Different Crystallization Behavior of Amorphous ITO Film by Rapid Infrared Annealing and Conventional Furnace Annealing Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Deposition of Amorphous ITO Films on Chemically Strengthened Glass Substrates
2.2. Annealing
2.3. Characterization
3. Results and Discussion
3.1. Structural Analysis
3.2. Morphological Analysis
3.3. Crystallization Kinetics Behavior Analysis
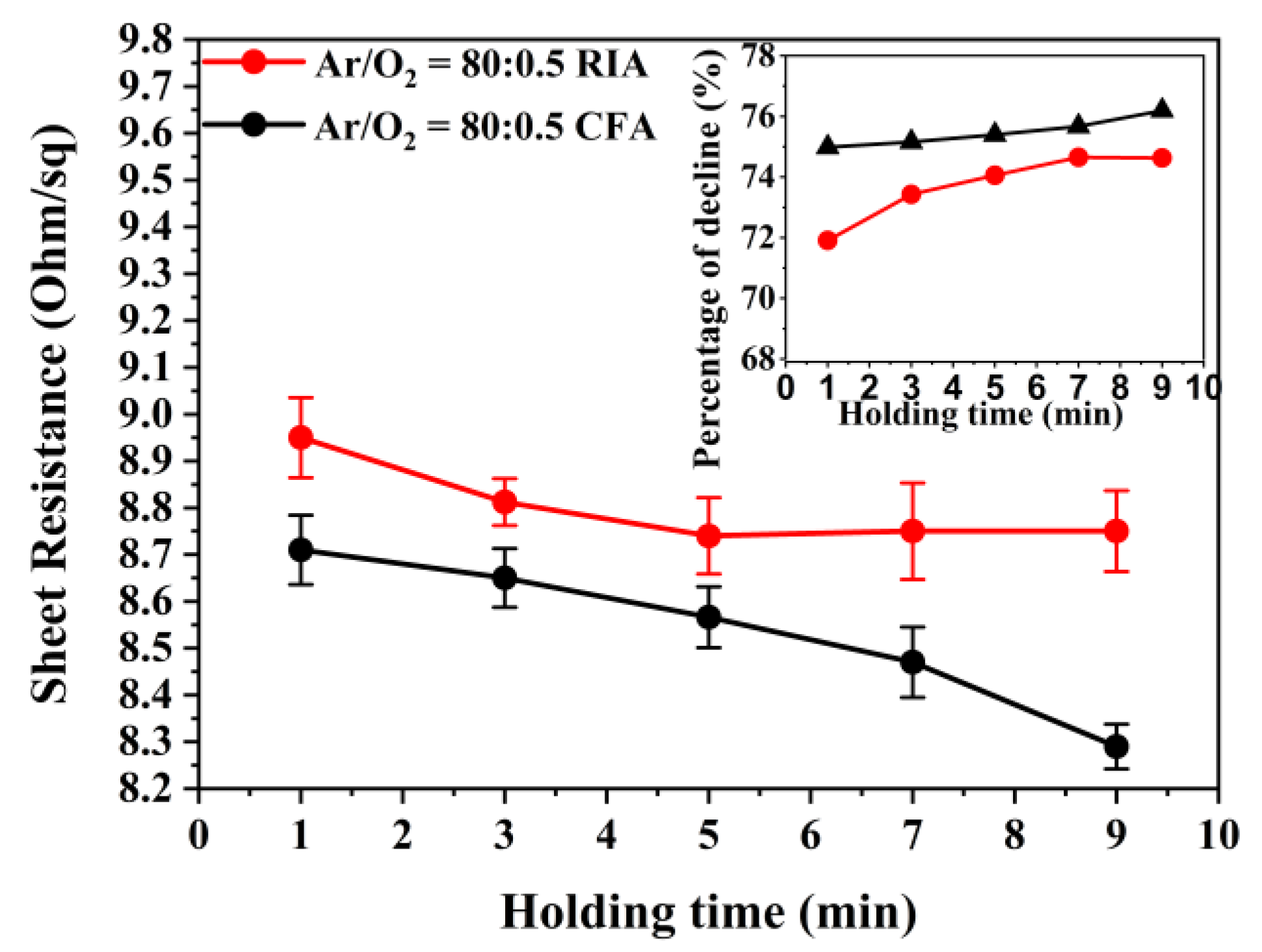
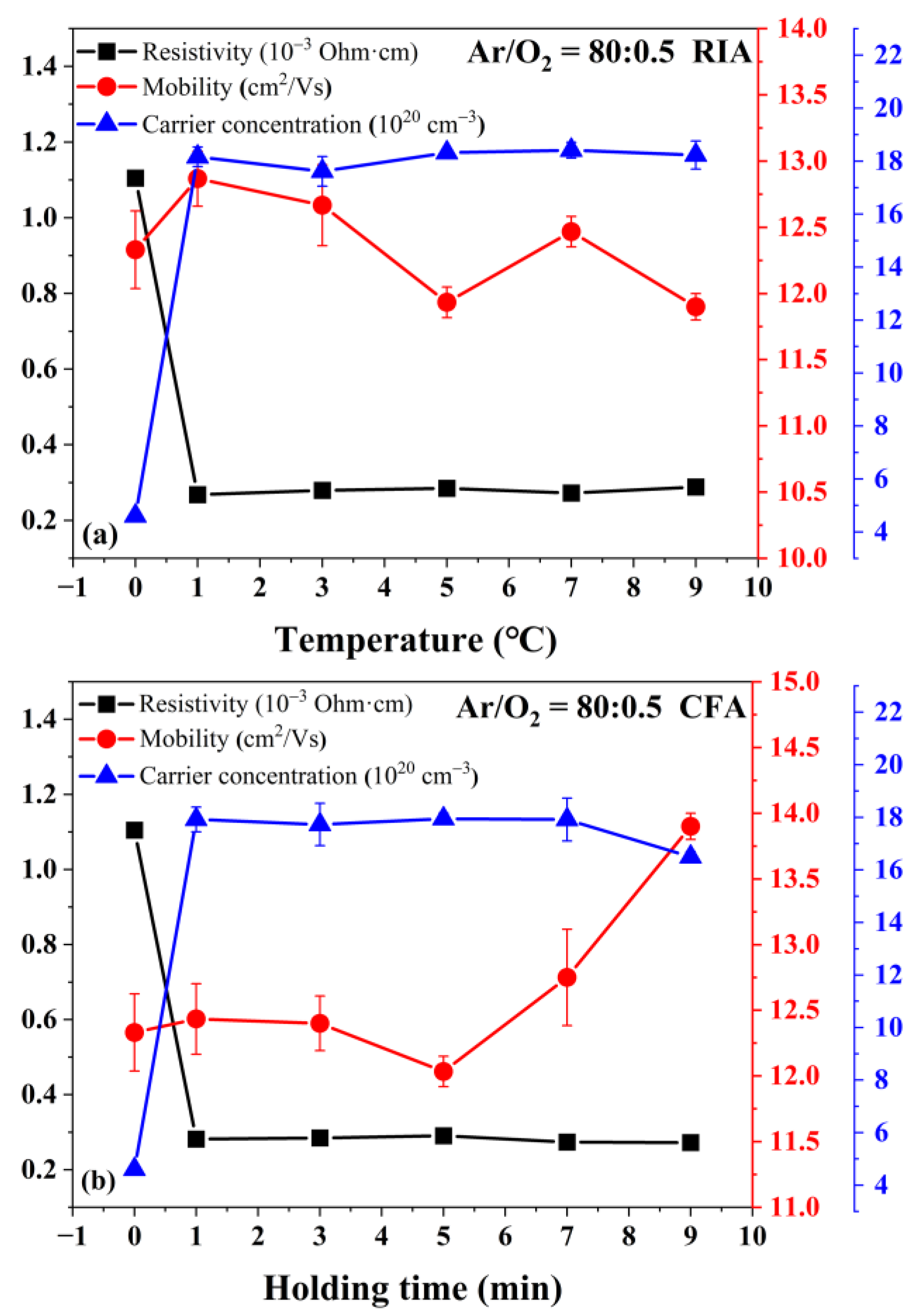
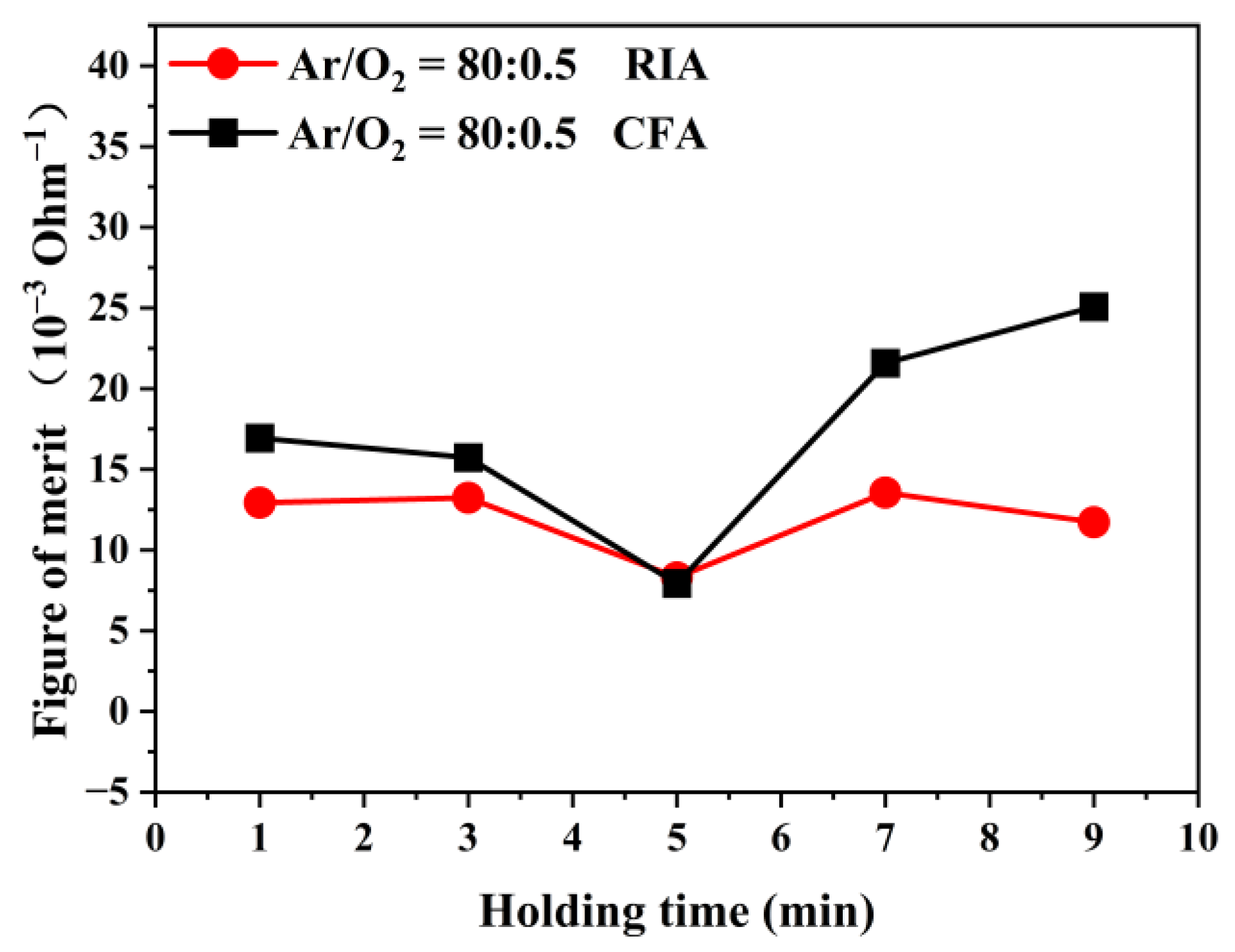
3.4. Electrical Properties Analysis
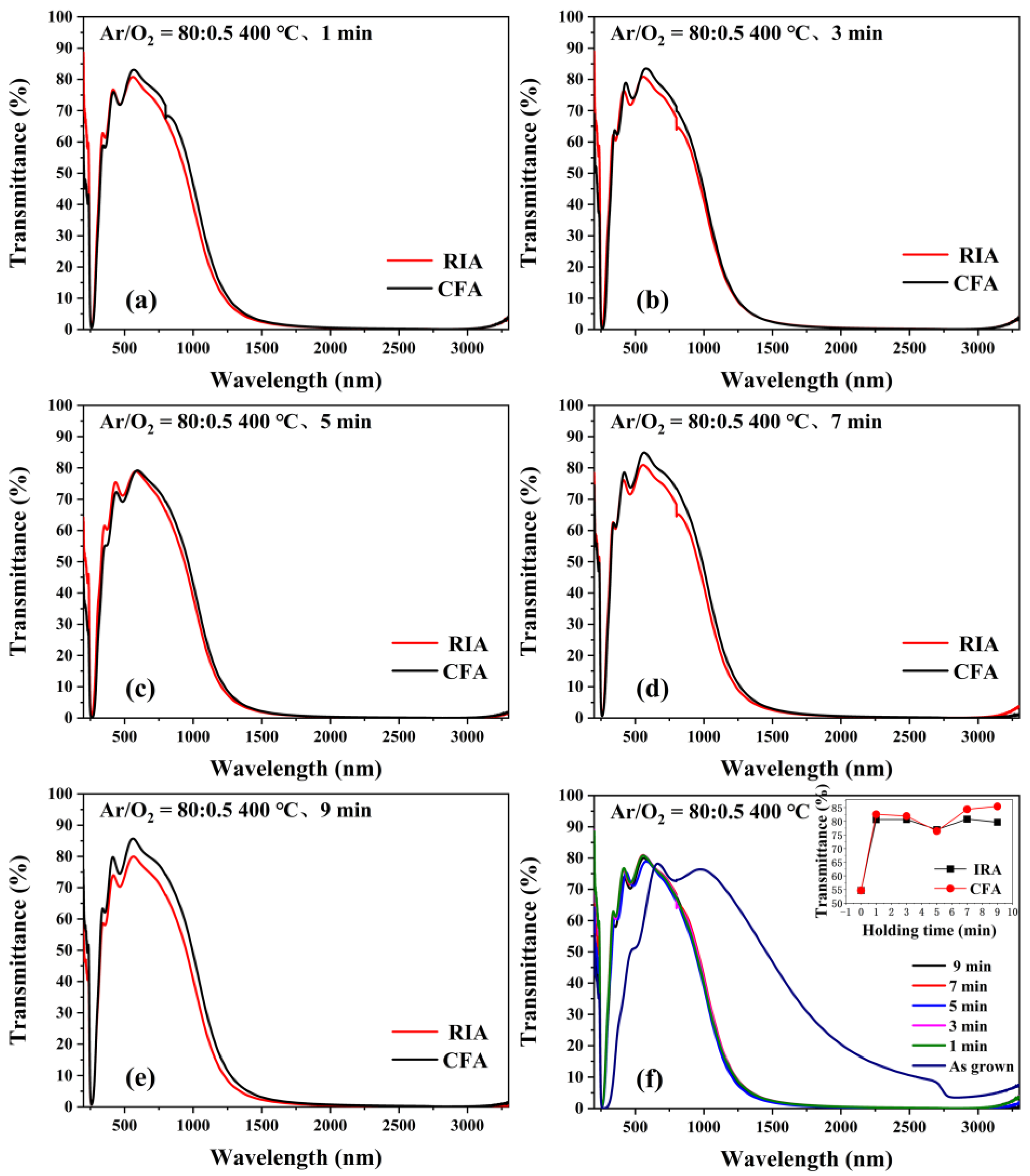
3.5. Optical Properties Analysis
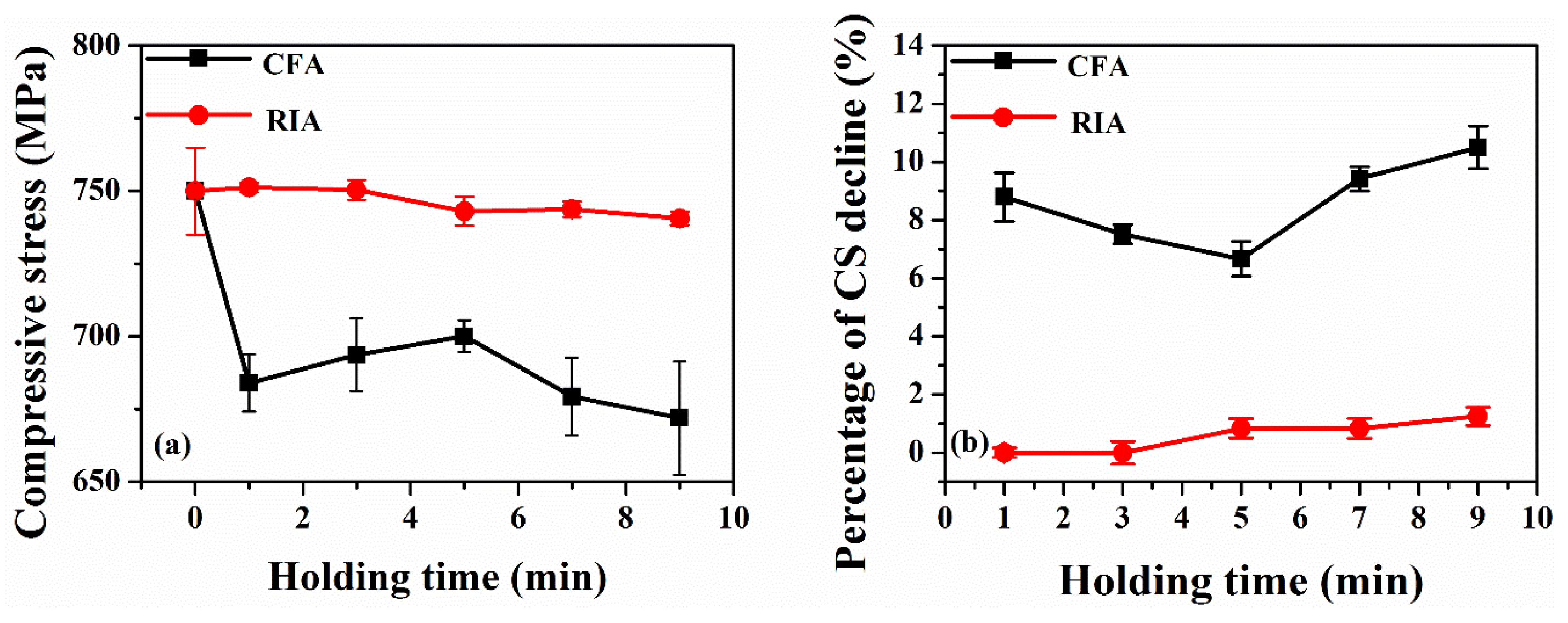
3.6. Compressive Stress Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.; Lee, D.; Song, S.; Cho, S.Y.; Bae, J.-S.; Kim, W.; Youn, B.; Kim, Y.; Lee, J.-S.; Bu, S.D.; et al. Surface chemistry modification in ITO films induced by Sn2+ ionic state variation. Curr. Appl. Phys. 2017, 17, 1415–1421. [Google Scholar] [CrossRef]
- KLi, K.D.; Chen, P.W.; Chang, K.S.; Hsu, S.C.; Jan, D.J. Indium-Zinc-Tin-Oxide film prepared by reactive magnetron sputtering for electrochromic ap-plications. Materials 2018, 11, 11112221. [Google Scholar]
- Zhao, S.; Lv, Z.; Guo, X.; Liu, C.; Wang, H.; Jiang, W.; Liu, S.; Wang, N.; Cui, Y.; Ding, W.; et al. The Diffusion of Low-Energy Methyl Group on ITO Film Surface and Its Impact on Optical-Electrical Properties. Materials 2018, 11, 1991. [Google Scholar] [CrossRef] [PubMed]
