Effects of Heat Treatment on the Physicochemical Properties and Electrochemical Behavior of Biochars for Electrocatalyst Support Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Biochars
2.2. Physicochemical Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sagarpa, U. Plan de Manejo de Residuos Generados en Actividades Agrícolas Primera Etapa: Diagnóstico Nacional. 2015, p. 145. Available online: http://www.sagarpa.gob.mx/ProgramasSAGARPA/2015/Productividad_y_competitividad_agroalimentaria/Programa_regional_de_desarrollo_previsto_en_el_PND/36incentivos/211PP064NUEVAAMERICAS.P.R.DER.L/5.PROYECTO/ManejoderesiduosDetallado.pdf (accessed on 29 March 2020).
- Herrera, E.; Feijoo, C.; Alfaro, R.; Solís, J.; Gómez, M.; Keiski, R.; Cruz, G. Biochar based on residual biomasses and its influence over seedling emergence and growth in vivarium of Capparis scabrida (Sapote). Sci. Agropecu. 2018, 9, 569–577. [Google Scholar] [CrossRef]
- Guida, M.; Hannioui, A. Renewable energy sources in Morocco: Comparative study of bio-oils from pyrolysis of lignocellulosic and algal biomass wastes. J. Mater. Environ. Sci. 2020, 11, 1976–1986. [Google Scholar]
- De Anda, A.; García, E.; Peña, A.; Seminario, J.; Nieto, A. Residuos Orgánicos: ¿Basura o recurso? Recur. Nat. Soc. 2021, 7, 19–42. Available online: https://www.cibnor.gob.mx/revista-rns/pdfs/vol1num3EE/3_RESIDUOS.pdf (accessed on 4 February 2022).
- Ćwieląg-Piasecka, I.; Jamroz, E.; Medyńska-Juraszek, A.; Bednik, M.; Kosyk, B.; Polláková, N. Deashed Wheat-Straw Biochar as a Potential Superabsorbent for Pesticides. Materials 2023, 16, 2185. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Cowie, A.L.; Cacho, O.; Kristiansen, P.; Anh Mai, T.L.; Joseph, S. Biochar addition in rice farming systems: Economic and energy benefits. Energy 2017, 140, 415–425. [Google Scholar] [CrossRef]
