Biogenic Fabrication of Silver Nanoparticles Using Calotropis procera Flower Extract with Enhanced Biomimetics Attributes
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material, Extract Preparation, and Phytochemical Analysis
2.2. Synthesis and Characterization of AgNPs
2.3. Biological Activities of CP-AgNPs
2.3.1. Antibacterial Activity
2.3.2. Antifungal Activity
2.3.3. Antioxidant Activity of CP-AgNPs
Hydrogen Peroxide Scavenging Activity
Reducing Power Activity of CP-AgNPs
2.3.4. Antidiabetic Activity of CP-AgNPs
2.3.5. Anti-Inflammatory Activity of AgNPs
Inhibition of Protein Denaturation
Membrane Stabilization Activity
3. Results
3.1. Phytochemical Analysis of the C. procera Flower Extract
3.2. Synthesis CP-AgNPs
3.2.1. Effect of Silver Nitrate Concentration on the Synthesis of AgNPs
3.2.2. Effect of Calotropis procera Flower Extract Volume on the Synthesis of AgNPs
3.2.3. Effect of Temperature on the Synthesis of CP-AgNPs
3.2.4. Effect of Reaction Time on the Synthesis of AgNPs
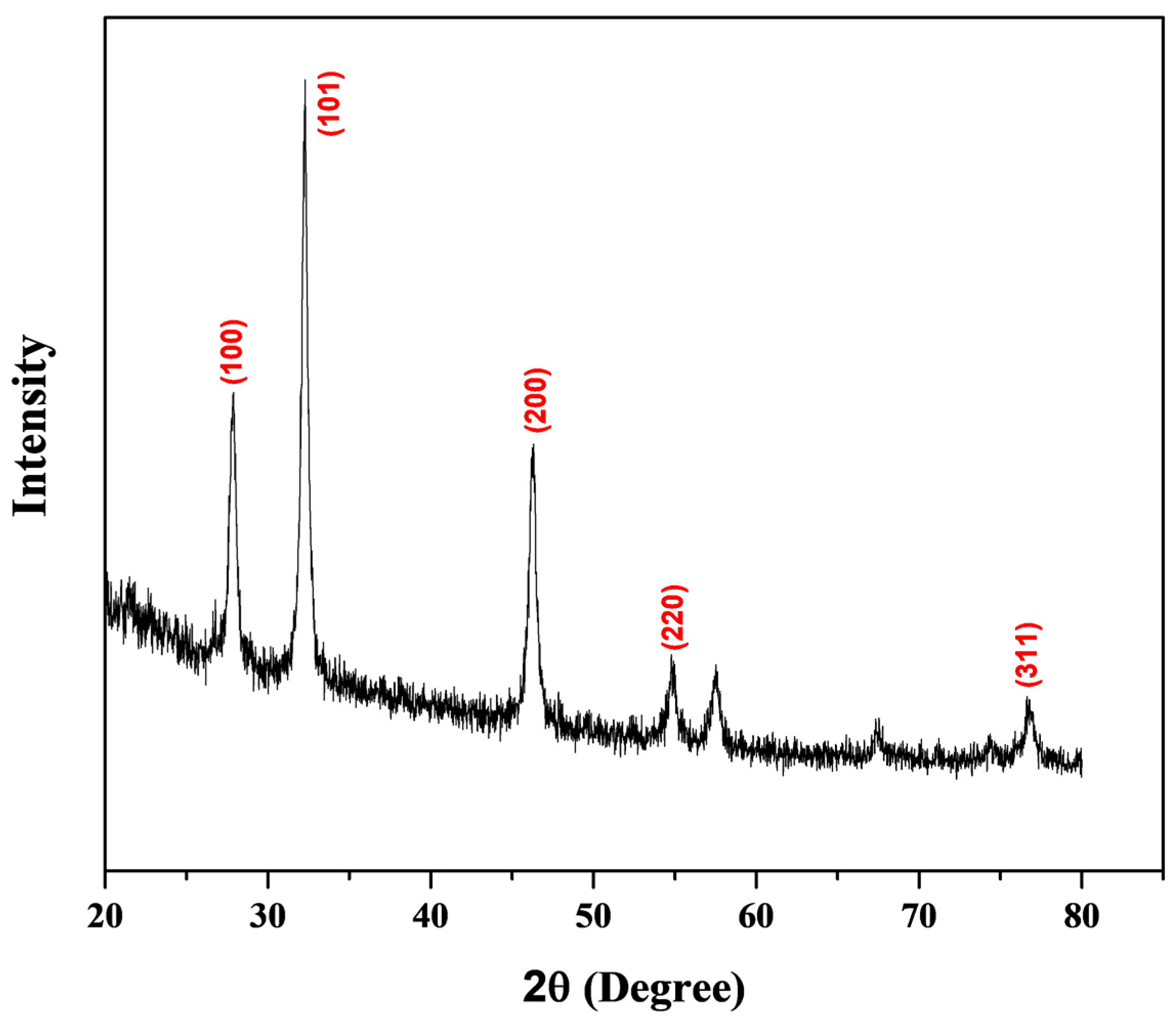
3.3. Structural and Morphological Characterization of CP-AgNPs
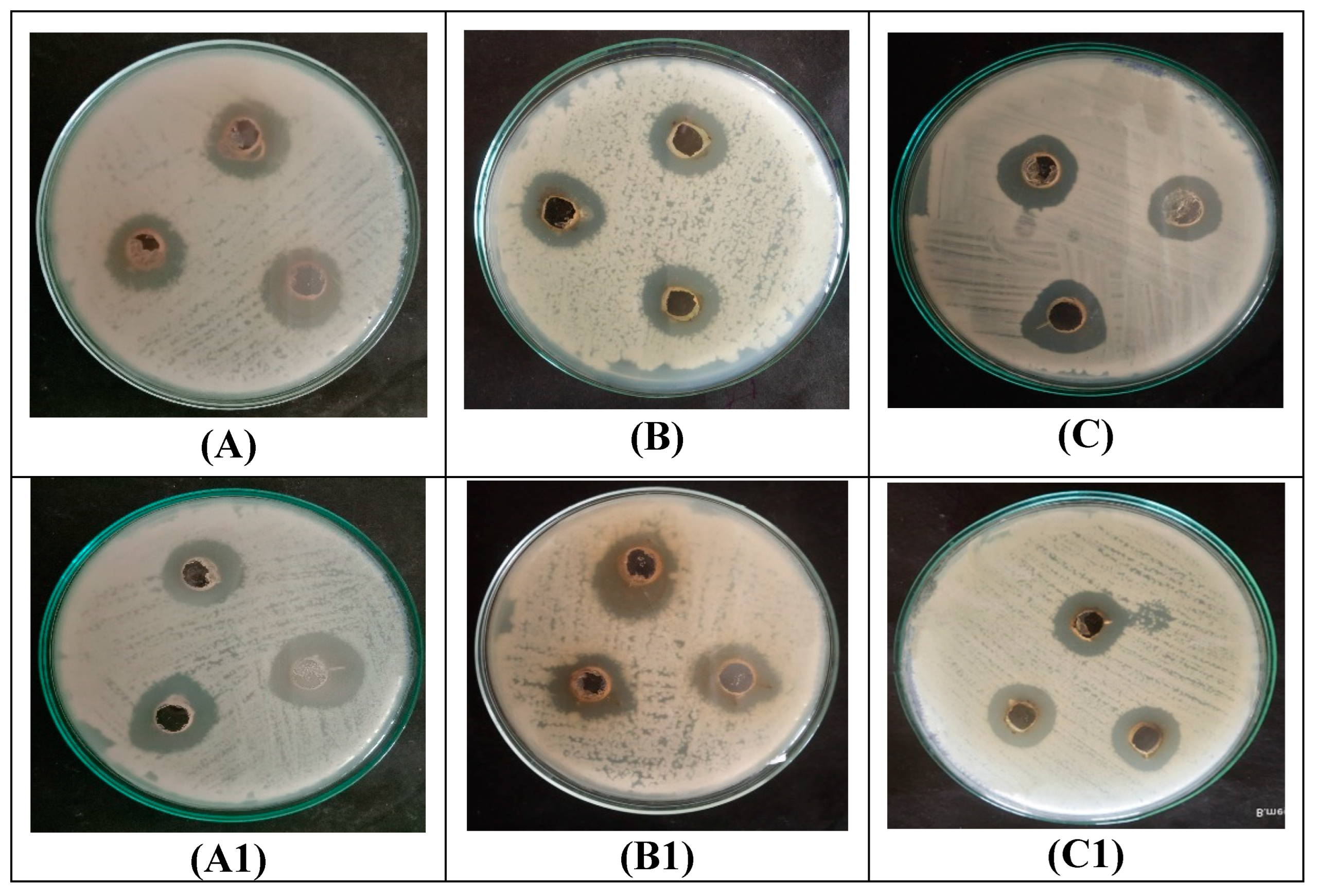
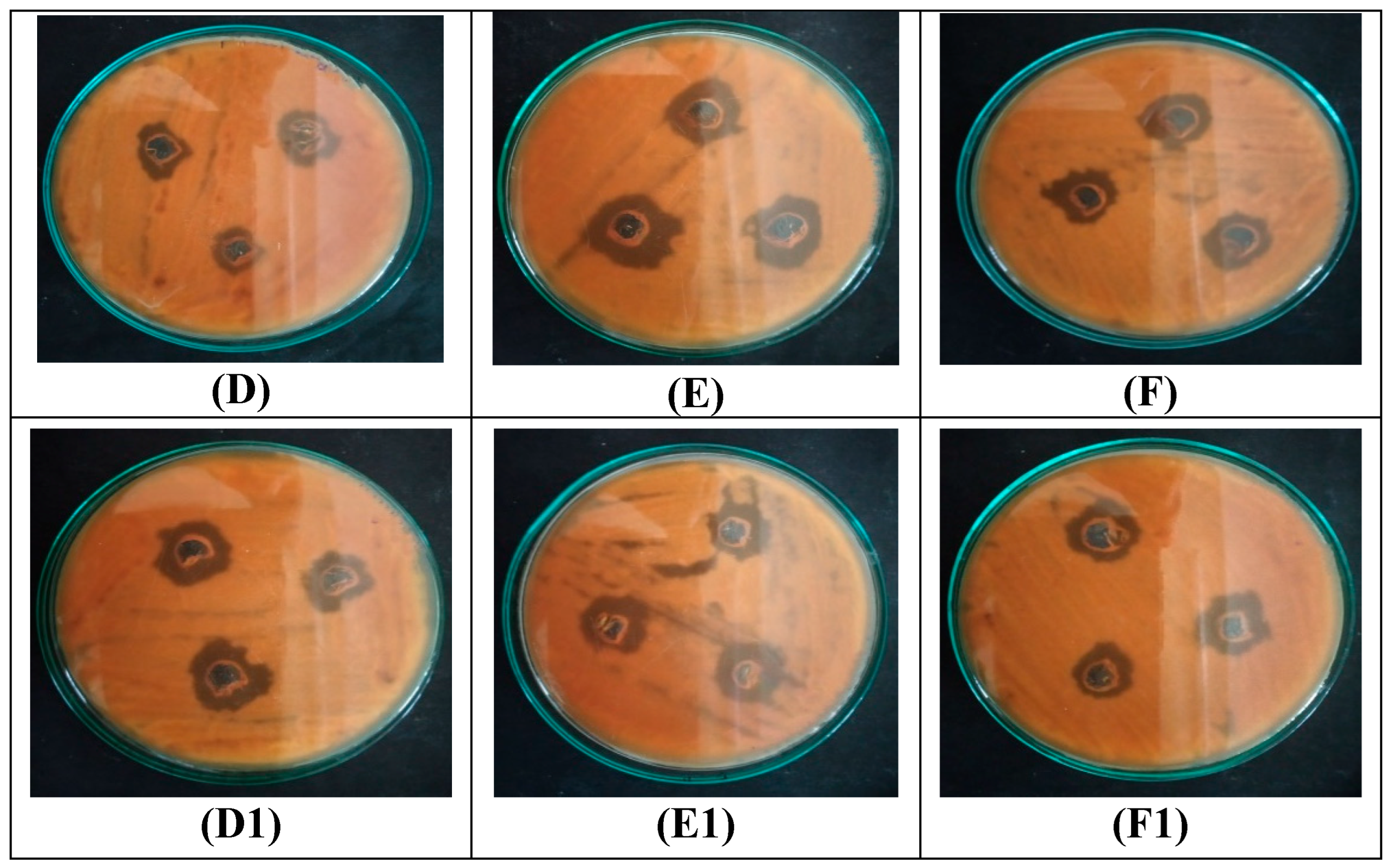
3.4. Antibacterial Activity of CP-AgNPs
3.5. Antifungal Activity of CP-AgNPs
3.6. Antioxidant Activity of CP-AgNPs
3.7. Antidiabetic Activity of CP-AgNPs
3.8. Anti-Inflammatory Activity of Synthesized AgNPs
3.8.1. Inhibition of Albumin Denaturation
3.8.2. Membrane Stabilization Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Nwabor, O.F.; Sukri, D.M.; Wunnoo, S.; Dumjun, K.; Lethongkam, S.; Kusolphat, P.; Hemtanon, N.; Klinprathum, K.; Sunghan, J.; et al. Poly (vinyl alcohol) copolymerized with xanthan gum/hypromellose/sodium carboxymethyl cellulose dermal dressings functionalized with biogenic nanostructured materials for antibacterial and wound healing application. Int. J. Biol. Macromol. 