- Szyszka, B.; Dewald, W.; Gurram, S.K.; Pflug, A.; Schulz, C.; Siemers, M.; Sittinger, V.; Ulrich, S. Recent developments in the field of transparent conductive oxide films for spectral selective coatings, electronics and photovoltaics. Curr. Appl. Phys. 2012, 12, S2–S11. [Google Scholar] [CrossRef]
- Vilca-Huayhua, C.; Paz-Corrales, K.; Aragón, F.; Mathpal, M.; Villegas-Lelovsky, L.; Coaquira, J.; Pacheco-Salazar, D. Growth and vacuum post-annealing effect on the structural, electrical and optical properties of Sn-doped In2O3 thin films. Thin Solid Film. 2020, 709, 138207. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, H.; Zhang, T.-H.; Yu, S.-J.; Guo, P.-C.; Wang, Y.-X.; Yang, Z.-S. Optical and electrical properties of ITO film on flexible fluorphlogopite substrate. Ceram. Int. 2021, 47, 16980–16985. [Google Scholar] [CrossRef]
- Hacini, A.; Ali, A.H.; Adnan, N.N. Optimization of ITO thin film properties as a function of deposition time using the swanepoel method. Opt. Mater. 2021, 120, 111411. [Google Scholar] [CrossRef]
- Gwamuri, J.; Marikkannan, M.; Mayandi, J.; Bowen, P.K.; Pearce, J.M. Influence of Oxygen Concentration on the Performance of Ultra-Thin RF Magnetron Sputter Deposited Indium Tin Oxide Films as a Top Electrode for Photovoltaic Devices. Materials 2016, 9, 63. [Google Scholar] [CrossRef]
