Investigation of Microstructure and Mechanical Properties of SAC105 Solders with Sb, In, Ni, and Bi Additions
Abstract
1. Introduction
2. Experimental Methods
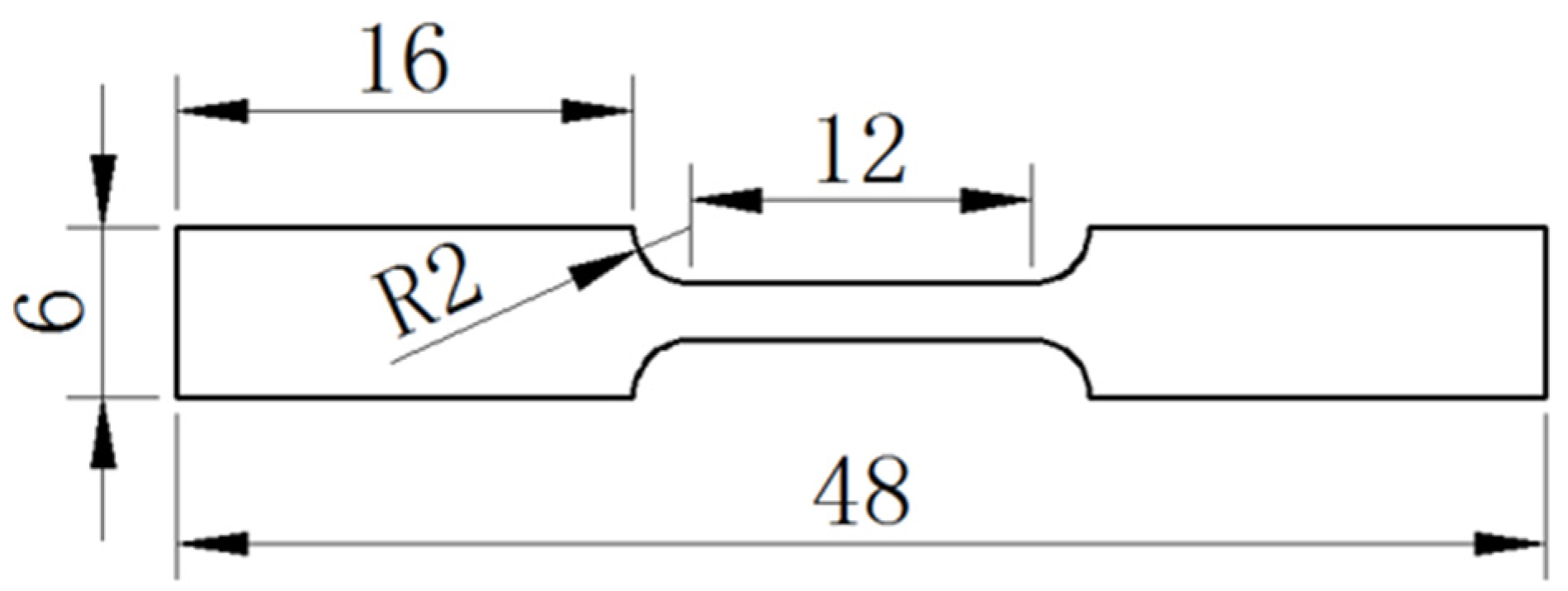
2.1. Processing of Lead-Free Solder
2.2. Mechanical Properties
2.3. Characterization of Physical Properties
