Biochar from Grapevine Pruning Residues as an Efficient Adsorbent of Polyphenolic Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Preparation
2.2. Biochar Characterization
2.3. Adsorption Capacity
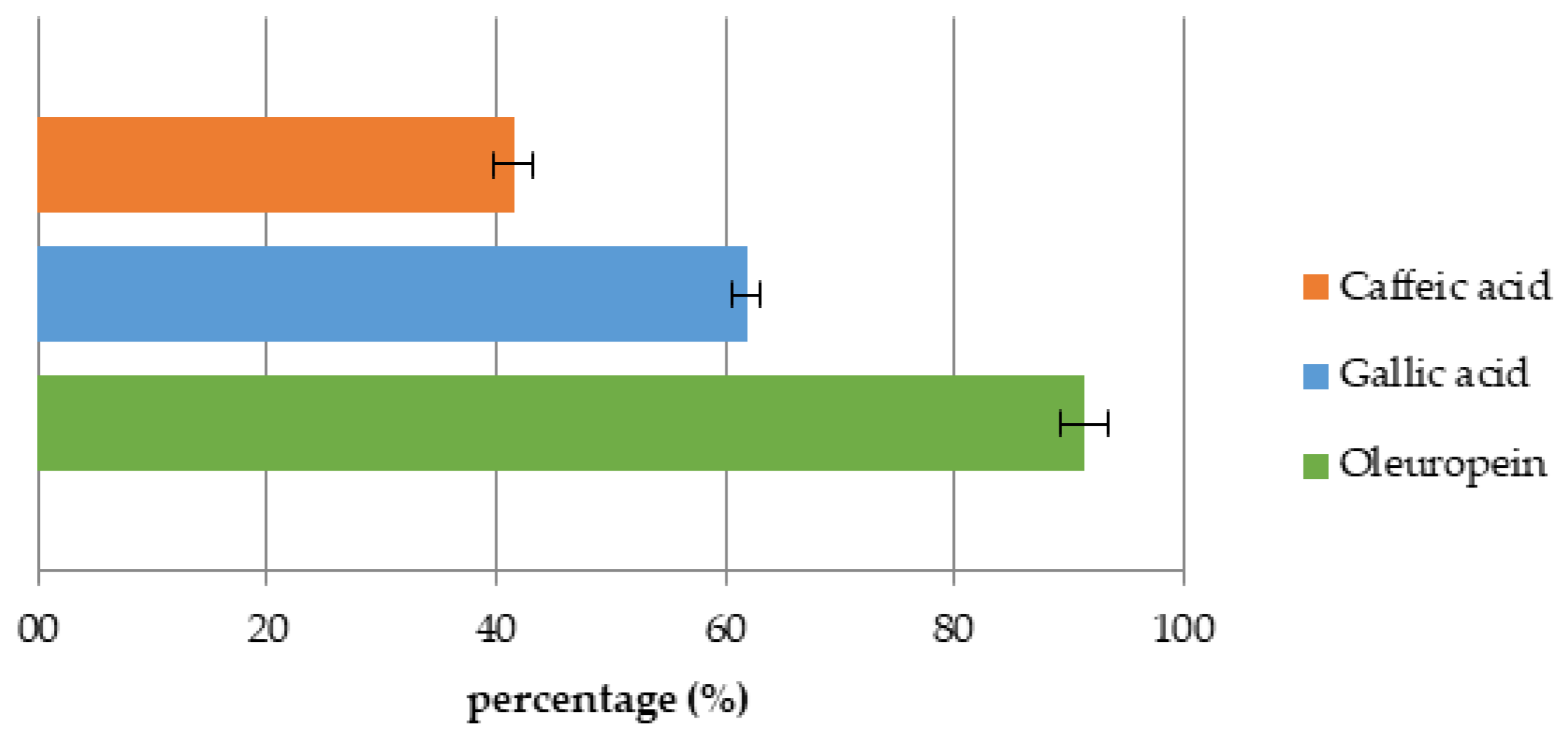