- Li, L.; Zou, D.; Xiao, Z.; Zeng, X.; Zhang, L.; Jiang, L.; Wang, A.; Ge, D.; Zhang, G.; Liu, F. Biochar as a Sorbent for Emerging Contaminants Enables Improvements in Waste Management and Sustainable Resource Use; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 210, ISBN 8673184673. [Google Scholar] [CrossRef]
- Prelac, M.; Palčić, I.; Cvitan, D.; Anđelini, D.; Repajić, M.; Ćurko, J.; Kovačević, T.K.; Goreta Ban, S.; Užila, Z.; Ban, D.; et al. Biochar from Grapevine Pruning Residues as an Efficient Adsorbent of Polyphenolic Compounds. Materials 2023, 16, 4716. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Strezov, V.; Nelson, P.F. Comparative Assessment of the Effect of Wastewater Sludge Biochar on Growth, Yield and Metal Bioaccumulation of Cherry Tomato. Pedosphere 2015, 25, 680–685. [Google Scholar] [CrossRef]
- Sadowska, U.; Zaleski, T.; Kuboń, M.; Latawiec, A.; Klimek-Kopyra, A.; Sikora, J.; Gliniak, M.; Kobyłecki, R.; Zarzycki, R. Effect of the Application of Sunflower Biochar and Leafy Trees Biochar on Soil Hydrological Properties of Fallow Soils and under Soybean Cultivation. Materials 2023, 16, 1737. [Google Scholar] [CrossRef]
- Major, J.; Steiner, C.; Downie, A.; Lehmann, J.; Joseph, S. Biochar effects on nutrient leaching. In Biochar for Environmental Management: Science and Technology; Routledge: London, UK, 2009; pp. 271–288. [Google Scholar]
- Magdziarz, A. Pyrolysis of Biomass Wastes into Carbon Materials. Energies 2022, 15, 1941. [Google Scholar] [CrossRef]
