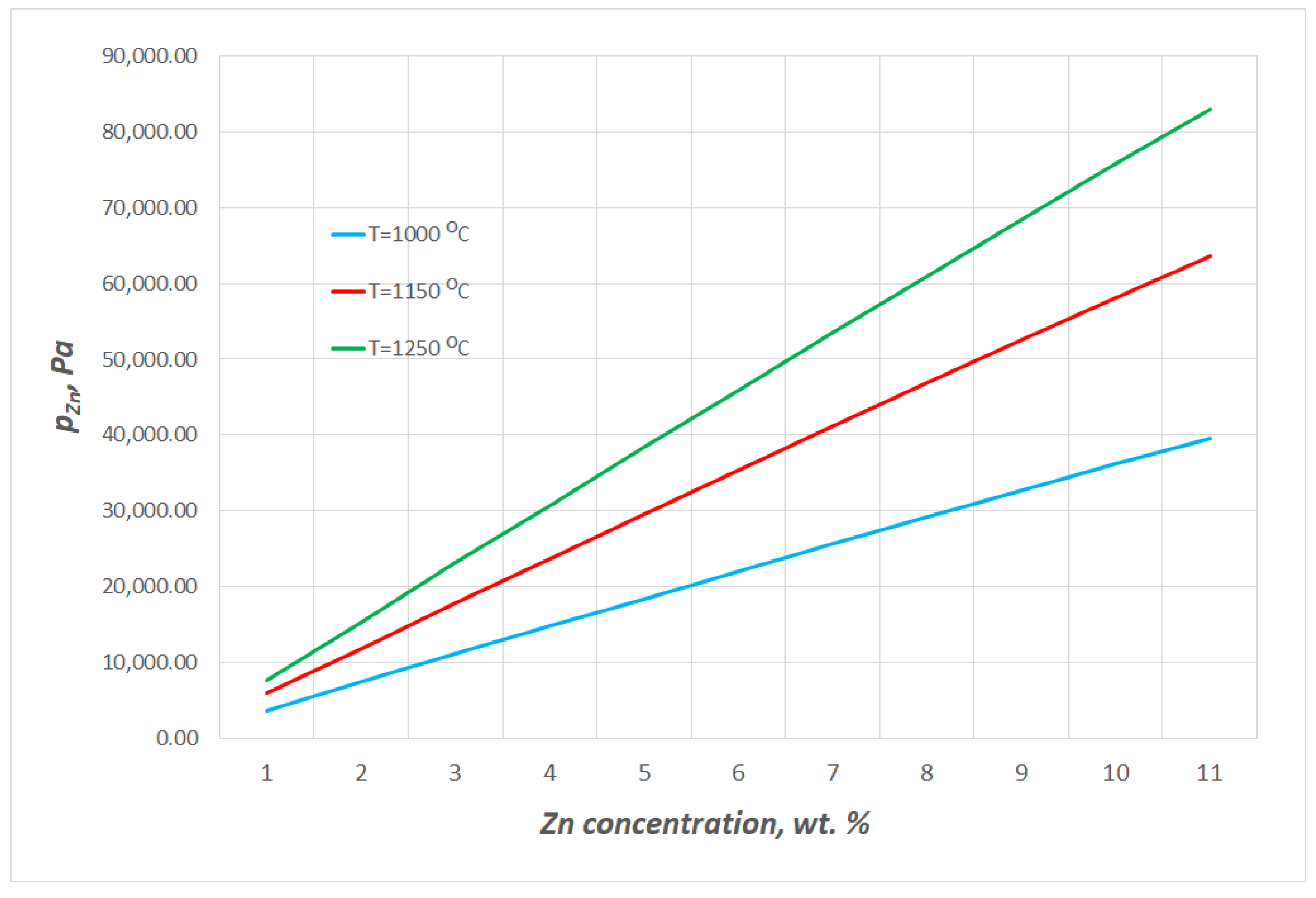
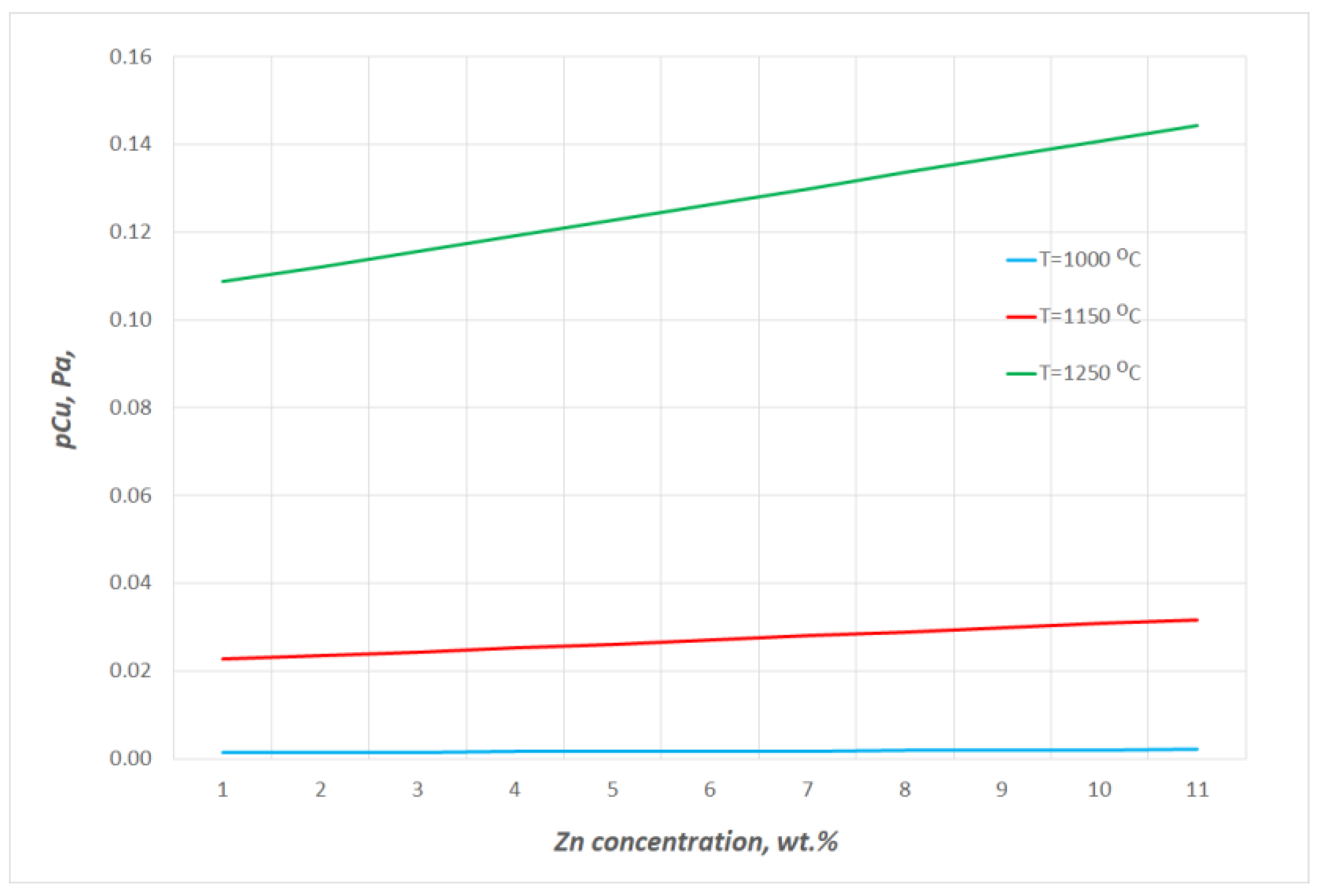
Equilibrium Pressure of Zinc over Cu–Zn Alloys
- ,—the zinc in the liquid phase and in the gas phase, respectively;
- , —the standard chemical potentials of the pure component “i” in the liquid and gas phases, respectively;
- —the partial equilibrium pressure of the component “i” over the solution.
- —the mole fraction of the component “i” in the alloy;
- —the vapour pressure of the component “i” over the pure liquid.
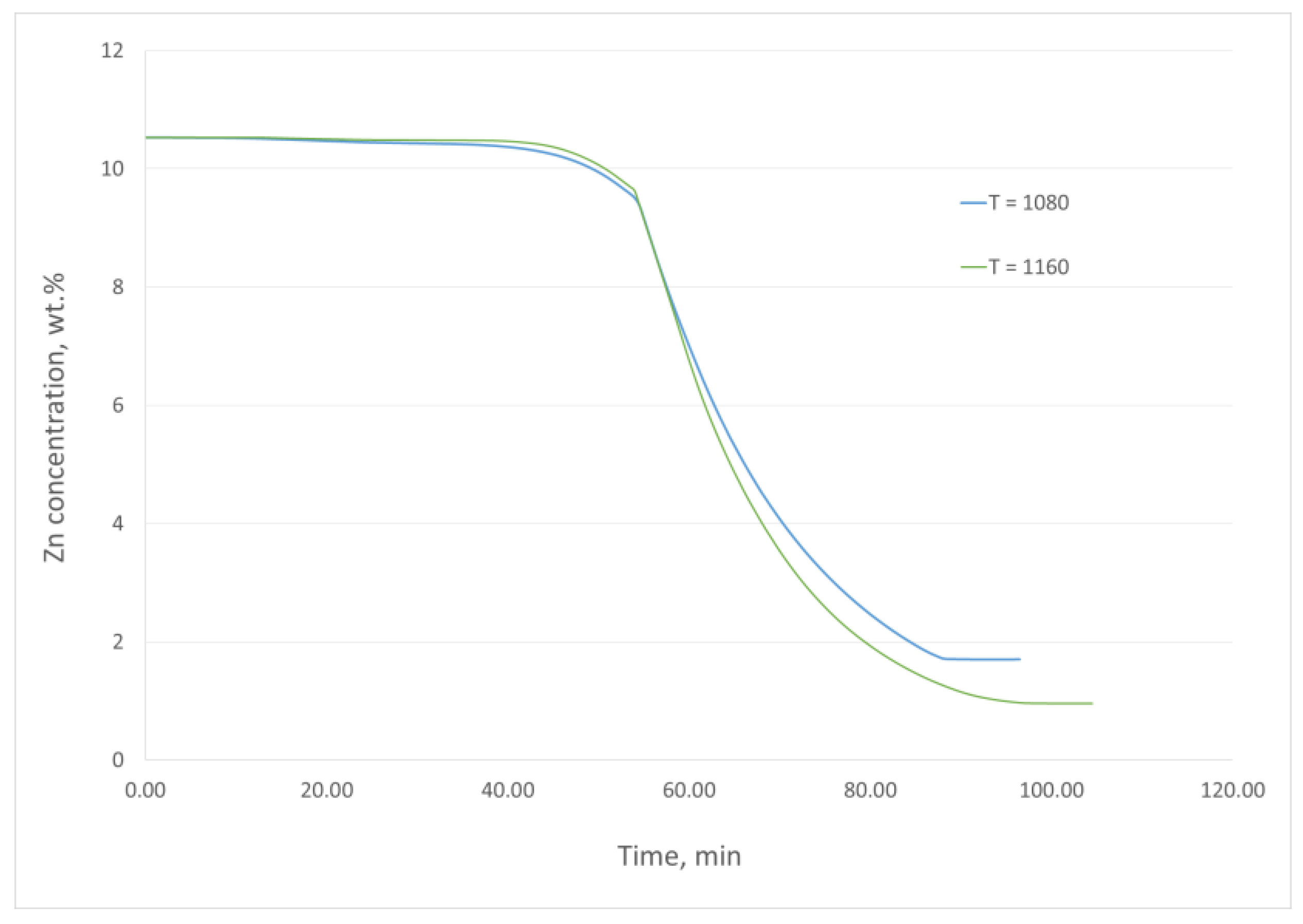
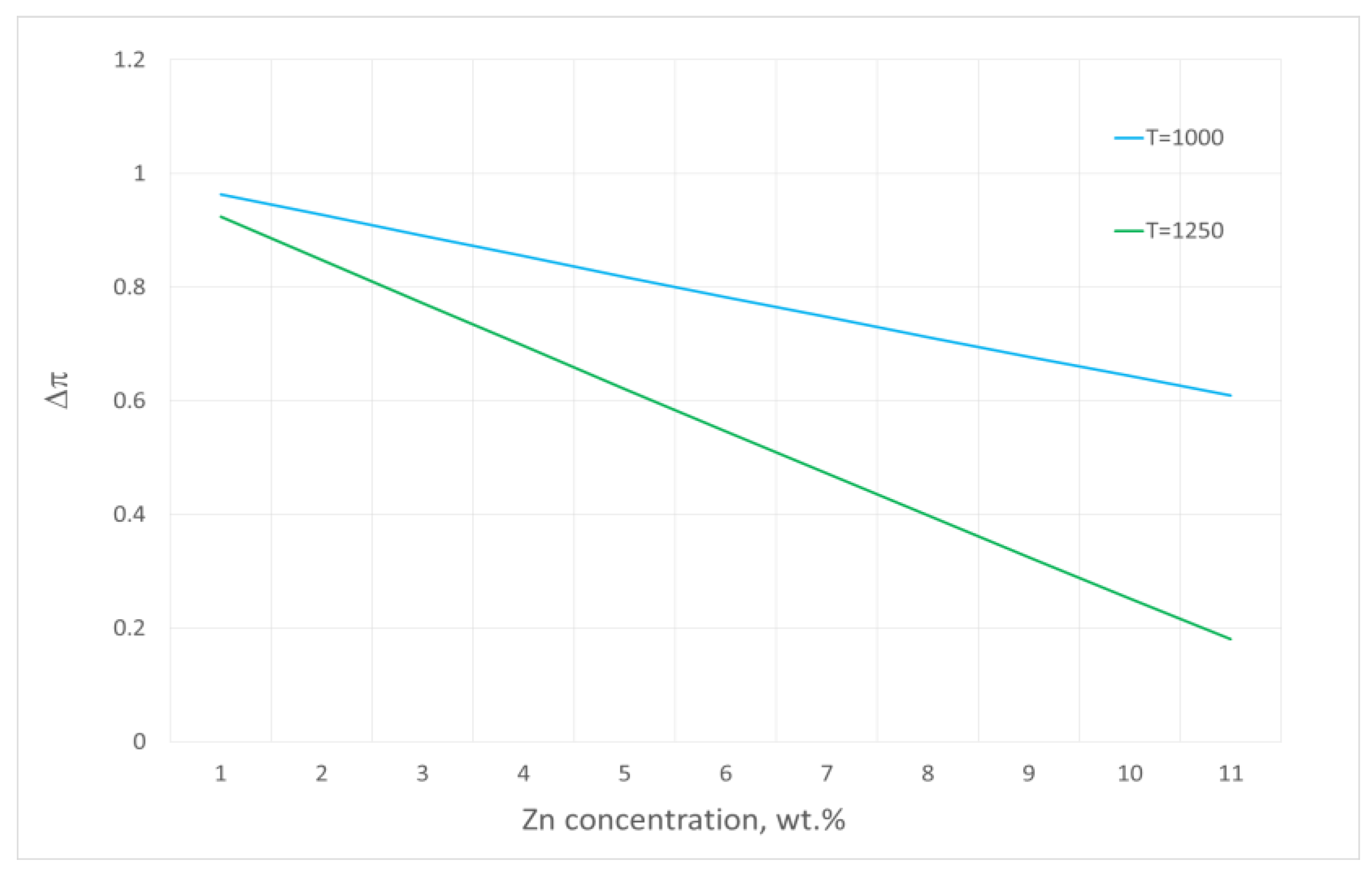