2.4. Recovery
2.5. Statistical Analysis
3. Results
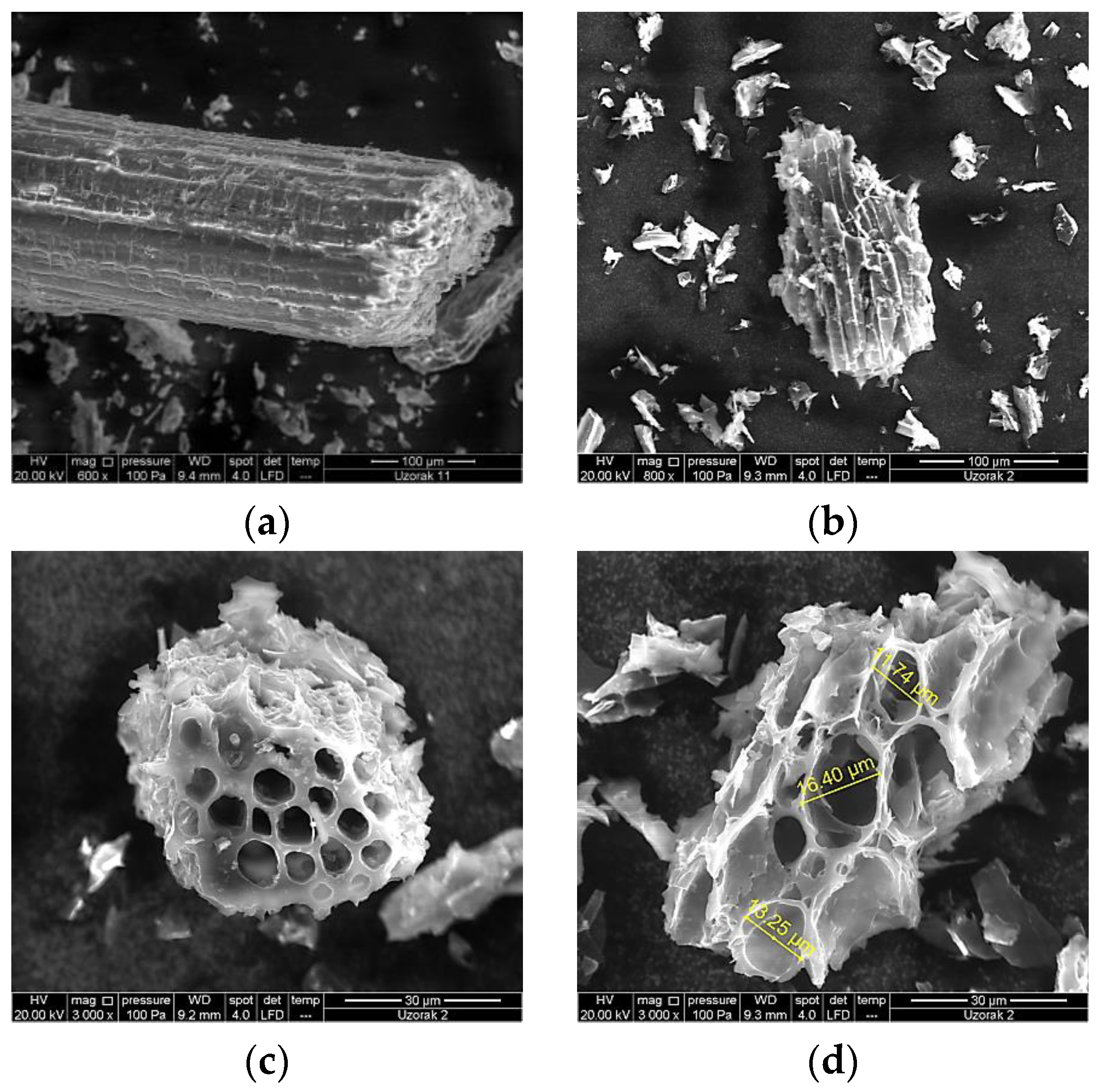
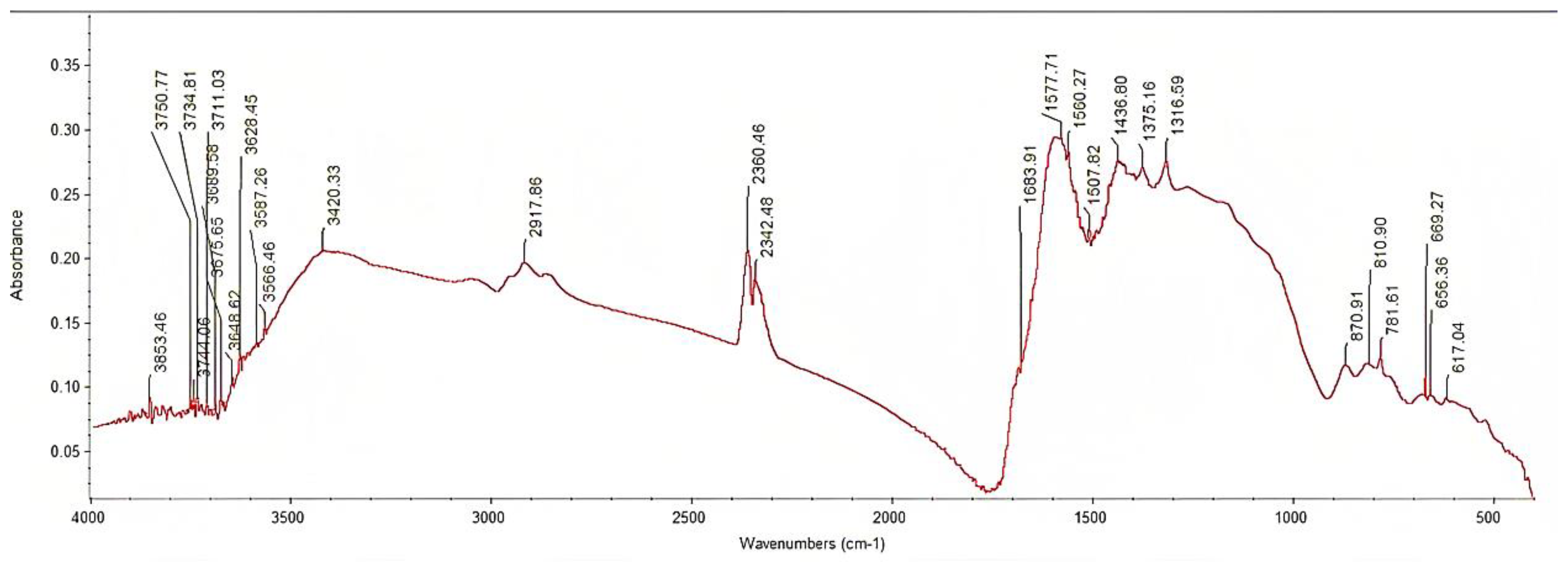
3.1. Physicochemical Characterization of GPRs Biochar
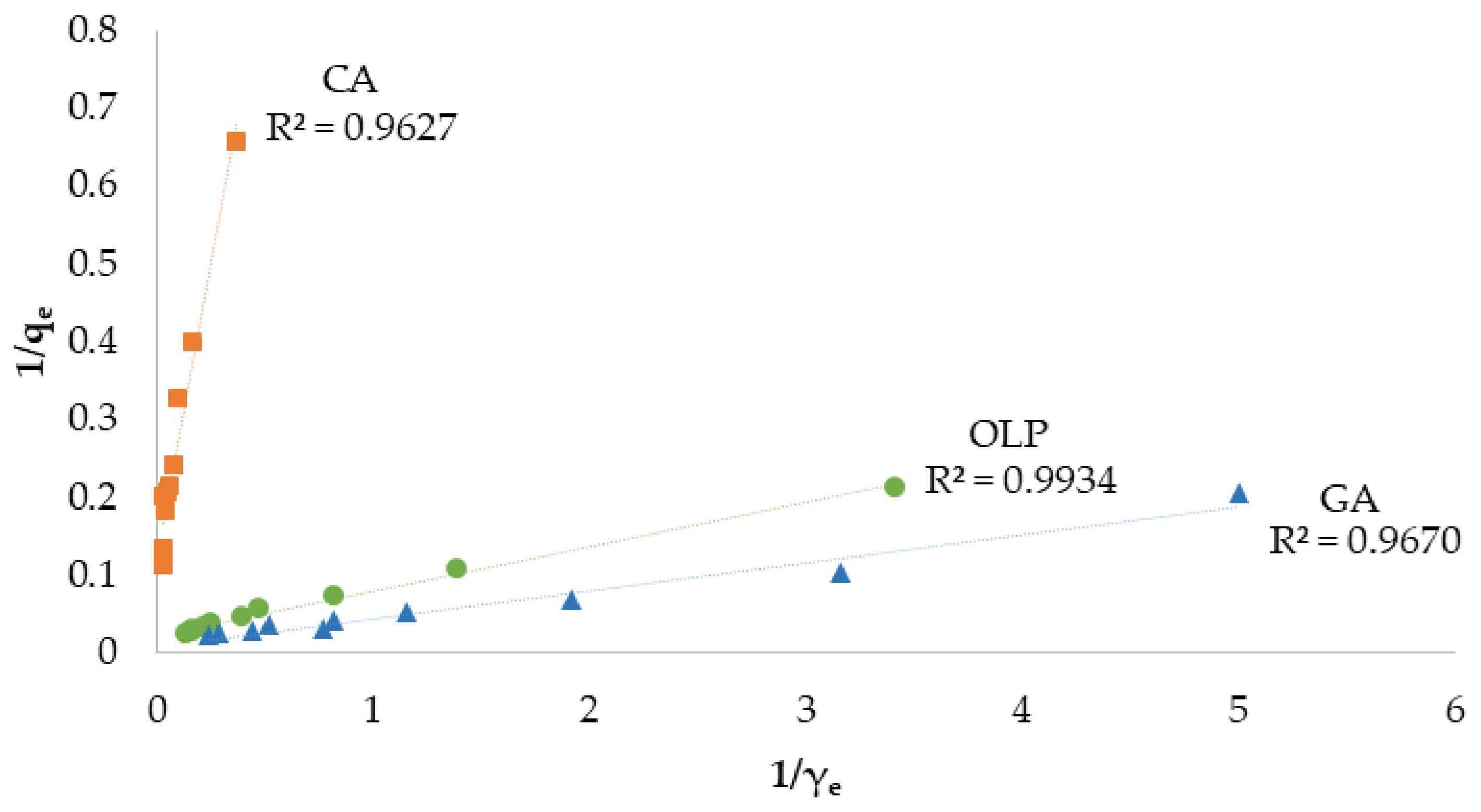