- Abioye, A.M.; Ani, F.N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review. Renew. Sustain. Energy Rev. 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619–620, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Z.; Edvinsson, T.; Kwong, P. Biochar for electrochemical applications. Curr. Opin. Green Sustain. Chem. 2020, 23, 25–30. [Google Scholar] [CrossRef]
- Huggins, T.M.; Pietron, J.J.; Wang, H.; Ren, Z.J.; Biffinger, J.C. Graphitic biochar as a cathode electrocatalyst support for microbial fuel cells. Bioresour. Technol. 2015, 195, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Pereira Lopes, R.; Astruc, D. Biochar as a support for nanocatalysts and other reagents: Recent advances and applications. Coord. Chem. Rev. 2021, 426, 213585. [Google Scholar]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Klinghoffer, N.B.; Castaldi, M.J.; Nzihou, A. Catalyst Properties and Catalytic Performance of Char from Biomass Gasification. Ind. Eng. Chem. Res. 2012, 51, 13113–13122. [Google Scholar] [CrossRef]
- Genovese, M.; Jiang, J.; Lian, K.; Holm, N. High capacitive performance of exfoliated biochar nanosheets from biomass waste corn cob. J. Mater. Chem. A 2015, 3, 2903–2913. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Cha, J.S.; Choi, J.C.; Ko, J.H.; Park, Y.K.; Park, S.H.; Jeong, K.E.; Kim, S.S.; Jeon, J.K. The low-temperature SCR of NO over rice straw and sewage sludge derived char. Chem. Eng. J. 2010, 156, 321–327. [Google Scholar] [CrossRef]
- Quintana, J.M.; Barrera, A.; Moreno, M.G.A. Pirólisis catalítica de coco a productos de carbono. Número Espec. Rev. Aristas Investig. Básica y Apl. 2017, 6, 238–245. [Google Scholar]
- Arnold, S.; Rodriguez-Uribe, A.; Misra, M.; Mohanty, A.K. Slow pyrolysis of bio-oil and studies on chemical and physical properties of the resulting new bio-carbon. J. Clean. Prod. 2018, 172, 2748–2758. [Google Scholar] [CrossRef]