- Marciel, A.; Graça, M.; Bastos, A.; Pereira, L.; Kumar, J.S.; Borges, J.; Vaz, F.; Peres, M.; Magalhães, S.; Lorenz, K.; et al. Molybdenum Oxide Thin Films Grown on Flexible ITO-Coated PET Substrates. Materials 2021, 14, 821. [Google Scholar] [CrossRef] [PubMed]
- Mei-Zhen, G.; Ke, X.; Fahrner, W.R. Study of the morphological change of amorphous ITO films after temperature-humidity treatment. J. Non-Cryst. Solids 2009, 355, 2682–2687. [Google Scholar] [CrossRef]
- Kaźmierczak-Bałata, A.; Bodzenta, J.; Dehbashi, M.; Mayandi, J.; Venkatachalapathy, V. Influence of post processing on thermal conductivity of ITO thin films. Materials 2023, 16, 362. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Song, L.; Zhang, T. Terahertz reflection and visible light transmission of ITO films affected by annealing temperature and applied in metamaterial absorber. Vacuum 2017, 149, 12–18. [Google Scholar] [CrossRef]
- Kurokawa, T.; Mori, R.; Norimasa, O.; Chiba, T.; Eguchi, R.; Takashiri, M. Influences of substrate types and heat treatment conditions on structural and thermoelectric properties of nanocrystalline Bi2Te3 thin films formed by DC magnetron sputtering. Vacuum 2020, 179, 109535. [Google Scholar] [CrossRef]
- Nandihalli, N. Thermoelectric films and periodic structures and spin Seebeck effect systems: Facets of performance optimi-zation. Mater. Today Energy 2022, 25, 100965. [Google Scholar] [CrossRef]