2.3.1. Microstructure Characterization
2.3.2. Melting Properties
2.3.3. Wettability Test
2.4. Creep Resistance
3. Results and Discussion
3.1. Mechanical Property
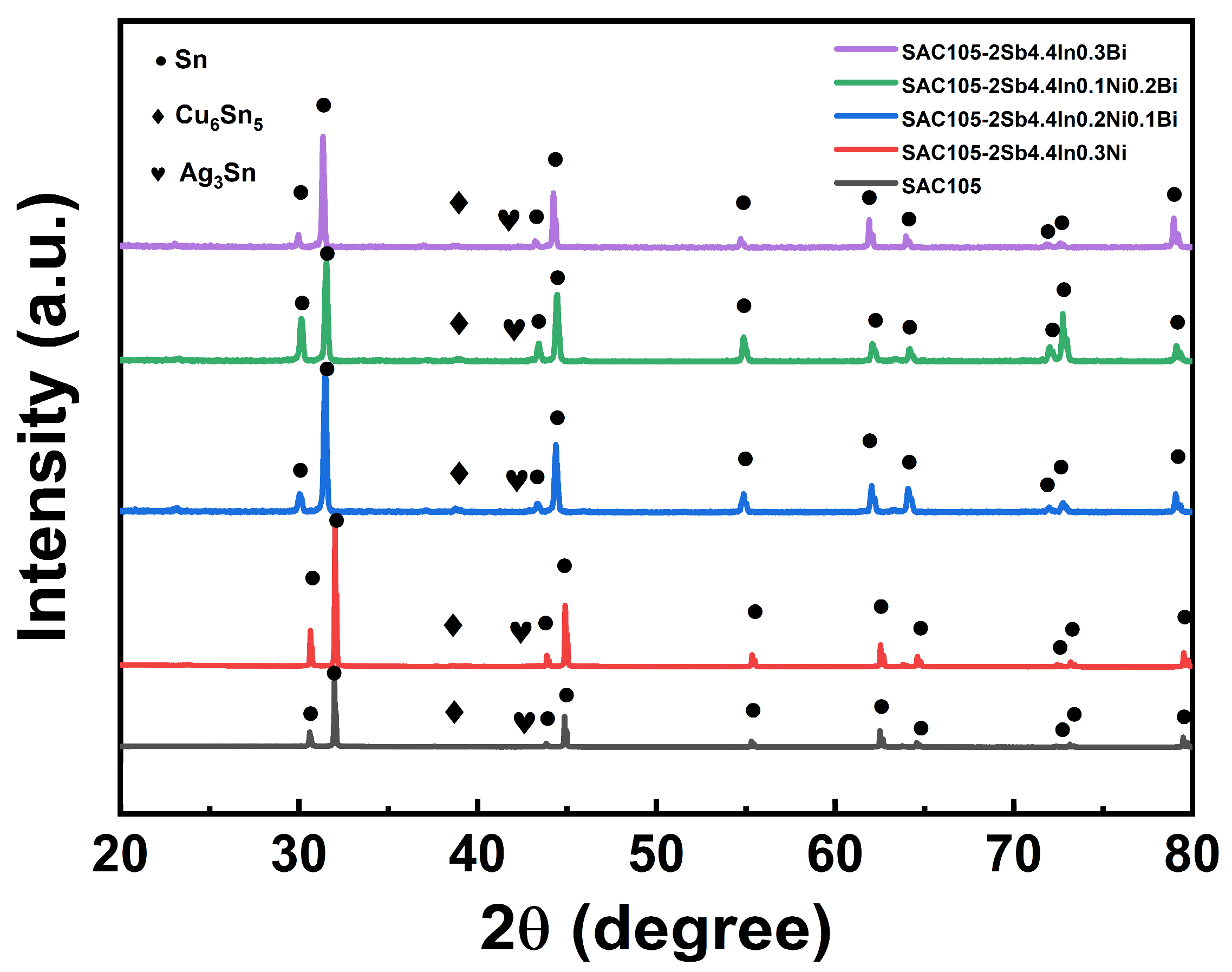
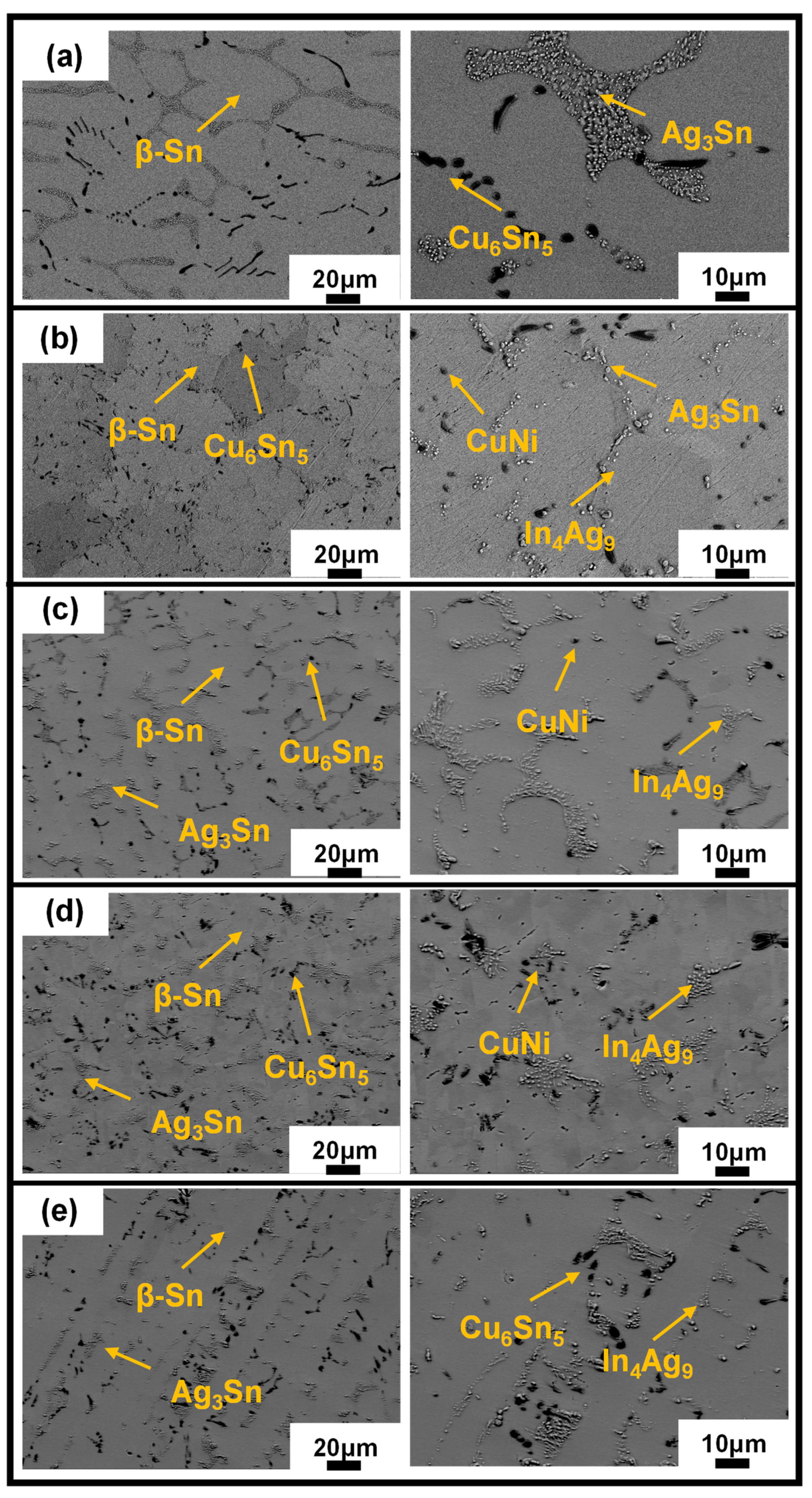
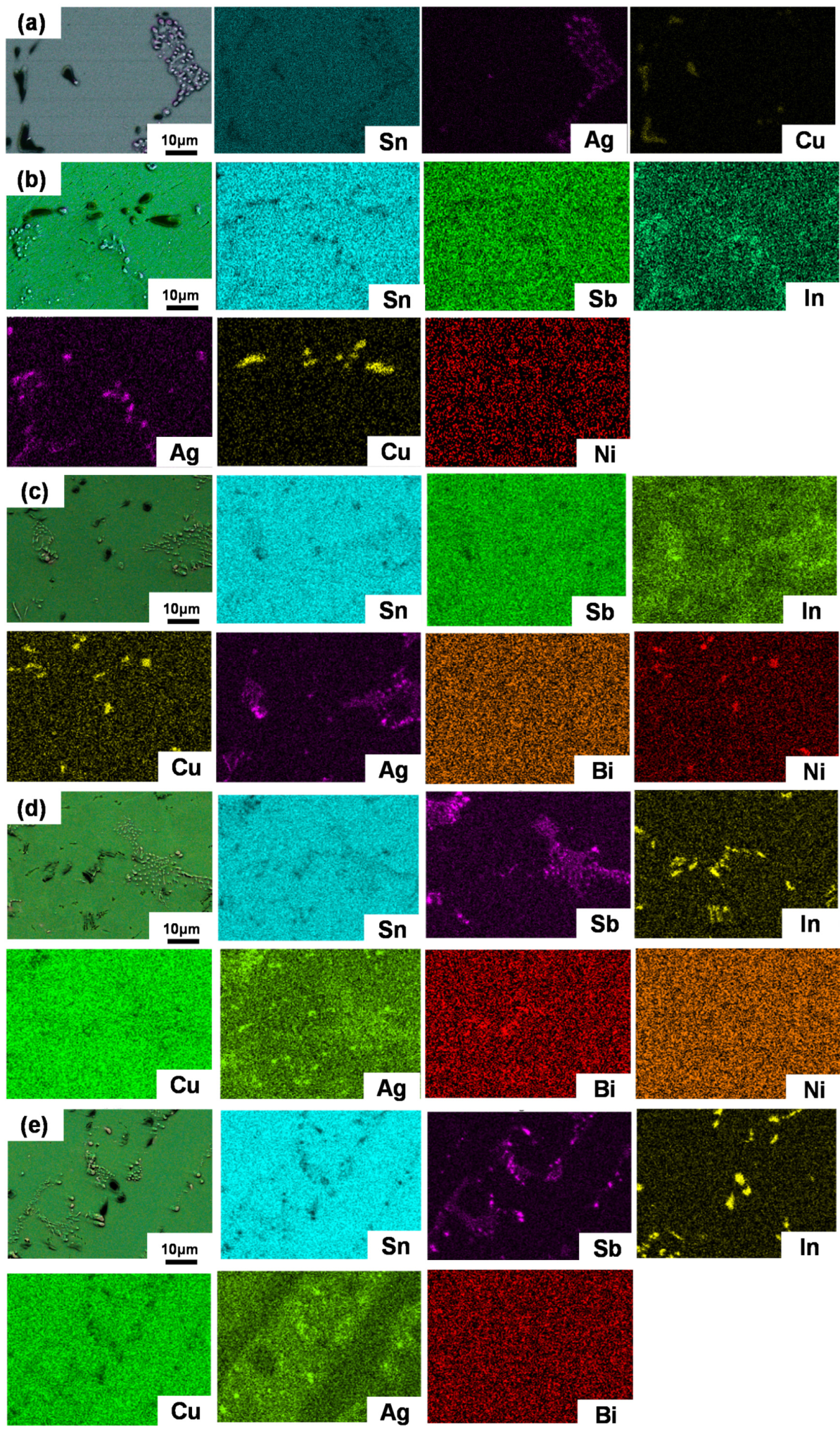
3.2. Microstructure Characterization