- Kane, S.; Warnat, S.; Ryan, C. Improvements in methods for measuring the volume conductivity of electrically conductive carbon powders. Adv. Powder Technol. 2021, 32, 702–709. [Google Scholar] [CrossRef]
- Hoffmann, V.; Correa, C.R.; Sautter, D.; Maringolo, E.; Kruse, A. Study of the electrical conductivity of biobased carbonaceous powder materials under moderate pressure for the application as electrode materials in energy storage technologies. GCB Bioenergy 2019, 11, 230–248. [Google Scholar] [CrossRef]
- Oh, H.S.; Nong, H.N.; Strasser, P. Preparation of mesoporous Sb-, F-, and in-doped SnO2 bulk powder with high surface area for use as catalyst supports in electrolytic cells. Adv. Funct. Mater. 2015, 25, 1074–1081. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martínez-Alonso, A.; Tascón, J.M.D. Raman microprobe studies on carbon materials. Carbon N. Y. 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Rodil, S.E.; Robertson, J.; Rodil, S.E.; Robertson, J. Interpretation of infrared and Raman spectra of amorphous carbon nitrides. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 67, 155306. [Google Scholar] [CrossRef]
- Wróblewska, A.; Dużyńska, A.; Judek, J.; Stobiński, L.; Żerańska, K.; Gertych, A.P.; Zdrojek, M. Statistical analysis of the reduction process of graphene oxide probed by Raman spectroscopy mapping. J. Phys. Condens. Matter 2017, 29, 475201. [Google Scholar] [CrossRef]
- Tascón, J.M.D. Materiales de carbono: Estructuras y formas. Opt. Pura Apl. 2007, 40, 149–159. [Google Scholar]
