Recent Advances in Magnetic Polymer Composites for BioMEMS: A Review
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. Evaluation Criteria
2.3. Searching Results
3. Recent Advances in Magnetic Polymer Composites
3.1. Self-Healing Magnetic Polymer Composites
3.2. Shape-Memory Magnetic Polymer Composites
3.3. Biodegradable Magnetic Polymer Composites
4. Recent Advances in Magnetic Polymer Composite-Based Biomedical MEMS
4.1. Magnetic Polymer Composite-Based Microactuators
4.2. Magnetic Polymer Composite-Based Micropumps
4.3. Magnetic Polymer Composite-Based Microvalves
4.4. Magnetic Polymer Composite-Based Micromixers
4.5. Magnetic Polymer Composite-Based Sensors
4.6. Other Magnetic Polymer Composite-Based Soft Robots
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perigo, E.A.; Weidenfeller, B.; Kollár, P.; Füzer, J. Past, present, and future of soft magnetic composites. Appl. Phys. Rev. 2018, 5, 031301. [Google Scholar] [CrossRef]
- Shie, M.-Y.; Shen, Y.-F.; Astuti, S.D.; Lee, A.K.-X.; Lin, S.-H.; Dwijaksara, N.L.B.; Chen, Y.-W. Review of polymeric materials in 4D printing biomedical applications. Polymers 2019, 11, 1864. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chung, S.E.; Choi, S.-E.; Lee, H.; Kim, J.; Kwon, S. Programming magnetic anisotropy in polymeric microactuators. Nat. Mater. 2011, 10, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A review of shape memory polymers and composites: Mechanisms, materials, and applications. Adv. Mater. 2021, 33, 2000713. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Heidarian, P.; Kouzani, A.Z. Starch-g-Acrylic Acid/Magnetic Nanochitin Self-Healing Ferrogels as Flexible Soft Strain Sensors. Sensors 2023, 23, 1138. [Google Scholar] [CrossRef]
- Cerdan, K.; Moya, C.; Van Puyvelde, P.; Bruylants, G.; Brancart, J. Magnetic self-healing composites: Synthesis and applications. Molecules 2022, 27, 3796. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, P.; Footer, C.; Stenning, G.B.; Darr, J.A.; Pancholi, K. Tuneable magnetic nanocomposites for remote self-healing. Sci. Rep. 2022, 12, 10180. [Google Scholar] [CrossRef]
- Tao, H.Q.; Yue, D.W.; Li, C.H. A Fast Self-Healing Magnetic Nanocomposite for Magnetic Actuators. Macromol. Mater. Eng. 2022, 307, 2100649. [Google Scholar] [CrossRef]
- Liu, K.; Pan, X.; Chen, L.; Huang, L.; Ni, Y.; Liu, J.; Cao, S.; Wang, H. Ultrasoft self-healing nanoparticle-hydrogel composites with conductive and magnetic properties. ACS Sustain. Chem. Eng. 2018, 6, 6395–6403. [Google Scholar] [CrossRef]
- Huang, J.; Cao, L.; Yuan, D.; Chen, Y. Design of novel self-healing thermoplastic vulcanizates utilizing thermal/magnetic/light-triggered shape memory effects. ACS Appl. Mater. Interfaces 2018, 10, 40996–41002. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zeng, Y.; Liu, G.; Tian, Y.; Wei, Y.; Zhao, L.; Yang, L.; Tao, L.J.A.M.L. Magnetic Self-Healing Hydrogel from Difunctional Polymers Prepared via the Kabachnik–Fields Reaction. ACS Macro Lett. 2021, 11, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Nardecchia, S.; Jimenez, A.; Morillas, J.R.; de Vicente, J. Synthesis and rheological properties of 3D structured self-healing magnetic hydrogels. Polymer 2021, 218, 123489. [Google Scholar] [CrossRef]
- Gang, F.; Yan, H.; Ma, C.; Jiang, L.; Gu, Y.; Liu, Z.; Zhao, L.; Wang, X.; Zhang, J.; Sun, X. Robust magnetic double-network hydrogels with self-healing, MR imaging, cytocompatibility and 3D printability. Chem. Commun. 2019, 55, 9801–9804. [Google Scholar] [CrossRef] [PubMed]
- Sanoh, N.C.; Salazar, G.M.; Penaloza, D. Magnetic biopolymeric hydrogel composite material with self-healing attribute. Biointerface Res. Appl. Chem 2021, 11, 14881–14888. [Google Scholar]
- Cheng, Y.; Chan, K.H.; Wang, X.Q.; Ding, T.; Li, T.; Zhang, C.; Lu, W.; Zhou, Y.; Ho, G.W. A fast autonomous healing magnetic elastomer for instantly recoverable, modularly programmable, and thermorecyclable soft robots. Adv. Funct. Mater. 2021, 31, 2101825. [Google Scholar] [CrossRef]
- Charlet, A.; Lutz-Bueno, V.; Mezzenga, R.; Amstad, E. Shape retaining self-healing metal-coordinated hydrogels. Nanoscale 2021, 13, 4073–4084. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wen, Y.; Zhong, Q.; Zhao, Y. Self-healable magnetic structural color hydrogels. ACS Appl. Mater. Interfaces 2020, 12, 7486–7493. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, D.; Ter-Yesayants, T.; Moreno-Mateos, M.A.; Lopez-Donaire, M.L. Hard-magnetic phenomena enable autonomous self-healing elastomers. Compos. Part B Eng. 2023, 248, 110357. [Google Scholar] [CrossRef]