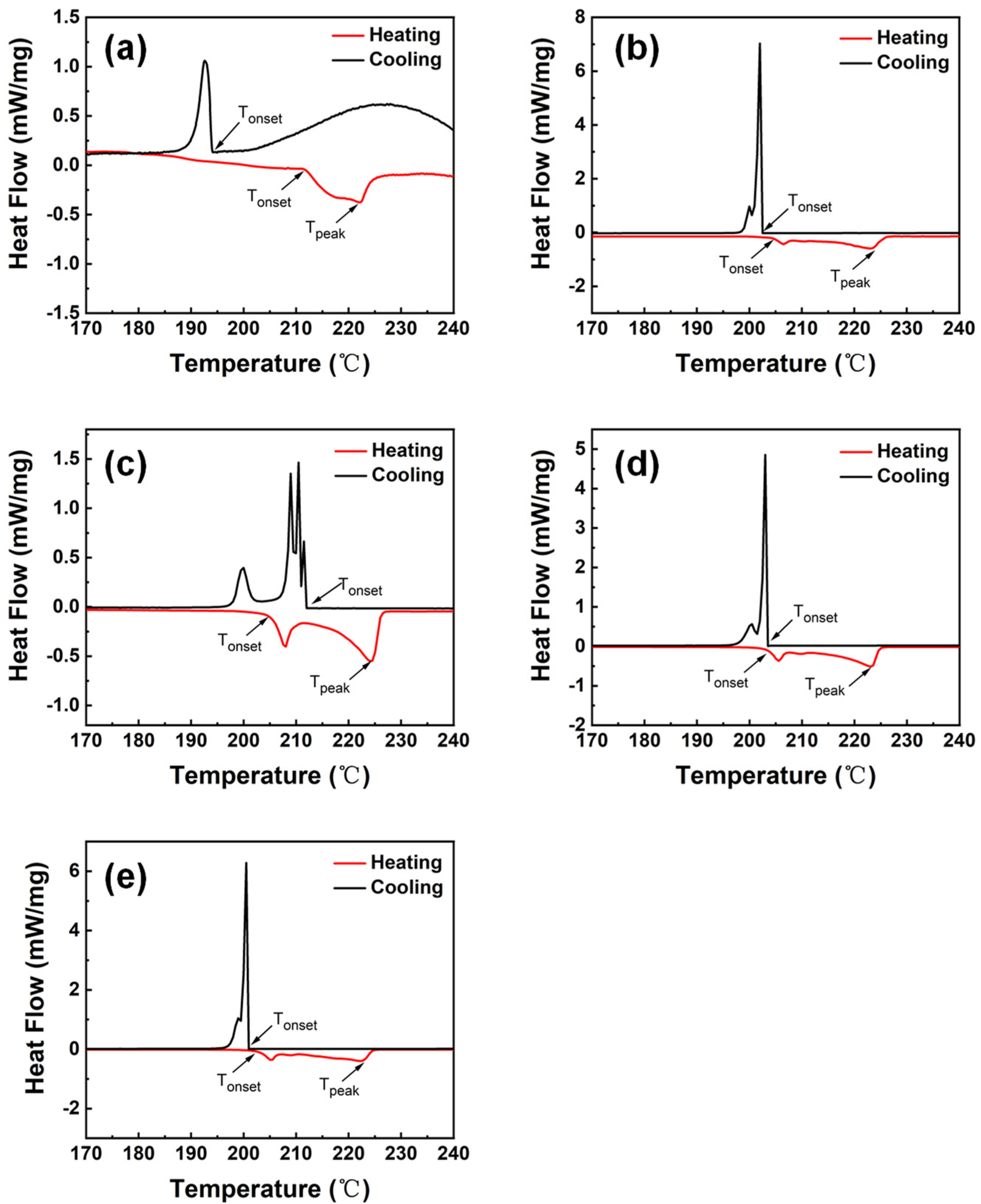
3.3. Thermal Property
3.4. Wettability
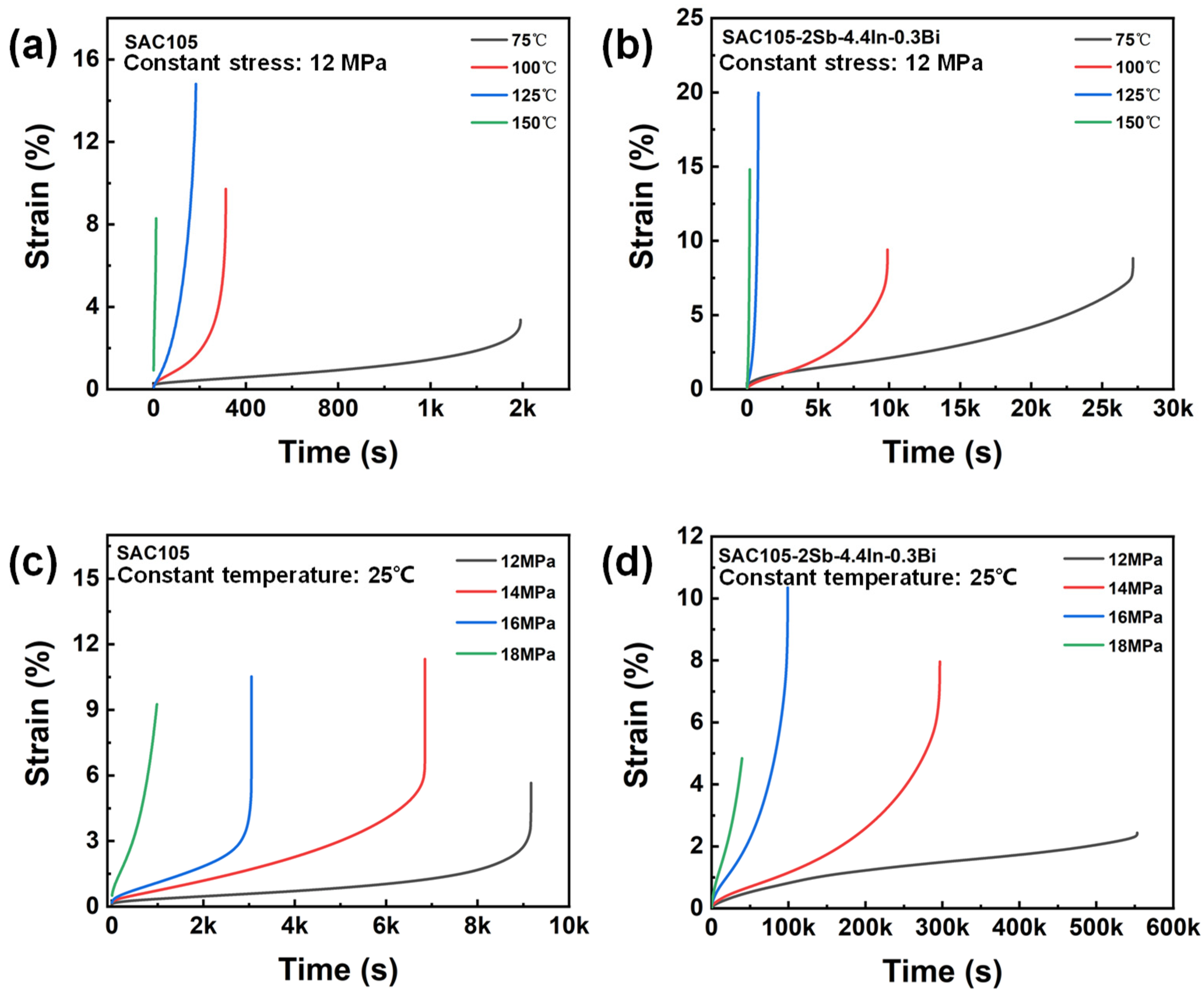
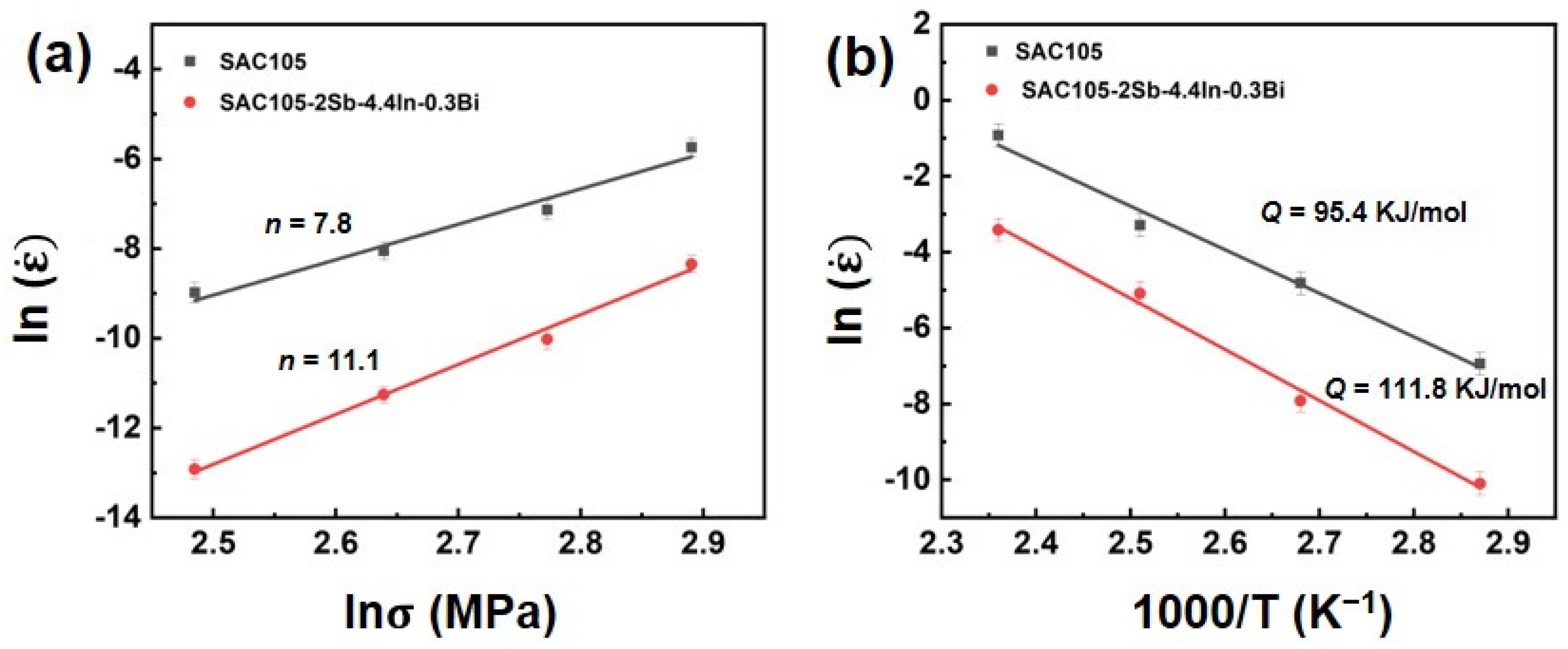
3.5. Creep Performance
4. Conclusions
- When Sb, In, Ni, and Bi were added to SAC105, the microstructure of solder alloys was refined. Sb and Bi can dissolve into the Sn matrix without forming any precipitations, which has a solid solution strengthening effect. The eutectic zones composed of Ag3Sn and Cu6Sn5 were refined and distributed more evenly in the SAC105-2Sb-4.4In-0.3Bi as compared with other compositions, implying Bi shows a better refinement effect.
