Fabrication of Mechanically Strong Silica Aerogels with the Thermally Induced Phase Separation (TIPS) Method of Poly(methyl methacrylate)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PMMA-Modified Aerogels via TIPS
2.3. Characterization
3. Results and Discussion
3.1. Sol-Gel Polymerizations
3.2. Turbidity Measurements of PMMA Solutions
3.3. Formation of PMMA-Modified Aerogels by TIPS
3.4. Aerogel Morphology
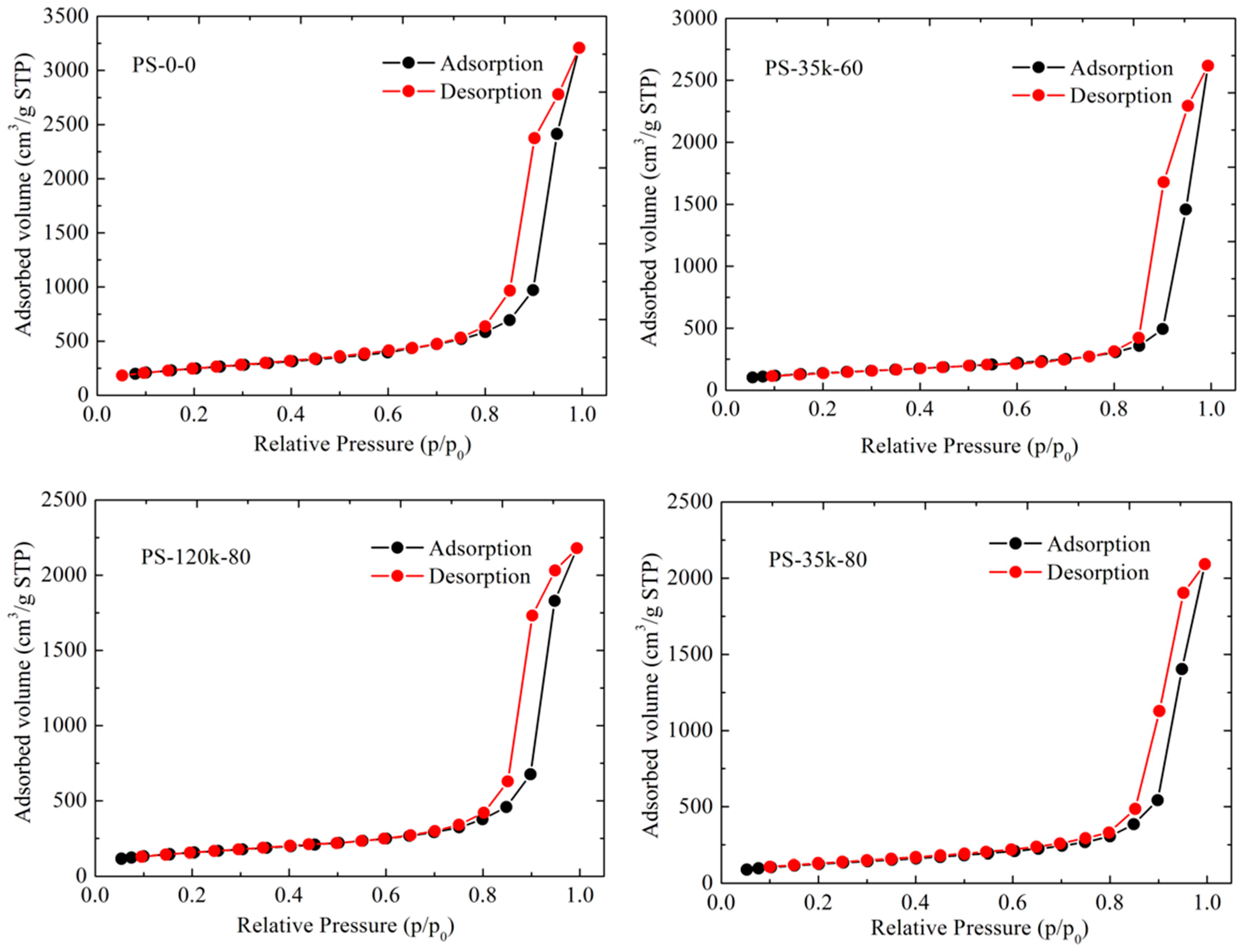
3.5. Pore Structure Analysis
3.6. Thermal Conductivity
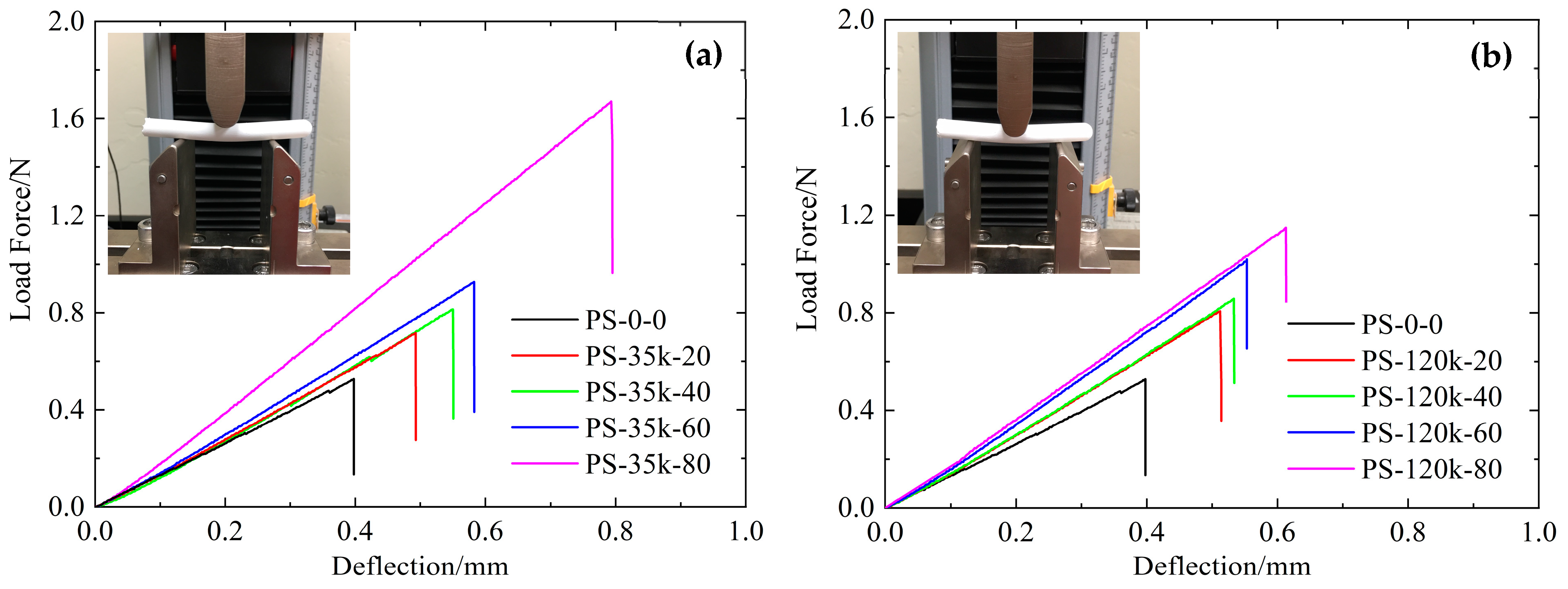
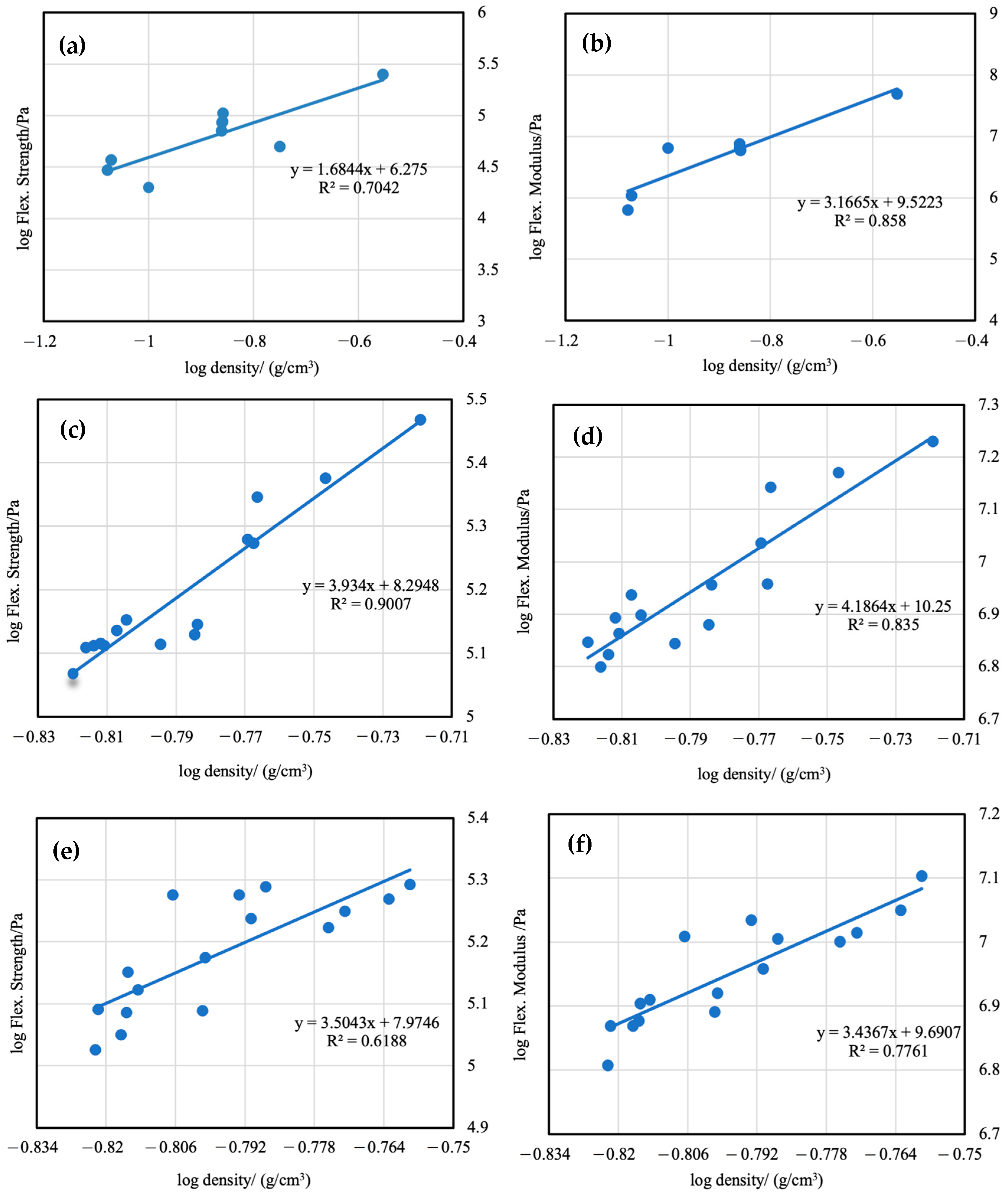
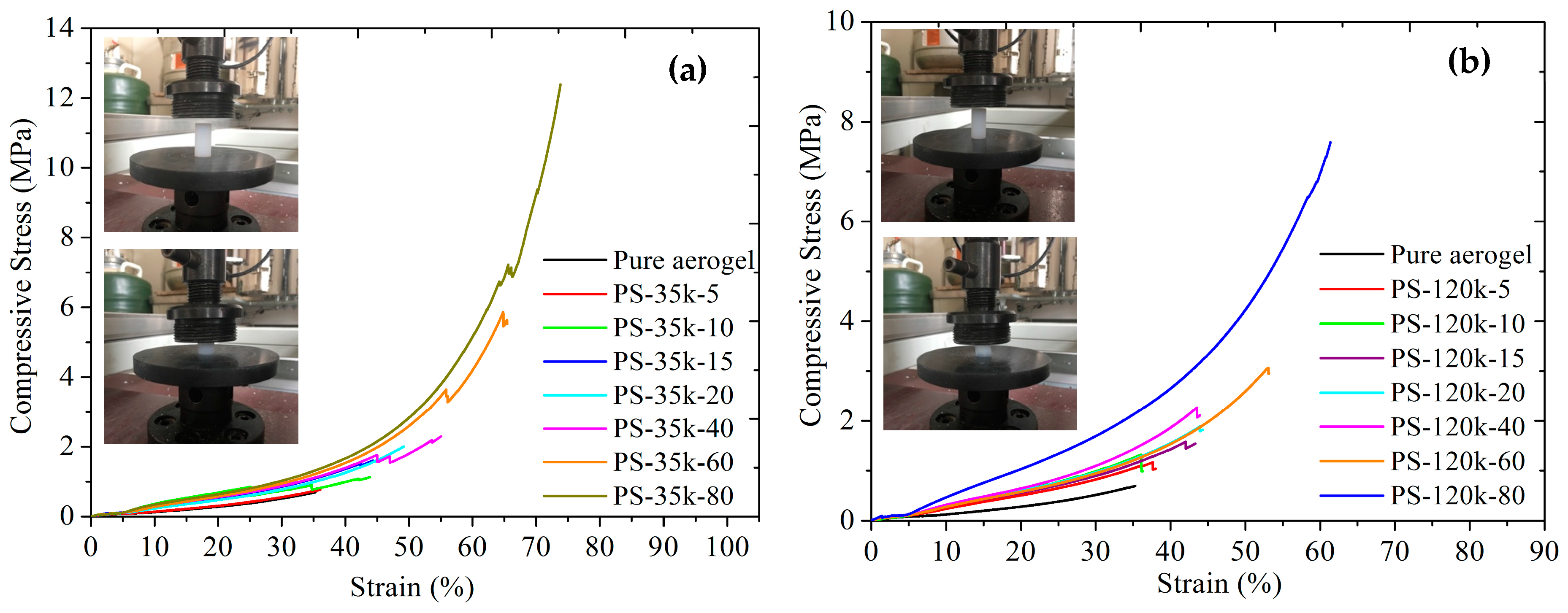
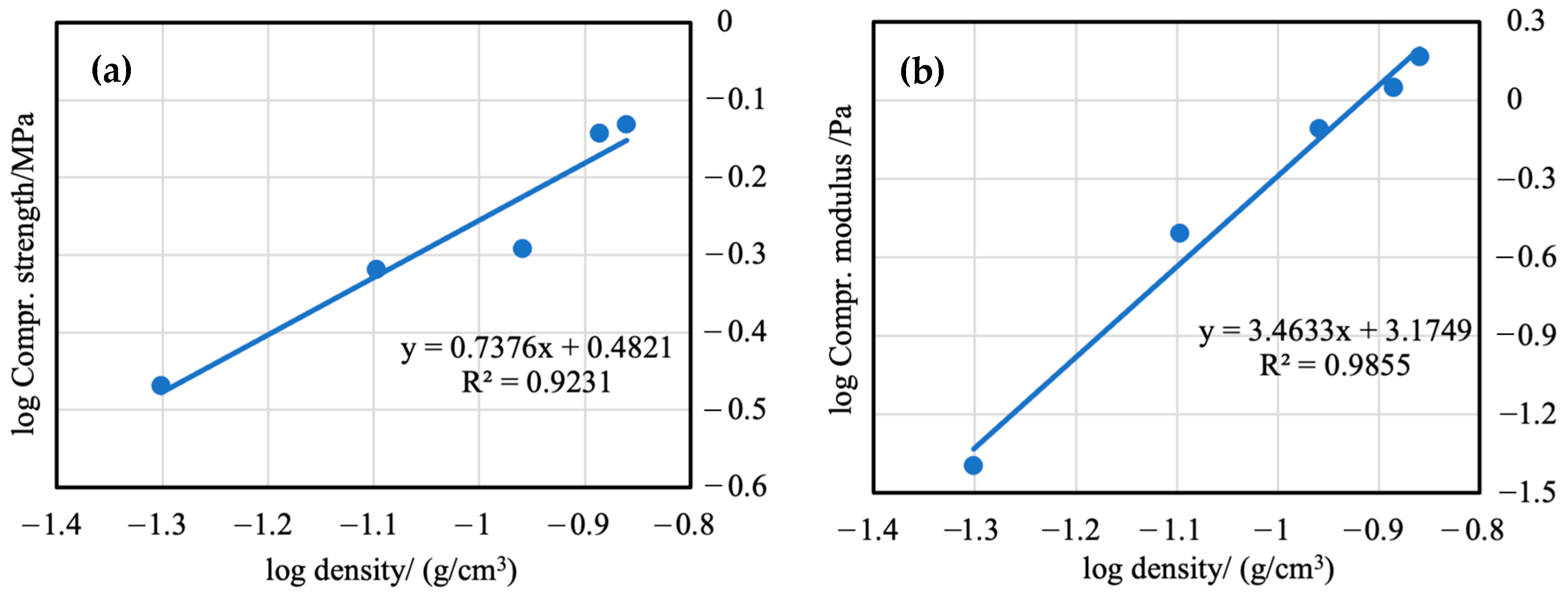
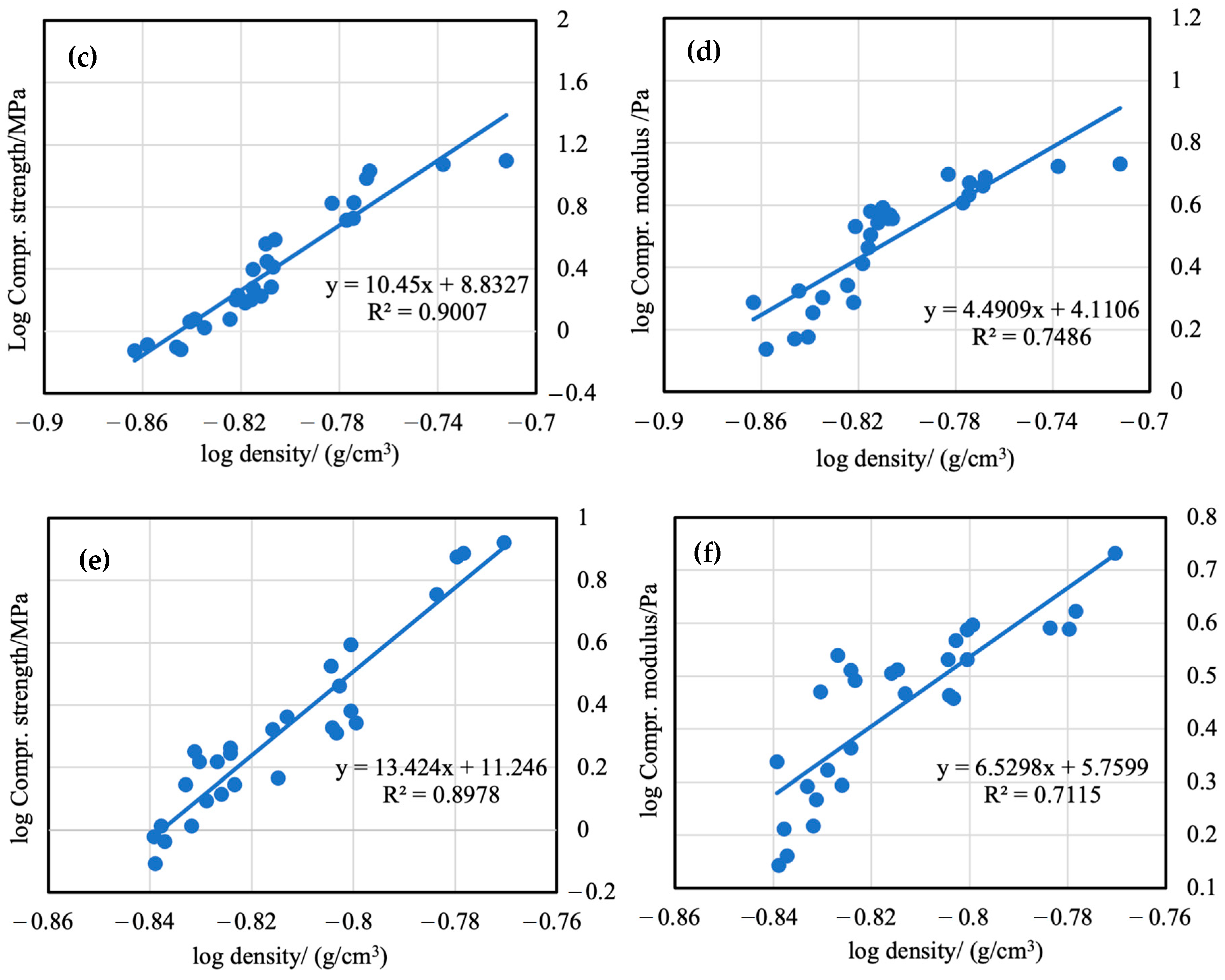
3.7. Mechanical Properties of Aerogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, S.M.; Sakamoto, J. Applications of aerogels in space exploration. In Aerogels Handbook; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Springer: New York, NY, USA, 2011; pp. 721–746. [Google Scholar]
- Kharzheev, Y.N. Use of silica aerogels in Cherenkov counters. Phys. Part. Nucl. 2008, 39, 107–135. [Google Scholar] [CrossRef]
- Yan, Z.G. A review of aerogels and their application as a multi-functional building material. Appl. Mech. Mater. 2013, 253, 564–567. [Google Scholar] [CrossRef]
- Zhao, S.; Siqueira, G.; Drdova, S.; Norris, D.; Ubert, C.; Bonnin, A.; Galmarini, S.; Ganobjak, M.; Pan, Z.; Brunner, S.; et al. Additive manufacturing of silica aerogels. Nature 2020, 584, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Iswar, S.; Galmarini, S.; Bonanomi, L.; Wernery, J.; Roumeli, E.; Nimalshantha, S.; Ben Ishai, A.M.; Lattuada, M.; Koebel, M.M.; Malfait, W.J. Dense and strong, but superinsulating silica aerogel. Acta Mater. 2021, 213, 116959. [Google Scholar] [CrossRef]
- Parmenter, K.E.; Milstein, F. Mechanical properties of silica aerogels. J. Non-Cryst. Solids 1998, 223, 179–189. [Google Scholar] [CrossRef]
- Babiarczuk, B.; Lewandowski, D.; Kierzek, K.; Detyna, J.; Jones, W.; Kaleta, J.; Krzak, J. Mechanical properties of silica aerogels controlled by synthesis parameters. J. Non-Cryst. Solids 2023, 606, 122171. [Google Scholar] [CrossRef]
- Lucas, E.M.; Doescher, M.S.; Ebenstein, D.M.; Wahl, K.J.; Rolison, D.R. Silica aerogels with enhanced durability, 30-nm mean pore-size, and improved immersibility in liquids. J. Non-Cryst. Solids 2004, 350, 244–252. [Google Scholar] [CrossRef]