- Cerdan, K.; Van Assche, G.; Van Puyvelde, P.; Brancart, J. A novel approach for the closure of large damage in self-healing elastomers using magnetic particles. Polymer 2020, 204, 122819. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Yin, Y.; Qi, H. A Facile Strategy to Fabricate Polysaccharide-Based Magnetic Hydrogel Based on Enamine Bond. Chin. J. Chem. 2020, 38, 1263–1268. [Google Scholar] [CrossRef]
- Chen, X.; Fan, M.; Tan, H.; Ren, B.; Yuan, G.; Jia, Y.; Li, J.; Xiong, D.; Xing, X.; Niu, X.; et al. Magnetic and self-healing chitosan-alginate hydrogel encapsulated gelatin microspheres via covalent cross-linking for drug delivery. Mater. Sci. Eng. C 2019, 101, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.V.; Madras, G.; Bose, S. Mussel-inspired self-healing polyurethane with “flower-like” magnetic MoS2 as efficient microwave absorbers. ACS Appl. Polym. Mater. 2019, 1, 2417–2429. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Smirnova, M.E.; Kessel, D.E.; Bedin, S.A.; Razumovskaya, I.V.; Philippova, O.E. Remotely self-healable, shapeable and pH-sensitive dual cross-linked polysaccharide hydrogels with fast response to magnetic field. Nanomaterials 2021, 11, 1271. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, M.; Lv, L.; Fu, H. Compounds. Self-healing, conductive and magnetic ZnFe2O4/MCNT/PPy ternary composite hydrogels. J. Alloy. Compd. 2021, 886, 161083. [Google Scholar] [CrossRef]
- Zheng, W.; Li, Y.; Wei, H.; Gao, G.; Zhang, D.; Jiang, Z. Rapidly self-healing, magnetically controllable, stretchable, smart, moldable nanoparticle composite gel. New J. Chem. 2020, 44, 10586–10591. [Google Scholar] [CrossRef]
- Zhao, B.; Li, S.; Liao, X.; Li, J.; Ma, W.; Dong, Y.; Zhou, X.; Liu, Y. Synthesis and Properties of Magnetic Self-Healing Polymers: An Effective Method for Improving Interface Compatibility of Doped Functional Polymers. Chemnanomat 2019, 5, 642–650. [Google Scholar] [CrossRef]
- Ko, E.S.; Kim, C.; Choi, Y.; Lee, K.Y. 3D printing of self-healing ferrogel prepared from glycol chitosan, oxidized hyaluronate, and iron oxide nanoparticles. Carbohydr. Polym. 2020, 245, 116496. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Q.; Su, G.; Cao, J.; Liu, J.; Zhang, X. Hierarchically structured self-healing actuators with superfast light-and magnetic-response. Adv. Funct. Mater. 2019, 29, 1906198. [Google Scholar] [CrossRef]
- Lalegani Dezaki, M.; Bodaghi, M. Soft magneto-responsive shape memory foam composite actuators. Macromol. Mater. Eng. 2022, 307, 2200490. [Google Scholar] [CrossRef]
- Chen, X.; He, Y. Thermo-magneto-mechanical coupling dynamics of magnetic shape memory alloys. Int. J. Plast. 2020, 129, 102686. [Google Scholar] [CrossRef]
- Hu, T.; Xuan, S.; Shu, Q.; Xu, Z.; Shen, L.; Li, J.; Gong, X. Triple-shape memory, magneto-response, and piezo-resistive flexible composites: Multiple-sensing and switchable actuating. J. Mater. Chem. C 2021, 9, 6568–6578. [Google Scholar] [CrossRef]
- Calvo-Correas, T.; Shirole, A.; Crippa, F.; Fink, A.; Weder, C.; Corcuera, M.A.; Eceiza, A. Biocompatible thermo-and magneto-responsive shape-memory polyurethane bionanocomposites. Mater. Sci. Eng. C 2019, 97, 658–668. [Google Scholar] [CrossRef]
- Hu, T.; Xuan, S.; Gao, Y.; Shu, Q.; Xu, Z.; Sun, S.; Li, J.; Gong, X. Smart Refreshable Braille Display Device Based on Magneto-Resistive Composite with Triple Shape Memory. Adv. Mater. Technol. 2022, 7, 2100777. [Google Scholar] [CrossRef]
- Yue, C.; Li, M.; Liu, Y.; Fang, Y.; Song, Y.; Xu, M.; Li, J. Three-dimensional printing of cellulose nanofibers reinforced PHB/PCL/Fe3O4 magneto-responsive shape memory polymer composites with excellent mechanical properties. Addit. Manuf. 2021, 46, 102146. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Wu, W.; Dong, X.; Sang, L. 4D printing of mechanically robust PLA/TPU/Fe3O4 magneto-responsive shape memory polymers for smart structures. Compos. Part B Eng. 2023, 248, 110382. [Google Scholar] [CrossRef]
- Lalegani Dezaki, M.; Bodaghi, M. Sustainable 4D printing of magneto-electroactive shape memory polymer composites. Int. J. Adv. Manuf. Technol. 2023, 126, 1–14. [Google Scholar] [CrossRef]
- Moghim, M.H.; Zebarjad, S.M.; Eqra, R. Effect of Fe3O4 nanoparticles on magneto-responsive shape memory behavior of polyurethane-carbon nanotube nanocomposites. J. Polym. Res. 2022, 29, 1–10. [Google Scholar] [CrossRef]
- Ze, Q.; Kuang, X.; Wu, S.; Wong, J.; Montgomery, S.M.; Zhang, R.; Kovitz, J.M.; Yang, F.; Qi, H.J.; Zhao, R. Magnetic shape memory polymers with integrated multifunctional shape manipulation. Adv. Mater. 2020, 32, 1906657. [Google Scholar] [CrossRef]
- Du, L.; Xu, Z.-Y.; Fan, C.-J.; Xiang, G.; Yang, K.-K.; Wang, Y.-Z. A fascinating metallo-supramolecular polymer network with thermal/magnetic/light-responsive shape-memory effects anchored by Fe3O4 nanoparticles. Macromolecules 2018, 51, 705–715. [Google Scholar] [CrossRef]