3.2. Adsorption Isotherms
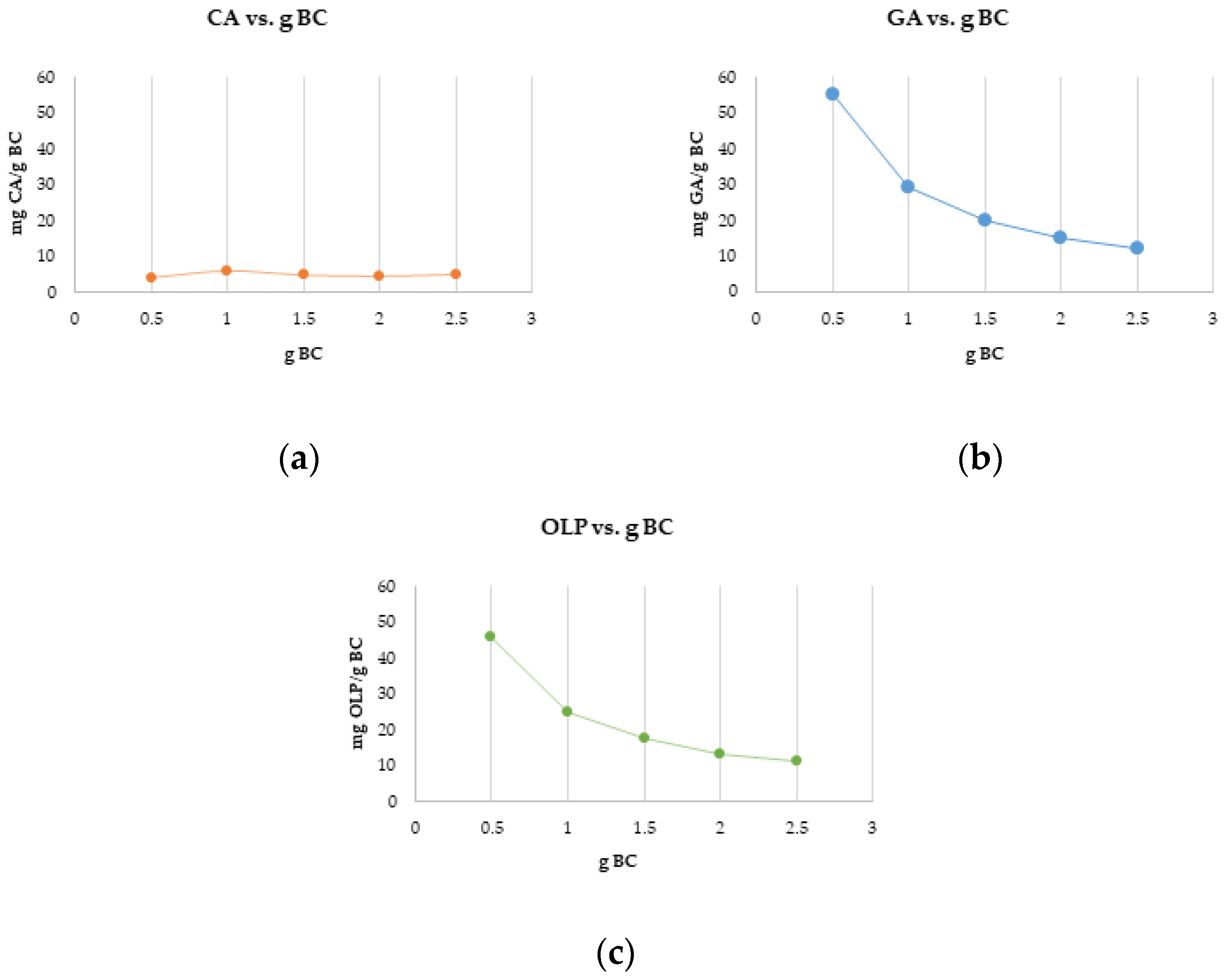
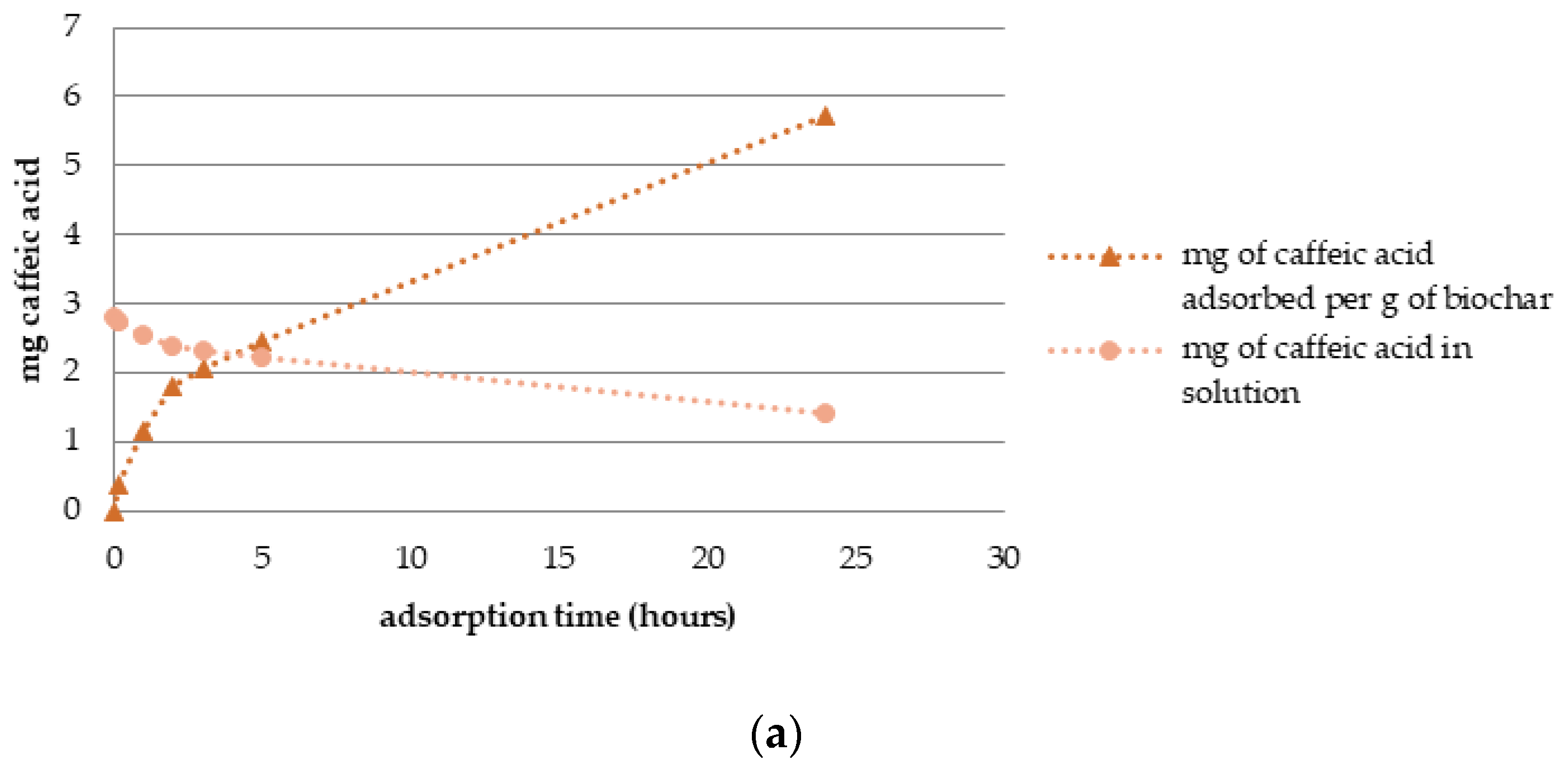
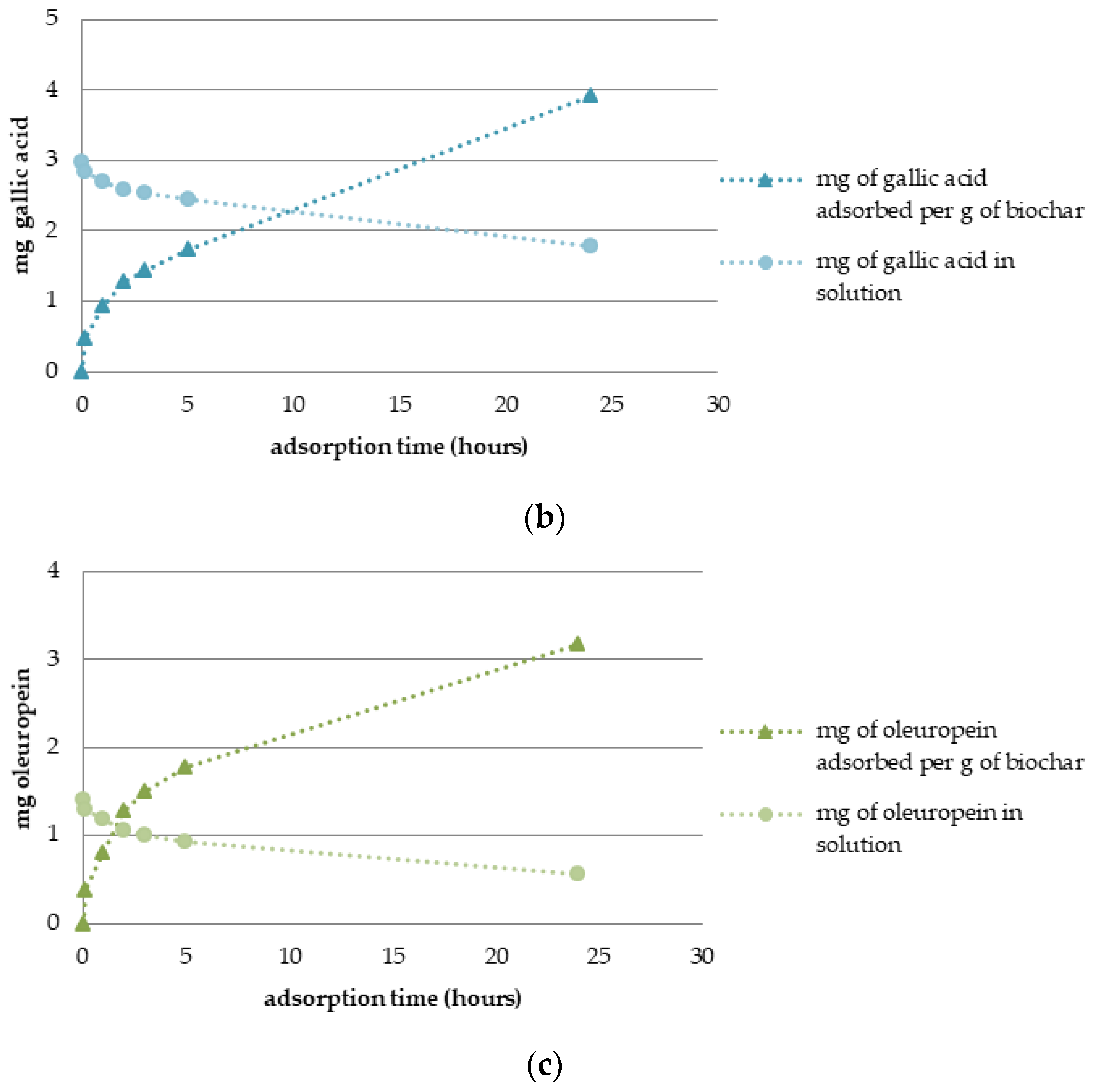
3.3. Adsorption Dynamics and Solid Phase Extraction Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat 2020-Share_of_EU_Area_under_Vines_(%,_2020). Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Vineyards_in_the_EU_-_statistics#million_hectares_of_vineyards_in_the_EU (accessed on 4 March 2023).