- Li, J.; Jiang, L.; Chen, M.; Li, X.; Wei, Y.; Ma, Y.; Fu, Z.; Yan, Y. Structure and physical properties evolution of ITO film during amorphous-crystalline transition using a highly effective annealing technique. Ceram. Int. 2019, 45, 16214–16225. [Google Scholar] [CrossRef]
- Li, X.; Jiang, L.; Wang, Y.; Mohagheghian, I.; Dear, J.P.; Li, L.; Yan, Y. Correlation between K+-Na+ diffusion coefficient and flexural strength of chemically tempered aluminosilicate glass. J. Non-Cryst. Solids 2017, 471, 72–81. [Google Scholar] [CrossRef]
- Kurdesau, F.; Khripunov, G.; da Cunha, A.; Kaelin, M.; Tiwari, A. Comparative study of ITO layers deposited by DC and RF magnetron sputtering at room temperature. J. Non-Cryst. Solids 2006, 352, 1466–1470. [Google Scholar] [CrossRef]
- Bahari, A.; Sadeghi-Nik, A.; Shaikh, F.U.A.; Sadeghi-Nik, A.; Cerro-Prada, E.; Mirshafiei, E.; Roodbari, M. Experimental studies on rheological, mechanical, and microstructure properties of self-compacting concrete containing perovskite nanomaterial. Struct. Concr. 2022, 23, 564–578. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Zhang, J.C.; Chen, Z.T.; Egawa, T. Demonstration on GaN-based light-emitting diodes grown on 3C-SiC/Si(111). J. Appl. Phys. 2009, 106, 124506. [Google Scholar] [CrossRef]