- Iswar, S.; Malfait, W.J.; Balog, S.; Winnefeld, F.; Lattuada, M.; Koebel, M.M. Effect of aging on silica aerogel properties. Microporous Mesoporous Mater. 2017, 241, 293–302. [Google Scholar] [CrossRef]
- Lin, J.; Li, G.; Liu, W.; Qiu, R.; Wei, H.; Zong, K.; Cai, X. A review of recent progress on the silica aerogel monoliths: Synthesis, reinforcement, and applications. J. Mater. Sci. 2021, 56, 10812–10833. [Google Scholar] [CrossRef]
- Nadargi, D.Y.; Rao, A.V. Methyltriethoxysilane: New precursor for synthesizing silica aerogels. J. Alloys Compd. 2009, 467, 397–404. [Google Scholar] [CrossRef]
- Cui, S.; Liu, Y.; Fan, M.; Cooper, A.T.; Lin, B.; Liu, X.; Han, G.; Shen, X. Temperature dependent microstructure of MTES modified hydrophobic silica aerogels. Mater. Lett. 2011, 65, 606–609. [Google Scholar] [CrossRef]
- Wong, J.C.H.; Kaymak, H.; Brunner, S.; Koebel, M.M. Mechanical properties of monolithic silica aerogels made from polyethoxydisiloxanes. Microporous Mesoporous Mater. 2014, 183, 23–29. [Google Scholar] [CrossRef]
- Zhang, G.H.; Dass, A.; Rawashdeh, A.M.M.; Thomas, J.; Counsil, J.A.; Sotiriou-Leventis, C.; Fabrizio, E.F.; Ilhan, F.; Vassilaras, P.; Scheiman, D.A.; et al. Isocyanate-crosslinked silica aerogel monoliths: Preparation and characterization. J. Non-Cryst. Solids 2004, 350, 152–164. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Zhang, G.; Rawashdeh, A.-M.M. Nanoengineering strong silica aerogels. Nano Lett. 2002, 2, 957–960. [Google Scholar] [CrossRef]
- Leventis, N. Three-Dimensional Core-Shell Superstructures: Mechanically strong aerogels. Acc. Chem. Res. 2007, 40, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.N.; Jana, S.C.; Lama, B.; Espe, M.P. Self-crosslinkable poly(urethane urea)-reinforced silica aerogels. RSC Adv. 2015, 5, 71551–71558. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Fabrizio, E.F.; Ilhan, F.; Dass, A.; Zhang, G.H.; Vassilaras, P.; Johnston, J.C.; Leventis, N. Cross-linking amine-modified silica aerogels with epoxies: Mechanically strong lightweight porous materials. Chem. Mater. 2005, 17, 1085–1098. [Google Scholar] [CrossRef]
- Ilhan, U.F.; Fabrizio, E.F.; McCorkle, L.; Scheiman, D.A.; Dass, A.; Palczer, A.; Meador, M.B.; Johnston, J.C.; Leventis, N. Hydrophobic monolithic aerogels by nanocasting polystyrene on amine-modified silica. J. Mater. Chem. 2006, 16, 3046–3054. [Google Scholar] [CrossRef]