- Kiciński, W.; Dyjak, S. Transition metal impurities in carbon-based materials: Pitfalls, artifacts and deleterious effects. Carbon N. Y. 2020, 168, 748–845. [Google Scholar] [CrossRef]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Macías-García, A.; Gómez-Serrano, V. Electrical conductivity of activated carbon–metal oxide nanocomposites under compression: A comparison study. Phys. Chem. Chem. Phys. 2014, 16, 25161–25175. [Google Scholar] [CrossRef] [PubMed]
- Kane, S.; Ulrich, R.; Harrington, A.; Stadie, N.P.; Ryan, C. Physical and chemical mechanisms that influence the electrical conductivity of lignin-derived biochar. Carbon Trends 2021, 5, 100088. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, N.; Xiao, Z.; Li, Z.; Zhang, D. Characterization of biochars derived from different materials and their effects on microbial dechlorination of pentachlorophenol in a consortium. RSC Adv. 2019, 9, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Quosai, P.; Anstey, A.; Mohanty, A.K.; Misra, M. Characterization of biocarbon generated by high- and low-temperature pyrolysis of soy hulls and coffee chaff: For polymer composite applications. R. Soc. Open Sci. 2018, 5, 171970. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Pilla, S. A comprehensive review on transforming lignocellulosic materials into biocarbon and its utilization for composites applications. Compos. Part C Open Access 2022, 7, 100225. [Google Scholar] [CrossRef]
- Pantea, D.; Darmstadt, H.; Kaliaguine, S.; Roy, C. Electrical conductivity of conductive carbon blacks: Influence of surface chemistry and topology. Appl. Surf. Sci. 2003, 217, 181–193. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Alaje, T.; Scott, K. Highly Stable Pt–Ru Nanoparticles Supported on Three-Dimensional Cubic Ordered Mesoporous Carbon (Pt–Ru/CMK-8) as Promising Electrocatalysts for Methanol Oxidation. J. Phys. Chem. C 2012, 116, 2630–2638. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Zhang, J.; Gao, Y. Comparative investigation of the resistance to electrochemical oxidation of carbon black and carbon nanotubes in aqueous sulfuric acid solution. Electrochim. Acta 2006, 51, 5853–5857. [Google Scholar] [CrossRef]
- Klüpfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2014, 48, 5601–5611. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.W.; Nagayama, M.; Matsuda, J.; Sasaki, K.; Hayashi, A. Mesoporous carbon fibers with tunable mesoporosity for electrode materials in energy devices. Molecules 2021, 26, 724. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; Ellis, N.; Gyenge, E. Electrosorption on activated biochar: Effect of thermo-chemical activation treatment on the electric double layer capacitance. J. Appl. Electrochem. 2014, 44, 141–157. [Google Scholar] [CrossRef]
- Ashton, S.J.; Arenz, M. A DEMS study on the electrochemical oxidation of a high surface area carbon black. Electrochem. Commun. 2011, 13, 1473–1475. [Google Scholar] [CrossRef]
- Solà-Hernández, L.; Claudel, F.; Maillard, F.; Beauger, C. Doped tin oxide aerogels as oxygen evolution reaction catalyst supports. Int. J. Hydrogen Energy 2019, 44, 24331–24341. [Google Scholar] [CrossRef]
- Disa, N.M.; Yuan, K.S.; Tan, K.L.; Tetsuo, S. The synthesized reduced graphene oxide enhanced the capacitive behavior of activated carbon/PVA as potential electrode materials. J. Nanostructures 2020, 10, 296–306. [Google Scholar] [CrossRef]