- Li, C.; Jiao, Y.; Zhang, Y.; Jiang, S.; Lv, X.; Wu, S.; Li, J.; Hu, Y.; Ye, J.; Liu, K. Noncontact All-In-Situ Reversible Reconfiguration of Femtosecond Laser-Induced Shape Memory Magnetic Microcones for Multifunctional Liquid Droplet Manipulation and Information Encryption. Adv. Funct. Mater. 2021, 31, 2100543. [Google Scholar] [CrossRef]
- Liu, J.A.C.; Evans, B.A.; Tracy, J.B. Photothermally reconfigurable shape memory magnetic cilia. Adv. Mater. Technol. 2020, 5, 2000147. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, Z.; Liu, L.; Wang, W.; Leng, J.; Liu, Y. Technology. Porous bone tissue scaffold concept based on shape memory PLA/Fe3O4. Compos. Sci. Technol. 2021, 203, 108563. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.; Yuan, D.; Chen, Y. Design of a high-strength XSBR/Fe3O4/ZDMA shape-memory composite with dual responses. Ind. Eng. Chem. Res. 2018, 57, 14527–14534. [Google Scholar] [CrossRef]
- Leungpuangkaew, S.; Amornkitbamrung, L.; Phetnoi, N.; Sapcharoenkun, C.; Jubsilp, C.; Ekgasit, S.; Rimdusit, S. Magnetic-and Light-Responsive Shape Memory Polymer Nanocomposites from Bio-Based Benzoxazine Resin and Iron Oxide Nanoparticles. Adv. Ind. Eng. Polym. Res. 2023, in press. [CrossRef]
- Liu, J.A.-C.; Gillen, J.H.; Mishra, S.R.; Evans, B.A.; Tracy, J.B. Photothermally and magnetically controlled reconfiguration of polymer composites for soft robotics. Sci. Adv. 2019, 5, eaaw2897. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Li, Y.; Jin, Z.-Y.; Yang, Y.; Yang, M.-B.; Yin, B.J. Light-and magnetic-responsive synergy controlled reconfiguration of polymer nanocomposites with shape memory assisted self-healing performance for soft robotics. J. Mater. Chem. C 2021, 9, 5515–5527. [Google Scholar] [CrossRef]
- Ma, C.; Wu, S.; Ze, Q.; Kuang, X.; Zhang, R.; Qi, H.J.; Zhao, R. Magnetic multimaterial printing for multimodal shape transformation with tunable properties and shiftable mechanical behaviors. ACS Appl. Mater. Interfaces 2020, 13, 12639–12648. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, F.; Leng, J.; Liu, Y. Personalized 4D printing of bioinspired tracheal scaffold concept based on magnetic stimulated shape memory composites. Compos. Sci. Technol. 2019, 184, 107866. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.; Zheng, Z.; Liu, Y.; Leng, J. Magnetic programming of 4D printed shape memory composite structures. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105571. [Google Scholar] [CrossRef]
- Noh, S.; Jeon, S.; Kim, E.; Oh, U.; Park, D.; Park, S.H.; Kim, S.W.; Pané, S.; Nelson, B.J.; Kim, J.y. A Biodegradable Magnetic Microrobot Based on Gelatin Methacrylate for Precise Delivery of Stem Cells with Mass Production Capability. Small 2022, 18, 2107888. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Kuang, H.; Deng, W.; Lei, Y. Immobilized copper nanoparticles on biodegradable magnetic starch composite: Investigation of its ovarian cancer, cytotoxicity, and antioxidant effects. J. Exp. Nanosci. 2022, 17, 496–508. [Google Scholar] [CrossRef]
- Mudhar, R.; Mucolli, A.; Ford, J.; Lira, C.; Yazdani Nezhad, H. Electrical and Magnetic Properties of 3D Printed Integrated Conductive Biodegradable Polymer Nanocomposites for Sustainable Electronics Development. J. Compos. Sci. 2022, 6, 345. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Yusoh, K. A review on the recent research of polycaprolactone (PCL). Adv. Mater. Res. 2016, 1134, 249–255. [Google Scholar] [CrossRef]
- Terzopoulou, A.; Wang, X.; Chen, X.Z.; Palacios-Corella, M.; Pujante, C.; Herrero-Martín, J.; Qin, X.H.; Sort, J.; deMello, A.J.; Nelson, B. Biodegradable metal–organic framework-based microrobots (MOFBOTs). Adv. Healthc. Mater. 2020, 9, 2001031. [Google Scholar] [CrossRef]
- Dong, M.; Wang, X.; Chen, X.Z.; Mushtaq, F.; Deng, S.; Zhu, C.; Torlakcik, H.; Terzopoulou, A.; Qin, X.H.; Xiao, X.J. 3D-printed soft magnetoelectric microswimmers for delivery and differentiation of neuron-like cells. Adv. Funct. Mater. 2020, 30, 1910323. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Jeon, S.; Lee, J.; Lee, S.; Lee, J.; Jeon, B.O.; Jang, J.E.; Choi, H. A simple and rapid fabrication method for biodegradable drug-encapsulating microrobots using laser micromachining, and characterization thereof. Sens. Actuators B Chem. 2018, 266, 276–287. [Google Scholar] [CrossRef]
- Rutkowski, S.; Si, T.; Gai, M.; Sun, M.; Frueh, J.; He, Q.J. Magnetically-guided hydrogel capsule motors produced via ultrasound assisted hydrodynamic electrospray ionization jetting. J. Colloid Interface Sci. 2019, 541, 407–417. [Google Scholar] [CrossRef]