- Mulik, S.; Sotiriou-Leventis, C.; Churu, G.; Lu, H.B.; Leventis, N. Cross-linking 3D assemblies of nanoparticles into mechanically strong aerogels by surface-initiated free-radical polymerization. Chem. Mater. 2008, 20, 5035–5046. [Google Scholar] [CrossRef]
- Boday, D.J.; Keng, P.Y.; Muriithi, B.; Pyun, J.; Loy, D.A. Mechanically reinforced silica aerogel nanocomposites via surface initiated atom transfer radical polymerizations. J. Mater. Chem. 2010, 20, 6863–6865. [Google Scholar] [CrossRef]
- Boday, D.J.; Loy, D.A. Strengthening silica aerogels with surface initiated ATRP cross-linked poly(methyl methacrylate). J. Non-Cryst. Solids 2015, 427, 114–119. [Google Scholar] [CrossRef]
- Boday, D.J.; Loy, D.A.; DeFriend, K.A.; Wilson, K.V.; Coder, D. Polymer-silica nanocomposite aerogels with enhanced mechanical properties using chemical vapor deposition (CVD) of cyanoacrylates. MRS Online Proc. Libr. 2007, 1007, 1–6. [Google Scholar] [CrossRef]
- Boday, D.J.; Stover, R.J.; Muriithi, B.; Keller, M.W.; Wertz, J.T.; Obrey, K.A.D.; Loy, D.A. Strong, low-density nanocomposites by chemical vapor deposition and polymerization of cyanoacrylates on aminated silica aerogels. ACS Appl. Mater. Interface 2009, 1, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Boday, D.J.; Stover, R.J.; Muriithi, B.; Loy, D.A. Strong, low density, hexylene- and phenylene-bridged polysilsesquioxane aerogel-polycyanoacrylate composites. J. Mater. Sci. 2011, 46, 6371–6377. [Google Scholar] [CrossRef]
- Choi, H.; Parale, V.G.; Kim, T.; Choi, Y.-S.; Tae, J.; Park, H.-H. Structural and mechanical properties of hybrid silica aerogel formed using triethoxy(1-phenylethenyl)silane. Microporous Mesoporous Mater. 2020, 298, 110092. [Google Scholar] [CrossRef]
- Sanli, D.; Erkey, C. Effect of polymer molecular weight and deposition temperature on the properties of silica aerogel/hydroxy-terminated poly(dimethylsiloxane) nanocomposites prepared by reactive supercritical deposition. J. Supercrit. Fluids 2015, 105, 99–107. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Vivod, S.L.; McCorkle, L.; Quade, D.; Sullivan, R.M.; Ghosn, L.J.; Clark, N.; Capadona, L.A. Reinforcing polymer cross-linked aerogels with carbon nanofibers. J. Mater. Chem. 2008, 18, 1843–1852. [Google Scholar] [CrossRef]
- Boday, D.J.; Muriithi, B.; Stover, R.J.; Loy, D.A. Polyaniline nanofiber-silica composite aerogels. J. Non-Cryst. Solids 2012, 358, 1575–1580. [Google Scholar] [CrossRef]