2022, 216, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Suriyakala, G.; Sathiyaraj, S.; Devanesan, S.; AlSalhi, M.S.; Rajasekar, A.; Maruthamuthu, M.K.; Babujanarthanam, R. Phytosynthesis of silver nanoparticles from Jatropha integerrima Jacq. flower extract and their possible applications as antibacterial and antioxidant agent. Saudi J. Biol. Sci. 2022, 29, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Ontong, J.C.; Singh, S.; Nwabor, O.F.; Chusri, S.; Voravuthikunchai, S.P. Potential of antimicrobial topical gel with synthesized biogenic silver nanoparticle using Rhodomyrtus tomentosa leaf extract and silk sericin. Biotechnol. Lett. 2020, 42, 2653–2664. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chunglok, W.; Nwabor, O.F.; Ushir, Y.V.; Singh, S.; Panpipat, W. Hydrophilic Biopolymer Matrix Antibacterial Peel-off Facial Mask Functionalized with Biogenic Nanostructured Material for Cosmeceutical Applications. J. Polym. Environ. 2022, 30, 938–953. [Google Scholar] [CrossRef]
- Jayeoye, T.J.; Eze, F.N.; Singh, S.; Olatunde, O.O.; Benjakul, S.; Rujiralai, T. Synthesis of gold nanoparticles/polyaniline boronic acid/sodium alginate aqueous nanocomposite based on chemical oxidative polymerization for biological applications. Int. J. Biol. Macromol. 2021, 179, 196–205. [Google Scholar] [CrossRef]
- Jayeoye, T.J.; Eze, F.N.; Olatunde, O.O.; Singh, S.; Zuo, J.; Olatunji, O.J. Multifarious Biological Applications and Toxic Hg(2+) Sensing Potentiality of Biogenic Silver Nanoparticles Based on Securidaca inappendiculata Hassk Stem Extract. Int. J. Nanomed. 2021, 16, 7557–7574. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Singh, S.; Wunnoo, S.; Lerwittayanon, K.; Voravuthikunchai, S.P. Facile deposition of biogenic silver nanoparticles on porous alumina discs, an efficient antimicrobial, antibiofilm, and antifouling strategy for functional contact surfaces. Biofouling 2021, 37, 538–554. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Singh, S.; Ontong, J.C.; Vongkamjan, K.; Voravuthikunchai, S.P. Valorization of Wastepaper Through Antimicrobial Functionalization with Biogenic Silver Nanoparticles, a Sustainable Packaging Composite. Waste Biomass Valorization 2021, 12, 3287–3301. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Singh, S.; Paosen, S.; Vongkamjan, K.; Voravuthikunchai, S.P. Enhancement of food shelf life with polyvinyl alcohol-chitosan nanocomposite films from bioactive Eucalyptus leaf extracts. Food Biosci. 2020, 36, 100609. [Google Scholar] [CrossRef]