- Baum, S.; Weih, M.; Busch, G.; Kroiher, F.; Bolte, A. The impact of short rotation coppice plantations on phytodiversity. Landbauforsch. Volkenrode 2009, 59, 163–170. [Google Scholar]
- Anđelini, D.; Cvitan, D.; Prelac, M.; Pasković, I.; Černe, M.; Nemet, I.; Major, N.; Ban, S.G.; Užila, Z.; Ferri, T.Z.; et al. Biochar from Grapevine-Pruning Residues Is Affected by Grapevine Rootstock and Pyrolysis Temperature. Sustainability 2023, 15, 4851. [Google Scholar] [CrossRef]
- Spinelli, R.; Magagnotti, N.; Nati, C. Harvesting Vineyard Pruning Residues for Energy Use. Biosyst. Eng. 2010, 105, 316–322. [Google Scholar] [CrossRef]
- Duca, D.; Toscano, G.; Pizzi, A.; Rossini, G.; Fabrizi, S.; Lucesoli, G.; Servili, A.; Mancini, V.; Romanazzi, G.; Mengarelli, C. Evaluation of the Characteristics of Vineyard Pruning Residues for Energy Applications: Effect of Different Copper-Based Treatments. J. Agric. Eng. 2016, 47, 22–27. [Google Scholar] [CrossRef]
- Jesus, M.; Romaní, A.; Mata, F.; Domingues, L. Current Options in the Valorisation of Vine Pruning Residue for the Production of Biofuels, Biopolymers, Antioxidants, and Bio-Composites Following the Concept of Biorefinery: A Review. Polymers 2022, 14, 1640. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.J.R.; Rodrigues, A.M.; Matias, J.C.O.; Ferraz, A.I.; Rodrigues, A.C. Production of Biochar from Vine Pruning: Waste Recovery in the Wine Industry. Agriculture 2021, 11, 489. [Google Scholar] [CrossRef]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of Feedstock and Pyrolysis Temperature on Biochar Adsorption of Ammonium and Nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of Metal Sorption by Biochars: Biochar Characteristics and Modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of Methylene Blue from Aqueous Solution by Sewage Sludge-Derived Biochar: Adsorption Kinetics, Equilibrium, Thermodynamics and Mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Maschio, G.; Koufopanos, C.; Lucchesi, A. Pyrolysis, a Promising Route for Biomass Utilization. Bioresour. Technol. 1992, 42, 219–231. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar Modification to Enhance Sorption of Inorganics from Water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent Advances in Utilization of Biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Demirbaş, A.; Arin, G. An Overview of Biomass Pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and Characterization of Slow Pyrolysis Biochar: Influence of Feedstock Type and Pyrolysis Conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Gęca, M.; Wiśniewska, M.; Nowicki, P. Biochars and Activated Carbons as Adsorbents of Inorganic and Organic Compounds from Multicomponent Systems—A Review. Adv. Colloid Interface Sci. 2022, 305, 102687. [Google Scholar] [CrossRef] [PubMed]
- Gurses, A.; Yalcin, M.; Sozbilir, M.; Dogar, C. The Investigation of Adsorption Thermodynamics and Mechanism of a Cationic Surfactant, CTAB, onto Powdered Active Carbon. Fuel Process. Technol. 2003, 81, 57–66. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A Critical Review on the Biochar Production Techniques, Characterization, Stability and Applications for Circular Bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Feng, D.; Guo, D.; Zhang, Y.; Sun, S.; Zhao, Y.; Shang, Q.; Sun, H.; Wu, J.; Tan, H. Functionalized Construction of Biochar with Hierarchical Pore Structures and Surface O-/N-Containing Groups for Phenol Adsorption. Chem. Eng. J. 2021, 410, 127707. [Google Scholar] [CrossRef]
- Liu, B.; Chen, T.; Wang, B.; Zhou, S.; Zhang, Z.; Li, Y.; Pan, X.; Wang, N. Enhanced Removal of Cd2+ from Water by AHP-Pretreated Biochar: Adsorption Performance and Mechanism. J. Hazard. Mater. 2022, 438, 129467. [Google Scholar] [CrossRef] [PubMed]
- Ćwieląg-Piasecka, I.; Jamroz, E.; Medyńska-Juraszek, A.; Bednik, M.; Kosyk, B.; Polláková, N. Deashed Wheat-Straw Biochar as a Potential Superabsorbent for Pesticides. Materials 2023, 16, 2185. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.A.L.; Pickler, T.B.; Segato, T.C.M.; Jozala, A.F.; Grotto, D. Biochar from Fungiculture Waste for Adsorption of Endocrine Disruptors in Water. Sci. Rep. 2022, 12, 6507. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Hong, S.H.; Hong, S.G.; Choi, J.W.; Park, S.J. Production of Biochar from Food Waste and Its Application for Phenol Removal from Aqueous Solution. Water Air Soil Pollut. 2019, 230, 70. [Google Scholar] [CrossRef]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of Dietary Polyphenols on Gut Microbiota, Their Metabolites and Health Benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Rosario, A.C.R.S.; Da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Chagas Monteiro, M. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 3–5. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of Polyphenols—A Review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Gołębiewska, E.; Świderski, G.; Męczyńska-Wielgosz, S.; Lewandowska, H.; Pietryczuk, A.; Cudowski, A.; Astel, A.; Świsłocka, R.; Samsonowicz, M.; et al. Plant-Derived and Dietary Hydroxybenzoic Acids-A Comprehensive Study of Structural, Anti-/Pro-Oxidant, Lipophilic, Antimicrobial, and Cytotoxic Activity in MDA-MB-231 and MCF-7 Cell Lines. Nutrients 2021, 13, 3107. [Google Scholar] [CrossRef] [PubMed]
- Sindona, G. A Marker of Quality of Olive Oils: The Expression of Oleuropein; Elsevier Inc.: Amsterdam, The Netherlands, 2010; ISBN 9780123744203. [Google Scholar]
- Soler-Rivas, C.; Espiń, J.C.; Wichers, H.J. Oleuropein and Related Compounds. J. Sci. Food Agric. 2000, 80, 1013–1023. [Google Scholar] [CrossRef]
- Rigacci, S.; Miceli, C.; Nediani, C.; Berti, A.; Cascella, R.; Pantano, D.; Nardiello, P.; Luccarini, I.; Casamenti, F.; Stefani, M. Oleuropein Aglycone Induces Autophagy via the AMPK/MTOR Signalling Pathway: A Mechanistic Insight. Oncotarget 2015, 6, 35344–35357. [Google Scholar] [CrossRef]
- Cornelissen, G.; Pandit, N.R.; Taylor, P.; Pandit, B.H.; Sparrevik, M.; Schmidt, H.P. Emissions and Char Quality of Flame-Curtain “Kon Tiki” Kilns for Farmer-Scale Charcoal/Biochar Production. PLoS ONE 2016, 11, e0154617. [Google Scholar] [CrossRef]
- DIN: DIN ISO 10390:2005-12; Soil Quality—Determination of pH. Beuth Verlag: Berlin, Germany, 2005.