- Jin, X.; Ma, B.; Zhao, K.; Zhang, Z.; Deng, J.; Luo, J.; Yuan, W. Effect of annealing on the thermoelectricity of indium tin oxide thin film thermocouples. Ceram. Int. 2020, 46, 4602–4609. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.; Kim, S.; Kim, B.-K.; Choi, Y.; Hwang, J.-H.; Kim, H.J. Flash lamp annealing of indium tin oxide thin-films deposited on polyimide backplanes. Thin Solid Film. 2017, 628, 88–95. [Google Scholar] [CrossRef]
- Park, J.; Buurma, C.; Sivananthan, S.; Kodama, R.; Gao, W.; Gessert, T. The effect of post-annealing on Indium Tin Oxide thin films by magnetron sputtering method. Appl. Surf. Sci. 2014, 307, 388–392. [Google Scholar] [CrossRef]
- Raoufi, D.; Kiasatpour, A.; Fallah, H.R.; Rozatian, A.S.H. Surface characterization and microstructure of ITO thin films at different annealing temperatures. Appl. Surf. Sci. 2007, 253, 9085–9090. [Google Scholar] [CrossRef]
- Mergel, D.; Stass, W.; Ehl, G.; Barthel, D. Oxygen incorporation in thin films of In2O3: Sn, prepared by radio frequency sputtering. J. Appl. Phys. 2000, 88, 2437–2442. [Google Scholar] [CrossRef]
- Shi, X.; Tian, Y.; Shen, C.; Wang, C.; Gao, H.J. Electrodeposition of Sb2Se3 on indium-doped tin oxides substrate: Nucleation and growth. Appl. Surf. Sci. 2012, 258, 2169–2173. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Xia, Y.; Ma, T.; Wang, P.W. Characteristics of ITO films fabricated on glass substrates by high intensity pulsed ion beam method. J. Non-Cryst. Solids 2007, 353, 2244–2249. [Google Scholar] [CrossRef]