- Syukri, D.M.; Nwabor, O.F.; Singh, S.; Ontong, J.C.; Wunnoo, S.; Paosen, S.; Munah, S.; Voravuthikunchai, S.P. Antibacterial-coated silk surgical sutures by ex situ deposition of silver nanoparticles synthesized with Eucalyptus camaldulensis eradicates infections. J. Microbiol. Methods 2020, 174, 105955. [Google Scholar] [CrossRef]
- Syukri, D.M.; Nwabor, O.F.; Singh, S.; Voravuthikunchai, S.P. Antibacterial functionalization of nylon monofilament surgical sutures through in situ deposition of biogenic silver nanoparticles. Surf. Coat. Technol. 2021, 413, 127090. [Google Scholar] [CrossRef]
- Kumar, A.; Shah, S.R.; Jayeoye, T.J.; Kumar, A.; Parihar, A.; Prajapati, B.G.; Singh, S.; Kapoor, D. Biogenic metallic nanoparticles: Biomedical, analytical, food preservation, and applications in other consumable products. Front. Nanotechnol. 2023, 5, 1175149. [Google Scholar] [CrossRef]
- Bhat, M.; Chakraborty, B.; Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Kotresha, D.; Pallavi, S.; Dhanyakumara, S.; Shashiraj, K.; Nayaka, S. Biogenic synthesis, characterization and antimicrobial activity of Ixora brachypoda (DC) leaf extract mediated silver nanoparticles. J. King Saud Univ.-Sci. 2021, 33, 101296. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385. [Google Scholar]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Badawi, M.S.; Ghatass, Z.; Mohamed, M.; Elkhatib, M. Synthesize of silver nanoparticles by arc discharge method using two different rotational electrode shapes. J. Clust. Sci. 2018, 29, 1169–1175. [Google Scholar] [CrossRef]