- By adding 2 wt.% Sb and 4.4 wt.% In, the tensile strength of SAC105 was increased from 27.9 MPa to 36.8 MPa. When 0.3 wt.% Ni was further added to SAC105, the tensile strength can be further increased to 54.4 MPa, which indicates that Ni has a significant effect on the improvement of the solder alloy. When Bi substitutes Ni gradually, the tensile strength was further improved. It was found that SAC105-2Sb-4.4In-0.3Bi alloy owns the highest strength ~64.8 MPa which is approximately 185% higher than that of SAC105. At the same time, the elongation of SAC105-2Sb-4.4In-0.3Bi remains at an acceptable level (>20%), which meets the actual production requirements.
- The alloying elements could improve the melting properties by considerably reducing the undercooling of solder alloys. It was found that SAC105-2Sb-4.4In-0.3Bi alloy has the smallest undercooling ~1.0 °C which is much smaller than that of SAC105. Although its pasty range was enlarged inevitably, the pasty range of SAC105-2Sb-4.4In-0.3Bi increased less as compared with other solder alloys, showing a relatively good melting performance.
- The wettability test conducted on the Cu substrate shows that the addition of Sb, In, and Bi can improve the wettability of the SAC105 alloy. The wetting angle of SAC105 is 36.6°, while the wetting angle of SAC105-2Sb-4.4In-0.3Bi decreases to 34.7°. Moreover, the addition of Bi has a stronger effect on improving wettability than Ni.
- Adding Sb, In, and Bi to SAC105 can improve the creep resistance significantly. Through DMA creep testing, it was found that SAC105-2Sb-4.4In-0.3Bi has a better creep resistance as compared with SAC105. This can be attributed to the synergistic alloying effect of Sb, In, and Bi, which improves the creep resistance through solid solution strengthening and precipitation strengthening mechanisms.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.Y.; Chen, C.; Tu, K.N. Electromigration in Sn–Pb solder strips as a function of alloy composition. J. Appl. Phys. 2000, 88, 5703–5709. [Google Scholar] [CrossRef]
- Boyce, B.L.; Brewer, L.N.; Neilsen, M.K.; Perricone, M.J. On the strain rate and temperature dependent tensile behavior of eutectic Sn–Pb solder. J. Electron. Packag. 2011, 133, 31009–31023. [Google Scholar] [CrossRef]
- The European Parliament. Directive 2002/95/EC of the European Parliament and of the Council of 27 Jan 2003 on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment. Off. J. Eur. Union. 2003, L37/19. [Google Scholar]
- Chen, G.; Wang, X.H.; Yang, J.; Xu, W.L.; Lin, Q. Effect of micromorphology on corrosion and mechanical properties of SAC305 lead-free solders. Microelectron Reliab. 2020, 108, 113634. [Google Scholar] [CrossRef]
- Liang, J.; Dariavach, N.; Shangguan, D. Solidification condition effects on microstructures and creep resistance of Sn3.8Ag0.7Cu lead-free solder. Metall. Mater. Trans. A 2007, 38, 1530–1538. [Google Scholar] [CrossRef]
- Neilsen, M.; Vianco, P. Unified creep plasticity damage (UCPD) model for SAC396 solder. J. Phys. 2011, 57, 71–93. [Google Scholar]
- Lin, F.; Bi, W.; Ju, G.; Wang, W.; Wei, X. Evolution of Ag3Sn at Sn-3.0Ag-0.3Cu-0.05Cr/Cu joint interfaces during thermal aging. J. Alloys Compd. 2011, 509, 6666–6672. [Google Scholar] [CrossRef]
- Chen, W.Y.; Yu, C.Y.; Duh, J.G. Suppressing the growth of interfacial Cu-Sn intermetallic compounds in the Sn-3.0Ag-0.5Cu-0.1Ni/Cu-15Zn solder joint during thermal aging. J. Mater. Sci. 2012, 47, 4012–4018. [Google Scholar] [CrossRef]
- Ju, G.; Lin, F.; Bi, W.; Han, Y.; Junjie, W.; Wei, X. Study of IMC at interfaces of Sn3.0Ag0.5Cu3.0Bi0.05Cr/Cu joints during thermal aging. Solder. Surf. Mt. Technol. 2014, 26, 173–179. [Google Scholar] [CrossRef]
- Sun, F.; Hochstenbach, P.; Driel, W.; Zhang, G.Q. Fracture morphology and mechanism of IMC in Low-Ag SAC Solder/UBM (Ni(P)-Au) for WLCSP. Microelectron. Reliab. 2008, 48, 1167–1170. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Fawzy, A.; Mansour, S.F.; Younis, M.J. Novel SiC nanoparticles containing Sn-1.0Ag-0.5Cu solder with good drop impact performance. Mater. Sci. Eng. A 2013, 578, 62–71. [Google Scholar] [CrossRef]
- Hammad, A.E. Evolution of microstructure, thermal and creep properties of Ni-doped Sn-0.5Ag-0.7Cu low-Ag solder alloys for electronic applications. Mater. Des. 2013, 52, 663–670. [Google Scholar] [CrossRef]
- Hammad, A.E. Investigation of microstructure and mechanical properties of novel Sn-0.5Ag-0.7Cu solders containing small amount of Ni. Mater. Des. 2013, 50, 108–116. [Google Scholar] [CrossRef]