- Bozuyuk, U.; Yasa, O.; Yasa, I.C.; Ceylan, H.; Kizilel, S.; Sitti, M. Light-triggered drug release from 3D-printed magnetic chitosan microswimmers. ACS Nano 2018, 12, 9617–9625. [Google Scholar] [CrossRef] [PubMed]
- Harmel, A.; Polley, T.; Voegele, G.; Weisener, T. Media-separated micropump consisting of magnetic shape memory alloy actuator. In Proceedings of the ACTUATOR 2022; International Conference and Exhibition on New Actuator Systems and Applications, Mannheim, Germany, 29–30 June 2022; VDE: Frankfurt, Germany, 2022; pp. 1–4. [Google Scholar]
- Park, J.; Jin, C.; Lee, S.; Kim, J.Y.; Choi, H.J. Magnetically actuated degradable microrobots for actively controlled drug release and hyperthermia therapy. Adv. Healthc. Mater. 2019, 8, 1900213. [Google Scholar] [CrossRef] [PubMed]
- Rashiku, M.; Bhattacharya, S. BioMEMS-Based Sensors for Future Diagnostic Applications. In MEMS Applications in Biology and Healthcare; AIP Publishing LLC: Melville, NY, USA, 2021; pp. 1–11. [Google Scholar]
- Quashie, D.; Benhal, P.; Chen, Z.; Wang, Z.; Mu, X.; Song, X.; Jiang, T.; Zhong, Y.; Cheang, U.K.; Ali, J. Magnetic bio-hybrid micro actuators. Nanoscale 2022, 14, 4364–4379. [Google Scholar] [CrossRef] [PubMed]
- Talbi, A.; Viard, R.; Ghouila-Houri, C.; Garnier, É.; Gallas, Q.; Kourta, A.; Sipp, D.; Merlen, A.; Pernod, P. IEMN/LEMAC-LICS Magneto-mechanical micro-actuators and active flow control: Review of the last 15 years results. In Proceedings of the 8th European Conference for Aeronautics and Space Sciences, Madrid, Spain, 1–4 July 2019. [Google Scholar]
- Zhang, S.; Wang, Y.; Onck, P.R.; den Toonder, J.M.J. Removal of Microparticles by Ciliated Surfaces—An Experimental Study. Adv. Funct. Mater. 2018, 29, 1806434. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, R.; Wang, Y.; Onck, P.R.; den Toonder, J.M.J. Controlled Multidirectional Particle Transportation by Magnetic Artificial Cilia. ACS Nano 2020, 14, 10313–10323. [Google Scholar] [CrossRef]
- Goudu, S.R.; Yasa, I.C.; Hu, X.; Ceylan, H.; Hu, W.; Sitti, M. Biodegradable Untethered Magnetic Hydrogel Milli-Grippers. Adv. Funct. Mater. 2020, 30, 2004975. [Google Scholar] [CrossRef]
- Qi, Z.; Zhou, M.; Li, Y.; Xia, Z.; Huo, W.; Huang, X. Reconfigurable Flexible Electronics Driven by Origami Magnetic Membranes. Adv. Mater. Technol. 2021, 6, 2001124. [Google Scholar] [CrossRef]
- Paknahad, A.A.; Tahmasebipour, M. An electromagnetic micro-actuator with PDMS-Fe3O4 nanocomposite magnetic membrane. Microelectron. Eng. 2019, 216, 111031. [Google Scholar] [CrossRef]
- Ninomiya, K.; Gaysornkaew, S.; Tsumori, F. Micro Magnetic Patterning on Extremely Tough Magnetic Gel Actuator. In Proceedings of the 2022 IEEE 35th International Conference on Micro Electro Mechanical Systems Conference (MEMS); IEEE: Piscataway, NJ, USA, 2022; pp. 235–238. [Google Scholar]
- Liu, L.; Song, W.; Jiang, S.; Duan, G.; Qin, X. Giving Penetrable Remote-Control Ability to Thermoresponsive Fibrous Composite Actuator with Fast Response Induced by Alternative Magnetic Field. Nanomaterials 2022, 12, 53. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Zhang, Z.; Yang, J.; Li, D. A phase change microactuator based on paraffin wax/expanded graphite/nickel particle composite with induction heating. Sens. Actuators A Phys. 2018, 275, 129–136. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; He, H. Design and fabrication of a soft micro-actuator based on distributed magnetic composite. In Proceedings of the 2021 22nd International Conference on Electronic Packaging Technology (ICEPT), Xiamen, China, 14–17 September 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–6. [Google Scholar]
- Mohith, S.; Karanth, P.N.; Kulkarni, S. Recent trends in mechanical micropumps and their applications: A review. Mechatronics 2019, 60, 34–55. [Google Scholar] [CrossRef]
- Qi, C.; Shinshi, T. A Disposable Bidirectional Micropump with Three Diaphragms Driven by a Rotating Multi-pole Magnet. In Proceedings of the 2021 IEEE 30th International Symposium on Industrial Electronics (ISIE), Kyoto, Japan, 20–23 June 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–4. [Google Scholar]
- Shinoda, H.; Tsumori, F. Development of micro pump using magnetic artificial cilia with metachronal wave. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 497–500. [Google Scholar]
- Saren, A.; Smith, A.; Ullakko, K. Integratable magnetic shape memory micropump for high-pressure, precision microfluidic applications. Microfluid. Nanofluidics 2018, 22, 1–10. [Google Scholar] [CrossRef]