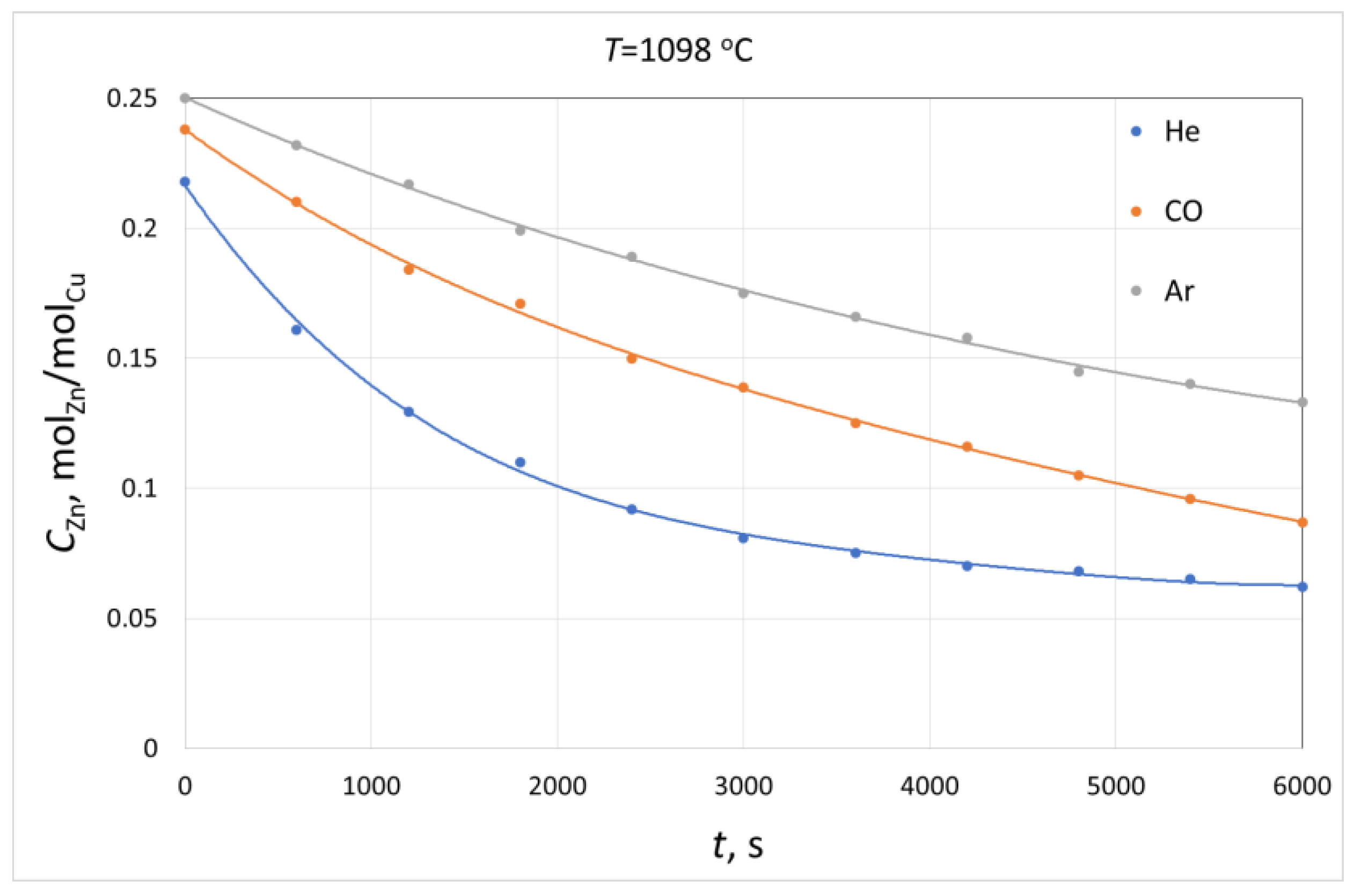
3. Results and Discussion
- m0—the initial sample mass;
- mk—the final sample mass.
- —the equilibrium concentration of zinc over the liquid Cu–Zn alloy.
- P—the overall pressure in the system.
- Zinc transfer from deep inside of the liquid alloy to the interface;
- Zinc evaporation from the liquid metal–gas phase interface;
- Zinc vapour transfer from the interface into the deeper part of the gas phase.
- kl—the mass transfer coefficient of Zn in the liquid phase;
- kg—the mass transfer coefficient of Zn in the gas phase;