- Finlay, K.A.; Gawryla, M.D.; Schiraldi, D.A. Effects of fiber reinforcement on clay aerogel composites. Materials 2015, 8, 5440–5451. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Yuan, B.; Ding, S.; Wang, D.; Wang, G.; Li, H. Heat insulation properties of silica aerogel/glass fiber composites fabricated by press forming. Mater. Lett. 2012, 75, 204–206. [Google Scholar] [CrossRef]
- Li, L.; Yalcin, B.; Nguyen, B.N.; Meador, M.A.B.; Cakmak, M. Flexible nanofiber-reinforced aerogel (xerogel) synthesis, manufacture, and characterization. ACS Appl. Mater. Interfaces 2009, 1, 2491–2501. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xie, S.; Sun, P.; Zhao, Z.; Li, L.; Shao, X.; Liu, X.; Xin, Z. Synergistic effect of graphene and silicon dioxide hybrids through hydrogen bonding self-assembly in elastomer composites. RSC Adv. 2018, 8, 17813–17825. [Google Scholar] [CrossRef]
- Loche, D.; Malfatti, L.; Carboni, D.; Alzari, V.; Mariani, A.; Casula, M.F. Incorporation of graphene into silica-based aerogels and application for water remediation. RSC Adv. 2016, 6, 66516–66523. [Google Scholar] [CrossRef]
- Karamikamkar, S.; Fashandi, M.; Naguib, H.E.; Park, C.B. In situ interface design in graphene-embedded polymeric silica aerogel with organic/inorganic hybridization. ACS Appl. Mater. Interfaces 2020, 12, 26635–26648. [Google Scholar] [CrossRef]
- Lei, Y.; Hu, Z.; Cao, B.; Chen, X.; Song, H. Enhancements of thermal insulation and mechanical property of silica aerogel monoliths by mixing graphene oxide. Mater. Chem. Phys. 2017, 187, 183–190. [Google Scholar] [CrossRef]
- Meti, P.; Mahadik, D.B.; Lee, K.-Y.; Wang, Q.; Kanamori, K.; Gong, Y.-D.; Park, H.-H. Overview of organic–inorganic hybrid silica aerogels: Progress and perspectives. Mater. Des. 2022, 222, 111091. [Google Scholar] [CrossRef]
- Dervin, S.; Lang, Y.; Perova, T.; Hinder, S.H.; Pillai, S.C. Graphene oxide reinforced high surface area silica aerogels. J. Non-Cryst. Solids 2017, 465, 31–38. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New trends in bio-based aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, J.; Mi, Q.; Yu, J.; Song, R.; Zhang, J. Transparent cellulose–silica composite aerogels with excellent flame retardancy via an in situ sol–gel process. ACS Sustain. Chem. Eng. 2017, 5, 11117–11123. [Google Scholar] [CrossRef]