- Wang, C.; Seo, S.-J.; Kim, J.-S.; Lee, S.-H.; Jeon, J.-K.; Kim, J.-W.; Kim, K.-H.; Kim, J.-K.; Park, J. Intravitreal implantable magnetic micropump for on-demand VEGFR-targeted drug delivery. J. Control. Release 2018, 283, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Park, J. Magnetic micropump embedded in contact lens for on-demand drug delivery. Micro Nano Syst. Lett. 2020, 8, 1–6. [Google Scholar] [CrossRef]
- Tahmasebipour, M.; Paknahad, A.A. Unidirectional and bidirectional valveless electromagnetic micropump with PDMS-Fe3O4 nanocomposite magnetic membrane. J. Micromechanics Microengineering 2019, 29, 075014. [Google Scholar] [CrossRef]
- Sadler, D.J.; Oh, K.W.; Ahn, C.H.; Bhansali, S.; Henderson, H.T. A new magnetically actuated microvalve for liquid and gas control applications. In Proceedings of the 10th International Conference on Solid-State Sensors and Actuators (Transducers ’99), Sendai, Japan, 7–10 June 1999; pp. 1812–1815. [Google Scholar]
- Pradeep, A.; Raj, S.V.; Stanley, J.; Babu, T.S. Design, simulation and fabrication of a normally-closed microvalve based on magnetic actuation. Mater. Today Proc. 2018, 5, 16059–16064. [Google Scholar] [CrossRef]
- Nakahara, T.; Suzuki, J.; Hosokawa, Y.; Shimokawa, F.; Kotera, H.; Suzuki, T. Fabrication of magnetically driven microvalve arrays using a photosensitive composite. Magnetochemistry 2018, 4, 7. [Google Scholar] [CrossRef]
- Kim, S.; Song, J.; Kim, R.; Lee, N.Y.; Kim, M.H.; Park, H.G. Ferrowax microvalves for fully automated serial dilution on centrifugal microfluidic platforms. Biotechnol. J. 2021, 16, 2100131. [Google Scholar] [CrossRef]
- Alam, M.W.; Samad, M.F. Design and Optimization of Electromagnetically Actuated Bidirectional Microvalve. In Proceedings of the 2019 3rd International Conference on Electrical, Computer & Telecommunication Engineering (ICECTE), Rajshahi, Bangladesh, 26–28 December 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 248–251. [Google Scholar]
- Liu, B.; Yang, J.; Yang, J.; Li, D.; Gao, G.; Wang, Y. A thermally actuated microvalve using paraffin composite by induction heating. Microsyst. Technol. 2019, 25, 3969–3975. [Google Scholar] [CrossRef]
- Lin, X.; Xie, W.; Lin, Q.; Cai, Y.; Hua, Y.; Lin, J.; He, G.; Chen, J. NIR-responsive metal-containing polymer hydrogel for light-controlled microvalve. Polym. Chem. 2021, 12, 3375–3382. [Google Scholar] [CrossRef]
- Dehghan, A.; Gholizadeh, A.; Navidbakhsh, M.; Sadeghi, H.; Pishbin, E. Integrated microfluidic system for efficient DNA extraction using on-disk magnetic stirrer micromixer. Sens. Actuators B Chem. 2022, 351, 130919. [Google Scholar] [CrossRef]
- Bahrami, D.; Nadooshan, A.A.; Bayareh, M. Effect of non-uniform magnetic field on mixing index of a sinusoidal micromixer. Korean J. Chem. Eng. 2022, 39, 1–12. [Google Scholar] [CrossRef]
- Buglie, W.; Tamrin, K.F. Enhanced mixing in dual-mode cylindrical magneto-hydrodynamic (MHD) micromixer. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2022, 236, 2491–2501. [Google Scholar] [CrossRef]
- Sun, J.; Shi, Z.; Li, M.; Sha, J.; Zhong, M.; Chen, S.; Liu, X.; Jia, S.J. Numerical and experimental investigation of a magnetic micromixer under microwires and uniform magnetic field. J. Magn. Magn. Mater. 2022, 551, 169141. [Google Scholar] [CrossRef]
- Azimi, N.; Rahimi, M.; Zangenehmehr, P. Numerical study of mixing and mass transfer in a micromixer by stimulation of magnetic nanoparticles in a magnetic field. Chem. Eng. Technol. 2021, 44, 1084–1093. [Google Scholar] [CrossRef]
- Baki, A.; Löwa, N.; Remmo, A.; Wiekhorst, F.; Bleul, R. Micromixer Synthesis Platform for a Tuneable Production of Magnetic Single-Core Iron Oxide Nanoparticles. Nanomaterials 2020, 10, 1845. [Google Scholar] [CrossRef]
- Ergin, F.G.; Kitenbergs, G.; Cēbers, A. Revisiting velocity, concentration and interface measurements in a magnetic micromixer. In Proceedings of the 13th International Symposium on Particle Image Velocimetry ISPIV 19, Munich, Germany, 2019, 22–24 July; pp. 22–24.
- Surendran, A.N.; Zhou, R. Active High-Throughput Micromixer Using Injected Magnetic Mixture Underneath Microfluidic Channel. In International Conference on Nanochannels, Microchannels, and Minichannels; American Society of Mechanical Engineers: New York, NY, USA, 2020. [Google Scholar]
- Becker, T.I.; Böhm, V.; Chavez Vega, J.; Odenbach, S.; Raikher, Y.L.; Zimmermann, K. Magnetic-field-controlled mechanical behavior of magneto-sensitive elastomers in applications for actuator and sensor systems. Arch. Appl. Mech. 2019, 89, 133–152. [Google Scholar] [CrossRef]
- Hu, T.; Xuan, S.; Ding, L.; Gong, X. Stretchable and magneto-sensitive strain sensor based on silver nanowire-polyurethane sponge enhanced magnetorheological elastomer. Mater. Des. 2018, 156, 528–537. [Google Scholar] [CrossRef]