- ke—the Zn evaporation rate coefficient.
- the initial zinc content in copper and its content after time t, wt%;
- F—the evaporation surface, m2;
- V—the volume of the liquid copper alloy, m3;
- (t – t0)—the duration of the process, s.
- α—the evaporation constant;
- MCu, MZn—the molar masses of copper and zinc, respectively;
- ρCu—the copper density.
- k—the reaction rate constant, m/s;
- A—the constant for the specific reaction, m/s;
- R—the gas constant, J/mol·K;
- T—the temperature, K.
4. Conclusions
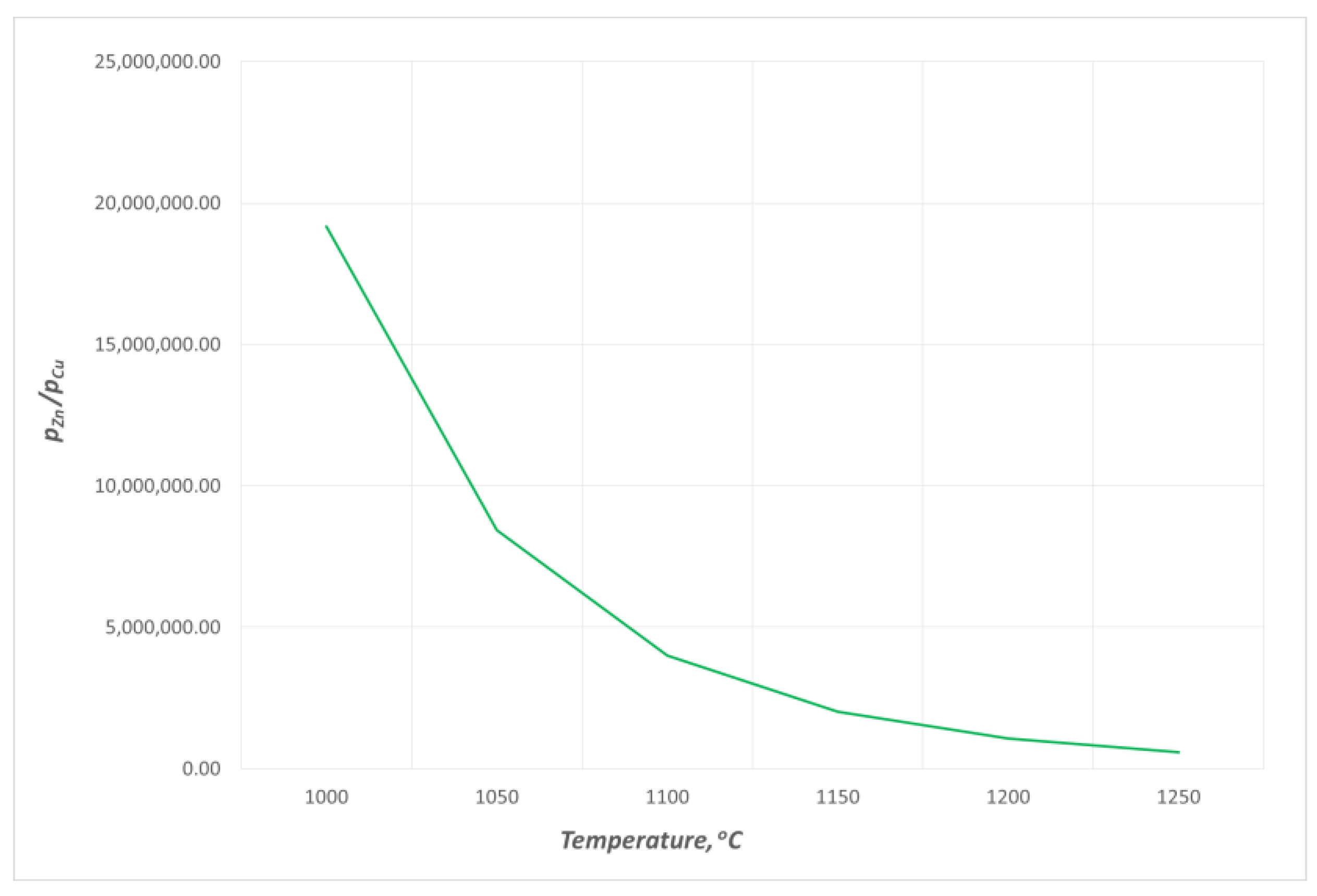