- Zakir, M.; Kasim, H.; Raya, I.; Lamba, Y.; Nuratisah; Jorge, A. Performance of Candlenut Shell (Alleuretus moluccana) Based Supercapacitor Electrode with Acid Electrolytes and Their Salts. IOP Conf. Ser. Mater. Sci. Eng. 2019, 619, 12042. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; Pastor, E.; Lázaro, M.J. Electrochemical behavior of the carbon black Vulcan XC-72R: Influence of the surface chemistry. Int. J. Hydrogen Energy 2018, 43, 7911–7922. [Google Scholar] [CrossRef]
- Husain, Z.; Shakeelur Raheman, A.R.; Ansari, K.B.; Pandit, A.B.; Khan, M.S.; Qyyum, M.A.; Lam, S.S. Nano-sized mesoporous biochar derived from biomass pyrolysis as electrochemical energy storage supercapacitor. Mater. Sci. Energy Technol. 2022, 5, 99–109. [Google Scholar] [CrossRef]


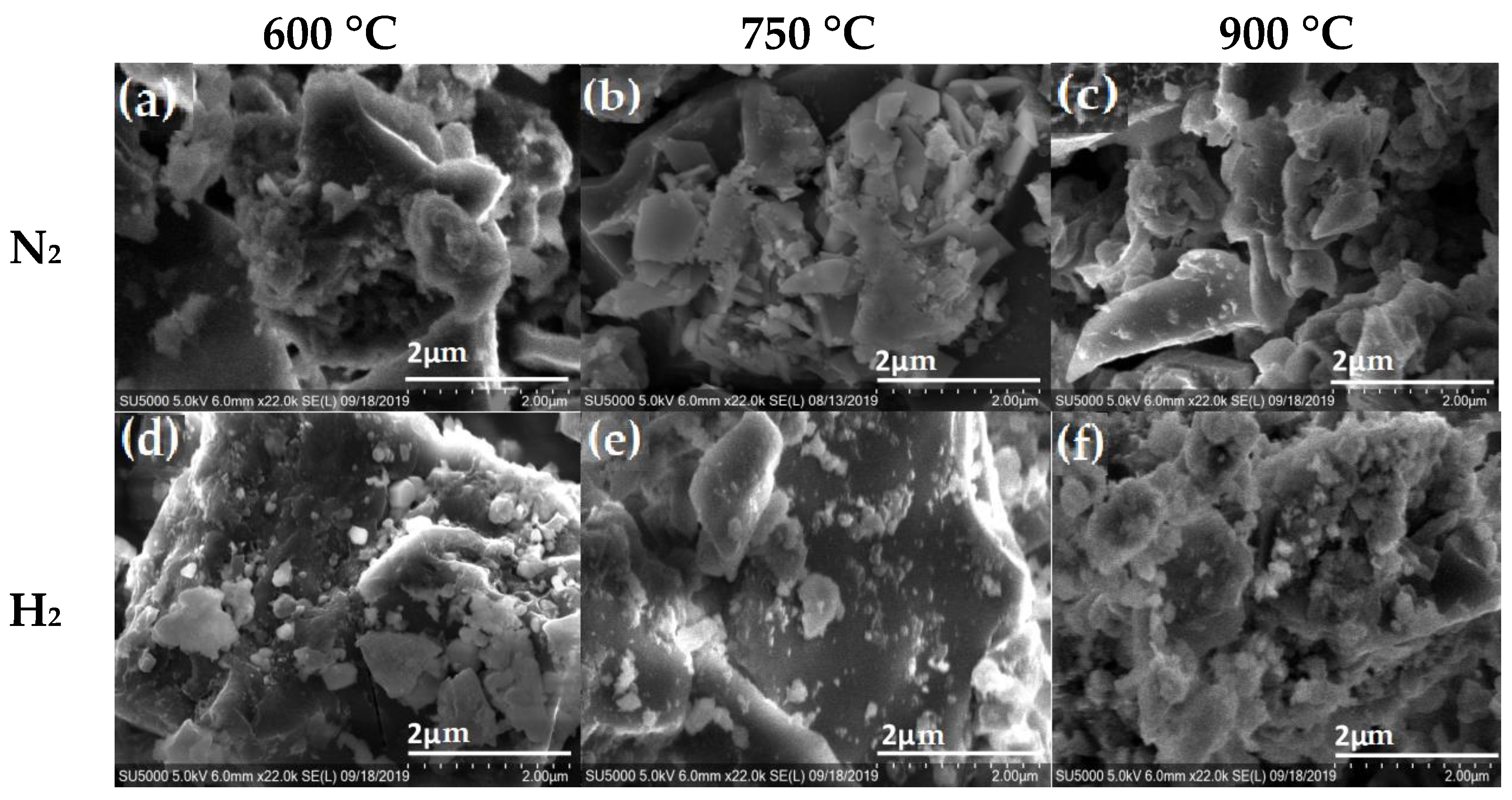
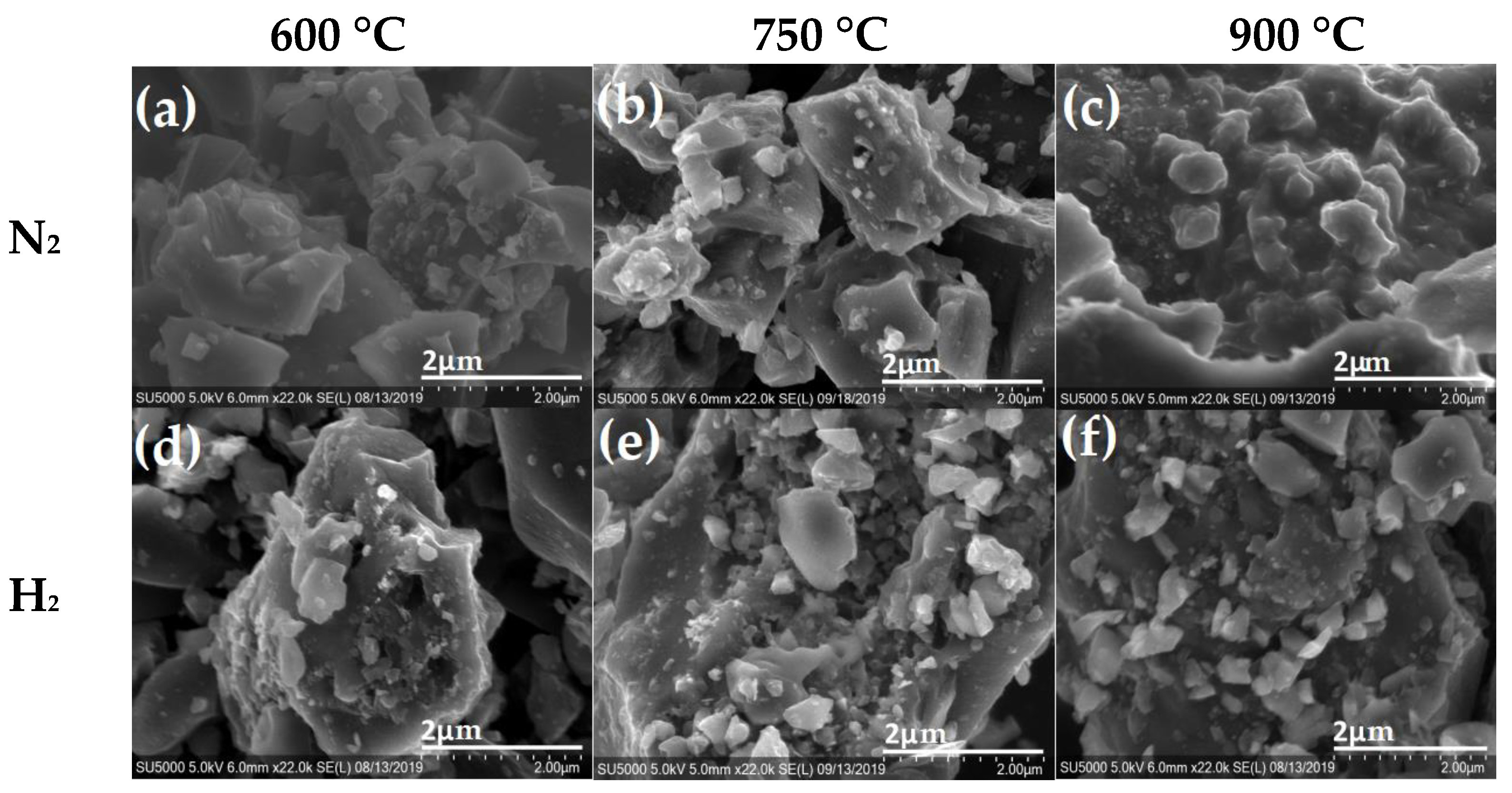
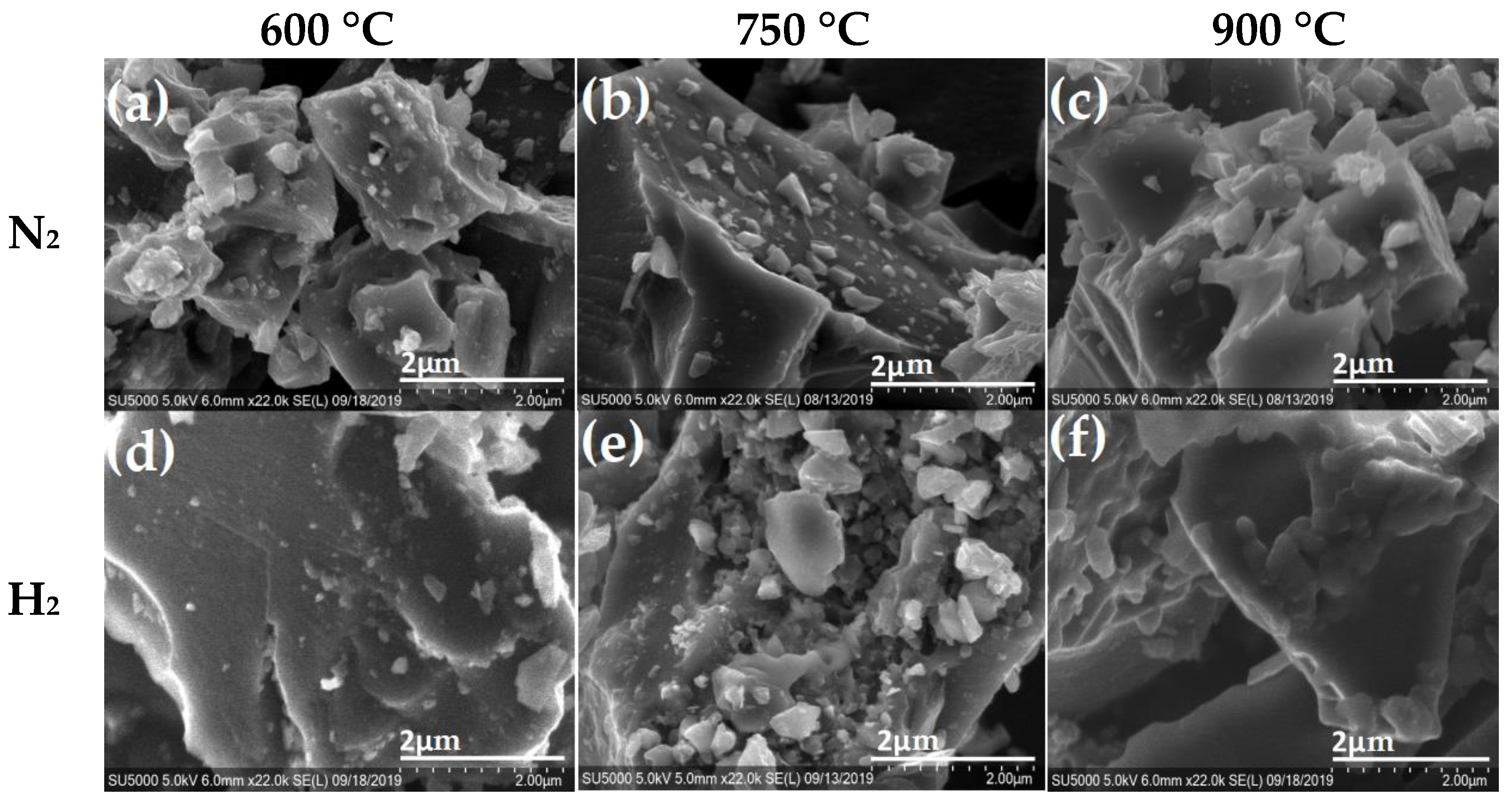
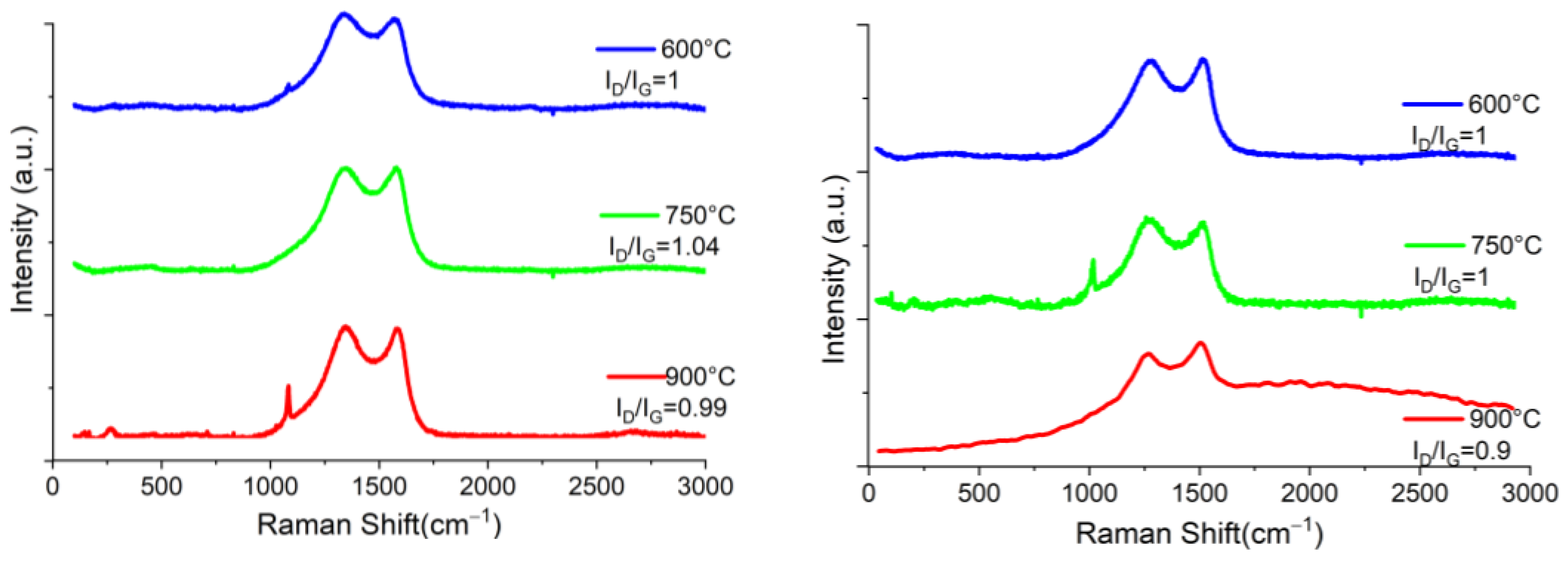
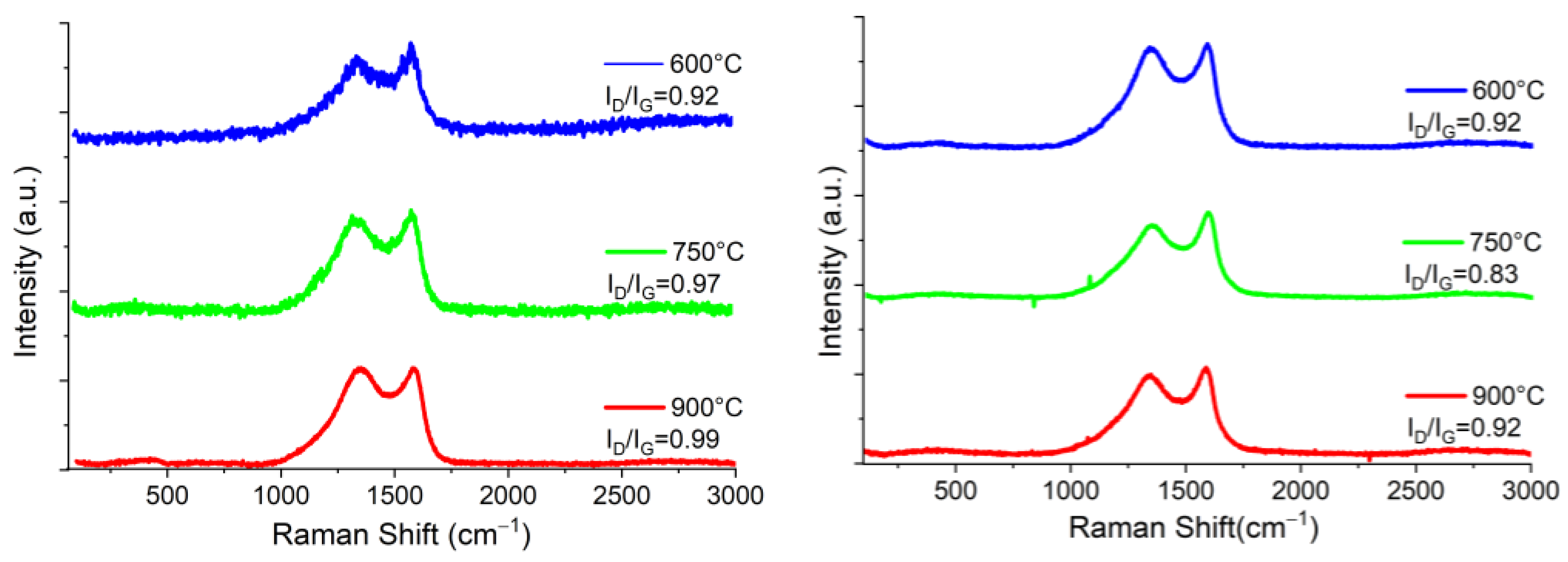
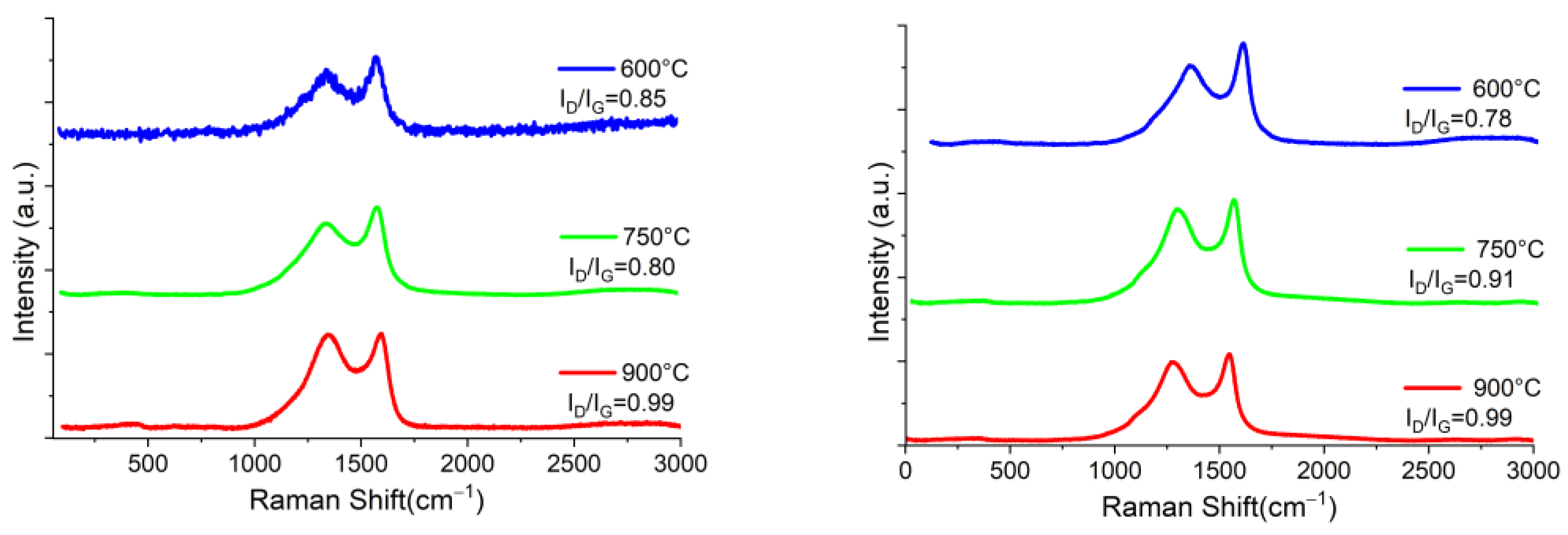
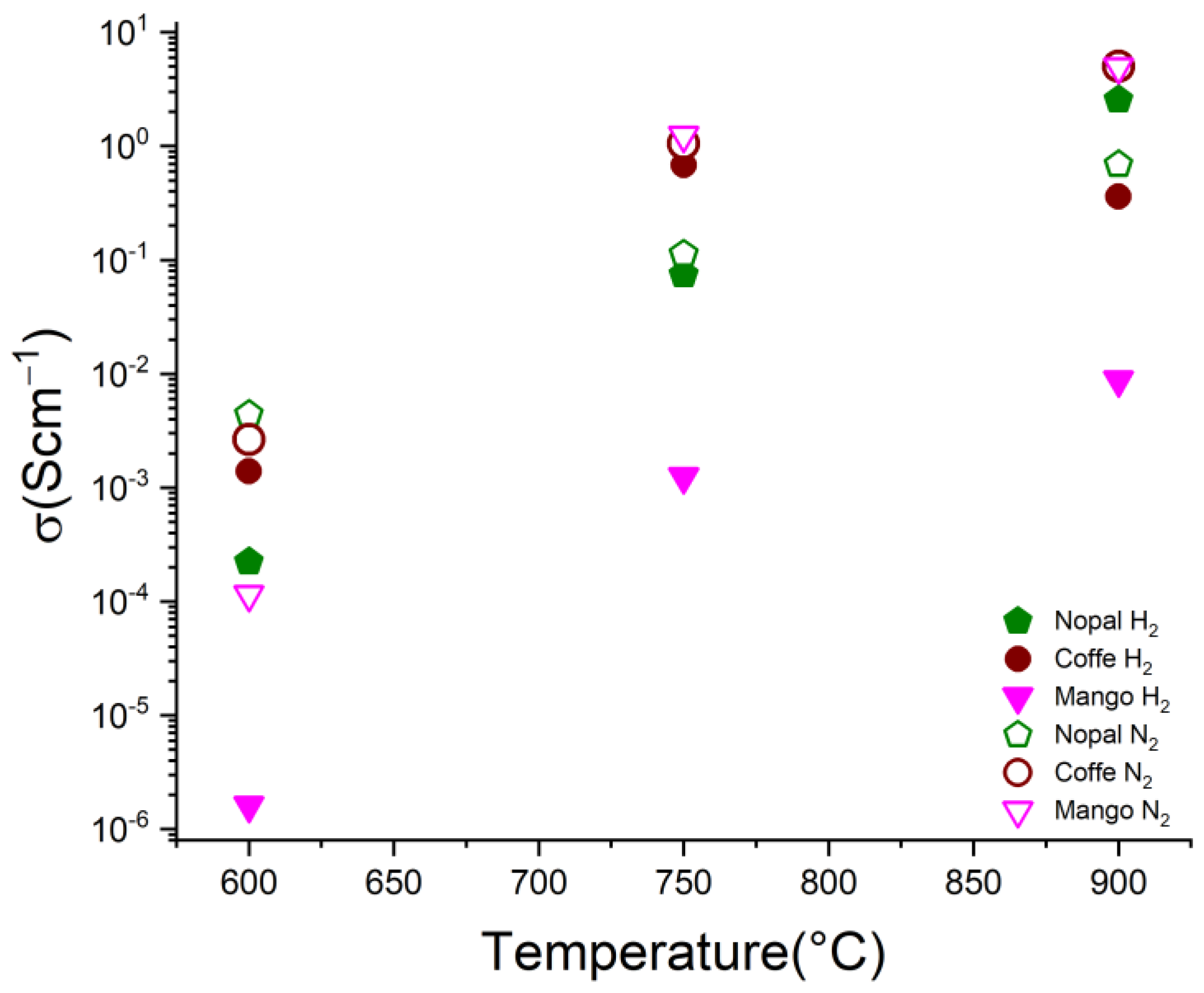


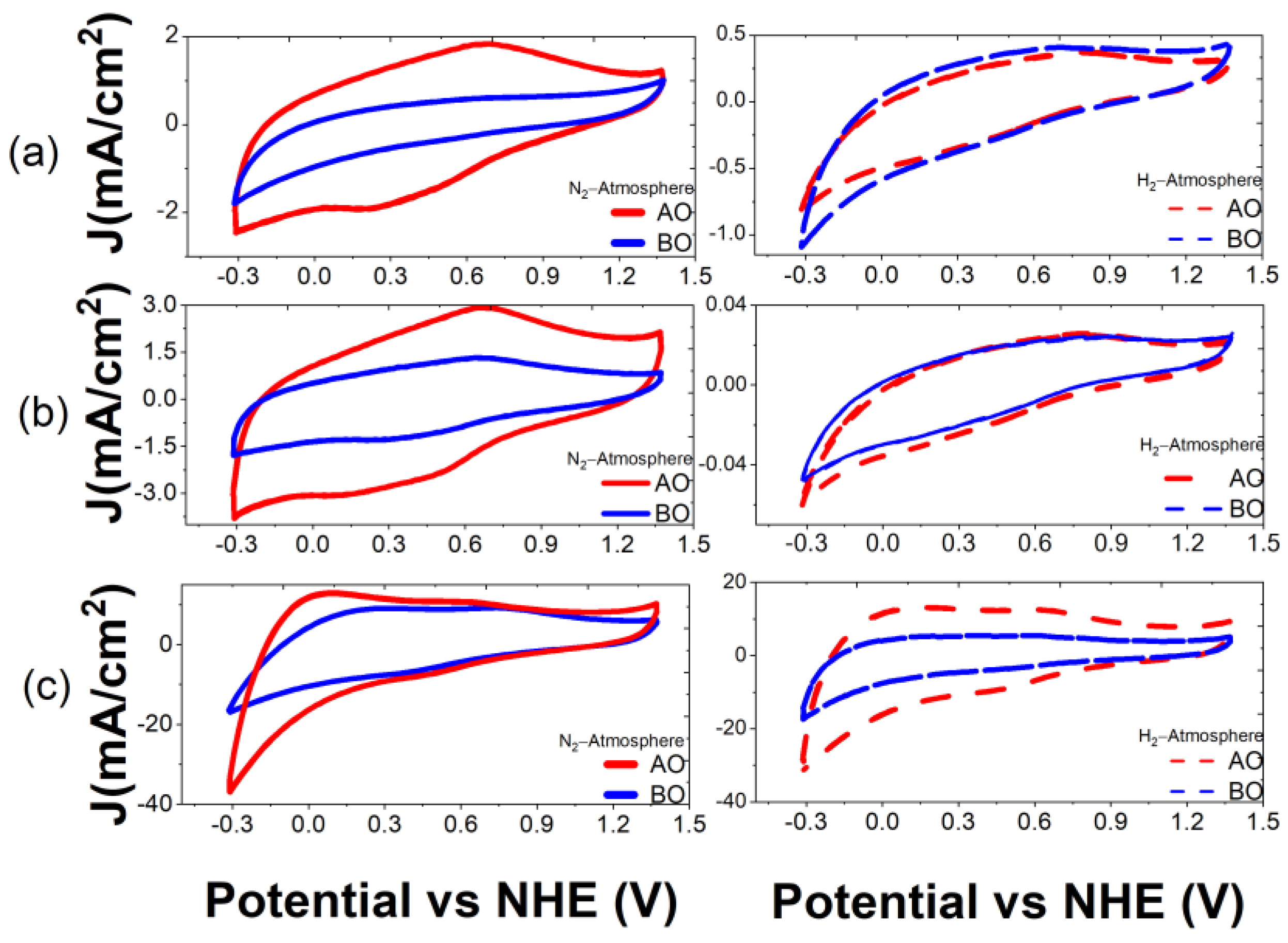

| La (nm) | ||||||
|---|---|---|---|---|---|---|
| Sample | 600 °C | 750 °C | 900 °C | |||
| N2 | H2 | N2 | H2 | N2 | H2 | |
| Mango | 5.83 | 6.34 | 6.20 | 5.44 | 5.07 | 5.07 |
| Coffee | 5.39 | 5.38 | 5.11 | 5.97 | 5.09 | 5.38 |
| Nopal | 4.77 | 4.96 | 5.00 | 4.96 | 4.96 | 5.51 |
| SSA (m2/g) | ||||||
|---|---|---|---|---|---|---|
| Sample | 600 °C | 750 °C | 900 °C | |||
| N2 | H2 | N2 | H2 | N2 | H2 | |
| Nopal | 766.7 | 568.2 | 263.5 | 550.6 | 179.3 | 462. 6 |
| Coffee | 171.0 | 126.0 | 231.0 | 699.3 | 276. 5 | 225.3 |
| Mango | 334.2 | 829.0 | 143.0 | 305.0 | 140.0 | 289. 1 |
| Capacitance (mF/g) | ||||||
|---|---|---|---|---|---|---|
| Sample | 600 °C | 750 °C | 900 °C | |||
| BO | AO | BO | AO | BO | AO | |
| Coffee H2 | 31.68 | 11.54 | 24.39 | 10.56 | 62.63 | 35.25 |