- Weerasinghe, J.; Li, W.; Zhou, R.; Zhou, R.; Gissibl, A.; Sonar, P.; Speight, R.; Vasilev, K.; Ostrikov, K. Bactericidal silver nanoparticles by atmospheric pressure solution plasma processing. Nanomaterials 2020, 10, 874. [Google Scholar] [CrossRef]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Chandraker, S.K.; Ghosh, M.K.; Lal, M.; Shukla, R. A review on plant-mediated synthesis of silver nanoparticles, their characterization and applications. Nano Express 2021, 2, 022008. [Google Scholar] [CrossRef]
- Bordiwala, R.V. Green Synthesis and Applications of Metal Nanoparticles—A Review article. Results Chem. 2023, 5, 100832. [Google Scholar] [CrossRef]
- Al-Rowaily, S.L.; Abd-ElGawad, A.M.; Assaeed, A.M.; Elgamal, A.M.; Gendy, A.E.-N.G.E.; Mohamed, T.A.; Dar, B.A.; Mohamed, T.K.; Elshamy, A.I. Essential oil of Calotropis procera: Comparative chemical profiles, antimicrobial activity, and allelopathic potential on weeds. Molecules 2020, 25, 5203. [Google Scholar] [CrossRef] [PubMed]
- Chidrawar, V.R.; Singh, S.; Jayeoye, T.J.; Dodiya, R.; Samee, W.; Chittasupho, C. Porous Swellable Hypromellose Composite Fortified with Eucalyptus camaldulensis Leaf Hydrophobic/Hydrophilic Phenolic-rich Extract to Mitigate Dermal Wound Infections. J. Polym. Environ. 2023, 1, 16. [Google Scholar] [CrossRef]
- Singh, S.; Chidrawar, V.R.; Hermawan, D.; Dodiya, R.; Samee, W.; Ontong, J.C.; Ushir, Y.V.; Prajapati, B.G.; Chittasupho, C. Hypromellose Highly Swellable Composite Fortified with Psidium Guajava Leaf Phenolic-rich Extract for Antioxidative, Antibacterial, Anti-inflammatory, Anti-melanogenesis, and Hemostasis Applications. J. Polym. Environ. 2023, 1, 18. [Google Scholar] [CrossRef]
- Paramasivam, D.; Balasubramanian, B.; Suresh, R.; Kumaravelu, J.; Vellingiri, M.M.; Liu, W.-C.; Meyyazhagan, A.; Alanazi, A.M.; Rengasamy, K.R.; Arumugam, V.A. One-Pot Synthesis of Silver Nanoparticles Derived from Aqueous Leaf Extract of Ageratum conyzoides and Their Biological Efficacy. Antibiotics 2023, 12, 688. [Google Scholar] [CrossRef]
- Ajaykumar, A.P.; Mathew, A.; Chandni, A.P.; Varma, S.R.; Jayaraj, K.N.; Sabira, O.; Rasheed, V.A.; Binitha, V.S.; Swaminathan, T.R.; Basheer, V.S. Green Synthesis of Silver Nanoparticles Using the Leaf Extract of the Medicinal Plant, Uvaria narum and Its Antibacterial, Antiangiogenic, Anticancer and Catalytic Properties. Antibiotics 2023, 12, 564. [Google Scholar] [CrossRef]