- Kjeldahl, J. A new method for nitrogen determination of organic matter. J. Anal. Chem. 1883, 22, 366–382. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Mica. J. Am. Chem. Soc. 1938, 40, 1361–1403. [Google Scholar] [CrossRef]
- Ćurko, J.; Matošić, M.; Crnek, V.; Stulić, V.; Mijatović, I. Adsorption Characteristics of Different Adsorbents and Iron(III) Salt for Removing As(V) from Water. Food Technol. Biotechnol. 2016, 54, 250–255. [Google Scholar] [CrossRef]
- Zhu, L.; Lei, H.; Wang, L.; Yadavalli, G.; Zhang, X.; Wei, Y.; Liu, Y.; Yan, D.; Chen, S.; Ahring, B. Biochar of Corn Stover: Microwave-Assisted Pyrolysis Condition Induced Changes in Surface Functional Groups and Characteristics; Elsevier: Amsterdam, The Netherlands, 2015; Volume 115, ISBN 5093727628. [Google Scholar]
- Tan, Z.; Zou, J.; Zhang, L.; Huang, Q. Morphology, Pore Size Distribution, and Nutrient Characteristics in Biochars under Different Pyrolysis Temperatures and Atmospheres. J. Mater. Cycles Waste Manag. 2018, 20, 1036–1049. [Google Scholar] [CrossRef]
- Marshall, J.; Muhlack, R.; Morton, B.J.; Dunnigan, L.; Chittleborough, D.; Kwong, C.W. Pyrolysis Temperature Effects on Biochar–Water Interactions and Application for Improved Water Holding Capacity in Vineyard Soils. Soil Syst. 2019, 3, 27. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of Pyrolysis Temperature on Properties and Environmental Safety of Heavy Metals in Biochars Derived from Municipal Sewage Sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The Forms of Alkalis in the Biochar Produced from Crop Residues at Different Temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, D.; Miao, L.; Jin, Z.; Dong, K.; Liu, W. A Series of Alkali Metal Elements Doped La2Ce2O7 Electrolytes for Solid Oxide Fuel Cells. Electrochem. commun. 2021, 126, 107026. [Google Scholar] [CrossRef]
- Bronstein, H.R.; Bredig, M.A. The Electrical Conductivity of Solutions of Alkali Metals in Their Molten Halides. J. Am. Chem. Soc. 1958, 80, 2077–2081. [Google Scholar] [CrossRef]
- Steiner, C. Considerations in Biochar Characterization. Agric. Environ. Appl. Biochar Adv. Barriers 2015, 63, 87–100. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative Distribution of Pb2+ Sorption Mechanisms by Sludge-Derived Biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Ion, V.A.; Bucharest, V.M.; Mot, A.; Popa, V.I.; Badulescu, L. Physicochemical Characterisation of Vine Waste Used For producing biochar. Sci. Pap. Ser. B. Hortic. 2021, 65, 268–273. [Google Scholar]
- Tangmankongworakoon, N. An Approach to Produce Biochar from Coffee Residue for Fuel and Soil Amendment Purpose. Int. J. Recycl. Org. Waste Agric. 2019, 8, 37–44. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural Residues as Precursors for Activated Carbon Production—A Review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Lu, S.; Zong, Y. Pore Structure and Environmental Serves of Biochars Derived from Different Feedstocks and Pyrolysis Conditions. Environ. Sci. Pollut. Res. 2018, 25, 30401–30409. [Google Scholar] [CrossRef]
- Wildman, J.; Derbyshire, F. Origins and Functions of Macroporosity in Activated Carbons from Coal and Wood Precursors. Fuel 1991, 70, 655–661. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Teng, H. Liquid-Phase Adsorption of Phenol onto Activated Carbons Prepared with Different Activation Levels. J. Colloid Interface Sci. 2000, 230, 171–175. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Cho, H.H.; Poster, D.L.; Ball, W.P. Evidence for a Pore-Filling Mechanism in the Adsorption of Aromatic Hydrocarbons to a Natural Wood Char. Environ. Sci. Technol. 2007, 41, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Vithanage, M.; Mayakaduwa, S.S.; Herath, I.; Ok, Y.S.; Mohan, D. Kinetics, Thermodynamics and Mechanistic Studies of Carbofuran Removal Using Biochars from Tea Waste and Rice Husks. Chemosphere 2016, 150, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Huggins, T.M.; Haeger, A.; Biffinger, J.C.; Ren, Z.J. Granular Biochar Compared with Activated Carbon for Wastewater Treatment and Resource Recovery. Water Res. 2016, 94, 225–232. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, J.M.; Paneque, M.; Miller, A.Z.; Knicker, H. Relating Physical and Chemical Properties of Four Different Biochars and Their Application Rate to Biomass Production of Lolium Perenne on a Calcic Cambisol during a Pot Experiment of 79 Days. Sci. Total Environ. 2014, 499, 175–184. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, L.; Yang, J.; Lin, D. Adsorption and Correlations of Selected Aromatic Compounds on a KOH-Activated Carbon with Large Surface Area. Sci. Total Environ. 2018, 618, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.F.; Zhu, S.S.; Wang, R.P.; Chen, Y.D.; Show, P.L.; Zhang, F.F.; Ho, S.H. Role of Biochar Surface Characteristics in the Adsorption of Aromatic Compounds: Pore Structure and Functional Groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Uchimiya, M.; Bannon, D.I.; Wartelle, L.H. Retention of Heavy Metals by Carboxyl Functional Groups of biochars in small arms range soil. J. Agric. Food Chem. 2012, 60, 1798–1809. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, S.; Pang, Y.; Li, J.; Xin, H. Effects of Coal Functional Groups on Adsorption Microheat of Coal Bed Methane. Energy Fuels 2015, 29, 1550–1557. [Google Scholar] [CrossRef]