| Mango H2 | 14.56 | 10.56 | 219.55 | 7.28 | 168.58 | 296.02 |
| Nopal H2 | 6.19 | 4.73 | 4.95 | 5.24 | 122.70 | 160.93 |
| Coffee N2 | 7.65 | 10.92 | 29.49 | 16.75 | 68.09 | 152.56 |
| Mango N2 | 15.29 | 9.47 | 25.12 | 48.06 | 273.08 | 158.02 |
| Nopal N2 | 10.92 | 13.84 | 8.37 | 5.10 | 99.04 | 61.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Rocha, R.; Durón-Torres, S.M.; Palomares-Sánchez, S.A.; Del Rio-De Santiago, A.; Rojas-de Soto, I.; Escalante-García, I.L. Effects of Heat Treatment on the Physicochemical Properties and Electrochemical Behavior of Biochars for Electrocatalyst Support Applications. Materials 2023, 16, 5571. https://doi.org/10.3390/ma16165571
García-Rocha R, Durón-Torres SM, Palomares-Sánchez SA, Del Rio-De Santiago A, Rojas-de Soto I, Escalante-García IL. Effects of Heat Treatment on the Physicochemical Properties and Electrochemical Behavior of Biochars for Electrocatalyst Support Applications. Materials. 2023; 16(16):5571. https://doi.org/10.3390/ma16165571
Chicago/Turabian StyleGarcía-Rocha, Rocío, Sergio M. Durón-Torres, Salvador A. Palomares-Sánchez, Antonio Del Rio-De Santiago, Ivone Rojas-de Soto, and Ismailia L. Escalante-García. 2023. "Effects of Heat Treatment on the Physicochemical Properties and Electrochemical Behavior of Biochars for Electrocatalyst Support Applications" Materials 16, no. 16: 5571. https://doi.org/10.3390/ma16165571
APA StyleGarcía-Rocha, R., Durón-Torres, S. M., Palomares-Sánchez, S. A., Del Rio-De Santiago, A., Rojas-de Soto, I., & Escalante-García, I. L. (2023). Effects of Heat Treatment on the Physicochemical Properties and Electrochemical Behavior of Biochars for Electrocatalyst Support Applications. Materials, 16(16), 5571. https://doi.org/10.3390/ma16165571