- Terashima, S.; Tanaka, M.; Tatsumi, K. Thermal fatigue properties and grain boundary character distribution in Sn-xAg-0.5Cu (x = 1, 1.2 and 3) lead free solder interconnects. Sci. Technol. Weld. Jol. 2008, 13, 61–65. [Google Scholar]
- Gao, L.L.; Xue, S.B.; Zhang, L.; Sheng, Z.; Ji, F.; Dai, W.; Yu, S.L.; Zeng, G. Effect of alloying elements on properties and microstructures of Sn-Ag-Cu solders. Microelectron. Eng. 2010, 87, 2025–2034. [Google Scholar] [CrossRef]
- Terashima, S.; Kariya, Y.; Hosoi, T.; Tanaka, M. Effect of silver content on thermal fatigue life of Sn-xAg-0.5Cu flip-chip interconnects. J. Electron. Mater. 2003, 32, 1527–1533. [Google Scholar] [CrossRef]
- Wang, Y.W.; Lin, Y.W.; Tu, C.T.; Kao, C.R. Effects of minor Fe, Co, and Ni additions on the reaction between SAC solder and Cu. J. Alloys Compd. 2009, 478, 121–127. [Google Scholar] [CrossRef]
- Witkin, D.B. Influence of microstructure on quasi-static and dynamic mechanical properties of bismuth-containing lead-free solder alloys. Mater. Sci. Eng. A 2012, 532, 212–220. [Google Scholar] [CrossRef]
- Kanlayasiri, K.; Mongkolwongrojn, M.; Ariga, T. Influence of indium addition on characteristics of Sn-0.3Ag-0.7Cu solder alloy. J. Alloys Compd. 2009, 485, 225–230. [Google Scholar] [CrossRef]
- Giuranno, D.; Delsante, S.; Borzone, G.; Novakovic, R. Effects of Sb addition on the properties of Sn-Ag-Cu/(Cu, Ni) solder systems. J. Alloys Compd. 2016, 689, 918–930. [Google Scholar] [CrossRef]
- Soares, D.; Sarmento, M.; Barros, D.; Peixoto, H.; Cerqueira, F. The effect of Bi addition on the electrical and microstructural properties of SAC405 soldered structure. Solder Surf. Mt. Technol. 2021, 33, 18–36. [Google Scholar] [CrossRef]
- Fine, M. Creep of tin, Sb-solution-strengthened tin, and Sb-Sn precipitate strengthened tin. Metall. Mater. Trans. 2002, 33, 1531–1539. [Google Scholar]
- Li, C.; Yan, Y.; Gao, T.; Xu, G. The microstructure, thermal, and mechanical properties of Sn-3.0Ag-0.5Cu-xSb high-temperature lead-free solder. Materials 2020, 13, 4443. [Google Scholar] [CrossRef]
- Chen, B.L.; Li, G.Y. Influence of Sb on IMC growth in Sn-Ag-Cu-Sb Pb-free solder joints in reflow process. Thin Solid Films 2004, 462, 395–401. [Google Scholar] [CrossRef]
- Korhonen, K.J. Thermodynamics of the Sn-In-Ag Solder system. J. Electron. Mater. 1998, 27, 149–158. [Google Scholar] [CrossRef]
- Huang, M.L. Effects of Cu, Bi, and In on microstructure and tensile properties of Sn-Ag-X (Cu, Bi, In) solders. Metall. Mater. Trans. 2005, 36, 1439–1446. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Hammad, A.E.; Fawzy, A.; Nasrallh, D.A. Microstructure, mechanical properties, and deformation behavior of Sn-1.0Ag-0.5Cu solder after Ni and Sb additions. Mater. Des. 2013, 43, 40–49. [Google Scholar] [CrossRef]
- Wang, C.H.; Shen, H.T. Effects of Ni addition on the interfacial reactions between Sn-Cu solders and Ni substrate. Intermetallics 2010, 18, 616–622. [Google Scholar] [CrossRef]
- Qi, L.; Huang, J.; Zhao, X.; Zhang, H. Growth behavior of compounds at Sn-3.5Ag-0.5Cu/Ni under thermal-shearing cycling. Trans. China Weld. Inst. 2007, 28, 61–64. [Google Scholar]
- Sharif, A.; Islam, M.N.; Chan, Y.C. Interfacial reactions of BGA Sn-3.5%Ag-0.5%Cu and Sn-3.5%Ag solders during high-temperature aging with Ni/Au metallization. Mater. Sci. Eng. 2004, 113, 184–189. [Google Scholar] [CrossRef]
- Hammad, A.E. Enhancing the ductility and mechanical behavior of Sn-1.0Ag-0.5Cu lead-free solder by adding trace amount of elements Ni and Sb. Microelectron. Reliab. 2018, 87, 133–141. [Google Scholar] [CrossRef]
- Kamal, M.; Gouda, E.S.; Marei, L.K. Effect of Bi-content on hardness and micro-creep behavior of Sn-3.5Ag rapidly solidified alloy. J. Exp. Ind. Crystallogr. 2010, 44, 1308–1312. [Google Scholar] [CrossRef]
- Ali, B.; Sabri, M.; Said, S.M.; Sukiman, N.L.; Jauhari, I.; Mahdavifard, M.H. Microstructural and tensile properties of Fe and Bi added Sn-1Ag-0.5Cu solder alloy under high temperature environment. Microelectron. Reliab. 2018, 82, 171–178. [Google Scholar] [CrossRef]
- Ali, B.; Sabri, M.; Jauhari, I.; Sukiman, N.L. Impact toughness, hardness and shear strength of Fe and Bi added Sn-1Ag-0.5Cu lead-free solders. Microelectron. Reliab. 2016, 63, 224–230. [Google Scholar] [CrossRef]
- Vianco, P.; Rejent, J. Properties of ternary Sn-Ag-Bi solder alloys: Part ii—Wettability and mechanical properties analyses. J. Electron. Mater. 1999, 28, 1138–1143. [Google Scholar] [CrossRef]