- Ali, A.H.; Shuhaimi, A.; Hassan, Z. Structural, optical and electrical characterization of ITO, ITO/Ag and ITO/Ni transparent conductive electrodes. Appl. Surf. Sci. 2014, 288, 599–603. [Google Scholar] [CrossRef]
- Han, B.; Chen, L.; Jin, S.; Guo, S.; Park, J.; Yoo, H.S.; Park, J.H.; Zhao, B.; Jung, Y.M. Modulating Mechanism of the LSPR and SERS in Ag/ITO Film: Carrier Density Effect. J. Phys. Chem. Lett. 2021, 12, 7612–7618. [Google Scholar] [CrossRef]
- Khachatryan, H.; Kim, D.J.; Kim, M.; Kim, H.K. Roll-to-Roll fabrication of ITO thin film for flexible optoelectronics applications: The role of post-annealing. Mater. Sci. Semicond. Process. 2018, 88, 51–56. [Google Scholar] [CrossRef]
- Shajari, D.; Bahari, A.; Gill, P.; Mohseni, M. Synthesis and tuning of gold nanorods with surface plasmon resonance. Opt. Mater. 2017, 64, 376–383. [Google Scholar] [CrossRef]
- Bhattacharyya, J.; Chaudhturi, S.; Pal, A.K. Studies on the optical properties and the Burstein-moss shift in indium tin oxide films. Phys. Status Solidi 2020, 95, 239–248. [Google Scholar] [CrossRef]
- Fujiwara, H.; Kondo, M. Effects of carrier concentration on the dielectric function of ZnO: Ga and In2O3: Sn studied by spec-troscopic ellipsometry: Analysis of free-carrier and band-edge absorption. Phys. Rev. B 2005, 71, 075109. [Google Scholar] [CrossRef]
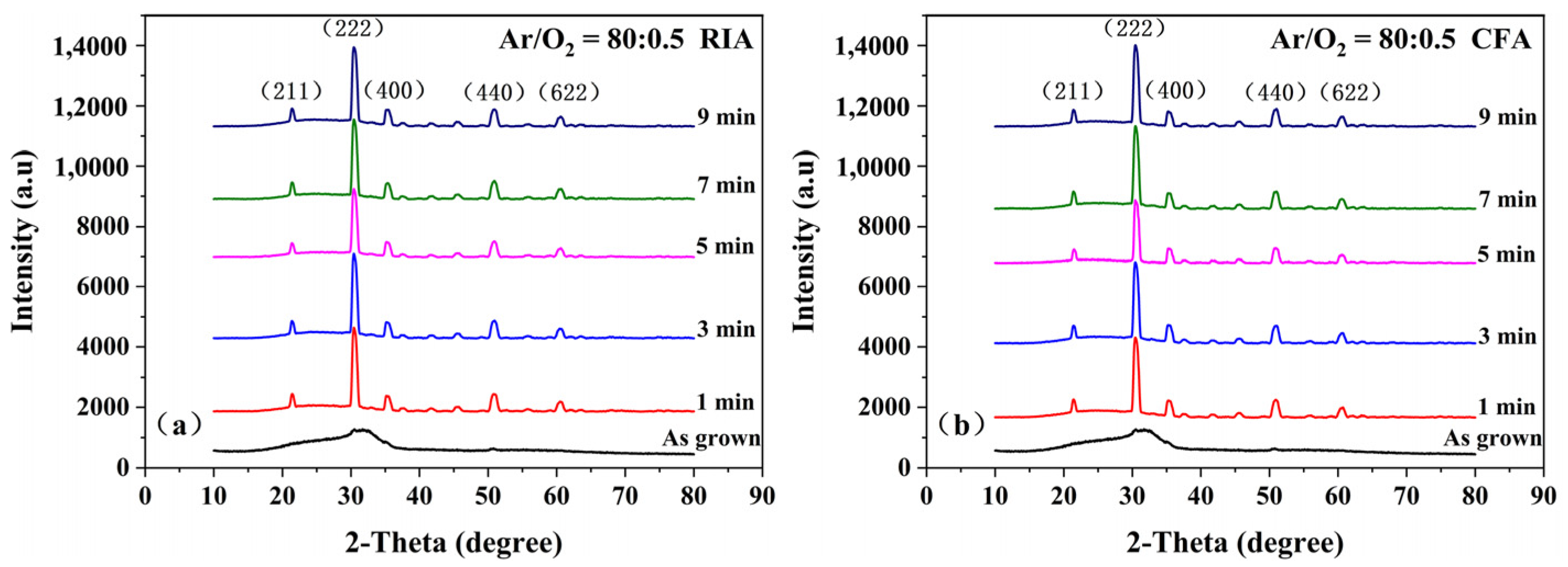
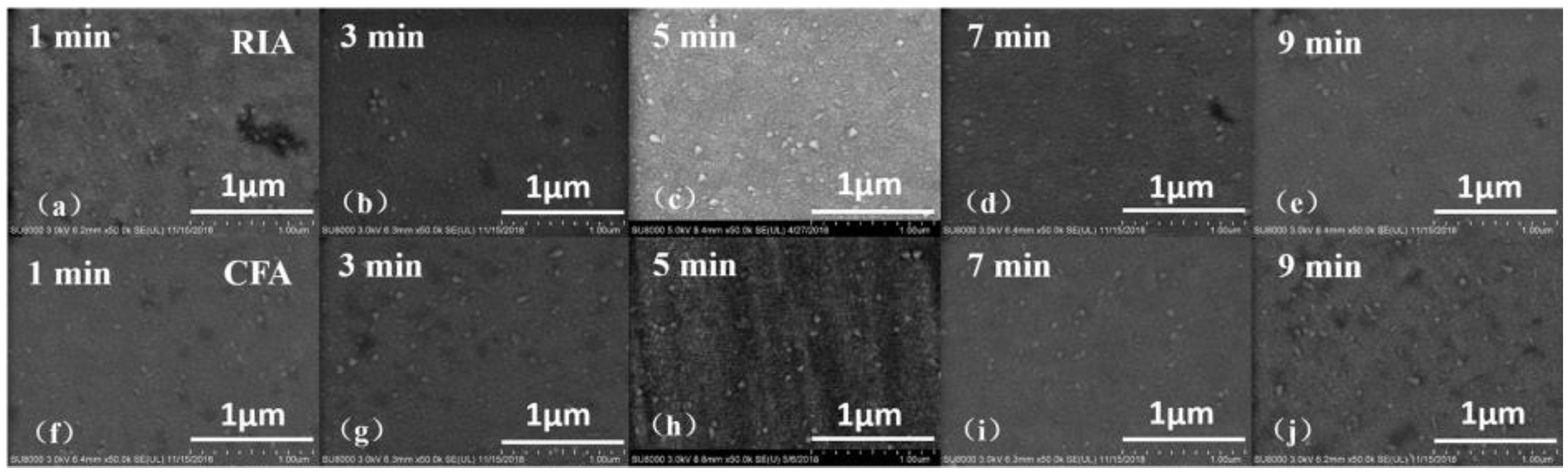