- Pirzada, T.; Ashrafi, Z.; Xie, W.; Khan, S.A. Cellulose silica hybrid nanofiber aerogels: From sol–gel electrospun nanofibers to multifunctional aerogels. Adv. Funct. Mater. 2020, 30, 1907359. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Demilecamps, A.; Zhang, Y.; Brunner, S.; Huber, L.; Tingaut, P.; Rigacci, A.; Budtova, T.; Koebel, M.M. Strong, thermally superinsulating biopolymer-silica aerogel hybrids by cogelation of silicic acid with pectin. Angew. Chem. 2015, 127, 14490–14494. [Google Scholar] [CrossRef]
- Maleki, H.; Huesing, N. Silica-silk fibroin hybrid (bio)aerogels: Two-step versus one-step hybridization. J. Sol-Gel Sci. Technol. 2021, 98, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Montes, S.; Hayati-Roodbari, N.; Putz, F.; Huesing, N. Compressible, thermally insulating, and fire retardant aerogels through self-assembling silk fibroin biopolymers inside a silica structure—An approach towards 3D printing of aerogels. ACS Appl. Mater. Interfaces 2018, 10, 22718–22730. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Shahbazi, M.-A.; Montes, S.; Hosseini, S.H.; Eskandari, M.R.; Zaunschirm, S.; Verwanger, T.; Mathur, S.; Milow, B.; Krammer, B.; et al. Mechanically strong silica-silk fibroin bioaerogel: A hybrid scaffold with ordered honeycomb micromorphology and multiscale porosity for bone regeneration. ACS Appl. Mater. Interfaces 2019, 11, 17256–17269. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Q.; Song, D.; Qi, B.; Zhang, Y.; Shao, Y.; Shao, Z. Chitosan–silica composite aerogels: Preparation, characterization and congo red adsorption. J. Sol-Gel Sci. Technol. 2015, 76, 501–509. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Jeong, E.; Fischer, B.; Zhang, Y.; Xu, H.; Angelica, E.; Risen, W.M., Jr.; Suggs, J.W.; Koebel, M.M. Facile one-pot synthesis of mechanically robust biopolymer–silica nanocomposite aerogel by cogelation of silicic acid with chitosan in aqueous media. ACS Sustain. Chem. Eng. 2016, 4, 5674–5683. [Google Scholar] [CrossRef]
- Takeshita, S.; Yoda, S. Translucent, hydrophobic, and mechanically tough aerogels constructed from trimethylsilylated chitosan nanofibers. Nanoscale 2017, 9, 12311–12315. [Google Scholar] [CrossRef]
- Okada, K.; Nandi, M.; Maruyama, J.; Oka, T.; Tsujimoto, T.; Kondoh, K.; Uyama, H. Fabrication of mesoporous polymer monolith: A template-free approach. Chem. Commun. 2011, 47, 7422–7424. [Google Scholar] [CrossRef]