- Hu, X.W.; Li, Y.L.; Liu, Y.; Min, Z.X. Developments of high strength Bi-containing Sn0.7Cu lead-free solder alloys prepared by directional solidification. J. Alloys Compd. 2015, 625, 241–250. [Google Scholar] [CrossRef]
- Suganuma, K. Advances in lead-free electronics soldering. Mater. Sci. 2002, 5, 55–64. [Google Scholar] [CrossRef]
- Pu, C.J.; Qiu, J.L.; Li, C.J.; Gao, P.; Peng, Y.Z.; He, Q.; Bai, H.L.; Yi, J.H. Effects of Bi addition on the solderability and mechanical properties of Sn-Zn-Cu lead-free Solder. J. Electron. Mater. 2022, 51, 4952–4963. [Google Scholar] [CrossRef]
- Tao, Q.B.; Benabou, L.; Vivet, L.; Le, V.N.; Ouezdou, F.B. Effect of Ni and Sb additions and testing conditions on the mechanical properties and microstructures of lead-free solder joints. MSEA 2016, 669, 403–416. [Google Scholar] [CrossRef]
- Kuo, J.C. Effects of bismuth additions on mechanical property and microstructure of SAC-Bi solder joint under current stressing. Microelectron. Reliab. 2021, 117, 114–141. [Google Scholar]
- Kariya, Y.; Otsuka, M. Mechanical fatigue characteristics of Sn-3.5Ag-X (X = Bi, Cu, Zn and In) solder alloys. J. Electron. Mater. 1998, 27, 1229–1235. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, K.N. Structure and properties of lead-free solders bearing micro and nano particles. Mater. Sci. Eng. R 2014, 82, 1–32. [Google Scholar] [CrossRef]
- Erer, A.M.; Oguz, S.; Türen, Y. Influence of bismuth (Bi) addition on wetting characteristics of Sn-3Ag-0.5Cu solder alloy on Cu substrate. Eng. Sci. Technol. Int. J. 2018, 21, 1159–1163. [Google Scholar] [CrossRef]
- Chou, T.T.; Song, R.W.; Chen, W.Y.; Duh, J.G. Enhancement of the mechanical strength of Sn-3.0Ag-0.5Cu/Ni joints via doping minor Ni into solder alloy. Mater. Lett. 2019, 235, 180–183. [Google Scholar] [CrossRef]
- Yang, Z.B.; Zhou, W.; Wu, P. Effects of Ni-coated carbon nanotubes addition on the microstructure and mechanical properties of Sn-Ag-Cu solder alloys. Mater. Sci. Eng. A 2014, 590, 295–300. [Google Scholar] [CrossRef]
- Moriuchi, M.; Kariya, Y.; Kondo, M.; Kanda, Y. Class I creep deformation of Sn-Ag-Cu containing solid solution elements and its effect on thermal fatigue life of solder joints. Mater. Trans. 2022, 63, 805–812. [Google Scholar] [CrossRef]
- Shen, L.; Septiwerdani, P.; Chen, Z. Elastic modulus, hardness and creep performance of SnBi alloys using nanoindentation. Mater. Sci. Eng. A 2012, 53, 2811–2822. [Google Scholar] [CrossRef]
- Sayyadi, R.; Naffakh-Moosavy, H. Physical and mechanical properties of synthesized low Ag/lead-free Sn-Ag-Cu-xBi (x = 0, 1,2.5, 5 wt%) solders. Mater. Sci. Eng. A 2018, 735, 367–377. [Google Scholar] [CrossRef]
- Wernicki, E.; Gu, Z. Effect of Sn nanoparticle additions on thermal properties of Sn-Ag-Cu lead-free solder paste. Thermochim. Acta 2020, 690, 178–642. [Google Scholar] [CrossRef]
- Uenishi, K.; Kohara, Y.; Sakatani, S.; Saeki, T.; Kobayashi, K.F.; Yamamoto, M. Lead-free electronics packaging: Melting and joining behavior of Sn/Ag and SnAg/Sn-Bi plating on Cu core ball. Mater. Trans. 2002, 43, 1833–1839. [Google Scholar] [CrossRef]
- El-Daly, A.A.; El-Taher, A.M.; Gouda, S. Novel Bi-containing Sn-1.5Ag-0.7Cu lead-free solder alloy with further enhanced thermal property and strength for mobile products. Mater. Des. 2015, 65, 796–805. [Google Scholar] [CrossRef]
- Celikin, M.; Maalekian, M.; Pekguleryuz, M. Effect of Bi additions on the creep behaviour of SAC solder alloys. J. Electron. Mater. 2018, 47, 5842–5849. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, K.N. Low melting point solders based on Sn, Bi, and In elements. Mater. Today Adv. 2020, 8, 100–115. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chan, Y.T.; Chen, C.C. Wettability and interfacial reactions between the molten Sn-3.0wt%Ag-0.5wt%Cu solder (SAC305) and Ni-Co alloys. J. Alloys Compd. 2010, 507, 419–424. [Google Scholar] [CrossRef]
- Hasnine, M.; Tolla, B.; Karasawa, M. Effect of Ge addition on wettability, copper dissolution, microstructural and mechanical behavior of SnCu–Ge solder alloy. J. Mater. Sci.-Mater. Electron. 2017, 28, 16106–16119. [Google Scholar] [CrossRef]
- Golan, O.; Arbel, A.; Eliezer, D.; Moreno, D. The applicability of norton’s creep power law and its modified version to a single-crystal superalloy type cmsx-2. Mater. Sci. Eng. A 1996, 216, 125–130. [Google Scholar] [CrossRef]
- Khodabakhshi, F.; Zareghomsheh, M.; Khatibi, G. Nanoindentation creep properties of lead-free nanocomposite solders reinforced by modified carbon nanotubes. Mater. Sci. Eng. A 2020, 797, 140203–140218. [Google Scholar] [CrossRef]