- The estimated zinc vapour pressure over the analysed Cu–Zn alloy at 1080 ÷ 1240 °C ranges from 8.80 × 108 Pa to 1.19 × 109 Pa. These values range from 5.12 × 103 Pa to 5.28 × 104 Pa for copper. The significant difference in the vapour pressures of both metals that form the alloy allows for the assumption to be made that during the liquid phase, practically only zinc is evaporated.
- For the assumed temperature range and the atmospheric pressure (the conditions of the experiments), the achieved level of zinc removal from the Cu–Zn alloy ranges from 83% to 99.9%.
- Based on the determined kinetic parameters of the zinc evaporation process, i.e., the overall zinc mass transfer coefficient kZn and the evaporation rate constant ke, it can be concluded that the analysed process is characterised by diffusion control and the stage that determines its rate is the mass transfer in the gas phase.
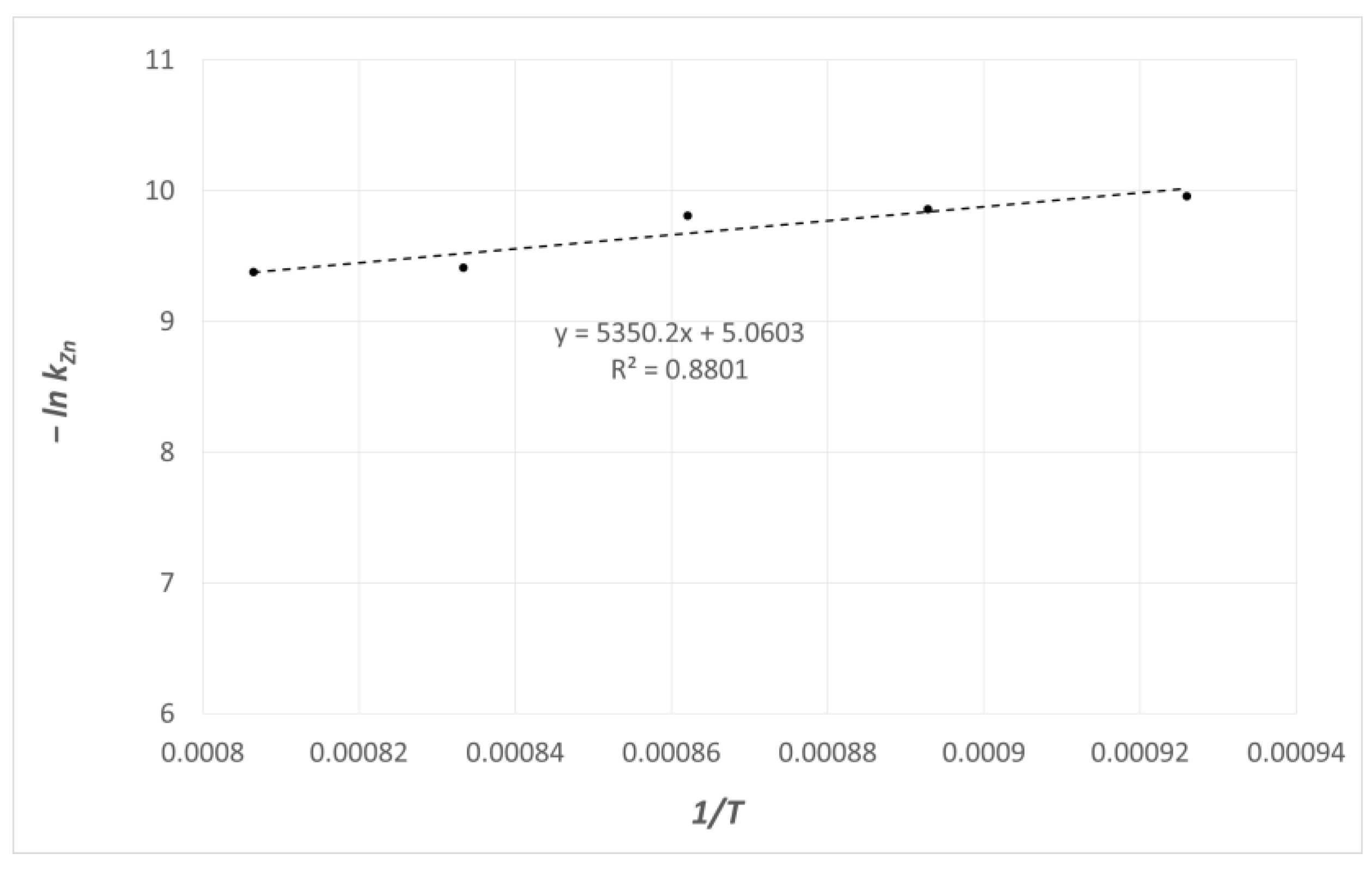
- The estimated value of apparent activation energy for the studied process of zinc evaporation is 69 kJ·mol−1. For comparison, the value of zinc diffusion activation energy in liquid copper, estimated based on the coefficients of diffusion of these metals, is 18 kJ·mol−1.