- Yoneda, S.; Han, W.; Hasegawa, U.; Uyama, H. Facile fabrication of poly(methyl methacrylate) monolith via thermally induced phase separation by utilizing unique cosolvency. Polymer 2014, 55, 3212–3216. [Google Scholar] [CrossRef]
- Wang, X.; Jana, S.C. Synergistic hybrid organic-inorganic aerogels. ACS Appl. Mater. Interface 2013, 5, 6423–6429. [Google Scholar] [CrossRef] [PubMed]
- Yolsal, U.; Horton, T.A.R.; Wang, M.; Shaver, M.P. Polymer-supported lewis acids and bases: Synthesis and applications. Prog. Polym. Sci. 2020, 111, 101313. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Mohsin, M.A.; Mcewen, I.J. Alcohol water cosolvent systems for poly(methyl methacrylate). Polymer 1987, 28, 1569–1572. [Google Scholar] [CrossRef]
- Wang, J.; Petit, D.; Ren, S. Transparent thermal insulation silica aerogels. Nanoscale Adv. 2020, 2, 5504–5515. [Google Scholar] [CrossRef] [PubMed]
- Venkateswara Rao, A.; Bhagat, S.D. Synthesis and physical properties of TEOS-based silica aerogels prepared by two step (acid–base) sol–gel process. Solid State Sci. 2004, 6, 945–952. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Becer, C.R.; Guerrero-Sanchez, C.; Hoeppener, S.; Schubert, U.S. Solubility and thermoresponsiveness of PMMA in alcohol-water solvent mixtures. Aust. J. Chem. 2010, 63, 1173–1178. [Google Scholar] [CrossRef]
- Rindfleisch, F.; DiNoia, T.P.; McHugh, M.A. Solubility of polymers and copolymers in supercritical CO2. J. Phys. Chem. 1996, 100, 15581–15587. [Google Scholar] [CrossRef]
- O’Neill, M.L.; Cao, Q.; Fang, M.; Johnston, K.P.; Wilkinson, S.P.; Smith, C.D.; Kerschner, J.L.; Jureller, S.H. Solubility of homopolymers and copolymers in carbon dioxide. Ind. Eng. Chem. Res. 1998, 37, 3067–3079. [Google Scholar] [CrossRef]
- Zhdanov, S.P.; Kosheleva, L.S.; Titova, T.I. Ir study of hydroxylated silica. Langmuir 1987, 3, 960–967. [Google Scholar] [CrossRef]
- El Rassy, H.; Pierre, A.C. NMR and IR spectroscopy of silica aerogels with different hydrophobic characteristics. J. Non-Cryst. Solids 2005, 351, 1603–1610. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Zu, G.; Shimizu, T.; Kanamori, K.; Zhu, Y.; Maeno, A.; Kaji, H.; Shen, J.; Nakanishi, K. Transparent, superflexible doubly cross-linked polyvinylpolymethylsiloxane aerogel superinsulators via ambient pressure drying. ACS Nano 2018, 12, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances.1. computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Yun, S.; Luo, H.; Gao, Y. Ambient-pressure drying synthesis of large resorcinol–formaldehyde-reinforced silica aerogels with enhanced mechanical strength and superhydrophobicity. J. Mater. Chem. A 2014, 2, 14542–14549. [Google Scholar] [CrossRef]