- Kassner, M.E. Fundamentals of Creep in Metals and Alloys; Elsevier: Amsterdam, The Netherlands, 2014; Volume 7, pp. 301–332. [Google Scholar]
- Dutta, I.; Park, C.; Choi, S. Impression creep characterization of rapidly cooled Sn-3.5Ag solders. Mater. Sci. Eng. A 2004, 379, 401–410. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Al-Ganainy, G.S.; Fawzy, A.; Younis, M.J. Structural characterization and creep resistance of nano-silicon carbide reinforced Sn-1.0Ag-0.5Cu lead-free solder alloy. Mater. Des. 2014, 55, 837–845. [Google Scholar] [CrossRef]



| Alloy (wt.%) | Ag | Cu | Sb | In | Ni | Bi | Sn |
|---|---|---|---|---|---|---|---|
| SAC105 | 1.0 | 0.5 | 2.0 | 4.4 | Bal. | ||
| SAC105-2Sb | 1.0 | 0.5 | 2.0 | Bal. | |||
| SAC105-4.4In | 1.0 | 0.5 | 4.4 | Bal. | |||
| SAC105-2Sb-4.4In | 1.0 | 0.5 | 2.0 | 4.4 | Bal. | ||
| SAC105-2Sb-4.4In-0.3Ni | 1.0 | 0.5 | 2.0 | 4.4 | 0.3 | Bal. | |
| SAC105-2Sb-4.4In-0.2Ni-0.1Bi | 1.0 | 0.5 | 2.0 | 4.4 | 0.2 | 0.1 | Bal. |
| SAC105-2Sb-4.4In-0.1Ni-0.2Bi | 1.0 | 0.5 | 2.0 | 4.4 | 0.1 | 0.2 | Bal. |
| SAC105-2Sb-4.4In-0.3Bi | 1.0 | 0.5 | 2.0 | 4.4 | 0.3 | Bal. |
| Component (wt.%) | UTS (MPa) | YS (MPa) | Elongation (%) |
|---|---|---|---|
| SAC105 | 22.7 ± 2.6 | 13.8 ± 1.8 | 47.1 ± 2.1 |
| SAC105-2Sb | 27.9 ± 4.9 | 20.1 ± 3.7 | 45.4 ± 4.4 |
| SAC105-4.4In | 49.6 ± 3.2 | 45.2 ± 2.9 | 36.4 ± 3.8 |
| SAC105-2Sb-4.4In | 36.8 ± 5.5 | 33.6 ± 4.3 | 43.4 ± 6.2 |
| SAC105-2Sb-4.4In-0.3Ni | 54.0 ± 3.4 | 37.9 ± 3.6 | 43.4 ± 4.5 |
| SAC105-2Sb-4.4In-0.2Ni-0.1Bi | 54.5 ± 4.9 | 39.1 ± 3.9 | 40.8 ± 3.7 |
| SAC105-2Sb-4.4In-0.1Ni-0.2Bi | 63.1 ± 2.8 | 41.4 ± 2.3 | 26.1 ± 2.9 |
| SAC105-2Sb-4.4In-0.3Bi | 64.8 ± 3.1 | 50.2 ± 2.6 | 25.0 ± 2.4 |
| Elements | Sn | Sb | In | Ni | Bi |
|---|---|---|---|---|---|
| Atomic radius (nm) | 0.158 | 0.160 | 0.166 | 0.124 | 0.170 |
| Alloys | Heating Tonset (°C) | Heating Tpeak (°C) | Cooling Tonset (°C) | Undercooling (°C) | Pasty Range (°C) |
|---|---|---|---|---|---|
| SAC105 | 211.5 ± 3.1 | 221.9 ± 1.8 | 194.0 ± 3.2 | 17.5 ± 1.9 | 10.4 ± 2.1 |
| SAC105-2Sb-4.4In-0.3Ni | 205.6 ± 2.5 | 224.7 ± 2.7 | 202.5 ± 2.4 | 3.1 ± 2.4 | 19.1 ± 3.7 |
| SAC105-2Sb-4.4In-0.2Ni-0.1Bi | 205.1 ± 2.9 | 224.5 ± 2.1 | 212.0 ± 3.7 | 6.9 ± 1.7 | 19.5 ± 4.5 |
| SAC105-2Sb-4.4In-0.1Ni-0.2Bi | 204.6 ± 3.4 | 223.7 ± 3.7 | 202.5 ± 2.1 | 2.1 ± 1.3 | 19.2 ± 3.2 |
| SAC105-2Sb-4.4In-0.3Bi | 202.0 ± 2.3 | 220.9 ± 2.6 | 201.0 ± 3.2 | 1.0 ± 0.8 | 18.9 ± 2.9 |
| Temperature (°C) | SAC105 | SAC105-2Sb-4.4In-0.3Bi | ||
|---|---|---|---|---|
| Creep Rate (s−1) | Creep Life (s) | Creep Rate (s−1) | Creep Life (s) | |
| 75 | 9.6 × 10−4 ± 3.1 × 10−8 | 1.6 × 103 ± 4.1 × 101 | 4.1 × 10−5 ± 9.2 × 10−8 | 2.7 × 104 ± 8.9 × 102 |
| 100 | 8.1 × 10−3 ± 5.7 × 10−7 | 3.1 × 102 ± 3.7 × 101 | 3.6 × 10−4 ± 7.9 × 10−7 | 9.9 × 103 ± 6.3 × 101 |
| 125 | 3.7 × 10−2 ± 6.4 × 10−5 | 1.8 × 102 ± 9.1 × 100 | 6.1 × 10−3 ± 4.2 × 10−5 | 7.9 × 102 ± 7.7 × 100 |
| 150 | 3.9 × 10−1 ± 4.9 × 10−3 | 1.1 × 101 ± 0.5 × 100 | 3.3 × 10−2 ± 3.7 × 10−4 | 1.8 × 102 ± 5.6 × 100 |
| Stress (MPa) | SAC105 | SAC105-2Sb-4.4In-0.3Bi | ||
|---|---|---|---|---|
| Creep Rate (s−1) | Creep Life (s) | Creep Rate (s−1) | Creep Life (s) | |
| 12 | 1.3 × 10−4 ± 4.2 × 10−8 | 9.2 × 103 ± 7.3 × 101 | 2.5 × 10−6 ± 5.1 × 10−10 | 5.5 × 105 ± 4.7 × 102 |
| 14 | 3.2 × 10−4 ± 4.9 × 10−7 | 6.9 × 103 ± 5.6 × 101 | 1.3 × 10−5 ± 2.1 × 10−8 | 2.9 × 105 ± 2.9 × 102 |
| 16 | 7.9 × 10−4 ± 4.1 × 10−7 | 3.1 × 103 ± 2.9 × 101 | 4.5 × 10−5 ± 6.7 × 10−8 | 9.9 × 104 ± 2.3 × 102 |
| 18 | 3.2 × 10−3 ± 3.4 × 10−5 | 9.8 × 102 ± 8.7 × 100 | 2.4 × 10−4 ± 5.2 × 10−8 | 3.9 × 104 ± 1.5 × 101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Bian, X.; Qiu, X.; Jia, Y.; Yi, J.; Wang, G. Investigation of Microstructure and Mechanical Properties of SAC105 Solders with Sb, In, Ni, and Bi Additions. Materials 2023, 16, 4059. https://doi.org/10.3390/ma16114059
Gao Y, Bian X, Qiu X, Jia Y, Yi J, Wang G. Investigation of Microstructure and Mechanical Properties of SAC105 Solders with Sb, In, Ni, and Bi Additions. Materials. 2023; 16(11):4059. https://doi.org/10.3390/ma16114059
Chicago/Turabian StyleGao, Yaxin, Xilei Bian, Xingbao Qiu, Yandong Jia, Jun Yi, and Gang Wang. 2023. "Investigation of Microstructure and Mechanical Properties of SAC105 Solders with Sb, In, Ni, and Bi Additions" Materials 16, no. 11: 4059. https://doi.org/10.3390/ma16114059
APA StyleGao, Y., Bian, X., Qiu, X., Jia, Y., Yi, J., & Wang, G. (2023). Investigation of Microstructure and Mechanical Properties of SAC105 Solders with Sb, In, Ni, and Bi Additions. Materials, 16(11), 4059. https://doi.org/10.3390/ma16114059