- Jang, D.; Park, J.-E.; Kim, Y.-K. Evaluation of (CNT@ CIP)-embedded magneto-resistive sensor based on carbon nanotube and carbonyl iron powder polymer composites. Polymers 2022, 14, 542. [Google Scholar] [CrossRef]
- Sang, M.; Wang, S.; Liu, M.; Bai, L.; Jiang, W.; Xuan, S.; Gong, X. Technology. Fabrication of a piezoelectric polyvinylidene fluoride/carbonyl iron (PVDF/CI) magnetic composite film towards the magnetic field and deformation bi-sensor. Compos. Sci. Technol. 2018, 165, 31–38. [Google Scholar] [CrossRef]
- Pradhan, S.; DESHMUKH, P.p.; Kambale, R.C.; Darvade, T.C.; Satapathy, S.; Majumder, S.K. Effect of nano-size on magnetostriction of BiFeO3 and exceptional magnetoelectric coupling properties of BiFeO3_P (VDF-TrFE) polymer composite films for magnetic field sensor application. Smart Mater. Struct. 2022, 32, 045017. [Google Scholar] [CrossRef]
- Karami, A.H.; Karami Horestani, F.; Kolahdouz, M.; Horestani, A.K.; Martín, F. 2D rotary sensor based on magnetic composite of microrods. J. Mater. Sci. Mater. Electron. 2020, 31, 167–174. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, S.; Jia, S.; Kang, X.; Yu, H.; Yang, S.; Wang, S.; Yang, Y. A highly sensitive magnetic field sensor based on FBG and magnetostrictive composite with oriented magnetic domains. Measurement 2022, 189, 110667. [Google Scholar] [CrossRef]
- Yang, W.; Tian, H.; Liao, J.; Wang, Y.; Liu, L.; Zhang, L.; Lu, A. Flexible and strong Fe3O4/cellulose composite film as magnetic and UV sensor. Appl. Surf. Sci. 2020, 507, 145092. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, F.; Li, H.Y.; Li, X.; Fan, Y.J.; Cao, J.W.; Yin, Y.M.; Wang, Y.; Wang, Z.L.; Zhu, G. A flexible and ultra-highly sensitive tactile sensor through a parallel circuit by a magnetic aligned conductive composite. ACS Nano 2022, 16, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, G.; Niu, S.; Chen, K.; Gan, T.; Wang, Z.; Wang, H.; Du, P.; Leung, C.W.; Qu, S. Magnetic-assisted transparent and flexible percolative composite for highly sensitive piezoresistive sensor via hot embossing technology. ACS Appl. Mater. Interfaces 2019, 11, 48331–48340. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pal, A.; Aghakhani, A.; Pena-Francesch, A.; Sitti, M. Soft actuators for real-world applications. Nat. Rev. Mater. 2022, 7, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Parada, G.A.; Liu, S.; Zhao, X.J.S.R. Ferromagnetic soft continuum robots. Sci. Robot. 2019, 4, eaax7329. [Google Scholar] [CrossRef]
- Alapan, Y.; Karacakol, A.C.; Guzelhan, S.N.; Isik, I.; Sitti, M.J.S.a. Reprogrammable shape morphing of magnetic soft machines. Sci. Adv. 2020, 6, eabc6414. [Google Scholar] [CrossRef]
- Song, H.; Lee, H.; Lee, J.; Choe, J.K.; Lee, S.; Yi, J.Y.; Park, S.; Yoo, J.-W.; Kwon, M.S.; Kim, J. Reprogrammable ferromagnetic domains for reconfigurable soft magnetic actuators. Nano Lett. 2020, 20, 5185–5192. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, J.; Salehizadeh, M.; Onaizah, O.; Diller, E. Millimeter-scale flexible robots with programmable three-dimensional magnetization and motions. Sci. Robot. 2019, 4, eaav4494. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xuan, S.; Sun, S.; Xu, Z.; Li, J.; Gong, X. 3D printing magnetic actuators for biomimetic applications. ACS Appl. Mater. 2021, 13, 30127–30136. [Google Scholar] [CrossRef] [PubMed]
- Lantean, S.; Roppolo, I.; Sangermano, M.; Hayoun, M.; Dammak, H.; Barrera, G.; Tiberto, P.; Pirri, C.F.; Bodelot, L.; Rizza, G. Magnetoresponsive Devices with Programmable Behavior Using a Customized Commercial Stereolithographic 3D Printer. Adv. Mater. 2022, 7, 2200288. [Google Scholar] [CrossRef]
- Lin, C.; Lv, J.; Li, Y.; Zhang, F.; Li, J.; Liu, Y.; Liu, L.; Leng, J. 4D-printed biodegradable and remotely controllable shape memory occlusion devices. Adv. Funct. Mater. 2019, 29, 1906569. [Google Scholar] [CrossRef]
- Qi, S.; Guo, H.; Fu, J.; Xie, Y.; Zhu, M.; Yu, M. 3D printed shape-programmable magneto-active soft matter for biomimetic applications. Compos. Sci. Technol. 2020, 188, 107973. [Google Scholar] [CrossRef]
- Roh, S.; Okello, L.B.; Golbasi, N.; Hankwitz, J.P.; Liu, J.A.C.; Tracy, J.B.; Velev, O.D. 3D-printed silicone soft architectures with programmed magneto-capillary reconfiguration. Adv. Mater. Technol. 2019, 4, 1800528. [Google Scholar] [CrossRef]
- Tang, J.; Yin, Q.; Shi, M.; Yang, M.; Yang, H.; Sun, B.; Guo, B.; Wang, T. Programmable shape transformation of 3D printed magnetic hydrogel composite for hyperthermia cancer therapy. Extrem. Mech. Lett. 2021, 46, 101305. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Q.; Yao, Y.; Liu, L.; Liu, Y.; Leng, J. Direct-write fabrication of 4D active shape-changing structures based on a shape memory polymer and its nanocomposite. ACS Appl. Mater. Interfaces 2017, 9, 876–883. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Yi, S.; Lin, Z.; Wang, C.; Chen, Z.; Jiang, L. 4D printing of magnetoactive soft materials for on-demand magnetic actuation transformation. ACS Appl. Mater. Interfaces 2021, 13, 4174–4184. [Google Scholar] [CrossRef]