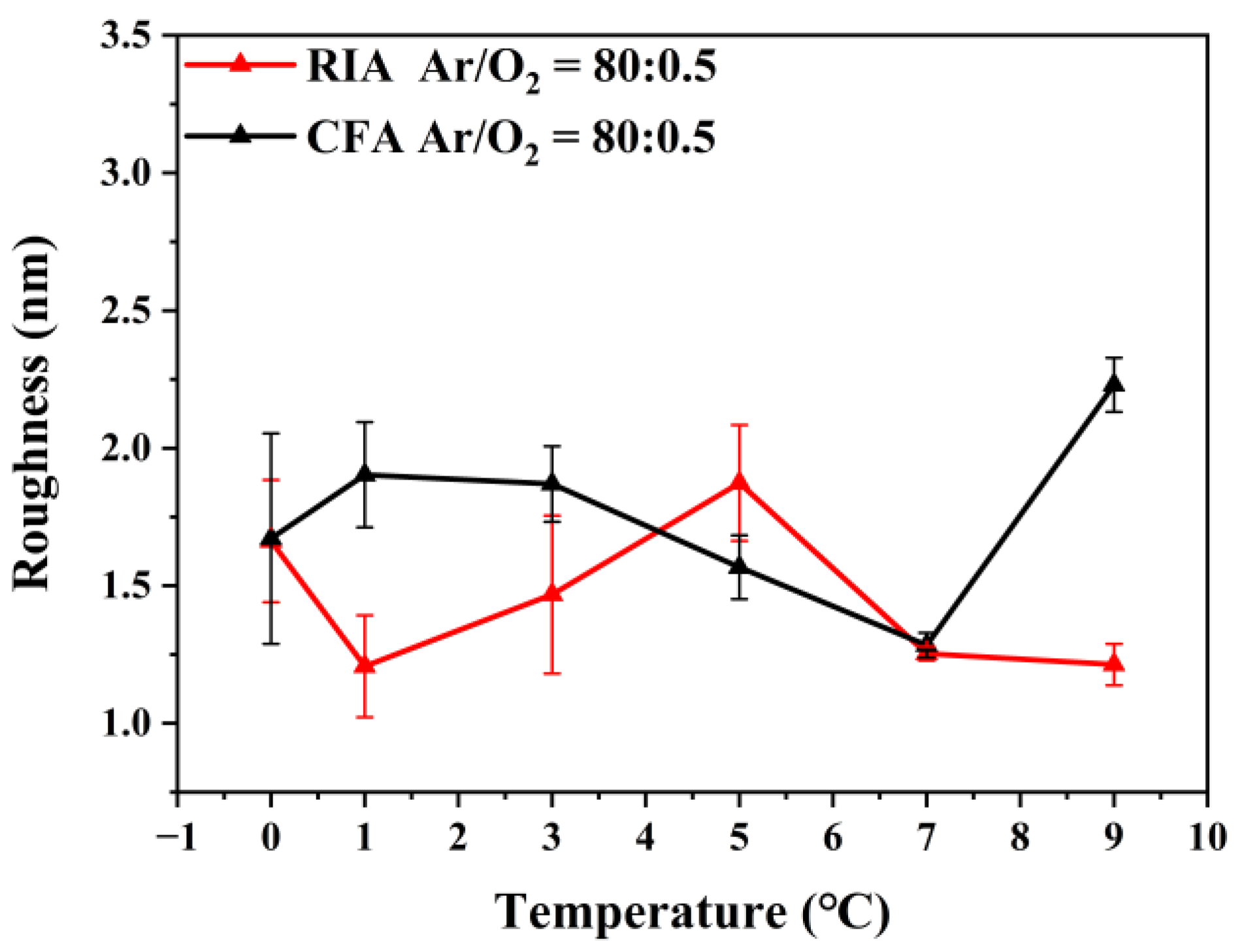
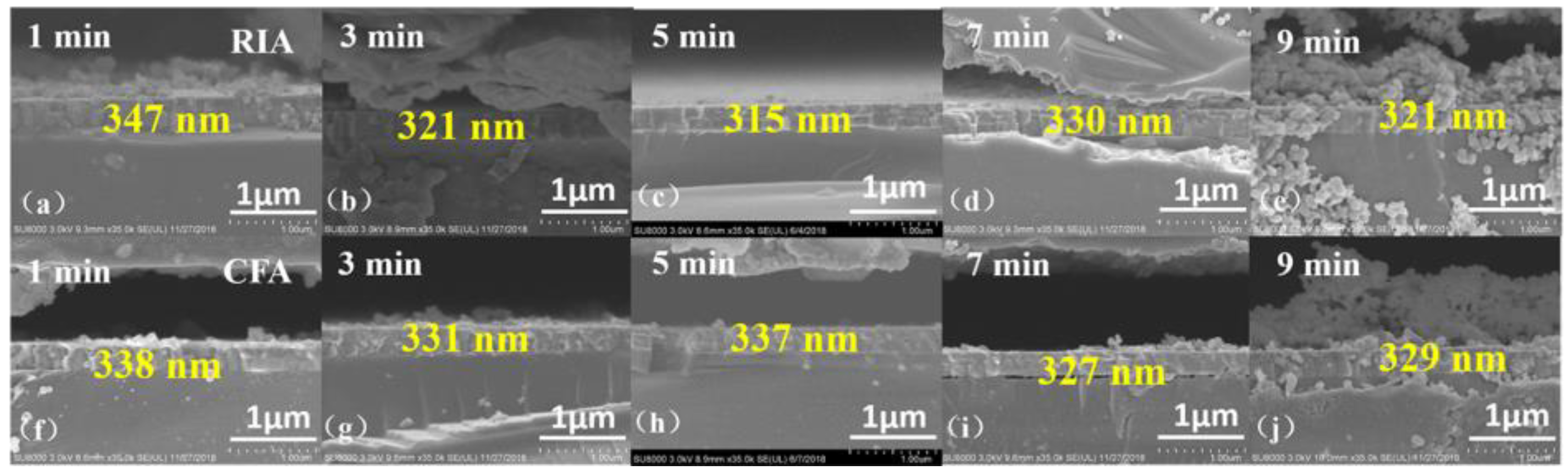


| Ar/O2 | Holding Time (min) | RIA | CFA | ||||
|---|---|---|---|---|---|---|---|
| 2θ Angle of (222) Peak | Grain Size of (222) Peak (nm) | Average Grain Size (nm) | 2θ Angle of (222) Peak | Grain Size of (222) Peak (nm) | Average Grain Size (nm) | ||
| 80:0.5 | 1 | 30.36 | 13.29 | 13.59 | 30.41 | 13.39 | 14.20 |
| 3 | 30.36 | 13.56 | 14.01 | 30.42 | 13.09 | 15.04 | |
| 5 | 30.34 | 13.27 | 14.11 | 30.32 | 12.91 | 14.42 | |
| 7 | 30.34 | 13.46 | 14.32 | 30.40 | 12.54 | 14.27 | |
| 9 | 30.34 | 13.39 | 13.58 | 30.36 | 11.18 | 14.22 | |
| Holding Time | Crystallization Method | |
|---|---|---|
| RIA | CFA | |
| 1 min | 54.39 nm | 54.16 nm |
| 3 min | 52.98 nm | 58.75 nm |
| 5 min | 53.67 nm | 52.00 nm |
| 7 min | 53.27 nm | 53.02 nm |
| 9 min | 56.22 nm | 54.95 nm |
| Holding Time | 1 min | 3 min | 5 min | 7 min | 9 min | |
|---|---|---|---|---|---|---|
| Technology | ||||||
| RIA | 347 nm | 321 nm | 315 nm | 330 nm | 321 nm | |
| CFA | 338 nm | 331 nm | 337 nm | 327 nm | 329 nm | |
| Eg (eV) | Holding Time | |||||
|---|---|---|---|---|---|---|
| Amorphous | 1 min | 3 min | 5 min | 7 min | 9 min | |
| RIA | 4.98 | 4.97 | 4.96 | 4.99 | 4.94 | 4.94 |
| CFA | 4.98 | 4.96 | 4.96 | 4.99 | 4.94 | 4.94 |
| Holding Time (min) | RIA | CFA | ||
|---|---|---|---|---|