- For all experiments, the determined fraction of resistance related to the process on the liquid alloy Re in the total resistance of the process ranges from 10% to 18%, which means that evaporation itself, which occurs on the interface, is not a factor that limits the rate of the analysed process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Xiang, D.; Cao, H.; Li, P. Life Cycle Energy Consumption and GHG Emissions of the Copper Production in China and the Influence of Main Factors on the above Performance. Processes 2022, 10, 2715. [Google Scholar] [CrossRef]
- Schäfer, P.; Schmidt, M. Discrete-Point Analysis of the Energy Demand of Primary versus Secondary Metal Production. Environ. Sci. Technol. 2020, 54, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Z.; Wu, Y.; Li, L.; Li, B.; Pan, D.; Zuo, T. Environmental Benefits of Secondary Copper from Primary Copper Based on Life Cycle Assessment in China. Resour. Conserv. Recycl. 2019, 146, 35–44. [Google Scholar] [CrossRef]
- HSC Chemistry 2022. Outocumpu Research Oy. Pori. Available online: http://www.hsc-chemistry.net/ (accessed on 22 May 2023).
- Lee, T.-H.; Joo, S.-H.; Nersisyan, H.H.; Kong, M.-S.; Lee, J.-W.; Park, K.-W.; Lee, J.-H. Reduction Kinetics of Zinc Powder from Brass Converter Slag by Pyrometallurgical Method Using Hydrogen Gas. KONA 2016, 33, 278–286. [Google Scholar] [CrossRef]
- Onyekwere Okwuchi Smith; Orji Chiawolamoke Ihenwokeleme; Uyanga Kindness Alfred Determination of Percentage Zinc Loss during Melting of Zinc Scrap in a Crucible Furnace Primary Tabs. Glob. J. Eng. Tech. Adv. 2019, 1, 22–26. [CrossRef]
- Trebukhov, S.; Volodin, V.; Nitsenko, A.; Burabaeva, N.; Ruzakhunova, G. Recovery of Zinc from the Concentrate of Domestic Waste Processing by Vacuum Distillation. Metals 2022, 12, 703. [Google Scholar] [CrossRef]
- Andersson, A.; Andersson, M.; Mousa, E.; Kullerstedt, A.; Ahmed, H.; Björkman, B.; Sundqvist-Ökvist, L. The Potential of Recycling the High-Zinc Fraction of Upgraded BF Sludge to the Desulfurization Plant and Basic Oxygen Furnace. Metals 2018, 8, 1057. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Cui, K.; Fu, T.; Gao, J.; Hussain, S.; AlGarni, T.S. Pyrometallurgical Recovery of Zinc and Valuable Metals from Electric Arc Furnace Dust—A Review. J. Clean. Prod. 2021, 298, 126788. [Google Scholar] [CrossRef]
- Stewart, D.J.C.; Barron, A.R. Pyrometallurgical Removal of Zinc from Basic Oxygen Steelmaking Dust—A Review of Best Available Technology. Resour. Conserv. Recycl. 2020, 157, 104746. [Google Scholar] [CrossRef]
- Xue, Y.; Hao, X.; Liu, X.; Zhang, N. Recovery of Zinc and Iron from Steel Mill Dust—An Overview of Available Technologies. Materials 2022, 15, 4127. [Google Scholar] [CrossRef]
- Nemchinova, N.V.; Patrushov, A.E.; Tyutrin, A.A. Pyrometallurgical Technology for Extracting Iron and Zinc from Electric Arc Furnace Dust. Appl. Sci. 2023, 13, 6204. [Google Scholar] [CrossRef]
- Sinaga, G.S.T.; Wismogroho, A.S.; Fitroturokhmah, A.; Kusumaningrum, R.; Widayatno, W.B.; Hadiko, G.; Amal, M.I. The Pyrometallurgical Recovery of Zinc from Electric Arc Furnace Dust (EAFD) with Active Carbon. IOP Conf. Ser. Mater. Sci. Eng. 2019, 578, 012068. [Google Scholar] [CrossRef]
- Nowinska, K. Mineralogical and Chemical Characteristics of Slags from the Pyrometallurgical Extraction of Zinc and Lead. Minerals 2020, 10, 371. [Google Scholar] [CrossRef]
- Ghayad, I.; Al Ansary, A.; Abdel Aziz, Z.; Al Akhar, A. Recovery of Zinc From Zinc Dross Using Pyrometallurgical and Electrochemical Methods. Egypt. J. Chem. 2018, 62, 373–384. [Google Scholar] [CrossRef]
- Baba, A.A.; Adekola, A.F.; Bale, R.B. Development of a Combined Pyro- and Hydro-Metallurgical Route to Treat Spent Zinc–Carbon Batteries. J. Hazard. Mater. 2009, 171, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Kumar, V.; Singh, R.J. Review of Hydrometallurgical Recovery of Zinc from Industrial Wastes. Resour. Conserv. Recycl. 2001, 33, 1–22. [Google Scholar] [CrossRef]
- Kendall, D.S. Toxicity Characteristic Leaching Procedure and Iron Treatment of Brass Foundry Waste. Environ. Sci. Technol. 2003, 37, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Radzymińska-Lenarcik, E.; Sulewski, M.; Urbaniak, W. Recovery of Zinc from Metallurgic Waste Sludges. Pol. J. Environ. Stud. 2015, 24, 1277–1282. [Google Scholar] [CrossRef]