- Alowaiesh, B.F.; Alhaithloul, H.A.S.; Saad, A.M.; Hassanin, A.A. Green Biogenic of Silver Nanoparticles Using Polyphenolic Extract of Olive Leaf Wastes with Focus on Their Anticancer and Antimicrobial Activities. Plants 2023, 12, 1410. [Google Scholar] [CrossRef]
- Meena, A.; Yadav, A.; Meda, M. Ayurvedic uses and pharmacological activities of Calotropis procera Linn. Asian J. Tradit. Med. 2011, 6, 45–53. [Google Scholar]
- Morsy, N.; Sherif, E.; Abdel-rassol, T. Phytochemical analysis of Calotropis procera with antimicrobial activity investigation. Main Group Chem. 2016, 15, 267–273. [Google Scholar] [CrossRef]
- Alzubaidi, A.K.; Al-Kaabi, W.J.; Ali, A.A.; Albukhaty, S.; Al-Karagoly, H.; Sulaiman, G.M.; Asiri, M.; Khane, Y. Green Synthesis and Characterization of Silver Nanoparticles Using Flaxseed Extract and Evaluation of Their Antibacterial and Antioxidant Activities. Appl. Sci. 2023, 13, 2182. [Google Scholar] [CrossRef]
- Mukaratirwa-Muchanyereyi, N.; Gusha, C.; Mujuru, M.; Guyo, U.; Nyoni, S. Synthesis of silver nanoparticles using plant extracts from Erythrina abyssinica aerial parts and assessment of their anti-bacterial and anti-oxidant activities. Results Chem. 2022, 4, 100402. [Google Scholar] [CrossRef]
- Melkamu, W.W.; Bitew, L.T. Green synthesis of silver nanoparticles using Hagenia abyssinica (Bruce) JF Gmel plant leaf extract and their antibacterial and anti-oxidant activities. Heliyon 2021, 7, e08459. [Google Scholar] [CrossRef]
- Patil, L.; Shet, A.; Tennalli, G.; Achappa, S.; Hombalimath, V.; Deshannavar, U. Optimization of Process Parameters for Synthesis of Silver Nanoparticles Using Leaf Extract of Tridax Procumbent and Its Biotechnological Applications. Int. J. Sci. Technol. Res. 2020, 9, 1050–1056. [Google Scholar]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef]