| Surface Compressive Stress (MPa) | Percentage of the Compressive Stress Decline (%) | Surface Compressive Stress (MPa) | Percentage of the Compressive Stress Decline (%) | |
| 1 | 751 | 0 | 684 | 8.8 |
| 3 | 750 | 0 | 693 | 7.6 |
| 5 | 743 | 0.9 | 700 | 6.7 |
| 7 | 743 | 0.9 | 679 | 9.4 |
| 9 | 740 | 1.3 | 671 | 10.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Jiang, L.; Li, X.; Luo, J.; Liu, J.; Wang, M.; Yan, Y. Different Crystallization Behavior of Amorphous ITO Film by Rapid Infrared Annealing and Conventional Furnace Annealing Technology. Materials 2023, 16, 3803. https://doi.org/10.3390/ma16103803
Li J, Jiang L, Li X, Luo J, Liu J, Wang M, Yan Y. Different Crystallization Behavior of Amorphous ITO Film by Rapid Infrared Annealing and Conventional Furnace Annealing Technology. Materials. 2023; 16(10):3803. https://doi.org/10.3390/ma16103803
Chicago/Turabian StyleLi, Jiaming, Liangbao Jiang, Xiaoyu Li, Junjie Luo, Jiaxi Liu, Minbo Wang, and Yue Yan. 2023. "Different Crystallization Behavior of Amorphous ITO Film by Rapid Infrared Annealing and Conventional Furnace Annealing Technology" Materials 16, no. 10: 3803. https://doi.org/10.3390/ma16103803
APA StyleLi, J., Jiang, L., Li, X., Luo, J., Liu, J., Wang, M., & Yan, Y. (2023). Different Crystallization Behavior of Amorphous ITO Film by Rapid Infrared Annealing and Conventional Furnace Annealing Technology. Materials, 16(10), 3803. https://doi.org/10.3390/ma16103803