- Schmauch, M.M.; Mishra, S.R.; Evans, B.A.; Velev, O.D.; Tracy, J.B. Chained iron microparticles for directionally controlled actuation of soft robots. ACS Appl. Mater. 2017, 9, 11895–11901. [Google Scholar] [CrossRef]

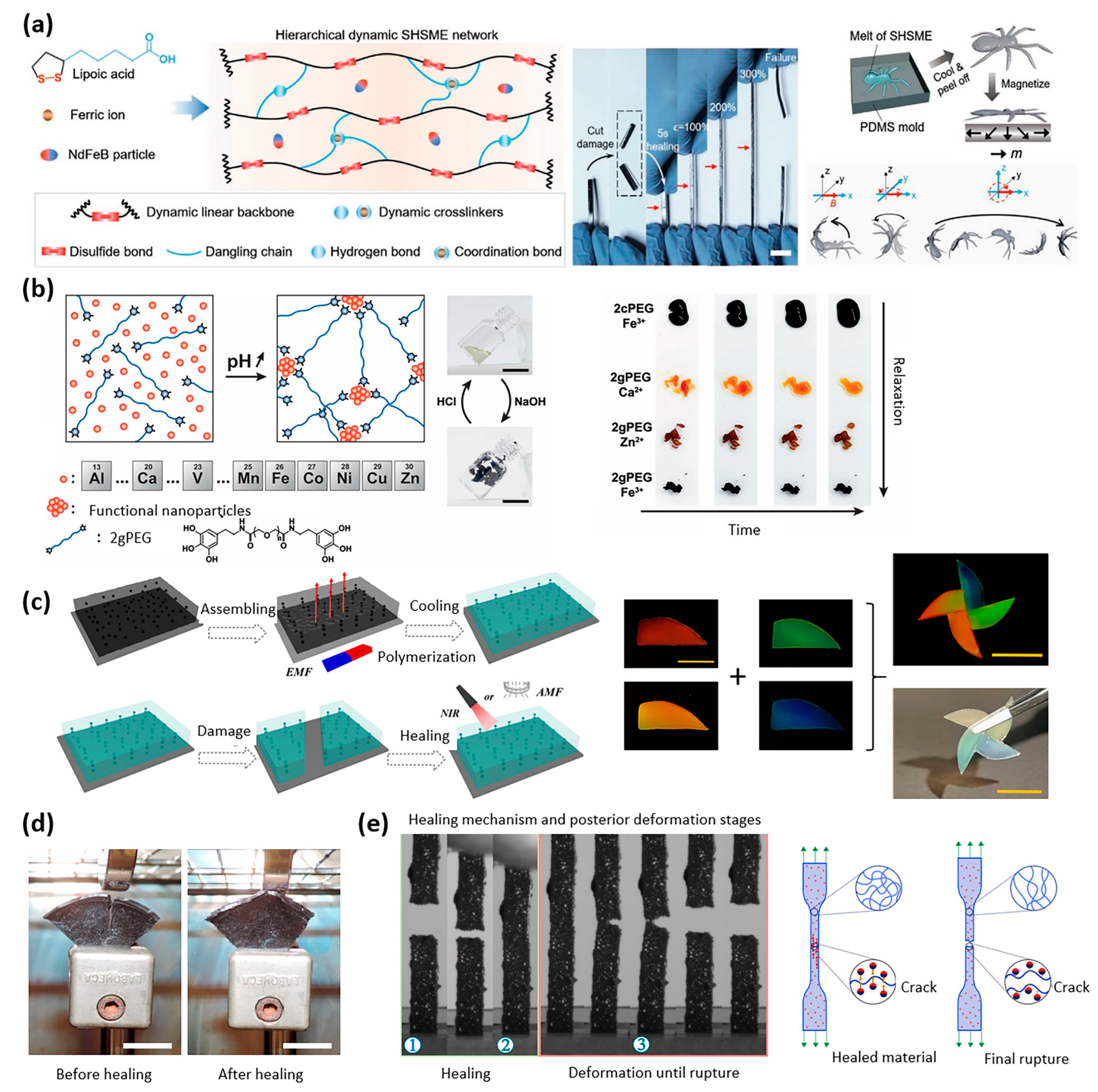
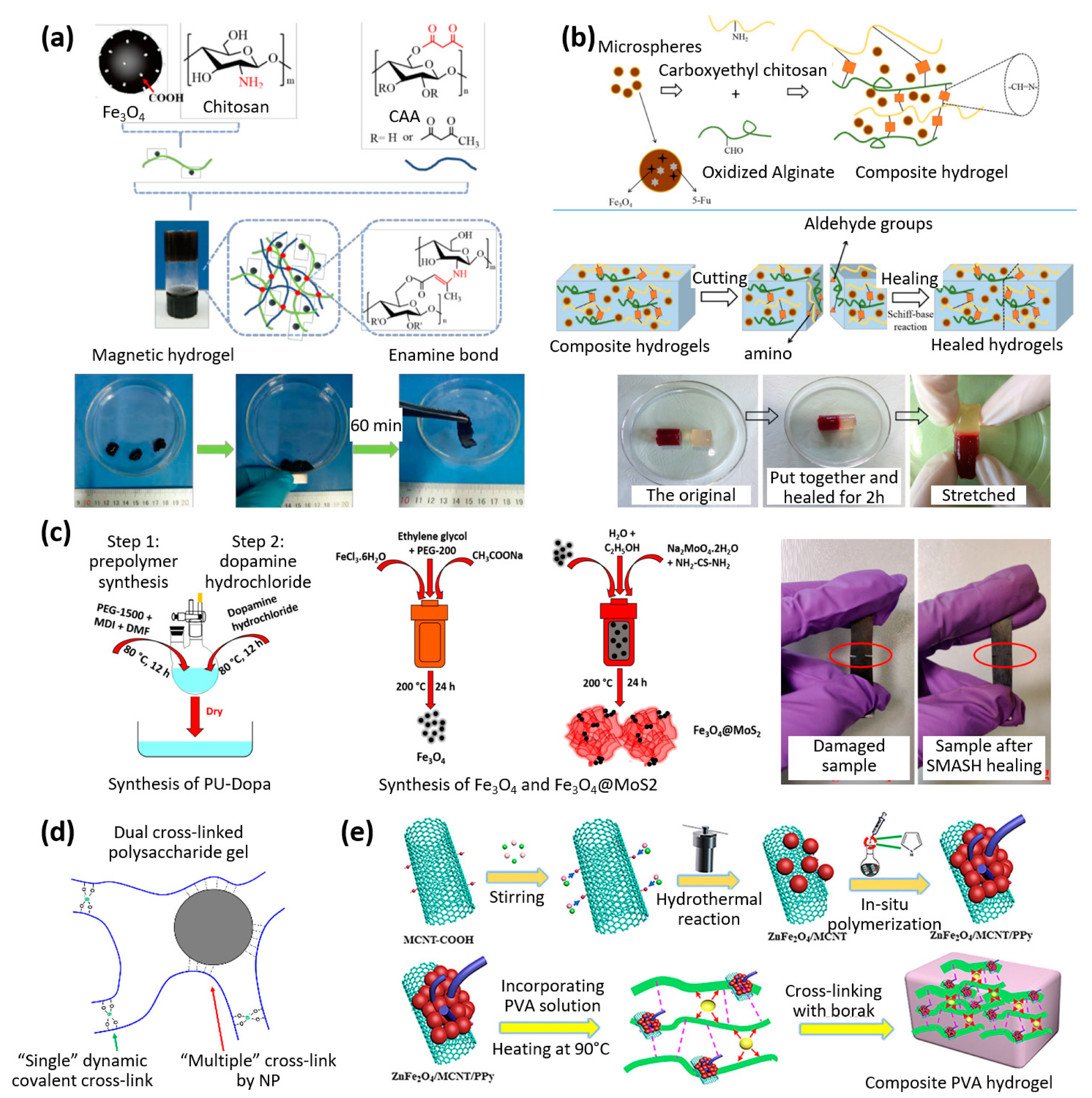
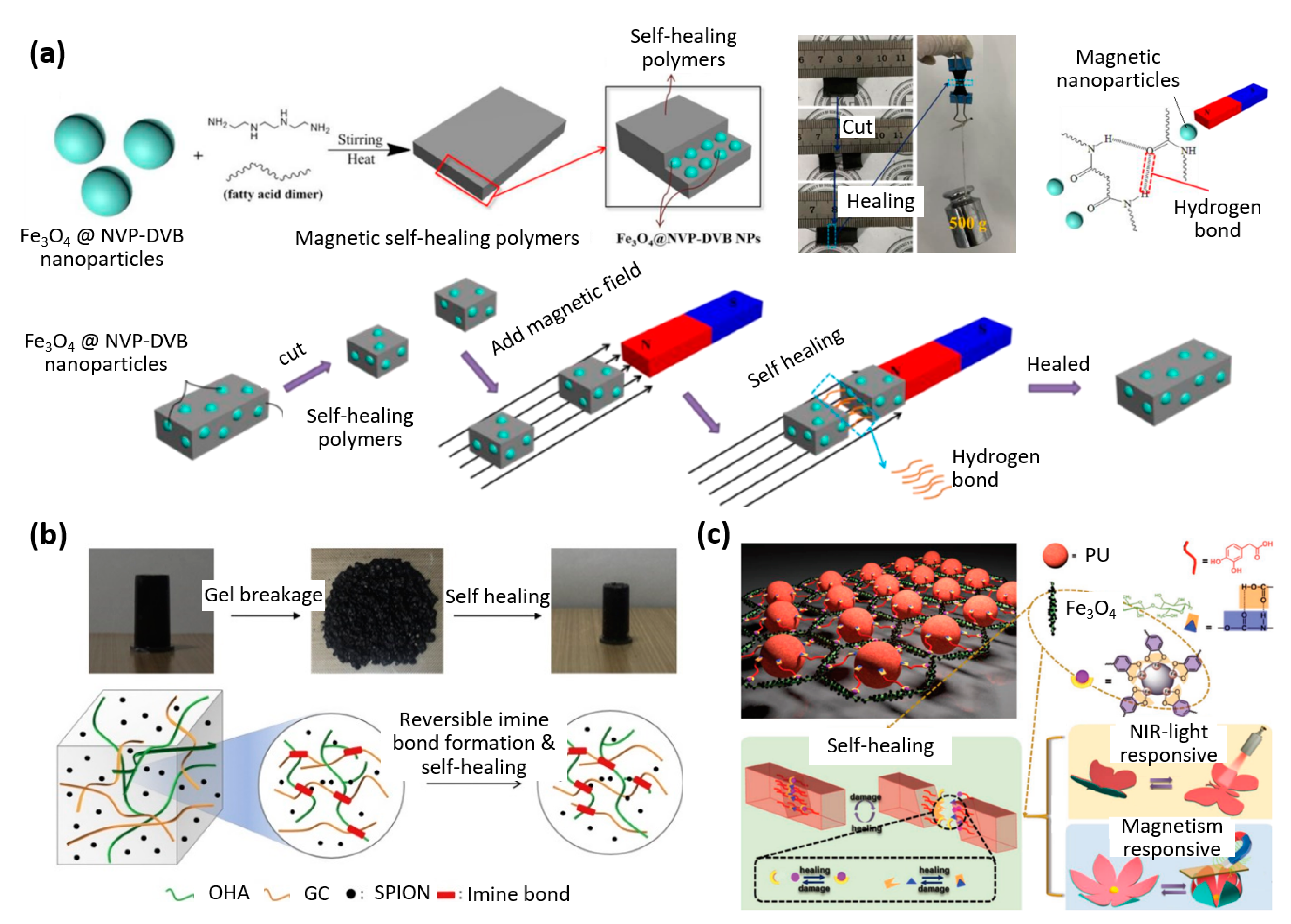
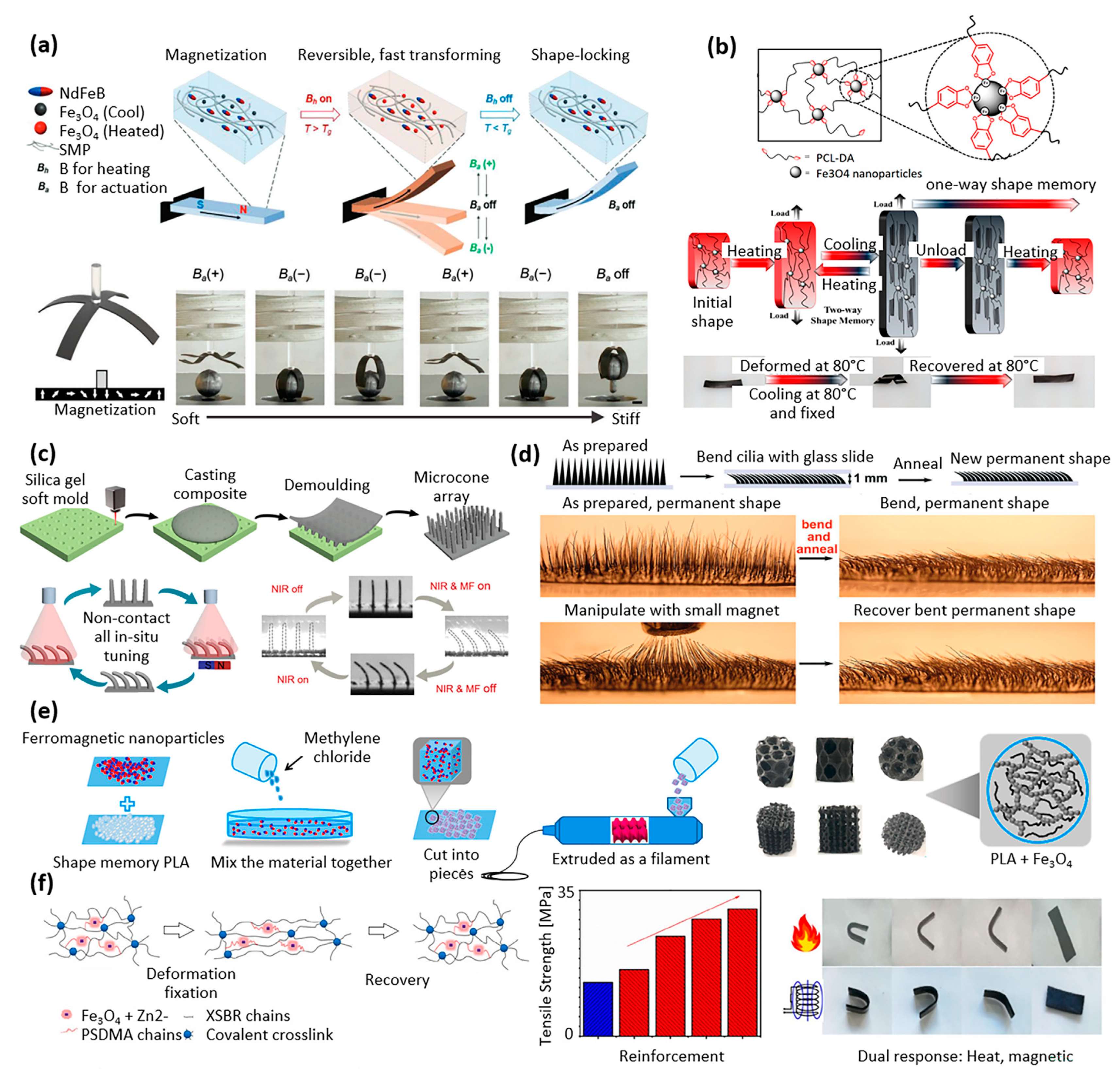
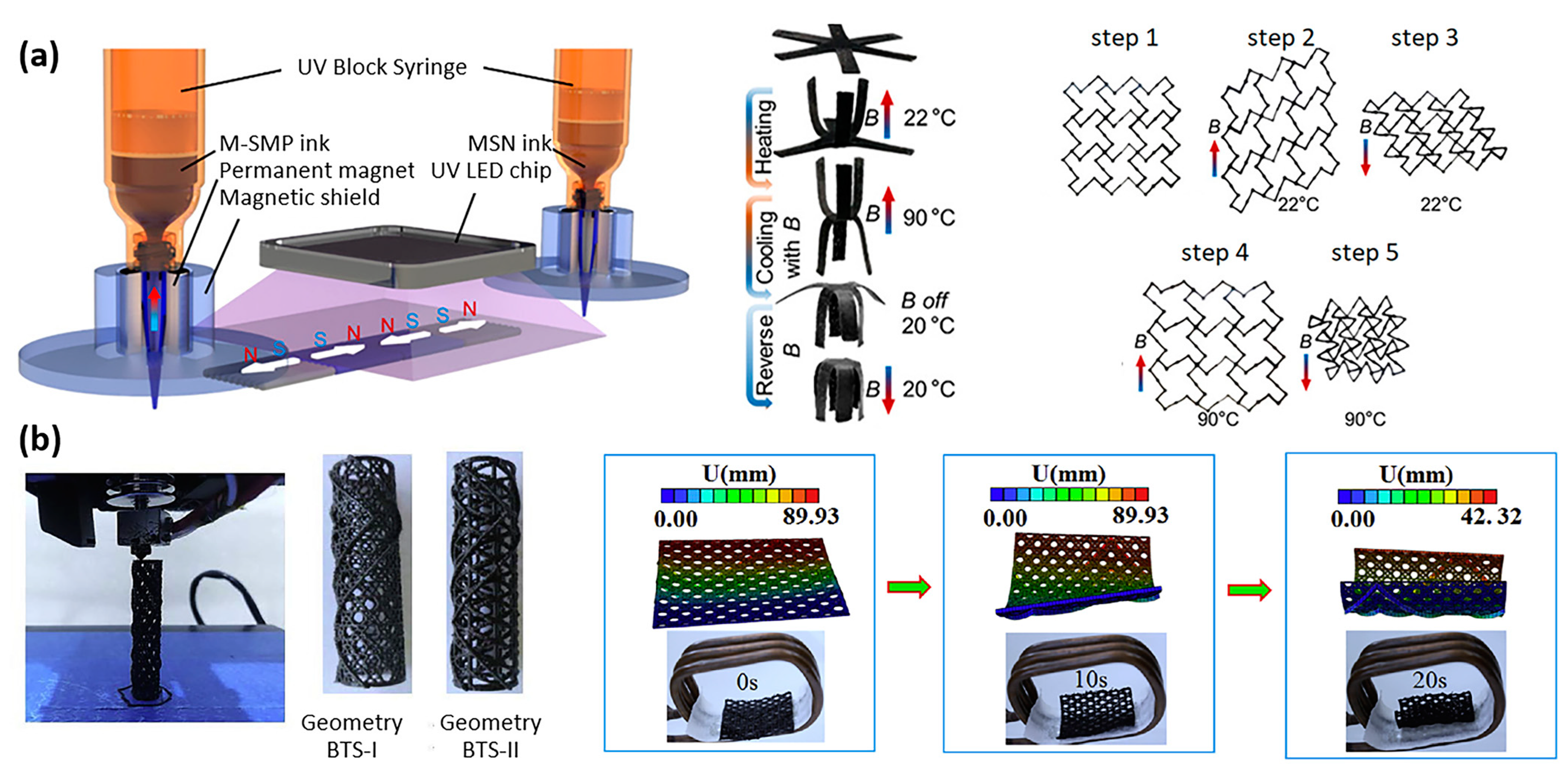
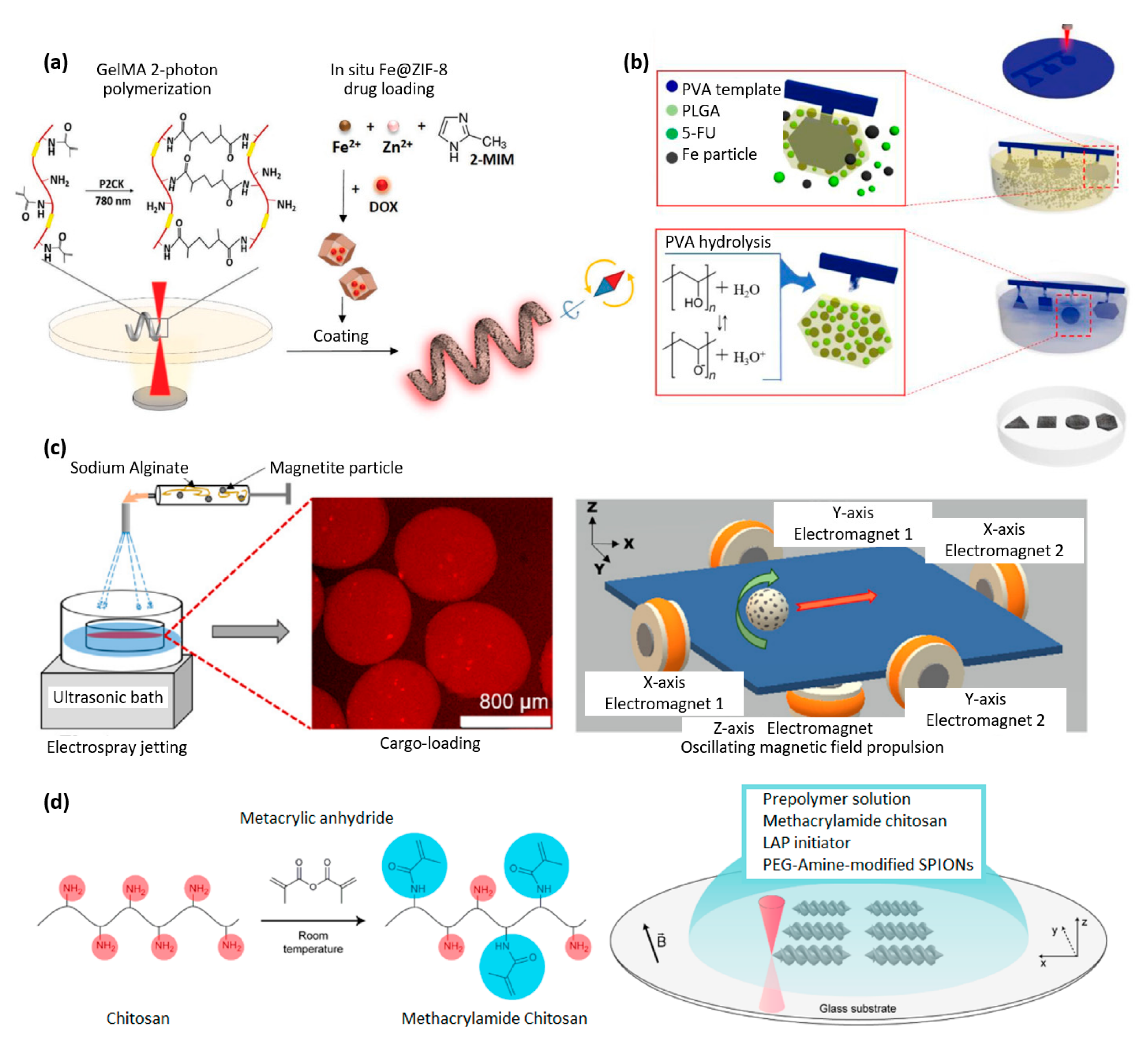
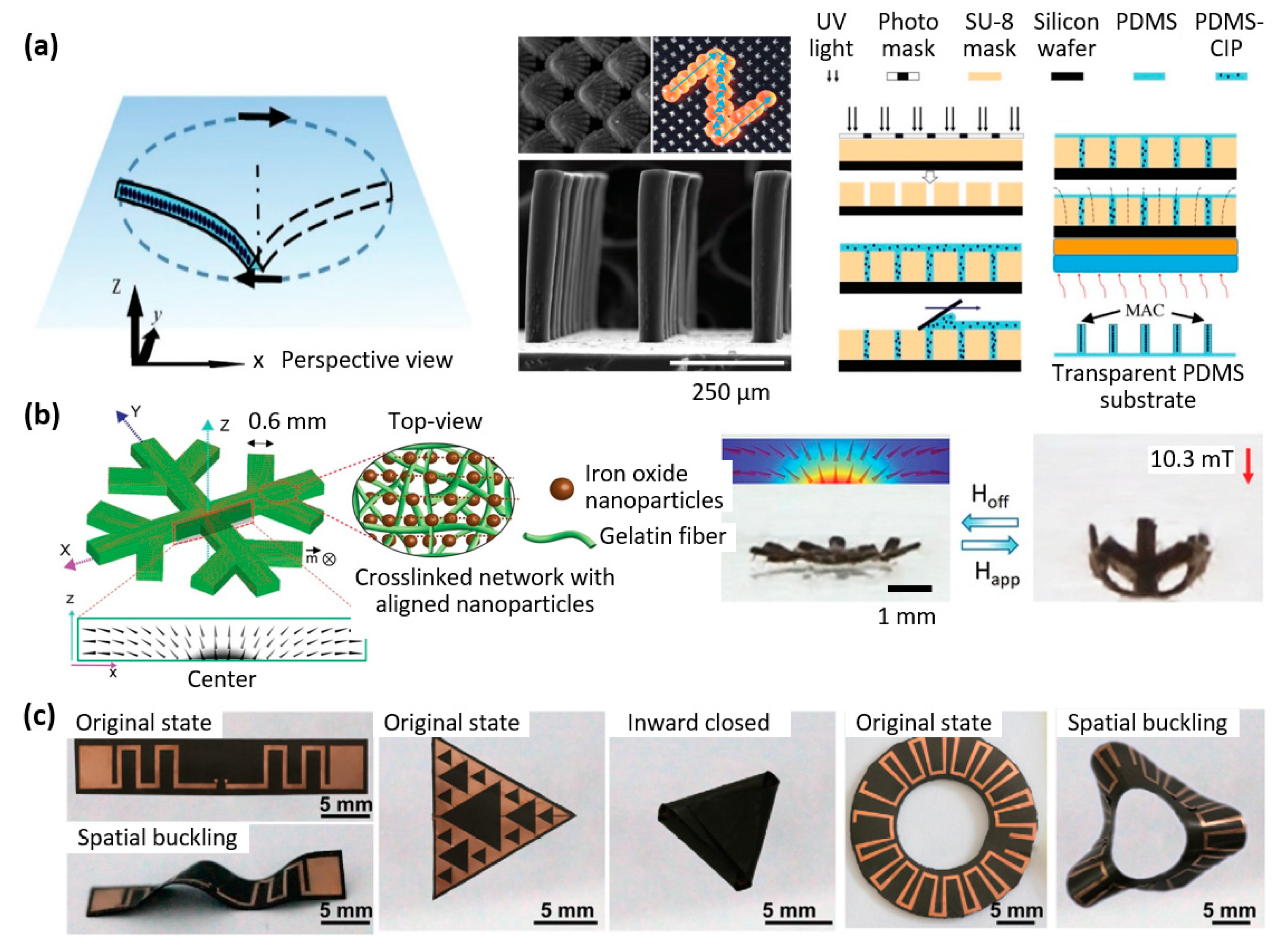

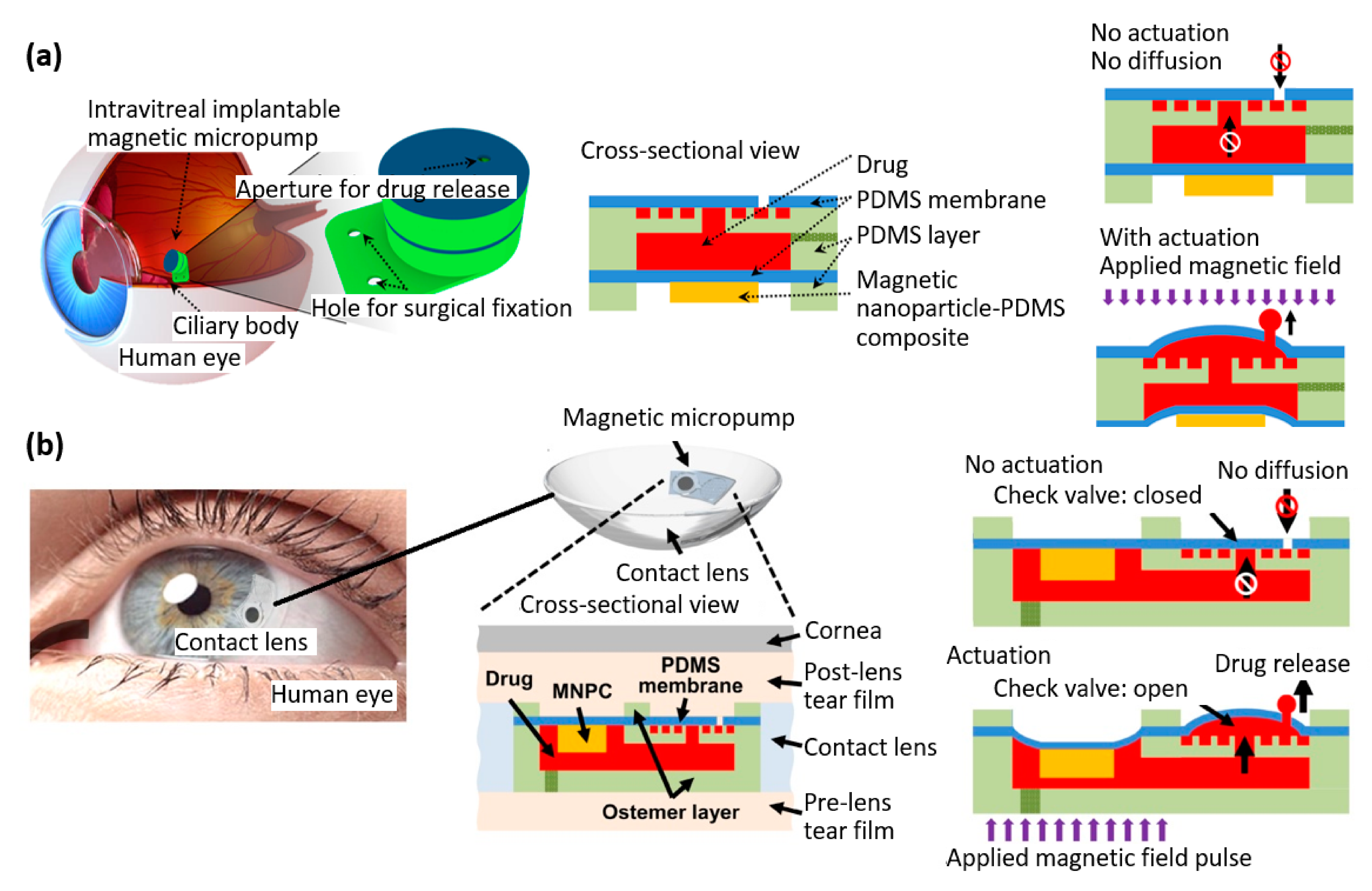
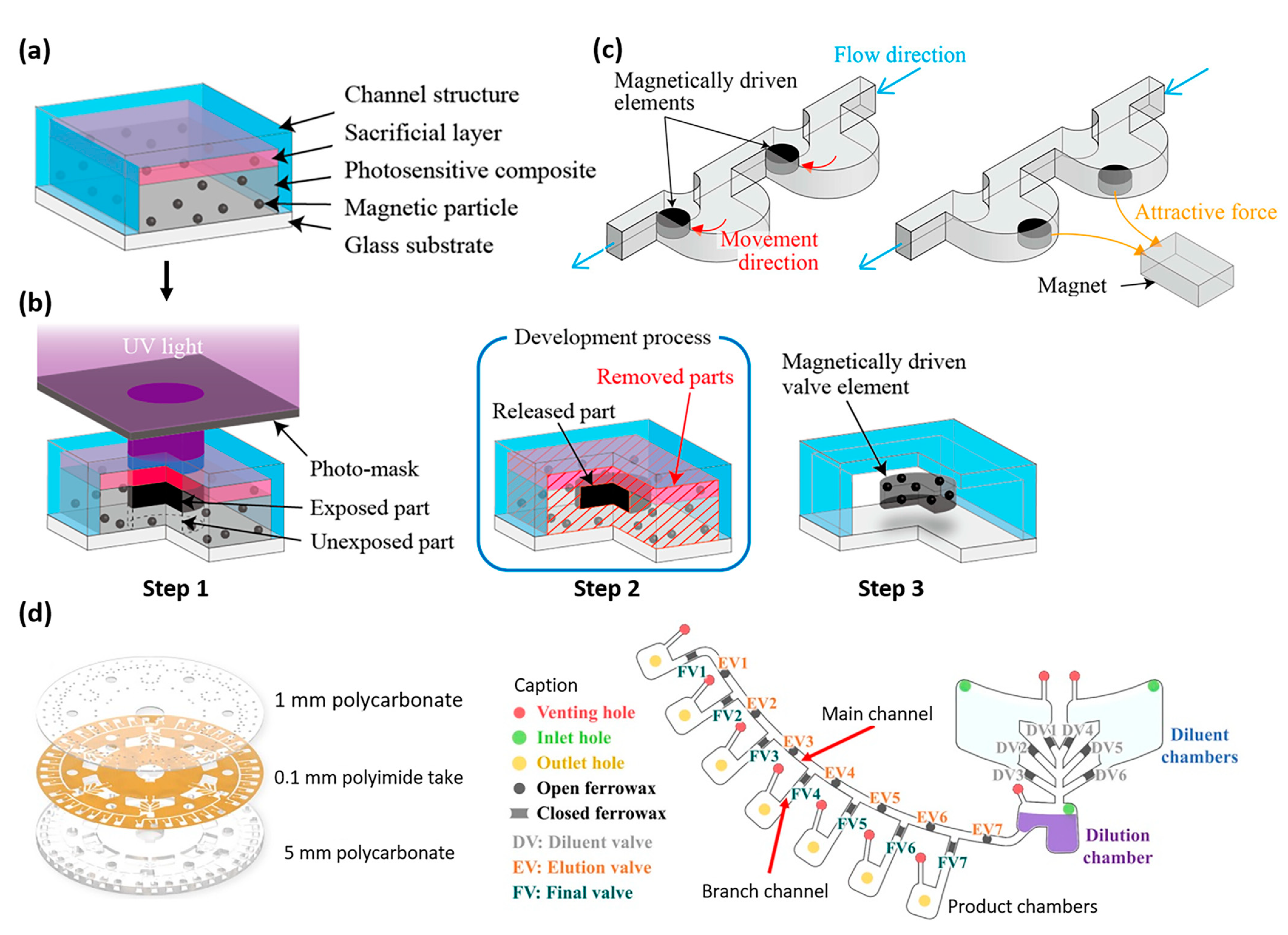
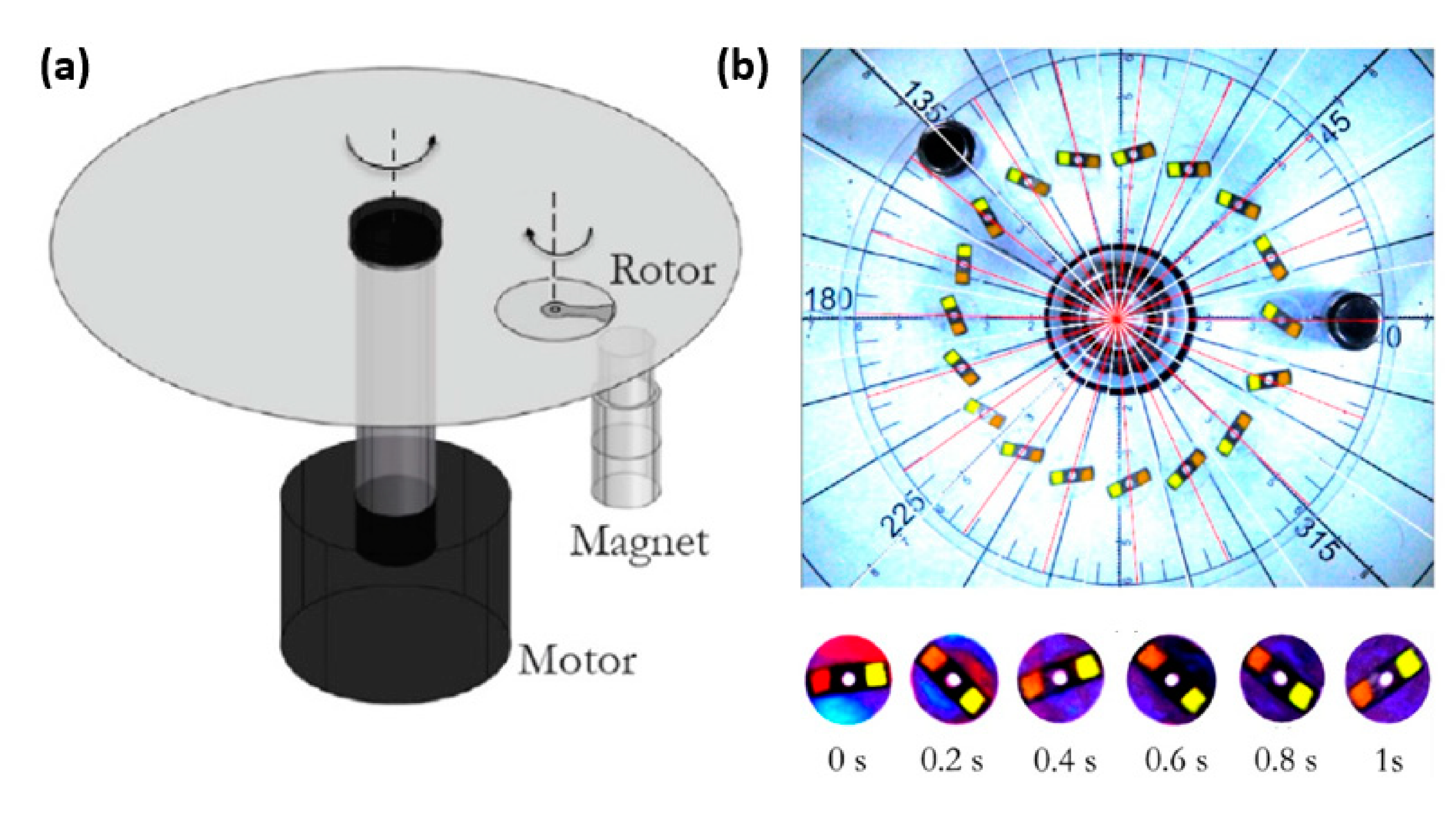
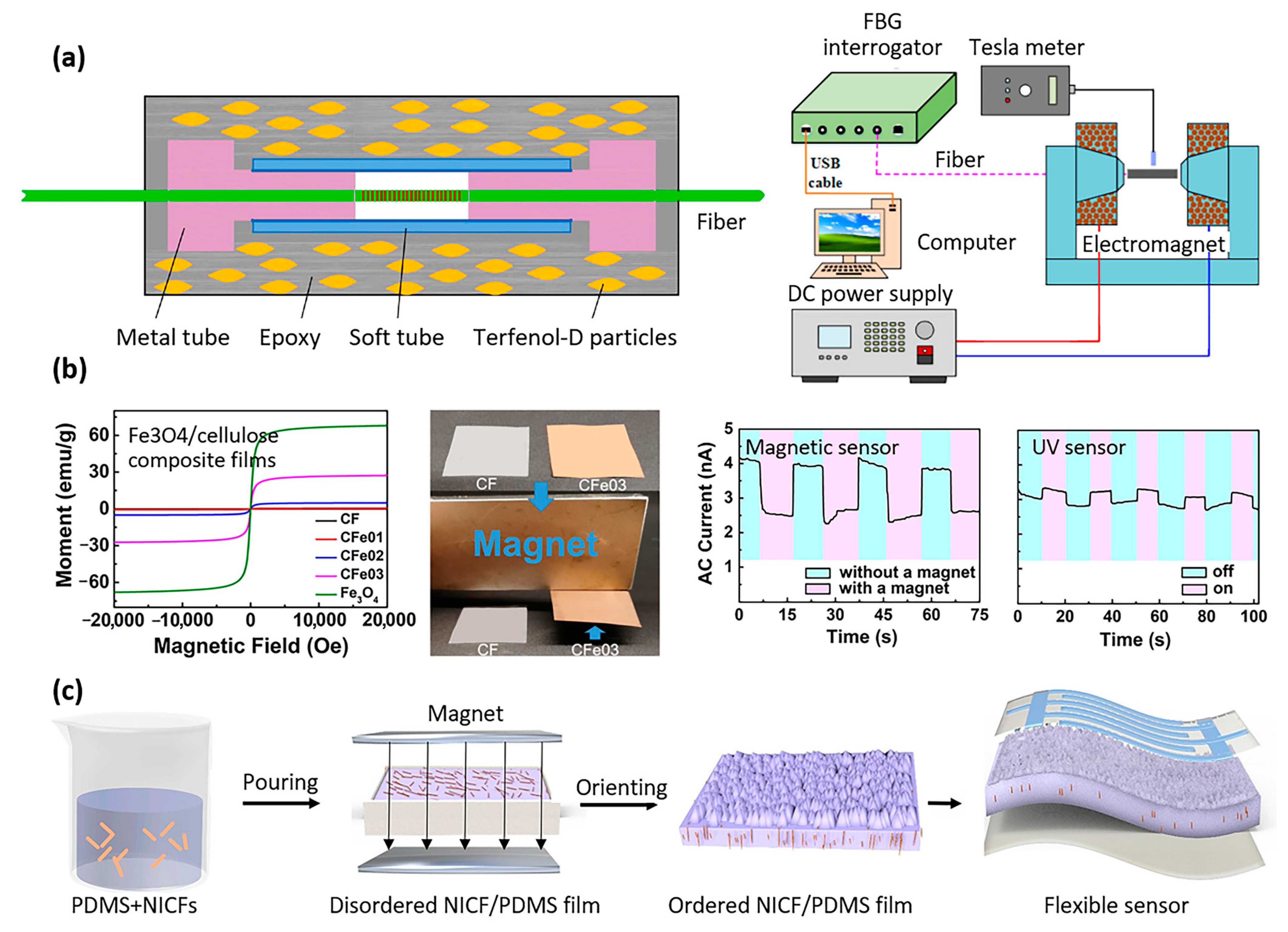
| Properties | Description | Non-Magnetic Polymer Composites | Magnetic Polymer Composites |
|---|---|---|---|
| Biocompatibility | No adverse reactions observed when the polymer composite comes into contact with biological tissue | Yes | Yes |
| Tailored properties | Polymer composites designed to have specific chemical, mechanical, electrical, and thermal properties | Yes | Yes |
| Lightweight | Lightweight and thin bioMEMS are important for medical devices that need to be small and portable | Yes | Yes |
| Easy processing | Polymer composites can be easily processed into complex shapes and structures using a variety of techniques (3D printing, molding, cleanroom microfabrication) | Yes | Yes |
| Magnetic properties at the macroscopic scale | Applying an external magnetic field on a device made of magnetic polymer composite (e.g., membrane, cantilever) allows (1) attracting the object by the generated magnetic force, (2) deforming/influencing the orientation of the object by the applied magnetic torque, with various applications in bioMEMS | No | Yes |
| Magnetic properties at the bulk material scale | Applying an external magnetic field on a magnetic polymer composite makes it possible to change the internal structure of the material, influencing:
| No | Yes |
| Applications in bioMEMS | Polymer composites are of particular interest for all applications that require devices that are mechanically compliant with cells or with the various organs of the human body. In addition, magnetic polymer composites allow for all bioMEMS applications in which an interaction with an external magnetic field allows for the control of, e.g., drug delivery devices, sensors, and actuators. | ||
| Self-Healing Mechanism | Matrix | Filler | Input | Output | Application | Ref. |
|---|---|---|---|---|---|---|
| Via direct mixing | ||||||
| Disulfide bonds | Polymerized alpha lipoic acid (ALA) | NdFeB | Magnetic Field | Actuation | Soft robotics | [17] |
| (Metal) ion bond | Functionalized PEG hydrogel with pyrogallols | Fe/Ca/Zn | Autonomous/close distance | Fast self-healing adhesive | Biocompatible wound sealant | [18] |
| Intermolecular diffusion | Ethylene glycol | Fe3O4 | Heat or magnetic field | Phase transformation self-healing | Cell engineering | [19] |
| Diels–Alder | Polyethertriamine Jeffamine | Fe3O4 | Magnetic field and heat | Closure of large gaps | Soft robotics | [21] |
| Dipole-interaction | Dowsil CY52-276 gel | NdFeB and CIP | Autonomous/residual magnetization | Instantaneous self-healing regardless of cycles | Bioengineering and soft robotics | [20] |
| Covalent bonds | Chitosan | Fe3O4 | Magnetic field | Actuation while shape shifting | Biocompatible injectable | [22] |
| Schiff base | Chitosan/alginate hydrogel and gelatin | Fe3O4 | Magnetic field | Self-healing under physiological conditions | Drug delivery | [23] |
| Metal ligand complex | Dopamine-functionalized polyurethane | Fe3O4 with MoS2 | Heat and shape-memory | EMI shielding | Stretchable electronics | [24] |
| Ionic bond (disulfide, hydrogen, iron–carboxylate) | Thioctic acid | Fe3O4 | Autonomous | 100% healing efficiency | Shape construction | [27] |
| Hydrogen bond | Carboxymethyl hydroxypropyl guar | CoFe2O4 | Magnetic field | 100% healing efficiency and actuation | Soft robotics | [25] |
| Boric acid ester and hydrogen bonds | Polypyrrole PPy | ZnFe2O4 and MWCNT | Autonomous | Self-healing | Electronic skins and drug delivery | [26] |
| Via surface engineering | ||||||
| Hydrogen bonds | NVP-DVB | Fe3O4 | Magnetic field | Self-healing and weight bearing | Soft robotics | [28] |
| Via additive manufacturing | ||||||
| Schiff base | Polysaccharides | Fe3O4 | Magnetic field | Printed structure alteration | 3D tissue engineering | [29] |