- Sengupta, B.; Bhakhar, M.S.; Sengupta, R. Extraction of Zinc and Copper–Zinc Mixtures from Ammoniacal Solutions into Emulsion Liquid Membranes Using LIX 84I®. Hydrometallurgy 2009, 99, 25–32. [Google Scholar] [CrossRef]
- Van Herck, P.; Vandecasteele, C.; Swennen, R.; Mortier, R. Zinc and Lead Removal from Blast Furnace Sludge with a Hydrometallurgical Process. Environ. Sci. Technol. 2000, 34, 3802–3808. [Google Scholar] [CrossRef]
- Oráč, D.; Klimko, J.; Klein, D.; Pirošková, J.; Liptai, P.; Vindt, T.; Miškufová, A. Hydrometallurgical Recycling of Copper Anode Furnace Dust for a Complete Recovery of Metal Values. Metals 2021, 12, 36. [Google Scholar] [CrossRef]
- Terrones-Saeta, J.M.; Suárez-Macías, J.; Moreno-López, E.R.; Corpas-Iglesias, F.A. Leaching of Zinc for Subsequent Recovery by Hydrometallurgical Techniques from Electric Arc Furnace Dusts and Utilisation of the Leaching Process Residues for Ceramic Materials for Construction Purposes. Metals 2021, 11, 1603. [Google Scholar] [CrossRef]
- de Soria-Aguilar, M.J.; Davila-Pulido, G.I.; Carrillo-Pedroza, F.R.; Gonzalez-Ibarra, A.A.; Picazo-Rodriguez, N.; de Lopez-Saucedo, F.J.; Ramos-Cano, J. Oxidative Leaching of Zinc and Alkalis from Iron Blast Furnace Sludge. Metals 2019, 9, 1015. [Google Scholar] [CrossRef]
- Palimąka, P.; Pietrzyk, S.; Stępień, M.; Ciećko, K.; Nejman, I. Zinc Recovery from Steelmaking Dust by Hydrometallurgical Methods. Metals 2018, 8, 547. [Google Scholar] [CrossRef]
- Zheng, X.; Li, S.; Liu, B.; Zhang, L.; Ma, A. A Study on the Mechanism and Kinetics of Ultrasound-Enhanced Sulfuric Acid Leaching for Zinc Extraction from Zinc Oxide Dust. Materials 2022, 15, 5969. [Google Scholar] [CrossRef] [PubMed]
- Skrzekut, T.; Piotrowicz, A.; Noga, P.; Wędrychowicz, M.; Bydałek, A.W. Studies of Selective Recovery of Zinc and Manganese from Alkaline Batteries Scrap by Leaching and Precipitation. Materials 2022, 15, 3966. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xia, H.; Zhang, Q.; Cai, W.; Jiang, G.; Zhang, L. Green and Efficient Recovery of Valuable Metals from By-Products of Zinc Hydrometallurgy and Reducing Toxicity. J. Clean. Prod. 2022, 380, 134993. [Google Scholar] [CrossRef]
- Luo, J.; Duan, N.; Xu, F.; Jiang, L.; Zhang, C.; Ye, W. System-Level Analysis of the Generation and Distribution for Pb, Cu, and Ag in the Process Network of Zinc Hydrometallurgy: Implications for Sustainability. J. Clean. Prod. 2019, 234, 755–766. [Google Scholar] [CrossRef]
- Katarína, P.; Jarmila, T.; Jaroslav, B.; Beatrice, P. Theoretical and Practical Evaluation of the Feasibility of Zinc Evaporation from the Bottom Zinc Dross as a Valuable Secondary Material. Materials 2022, 15, 8843. [Google Scholar] [CrossRef]
- Ali, S.T.; Srinivas Rao, K.; Laxman, C.; Munirathnam, N.R.; Prakash, T.L. Preparation of High Pure Zinc for Electronic Applications Using Selective Evaporation under Vacuum. Sep. Purif. Technol. 2012, 85, 178–182. [Google Scholar] [CrossRef]
- Ji, W.; Xie, K.; Yan, S. Separation and Recovery of Heavy Metals Zinc and Lead from Phosphorus Flue Dust by Vacuum Metallurgy. J. Environ. Manag. 2021, 294, 113001. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, Z.; Xing, X.; Xu, S.; Li, X. Kinetic Analysis of Recovering Zinc from Electric Arc Furnace Dust by Vacuum Carbothermic Reduction at 20 Pa. Minerals 2022, 12, 261. [Google Scholar] [CrossRef]
- Deng, X.; Huang, R.; Lv, X.; Yang, J.; Yang, J. Separation and Recovery of Metallic Zinc and Iron Concentrate from Blast Furnace Dust by Vacuum Carbothermal Reduction. Process Saf. Environ. Prot. 2022, 162, 746–751. [Google Scholar] [CrossRef]
- Smalcerz, A.; Blacha, L. Removal of Lead From Blister Copper by Melting in the Induction Vacuum Furnace. Arch. Foundry Eng. 2020, 2, 84–88. [Google Scholar] [CrossRef]
- Siwiec, G.; Buliński, P.; Palacz, M.; Smołka, J.; Blacha, L. Investigations on the Process of Lead Removal from Cu-Pb Alloys During Their Melting in Vacuum Induction Furnace. Arch. Metall. Mater. 2017, 62, 2449–2453. [Google Scholar] [CrossRef]