- Komnitsas, K.A.; Zaharaki, D. Morphology of Modified Biochar and Its Potential for Phenol Removal from Aqueous Solutions. Front. Environ. Sci. 2016, 4, 26. [Google Scholar] [CrossRef]
- Oh, S.Y.; Seo, Y.D. Sorption of Halogenated Phenols and Pharmaceuticals to Biochar: Affecting Factors and Mechanisms. Environ. Sci. Pollut. Res. 2016, 23, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhou, L.; Huang, Y.; Song, Z.; Qiu, W. Effects of a Manganese Oxide-Modified Biochar Composite on Adsorption of Arsenic in Red Soil. J. Environ. Manag. 2015, 163, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Song, W.; Tian, J. Biochar-Facilitated Soil Remediation: Mechanisms and Efficacy Variations. Front. Environ. Sci. 2020, 8, 183. [Google Scholar] [CrossRef]
- Jia, L.; Fan, B.G.; Yao, Y.X.; Han, F.; Huo, R.P.; Zhao, C.W.; Jin, Y. Study on the Elemental Mercury Adsorption Characteristics and Mechanism of Iron-Based Modified Biochar Materials. Energy Fuels 2018, 32, 12554–12566. [Google Scholar] [CrossRef]
- Tessmer, C.H.; Vidic, R.D.; Uranowski, L.I. Impact of Oxygen-Containing Surface Functional Groups on Activated Carbon Adsorption of Phenols. Environ. Sci. Technol. 1997, 31, 1872–1878. [Google Scholar] [CrossRef]
- Othmani, A.; John, J.; Rajendran, H.; Mansouri, A.; Sillanpää, M.; Velayudhaperumal Chellam, P. Biochar and Activated Carbon Derivatives of Lignocellulosic Fibers towards Adsorptive Removal of Pollutants from Aqueous Systems: Critical Study and Future Insight. Sep. Purif. Technol. 2021, 274, 119062. [Google Scholar] [CrossRef]
- Fernandes, B.C.C.; Mendes, K.F.; Júnior, A.F.D.; da Silva Caldeira, V.P.; da Silva Teófilo, T.M.; Silva, T.S.; Mendonça, V.; de Freitas Souza, M.; Silva, D.V. Impact of Pyrolysis Temperature on the Properties of Eucalyptus Wood-Derived Biochar. Materials 2020, 13, 5841. [Google Scholar] [CrossRef]
- Gámiz, B.; Hall, K.; Spokas, K.A.; Cox, L. Understanding Activation Effects on Low-Temperature Biochar for Optimization of Herbicide Sorption. Agronomy 2019, 9, 588. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of Emerging Contaminants from Water and Wastewater by Modified Biochar: A Review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Meng, J.; Dang, S.; Gao, M.C.; Che, W.F. Research on Cadmium Adsorption-Desorption Dynamics of Biochar. Adv. Mater. Res. 2013, 726–731, 179–183. [Google Scholar] [CrossRef]
- Dong, F.X.; Yan, L.; Zhou, X.H.; Huang, S.T.; Liang, J.Y.; Zhang, W.X.; Guo, Z.W.; Guo, P.R.; Qian, W.; Kong, L.J.; et al. Simultaneous Adsorption of Cr(VI) and Phenol by Biochar-Based Iron Oxide Composites in Water: Performance, Kinetics and Mechanism. J. Hazard. Mater. 2021, 416, 125930. [Google Scholar] [CrossRef]
- Lawal, A.A.; Hassan, M.A.; Farid, M.A.A.; Yasim-Anuar, T.A.T.; Samsudin, M.H.; Yusoff, M.Z.M.; Zakaria, M.R.; Mokhtar, M.N.; Shirai, Y. Adsorption Mechanism and Effectiveness of Phenol and Tannic Acid Removal by Biochar Produced from Oil Palm Frond Using Steam Pyrolysis. Environ. Pollut. 2021, 269, 116197. [Google Scholar] [CrossRef]
- Hamzah, S.; Ramli, S.Z.; Mohammad, N.A.; Rasit, N.; Alyza; Azmi, A.A.R.; Hairom, N.H.H.; Mohamad, A.W. Acid–Treated Activated Carbon for Phenolic Compound Removal in Acid Pre-Treatment of Lignocellulosic Biomass for Biogas Production. Biointerface Res. Appl. Chem. 2020, 10, 5466–5471. [Google Scholar] [CrossRef]
- Şahin, S.; Bilgin, M.; Dramur, M.U. Investigation of Oleuropein Content in Olive Leaf Extract Obtained by Supercritical Fluid Extraction and Soxhlet Methods. Sep. Sci. Technol. 2011, 46, 1829–1837. [Google Scholar] [CrossRef]
- Kfoury, M.; Geagea, C.; Ruellan, S.; Greige-Gerges, H.; Fourmentin, S. Effect of Cyclodextrin and Cosolvent on the Solubility and Antioxidant Activity of Caffeic Acid. Food Chem. 2019, 278, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Núñez, M.D.L.D.; Schweich, D. Adsorption of Complex Phenolic Compounds on Active Charcoal: Adsorption Capacity and Isotherms. Chem. Eng. J. 2009, 148, 1–7. [Google Scholar] [CrossRef]
- Ekinci, R. Effect of Activated Charcoal on Some Phenolic Compounds of Apple Juice. Asian J. Chem. 2013, 25, 2905–2908. [Google Scholar] [CrossRef]