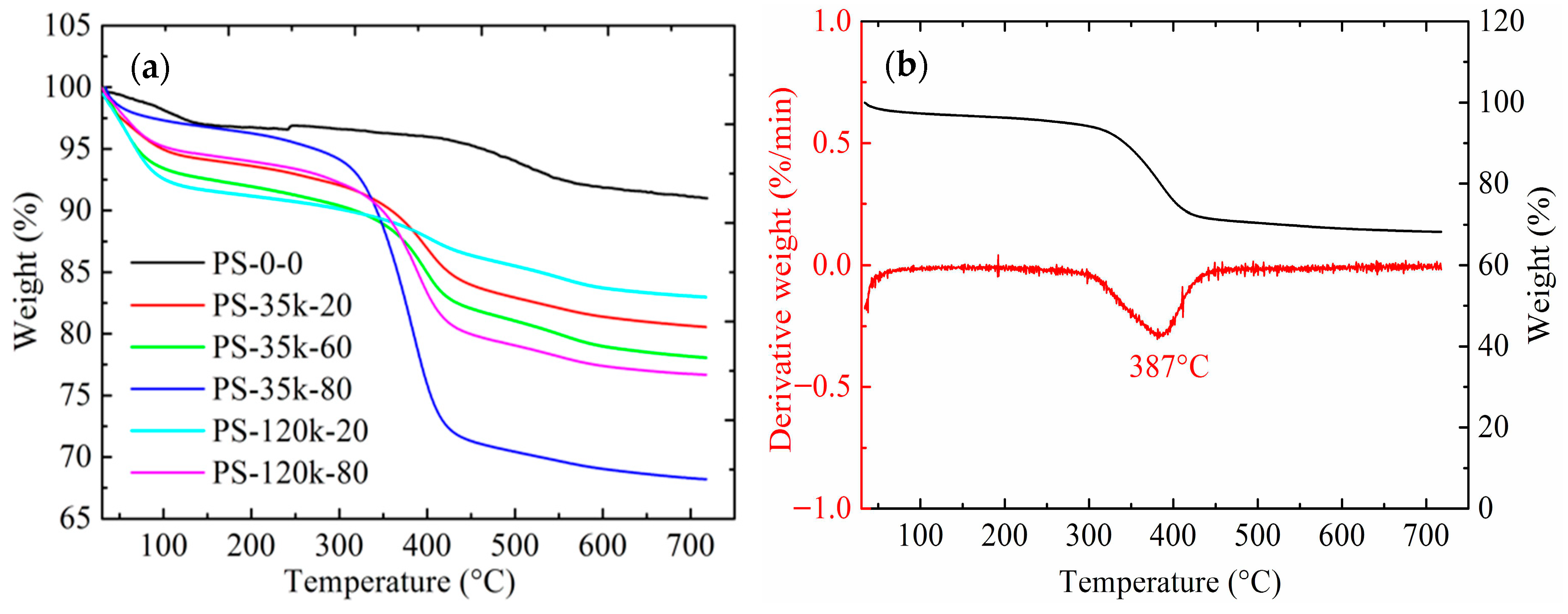
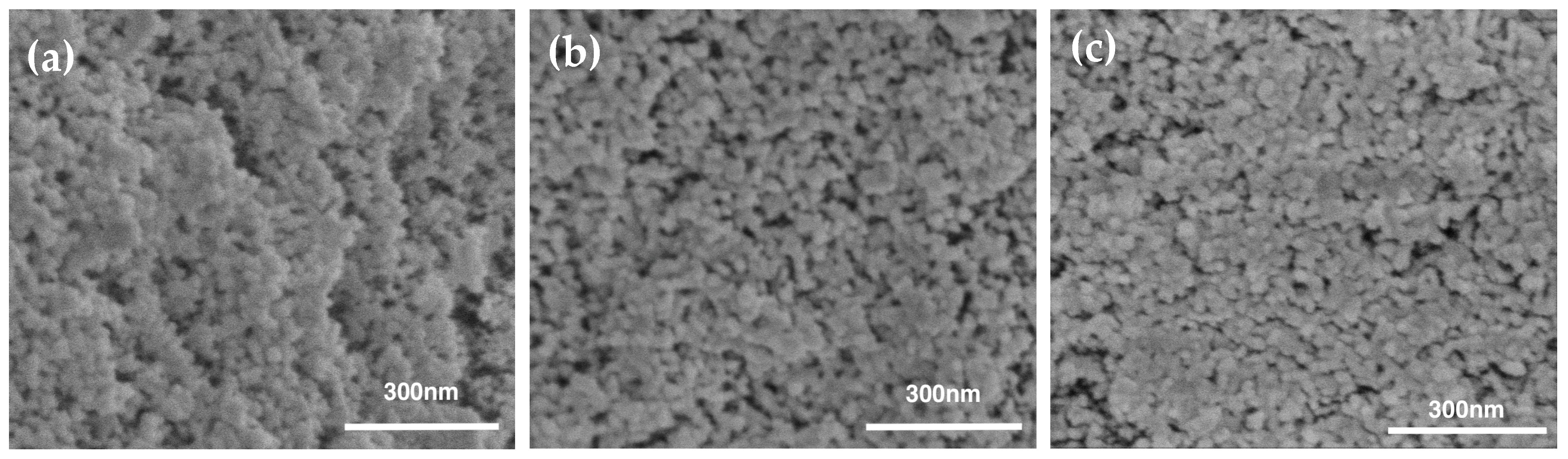
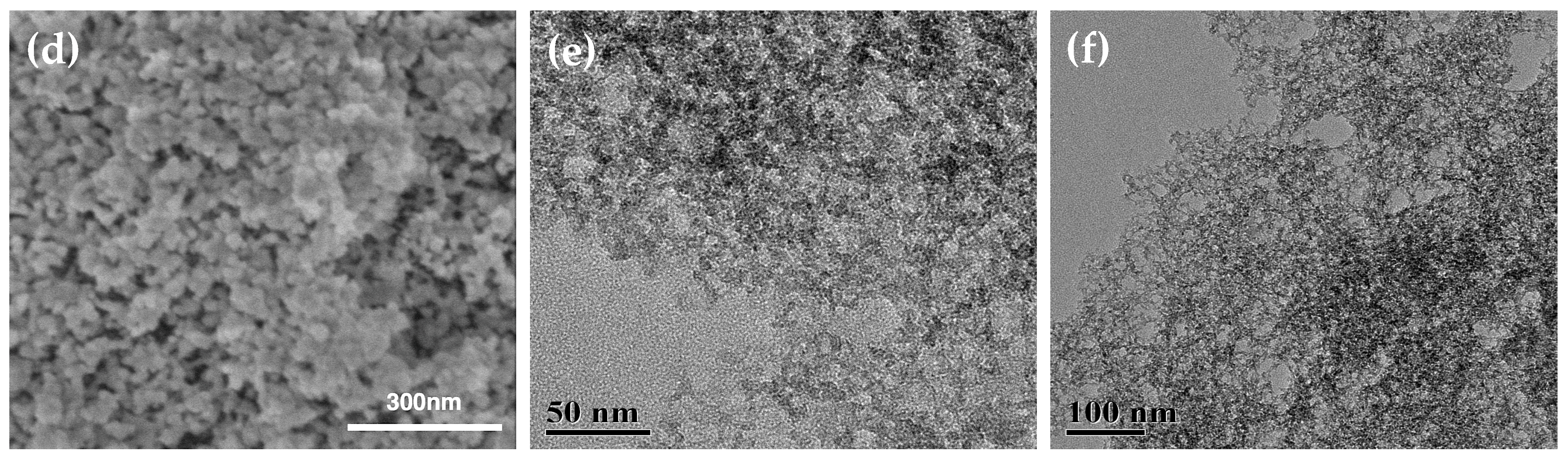
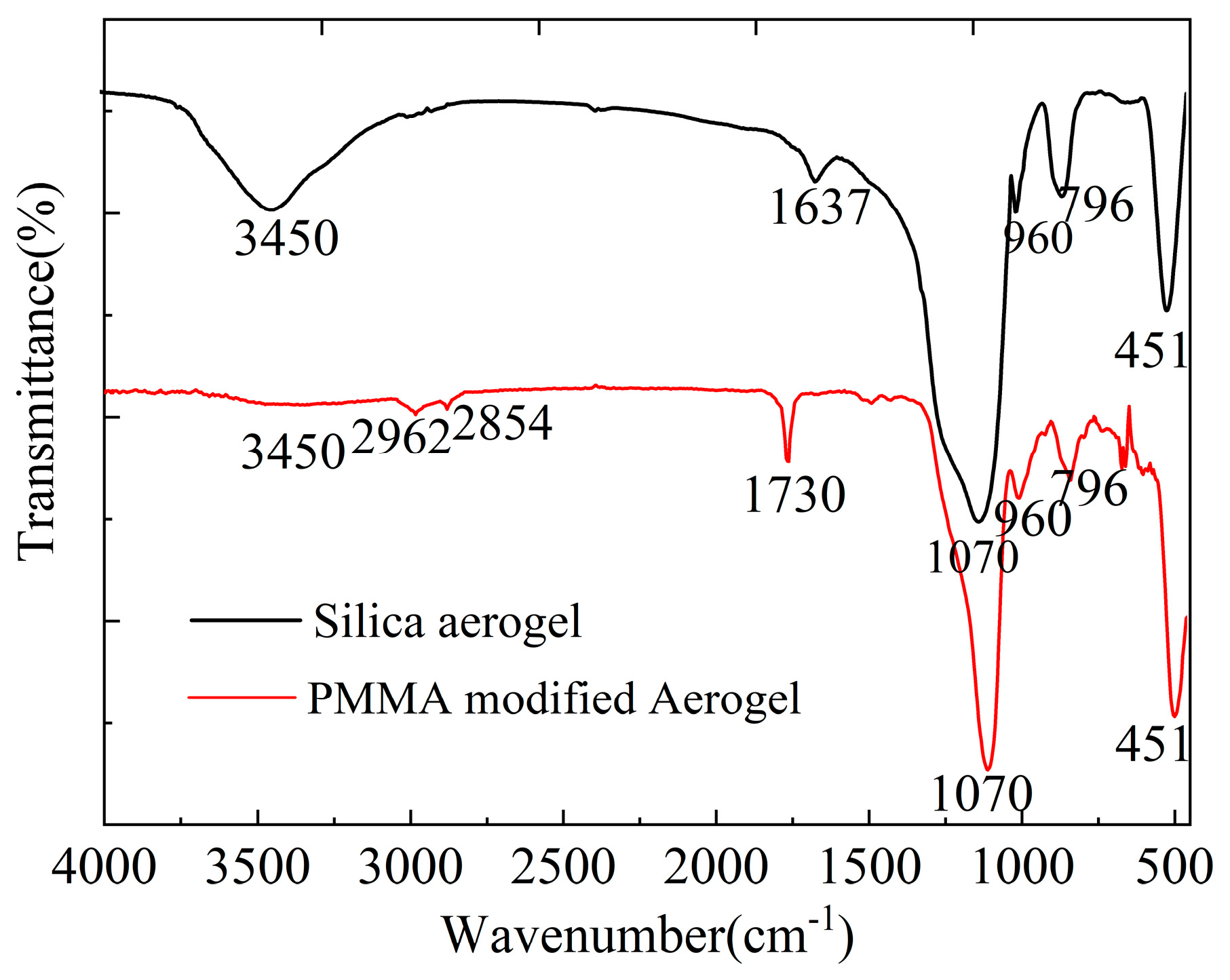


| Samples * | Bulk Density (g/cm3) | Porosity (%) | Percent PMMA Incorp (%) ** | PMMA Content Densities (g/g) *** | Mass Loss TGA (%) | PMMA Content TGA (g/g) |
|---|---|---|---|---|---|---|
| PS-0-0 | 0.139 ± 0.002 | 93.7 | 0 | 0 | 9.1 | 0 |
| PS-35k-5 | 0.140 ± 0.002 | 93.4 | 20.0 | 0.0071 | 12.2 | 0.0273 |
| PS-35k-10 | 0.147 ± 0.004 | 93.0 | 80.0 | 0.0576 | 14.6 | 0.0459 |
| PS-35k-15 | 0.152 ± 0.003 | 92.8 | 86.6 | 0.0855 | 15.3 | 0.0587 |
| PS-35k-20 | 0.153 ± 0.004 | 92.5 | 70.0 | 0.0915 | 19.5 | 0.0912 |
| PS-35k-40 | 0.154 ± 0.001 | 92.4 | 37.5 | 0.1080 | 21.0 | 0.1070 |
| PS-35k-60 | 0.165 ± 0.004 | 91.7 | 43.3 | 0.1580 | 22.9 | 0.1142 |
| PS-35k-80 | 0.178 ± 0.010 | 90.1 | 48.7 | 0.2190 | 31.8 | 0.2370 |
| PS-120k-5 | 0.143 ± 0.002 | 93.2 | 80.0 | 0.0280 | 12.5 | 0.0282 |
| PS-120k-10 | 0.145 ± 0.003 | 93.1 | 60.0 | 0.0414 | 13.1 | 0.0329 |
| PS-120k-15 | 0.151 ± 0.005 | 92.8 | 80.0 | 0.0795 | 13.2 | 0.0354 |
| PS-120k-20 | 0.153 ± 0.005 | 92.7 | 70.0 | 0.0920 | 17.0 | 0.0461 |
| PS-120k-40 | 0.156 ± 0.003 | 92.4 | 42.5 | 0.1090 | 18.1 | 0.0552 |
| PS-120k-60 | 0.159 ± 0.003 | 92.0 | 33.3 | 0.1260 | 19.8 | 0.1029 |
| PS-120k-80 | 0.162 ± 0.002 | 91.7 | 28.7 | 0.1420 | 23.4 | 0.1491 |
| Samples | Mass Loss TGA (%) | Mass Loss between 50–200 °C | Mass Loss between 200–500 °C | Mass Loss over 500 °C |
|---|---|---|---|---|
| PS-35k-20 | 19.5 | 5.0 | 9.1 | 5.2 |
| PS-35k-40 | 21.0 | 5.2 | 7.8 | 7.9 |
| PS-35k-60 | 22.9 | 5.3 | 9.3 | 7.5 |
| PS-35k-80 | 31.8 | 6.1 | 23.7 | 4.3 |
| PS-120k-20 | 17.0 | 3.8 | 4.6 | 4.6 |
| PS-120k-40 | 18.1 | 7.8 | 5.5 | 7.3 |
| PS-120k-60 | 19.8 | 5.3 | 10.2 | 4.8 |
| PS-120k-80 | 23.4 | 4.8 | 13.2 | 4.4 |
| Sample | SBET (m2/g) | SNLDFT (m2/g) | Vpore,BJH (cm3/g) | Vpore,NLDFT (cm3/g) | Dpore,BJH (nm) |
|---|---|---|---|---|---|
| PS-0-0 | 880 | 933 | 5.076 | 4.758 | 17.67 |
| PS-35k-15 | 731 | 775 | 4.944 | 4.286 | 17.68 |
| PS-35k-20 | 639 | 700 | 4.210 | 3.769 | 17.72 |