- Oves, M.; Rauf, M.A.; Aslam, M.; Qari, H.A.; Sonbol, H.; Ahmad, I.; Zaman, G.S.; Saeed, M. Green synthesis of silver nanoparticles by Conocarpus lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J. Biol. Sci. 2022, 29, 460–471. [Google Scholar] [CrossRef]
- Hemmati, S.; Rashtiani, A.; Zangeneh, M.M.; Mohammadi, P.; Zangeneh, A.; Veisi, H. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron 2019, 158, 8–14. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Facile green synthesis of silver nanoparticles using aqueous leaf extract of Origanum majorana with potential bioactivity against multidrug resistant bacterial strains. Crystals 2022, 12, 603. [Google Scholar] [CrossRef]
- Mohamed, N.H.; Ismail, M.A.; Abdel-Mageed, W.M.; Shoreit, A.A.M. Antimicrobial activity of latex silver nanoparticles using Calotropis procera. Asian Pac. J. Trop. Biomed. 2014, 4, 876–883. [Google Scholar] [CrossRef]
- Rajkuberan, C.; Sudha, K.; Sathishkumar, G.; Sivaramakrishnan, S. Antibacterial and cytotoxic potential of silver nanoparticles synthesized using latex of Calotropis gigantea L. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 924–930. [Google Scholar] [CrossRef]
- He, W.; Zhou, Y.-T.; Wamer, W.G.; Boudreau, M.D.; Yin, J.-J. Mechanisms of the pH dependent generation of hydroxyl radicals and oxygen induced by Ag nanoparticles. Biomaterials 2012, 33, 7547–7555. [Google Scholar] [CrossRef]
- Bhakya, S.; Muthukrishnan, S.; Sukumaran, M.; Muthukumar, M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- Malapermal, V.; Botha, I.; Krishna, S.B.N.; Mbatha, J.N. Enhancing antidiabetic and antimicrobial performance of Ocimum basilicum, and Ocimum sanctum (L.) using silver nanoparticles. Saudi J. Biol. Sci. 2017, 24, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, A.; Mani, N. Anti-inflammatory activity of silver nanoparticles synthesized from Eichhornia crassipes: An in vitro study. J. Pharmacogn. Phytochem. 2019, 8, 2556–2558. [Google Scholar]