- Abid, N.; Masmoudi, M.A.; Megdiche, M.; Barakat, A.; Ellouze, M.; Chamkha, M.; Ksibi, M.; Sayadi, S. Biochar from Olive Mill Solid Waste as an Eco-Friendly Adsorbent for the Removal of Polyphenols from Olive Mill Wastewater. Chem. Eng. Res. Des. 2022, 181, 384–398. [Google Scholar] [CrossRef]
- Abid, N.; Karray, F.; Kallel, I.; Slim, M.; Barakat, A.; Mhiri, N.; Chamkha, M.; Sayadi, S. Role of Biochar in Anaerobic Microbiome Enrichment and Methane Production Enhancement during Olive Mill Wastewater Biomethanization. Front. Bioeng. Biotechnol. 2023, 10, 1100533. [Google Scholar] [CrossRef] [PubMed]
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R.Z.; Almalki, W.H. Treatment Technologies for Olive Mill Wastewater with Impacts on Plants. Environ. Res. 2023, 216, 114399. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Value | Unit |
|---|---|---|
| yield | 30.8 ± 0.02 | % |
| pH | 8.67 ± 0.04 | - |
| EC | 2.63 ± 0.29 | mS cm−1 |
| TC | 77.15 ± 0.52 | % |
| N | 1.11 ± 0.00 | % |
| P | 2.90 ± 0.13 | g kg−1 |
| K | 27.1 ± 1.26 | g kg−1 |
| Ca | 17.8 ± 0.56 | g kg−1 |
| S | 0.76 ± 0.02 | g kg−1 |
| Mg | 2.89 ± 0.09 | g kg−1 |
| Na | 0.03 ± 0.01 | g kg−1 |
| Fe | 230 ± 8.70 | mg kg−1 |
| Al | 295 ± 15.5 | mg kg−1 |
| Cu | 19.9 ± 0.95 | mg kg−1 |
| Mn | 58.8 ± 2.01 | mg kg−1 |
| Mo | 2.15 ± 0.73 | mg kg−1 |
| Si | 1706 ± 25.8 | mg kg−1 |
| Zn | 62.7 ± 1.75 | mg kg−1 |
| Surface area (BET) | 1.89 ± 0.01 | m2 g−1 |
| Wave Number (cm−1) | Range (cm−1) | Corresponding Vibration |
|---|---|---|
| 617.04–656.36 | 460–800 | Si-O stretching bands |
| 781.61 | 460–800 | Si-O stretching bands |
| 720–750 | C-C alkanes skeletal vibrations | |
| 675–1000 | =C-H bending | |
| 750 | C-H out of plane bending | |
| 650–770 | O-H out of plane bending | |
| 810.90 | 810–850 | C-O-C aromatic ethers, symmetric stretch |
| 675–1000 | =C-H bending | |
| 870.91 | 885 | symmetric C-O stretching and aromatic C-H groups |
| 875 | C-O-C stretch | |
| 675–1000 | =C-H bending | |
| 830–874 | C-H out of plane bending | |
| 1316.59 | 1260–1350 | Phenyl group-CHR-OH deformation |
| 1000–1300 | C-O | |
| 1375.16 | 1372 | CH deformation (cellulose and hemicellulose) |
| 1436.80 | 1430–1485 | C-H deformation, asymmetric in –CH3 and |
| –CH2– (cellulose) | ||
| 1440 | aromatic C=C stretching | |
| 1400–1600 | C=C stretch | |
| 1507.82 | 1514 | Secondary aromatic amines |
| 1400–1600 | C=C stretch | |
| 1560.27 | 1580–1590 | COO- asymmetric stretching |
| 1400–1600 | C=C stretch | |
| 1560 | COO-asymmetric stretching vibrations | |
| 1577.71 | 1580–1590 | COO- asymmetric stretching |
| 1400–1600 | C=C stretch | |
| 1587 | C=O | |
| 1597 | Aromatic skeletal vibration with C=O stretching vibration | |
| 1683.91 | 1620–1650 | Aromatic and olefinic C=C vibrations, C=O amide(I), ketone and quinone groups |
| 1676 | C=O vibration in carboxylic group of phenolic acids (p-hydroxybenzoic acid) | |
| 1600–1700 | C=C and C=O stretching | |
| 2342.48 | 2332 | CO2 adsorption, Carbonyl bond group |
| 2360.46 | 2332 | CO2 adsorption, Carbonyl bond group |
| 2917.86 | 2850–2950 | C-H stretching of alkyl structures |
| 2916–2936 | –CH2– asymmetric stretch | |
| 3420.33 | 3400–3410 | H-bonded O-H stretching vibrations of hydroxyl groups from alcohols, phenols, and organic acids |
| 3428–3437 | O-H stretch | |
| 3566.46–3853.46 | 3200–3550 | O-H |
| Type of Isotherm | Parameters | CA | GA | OLP |
|---|---|---|---|---|
| Langmuir | qmax (mg g−1) | 8.21 | 118 | 45.5 |
| KL (L mg−1) | 0.08 | 0.24 | 0.39 | |
| RL R2 | 0.02–0.71 0.9627 | 0.04–0.45 0.9670 | 0.05–0.34 0.9934 | |
| Freundlich | KF (mg g−1) × (L g−1)n 1/n | 0.80 0.62 | 20.6 0.70 | 11.3 0.66 |
| R2 | 0.9380 | 0.9373 | 0.9928 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prelac, M.; Palčić, I.; Cvitan, D.; Anđelini, D.; Repajić, M.; Ćurko, J.; Kovačević, T.K.; Goreta Ban, S.; Užila, Z.; Ban, D.; et al. Biochar from Grapevine Pruning Residues as an Efficient Adsorbent of Polyphenolic Compounds. Materials 2023, 16, 4716. https://doi.org/10.3390/ma16134716
Prelac M, Palčić I, Cvitan D, Anđelini D, Repajić M, Ćurko J, Kovačević TK, Goreta Ban S, Užila Z, Ban D, et al. Biochar from Grapevine Pruning Residues as an Efficient Adsorbent of Polyphenolic Compounds. Materials. 2023; 16(13):4716. https://doi.org/10.3390/ma16134716
Chicago/Turabian StylePrelac, Melissa, Igor Palčić, Danko Cvitan, Dominik Anđelini, Maja Repajić, Josip Ćurko, Tvrtko Karlo Kovačević, Smiljana Goreta Ban, Zoran Užila, Dean Ban, and et al. 2023. "Biochar from Grapevine Pruning Residues as an Efficient Adsorbent of Polyphenolic Compounds" Materials 16, no. 13: 4716. https://doi.org/10.3390/ma16134716
APA StylePrelac, M., Palčić, I., Cvitan, D., Anđelini, D., Repajić, M., Ćurko, J., Kovačević, T. K., Goreta Ban, S., Užila, Z., Ban, D., & Major, N. (2023). Biochar from Grapevine Pruning Residues as an Efficient Adsorbent of Polyphenolic Compounds. Materials, 16(13), 4716. https://doi.org/10.3390/ma16134716