| Via self-assembly | ||||||
| Metal–ligand complex | Polyurethane | Fe3O4 | Autonomous | Fast and efficient self-healing during actuation | Smart biomimetic devices | [30] |
| Matrix | Filler | Input | Output | Application | Ref. |
|---|---|---|---|---|---|
| Via solvent casting | |||||
| Amorphous acrylate-based polymers | Fe3O4 and NdFeB particles | AC magnetic field | Carry a weight 64x heavier than own weight | Autonomous soft robots, minimally invasive surgery, digital logic circuits | [40] |
| Bio-based benzoxazine resin | Fe3O4 particles | AC magnetic field Light | Highest shape recovery of 99% within 26 s for 5 wt% Fe3O4 | Soft robotics | [46] |
| Poly(ε-caprolactone) (PCL) | Fe3O4 particle | AC magnetic field Light Direct heating | Fixity ratio >99%, recovery ratio >95% | Soft robotics | [41] |
| Epoxy resin (EP) | Fe particles | AC magnetic field Near-infrared | Microconed surface with anisotropic droplet sliding ability | Surfaces with tunable wettability, liquid transport, temperature switch | [42] |
| Thermoplastic polyurethane | Carbonyl iron particles | AC magnetic field Light | Recovery of permanent shape of the cilia after 30 s | Biomedical applications, microrobot | [43] |
| Thermoplastic polyurethane | Fe particles | AC magnetic field Light | Shape recovery >95.5% Excellent biocompatibility in vitro | Reconfigurable optics, surfaces with tunable wettability | [47] |
| Polylactic acid (PLA) | Fe3O4 particle | AC magnetic field | Snapper transition extremely sharp (>90%) | Scaffolds for bone repair | [44] |
| Carboxylic styrene-butadiene rubber (XSBR) | Fe3O4 particle, zinc dimetha-crylate (ZDMA) | AC magnetic field Heat | Fixity ratio >99%, recovery ratio >99%, tensile strength reached >30 MPa | Biomedical applications | [45] |
| Poly(ε-caprolactone) (PCL), thermoplastic polyurethane (TPU) | Fe3O4 particle, polydopamine | AC magnetic field Light | Fast light- and magnetic-responsive ability (magnetic field: 1 s; light: 5 s) | Soft robotics, artificial muscles | [48] |
| Via 3D printing | |||||
| Acrylate-based amorphous polymers | NdFeB particle | AC magnetic field Heat | Surfaces with controllable varying Poisson’s ratio | Soft robotic, biomedical applications | [49] |
| Polylactic acid (PLA) | Fe3O4 particle | AC magnetic field Heat | Fe3O4 enhances the mechanical strength of PLA by 17% | Personalized bio-designed tracheal scaffolds | [50] |
| Polylactic acid (PLA) | Fe3O4 particle | AC magnetic field Heat | Shape recovery in few seconds. Shape recovery at 40 °C, relevant in vivo | Bone tissue repair, biomedical applications | [51] |
| Matrix | Filler | Fabrication | Output | Application | Ref. |
|---|---|---|---|---|---|
| Polylactic acid (PLA) | Fe3O4 particles | Extrusion 3D printing | PLA is biodegradable within up to two years | Scaffolds for bone repair, tracheal scaffolds | [44,50,51] |
| Poly(ε-caprolactone), polyurethane | Fe3O4 particles, polydopamine | Solvent casting | PCL is biodegradable within up to two years | Soft robotics, artificial muscles | [48] |
| Gelatin methacryloyl | Fe3O4 particles | Two-photon polymerization | Complete degradation after two weeks | Helicoidal microrobots for drug delivery | [57,58] |
| Poly(ethylene glycol) diacrylate (PEGDA), pentaerythritol triacrylate (PETA) | Fe3O4 particles, 5-fluorou- racil | Micro machining + dip coating | Drug release from the microrobots investigated over 6 weeks | Multi-shape microrobots for drug delivery | [59,63] |
| Calcium alginate hydrogel (Ca-Alg) | Fe3O4 particles | Hydrodynamic electrospray ionization jetting | Ultrasound actuation used for on-demand drug release | Loaded capsules for drug delivery | [60] |
| Methacrylamide Chitosan (chMA) | Fe3O4 particles | Two-photon polymerization | Delivery of drugs and other cargo (gene, stem cell, imaging agent) | Helicoidal microrobots for drug delivery | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Z.; Zoumhani, O.; Boutry, C.M. Recent Advances in Magnetic Polymer Composites for BioMEMS: A Review. Materials 2023, 16, 3802. https://doi.org/10.3390/ma16103802
Liao Z, Zoumhani O, Boutry CM. Recent Advances in Magnetic Polymer Composites for BioMEMS: A Review. Materials. 2023; 16(10):3802. https://doi.org/10.3390/ma16103802
Chicago/Turabian StyleLiao, Zhengwei, Oualid Zoumhani, and Clementine M. Boutry. 2023. "Recent Advances in Magnetic Polymer Composites for BioMEMS: A Review" Materials 16, no. 10: 3802. https://doi.org/10.3390/ma16103802
APA StyleLiao, Z., Zoumhani, O., & Boutry, C. M. (2023). Recent Advances in Magnetic Polymer Composites for BioMEMS: A Review. Materials, 16(10), 3802. https://doi.org/10.3390/ma16103802