- Blacha, L.; Burdzik, R.; Smalcerz, A.; Matuła, T. Effects of Pressure on the Kinetics of Manganese Evaporation from the Ot4 Alloy / Wpływ Cisnienia Na Kinetyke Procesu Odparowania Manganu Ze Stopu Ot4. Arch. Metall. Mater. 2013, 58, 197–201. [Google Scholar] [CrossRef]
- Łabaj, J. Kinetics of Coper Evaporation from the Fe-Cu Alloys Under Reduced Pressure. Arch. Metall. Mater. 2012, 57, 165–172. [Google Scholar] [CrossRef]
- Grieveson, P.; Turkdogan, E.T. Determination of Interdiffusivities of Argon and Metal Vapor Mixtures at Elevated Temperatures. J. Phys. Chem. 1964, 68, 1547–1552. [Google Scholar] [CrossRef]
- Fromm, E. Reduction of Metal Evaporation Losses by Inert Gas Atmospheres. Metall. Mater. Trans. A 1978, 9, 1835–1838. [Google Scholar] [CrossRef]
- Cai, X. Vacuum distillation of zinc from brass. J. Kunming Univ. Sci. Technol. 1996, 21, 66–70. [Google Scholar]
- Ma, Y.; Qiu, K. Separation and Recovery of Zinc from Copper-Based Alloy Scraps under Vacuum Conditions. Vacuum 2014, 106, 5–10. [Google Scholar] [CrossRef]
- Zha, G.; Yang, B.; Yang, C.; Guo, X.; Jiang, W. Selective Separation and Recovery of Valuable Metals by Vacuum Distillation of Copper Anode Slime Flotation Tailings. JOM 2019, 71, 2413–2419. [Google Scholar] [CrossRef]
- Handzlik, P. Separation and recovery of zinc from electronic copper-based alloy scrap under vacuum. Int. Multidiscip. Sci. GeoConference SGEM 2019, 19, 143–150. [Google Scholar]
- Smalcerz, A.; Wecki, B.; Blacha, L.; Labaj, J.; Jodkowski, M.; Smagor, A. Kinetics of Zinc Evaporation from Aluminium Alloys Melted Using VIM and ISM Technologies. Materials 2021, 14, 6641. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Comas-Vives, A.; Copéret, C. Ga and Zn Increase the Oxygen Affinity of Cu-Based Catalysts for the COx Hydrogenation According to Ab Initio Atomistic Thermodynamics. Chem. Sci. 2022, 13, 13442–13458. [Google Scholar] [CrossRef] [PubMed]
- Plewa, J.; Blacha, L.; Wojtala, J. Własności Termodynamiczne Ciekłych Roztworów Zn-Cu. Rudy Metale 1984, 29, 234–239. [Google Scholar]
- Gerling, U.; Predel, B. Zur Kenntnis Thermodynamischer Eigenschaften Flüssiger Kupfer- Zink-Legierungen. Int. J. Mater. Res. 1980, 71, 158–164. [Google Scholar] [CrossRef]
- Richardson, F.D. Physical Chemistry of Melts in Metallurgy; Academic Press: London, UK; New York, NY, USA, 1974; ISBN 978-0-12-587901-9. [Google Scholar]
- Safarian, J.; Engh, T.A. Vacuum Evaporation of Pure Metals. Metall. Mater. Trans. A 2013, 44, 747–753. [Google Scholar] [CrossRef]
- Labaj, J. Zjawiska Parowania w Procesach Rafinacji Metali; Wydawnictwo Komisja Odlewnictwa PAN: Katowice, Poland, 2019; ISBN 978-83-63605-38-4. [Google Scholar]






| Component | Zn | Fe | Al | Ni | Sn | Cu |
|---|---|---|---|---|---|---|
| Content, % | 10.53 | 0.18 | 0.01 | 0.3 | 0.08 | balance |
| No. | Temperature, °C | Sample Mass Loss during Its Heating, mg | Sample Mass Loss during Its Isothermal Holding, mg | The Total Weight Loss of the Sample during the Heating Process, mg | Level of Zn Removal, % |
|---|---|---|---|---|---|
| 1 | 1080 | 18.67 | 69.41 | 89.64 | 83.82 |
| 2 | 1120 | 28.03 | 65.67 | 95.71 | 89.88 |
| 3 | 1160 | 38.06 | 56.71 | 96.74 | 90.90 |
| 4 | 1200 | 57.40 | 45.89 | 103.89 | 98.19 |
| 5 | 1240 | 80.09 | 27.90 | 108.30 | 99.87 |
| T, °C | kZn, m s−1 | ke, m s−1 |
|---|---|---|
| 1080 | 4.74 × 10−5 | 4.72 × 10−4 |
| 1120 | 5.22 × 10−5 | 4.65 × 10−4 |
| 1160 | 5.48 × 10−5 | 4.58 × 10−4 |
| 1200 | 8.18 × 10−5 | 4.52 × 10−4 |
| 1240 | 8.46 × 10−5 | 4.46 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilk, M.; Matula, T.; Blacha, L.; Smalcerz, A.; Labaj, J. Zinc Evaporation from Brass Scraps in the Atmosphere of Inert Gas. Materials 2023, 16, 5178. https://doi.org/10.3390/ma16145178
Wilk M, Matula T, Blacha L, Smalcerz A, Labaj J. Zinc Evaporation from Brass Scraps in the Atmosphere of Inert Gas. Materials. 2023; 16(14):5178. https://doi.org/10.3390/ma16145178
Chicago/Turabian StyleWilk, Magdalena, Tomasz Matula, Leszek Blacha, Albert Smalcerz, and Jerzy Labaj. 2023. "Zinc Evaporation from Brass Scraps in the Atmosphere of Inert Gas" Materials 16, no. 14: 5178. https://doi.org/10.3390/ma16145178
APA StyleWilk, M., Matula, T., Blacha, L., Smalcerz, A., & Labaj, J. (2023). Zinc Evaporation from Brass Scraps in the Atmosphere of Inert Gas. Materials, 16(14), 5178. https://doi.org/10.3390/ma16145178