- Kedi, P.B.E.; Meva, F.E.; Kotsedi, L.; Nguemfo, E.L.; Zangueu, C.B.; Ntoumba, A.A.; Mohamed, H.E.A.; Dongmo, A.B.; Maaza, M. Eco-friendly synthesis, characterization, in vitro and in vivo anti-inflammatory activity of silver nanoparticle-mediated Selaginella myosurus aqueous extract. Int. J. Nanomed. 2018, 13, 8537–8548. [Google Scholar] [CrossRef] [PubMed]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.-K.; Rai, V.R.; Ramachandra, Y. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Phys. 2018, 9, 400–408. [Google Scholar] [CrossRef]
- Hebeish, A.; Ramadan, M.; Montaser, A.; Farag, A.M. Preparation, characterization and antibacterial activity of chitosan-g-poly acrylonitrile/silver nanocomposite. Int. J. Biol. Macromol. 2014, 68, 178–184. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagime, P.V.; Singh, S.; Shaikh, N.M.; Gomare, K.S.; Chitme, H.; Abdel-Wahab, B.A.; Alqahtany, Y.S.; Khateeb, M.M.; Habeeb, M.S.; Bakir, M.B. Biogenic Fabrication of Silver Nanoparticles Using Calotropis procera Flower Extract with Enhanced Biomimetics Attributes. Materials 2023, 16, 4058. https://doi.org/10.3390/ma16114058
Nagime PV, Singh S, Shaikh NM, Gomare KS, Chitme H, Abdel-Wahab BA, Alqahtany YS, Khateeb MM, Habeeb MS, Bakir MB. Biogenic Fabrication of Silver Nanoparticles Using Calotropis procera Flower Extract with Enhanced Biomimetics Attributes. Materials. 2023; 16(11):4058. https://doi.org/10.3390/ma16114058
Chicago/Turabian StyleNagime, Pooja V., Sudarshan Singh, Nishat M. Shaikh, Komal S. Gomare, Havagiray Chitme, Basel A. Abdel-Wahab, Yahya S. Alqahtany, Masood Medleri Khateeb, Mohammed Shafiuddin Habeeb, and Marwa B. Bakir. 2023. "Biogenic Fabrication of Silver Nanoparticles Using Calotropis procera Flower Extract with Enhanced Biomimetics Attributes" Materials 16, no. 11: 4058. https://doi.org/10.3390/ma16114058
APA StyleNagime, P. V., Singh, S., Shaikh, N. M., Gomare, K. S., Chitme, H., Abdel-Wahab, B. A., Alqahtany, Y. S., Khateeb, M. M., Habeeb, M. S., & Bakir, M. B. (2023). Biogenic Fabrication of Silver Nanoparticles Using Calotropis procera Flower Extract with Enhanced Biomimetics Attributes. Materials, 16(11), 4058. https://doi.org/10.3390/ma16114058