| PS-35k-40 | 519 | 579 | 3.668 | 3.767 | 17.66 |
| PS-35k-60 | 495 | 583 | 3.326 | 3.607 | 17.69 |
| PS-35k-80 | 455 | 541 | 3.320 | 2.929 | 17.71 |
| PS-120k-15 | 706 | 825 | 4.497 | 4.306 | 17.68 |
| PS-120k-20 | 695 | 804 | 4.635 | 4.225 | 17.65 |
| PS-120k-40 | 663 | 773 | 5.069 | 4.100 | 17.67 |
| PS-120k-60 | 607 | 676 | 4.185 | 3.816 | 17.71 |
| PS-120k-80 | 548 | 652 | 3.535 | 3.202 | 17.70 |
| Sample | Density (g/cm3) | Flex. Strength (105 Pa) | Flex. Modulus (MPa) | Compr. Strength (MPa) | Compr. Modulus (MPa) |
|---|---|---|---|---|---|
| PS-0-0 | 0.139 ± 0.002 | 0.98 ± 0.20 | 5.61 ± 0.24 | 0.74 ± 0.17 | 0.78 ± 0.31 |
| PS-35k-20 | 0.153 ± 0.004 | 1.13 ± 0.13 | 7.10 ± 0.71 | 1.97 ± 0.41 | 3.53 ± 0.59 |
| PS-35k-40 | 0.154 ± 0.001 | 1.25 ± 0.11 | 6.98 ± 0.96 | 3.21 ± 0.67 | 3.75 ± 0.83 |
| PS-35k-60 | 0.165 ± 0.004 | 1.39 ± 0.24 | 7.58 ± 0.17 | 5.96 ± 0.82 | 4.52 ± 0.47 |
| PS-35k-80 | 0.178 ± 0.010 | 2.15 ± 0.46 | 12.60 ± 0.25 | 11.15 ± 0.127 | 5.05 ± 0.82 |
| PS-120k-20 | 0.153 ± 0.002 | 1.37 ± 0.30 | 8.01 ± 0.23 | 1.79 ± 0.35 | 3.22 ± 0.22 |
| PS-120k-40 | 0.156 ± 0.005 | 1.48 ± 0.22 | 8.66 ± 0.11 | 2.91 ± 0.43 | 3.33 ± 0.19 |
| PS-120k-60 | 0.159 ± 0.005 | 1.52 ± 0.35 | 8.63 ± 0.16 | 3.07 ± 0.76 | 3.54 ± 0.30 |
| PS-120k-80 | 0.171 ± 0.003 | 1.69 ± 0.27 | 11.06 ± 0.12 | 7.31 ± 0.114 | 4.34 ± 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Wang, B.; Qi, J.; Pan, Y.; Chen, C. Fabrication of Mechanically Strong Silica Aerogels with the Thermally Induced Phase Separation (TIPS) Method of Poly(methyl methacrylate). Materials 2023, 16, 3778. https://doi.org/10.3390/ma16103778
Ma H, Wang B, Qi J, Pan Y, Chen C. Fabrication of Mechanically Strong Silica Aerogels with the Thermally Induced Phase Separation (TIPS) Method of Poly(methyl methacrylate). Materials. 2023; 16(10):3778. https://doi.org/10.3390/ma16103778
Chicago/Turabian StyleMa, Hainan, Baomin Wang, Jiarui Qi, Yiheng Pan, and Chao Chen. 2023. "Fabrication of Mechanically Strong Silica Aerogels with the Thermally Induced Phase Separation (TIPS) Method of Poly(methyl methacrylate)" Materials 16, no. 10: 3778. https://doi.org/10.3390/ma16103778
APA StyleMa, H., Wang, B., Qi, J., Pan, Y., & Chen, C. (2023). Fabrication of Mechanically Strong Silica Aerogels with the Thermally Induced Phase Separation (TIPS) Method of Poly(methyl methacrylate). Materials, 16(10), 3778. https://doi.org/10.3390/ma16103778







