The Effect of Ru on the Evolution of the γ′ Phase in Ni-Al-Ru Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
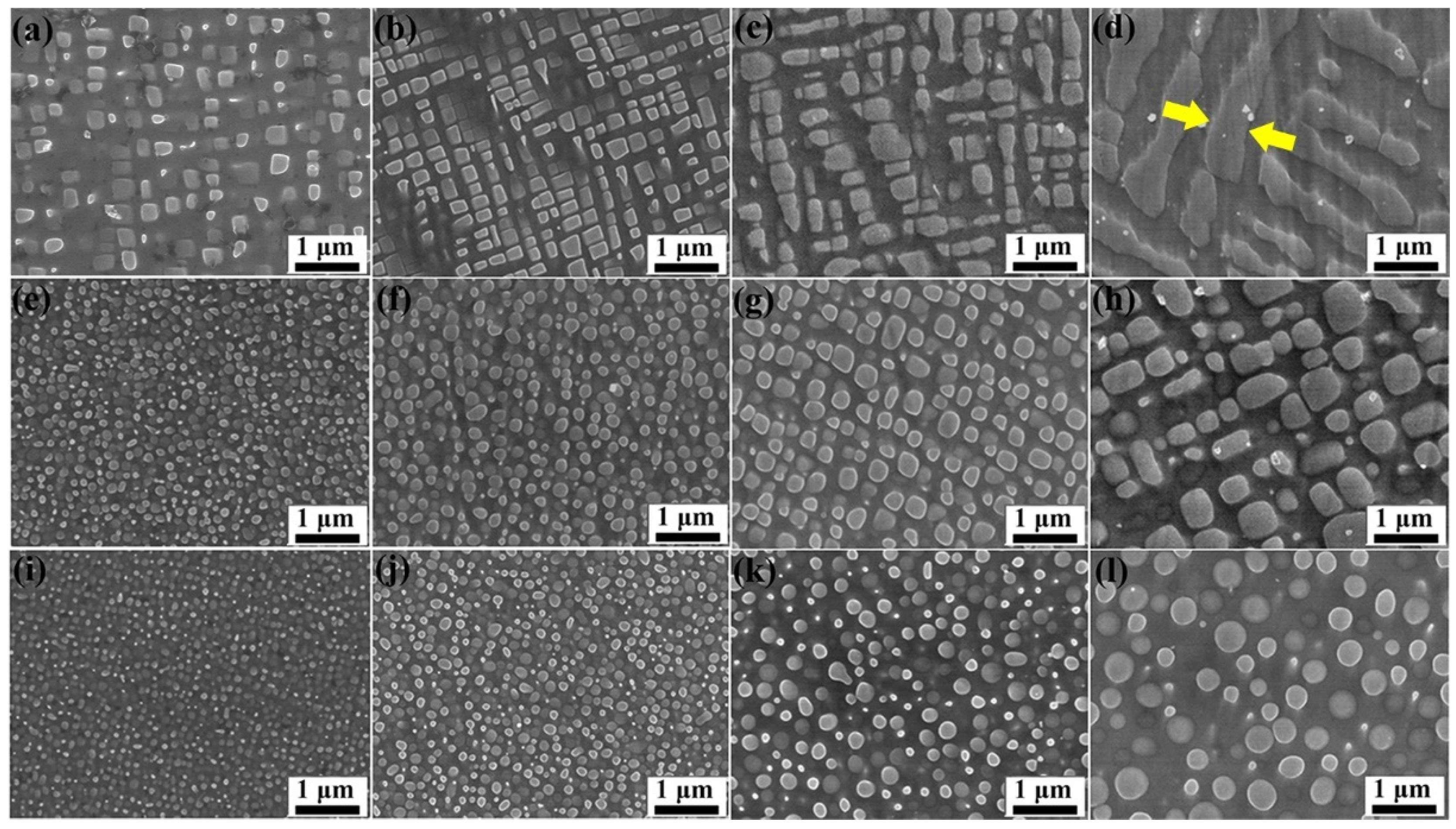
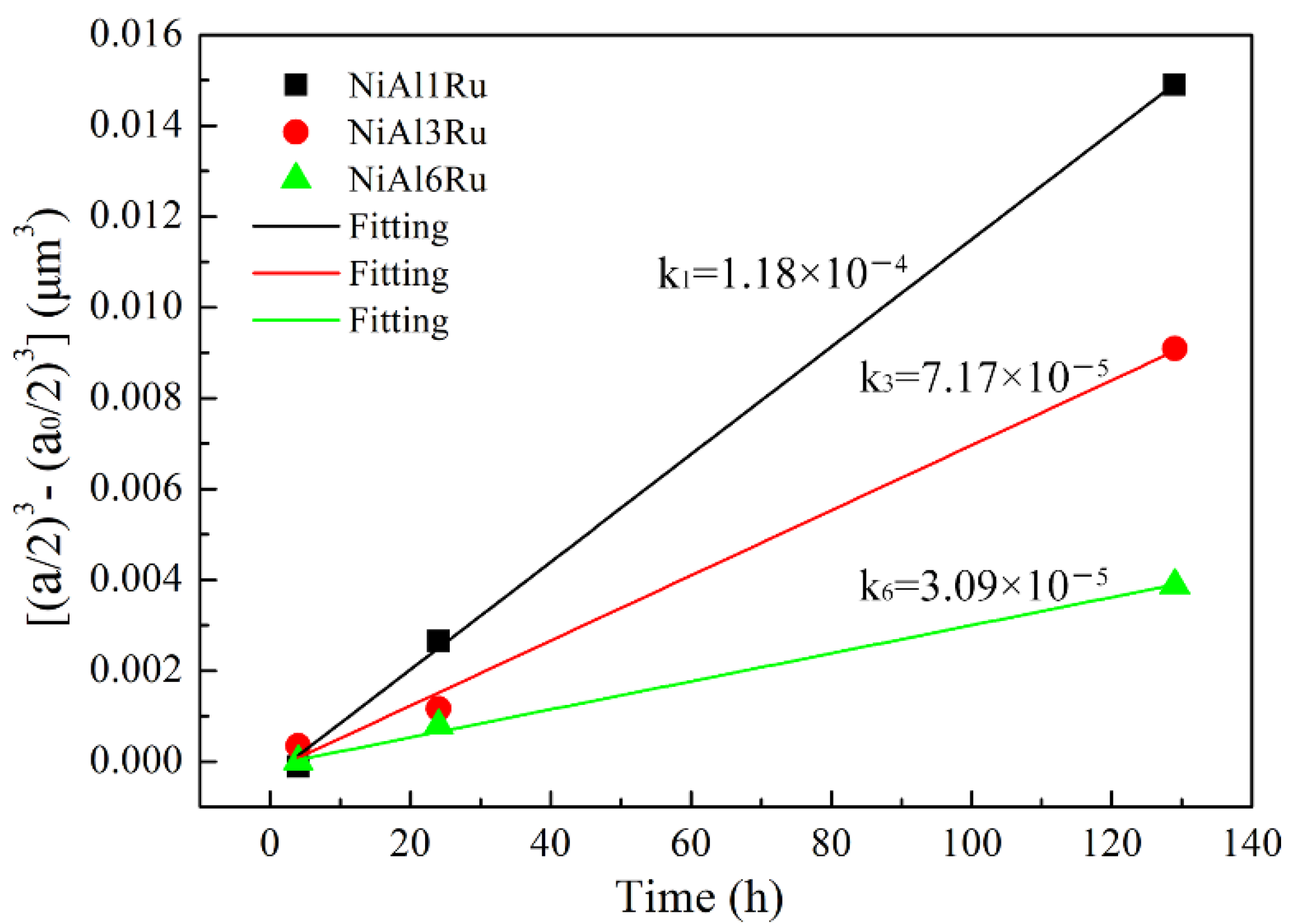
3.1. Microstructural Characteristics
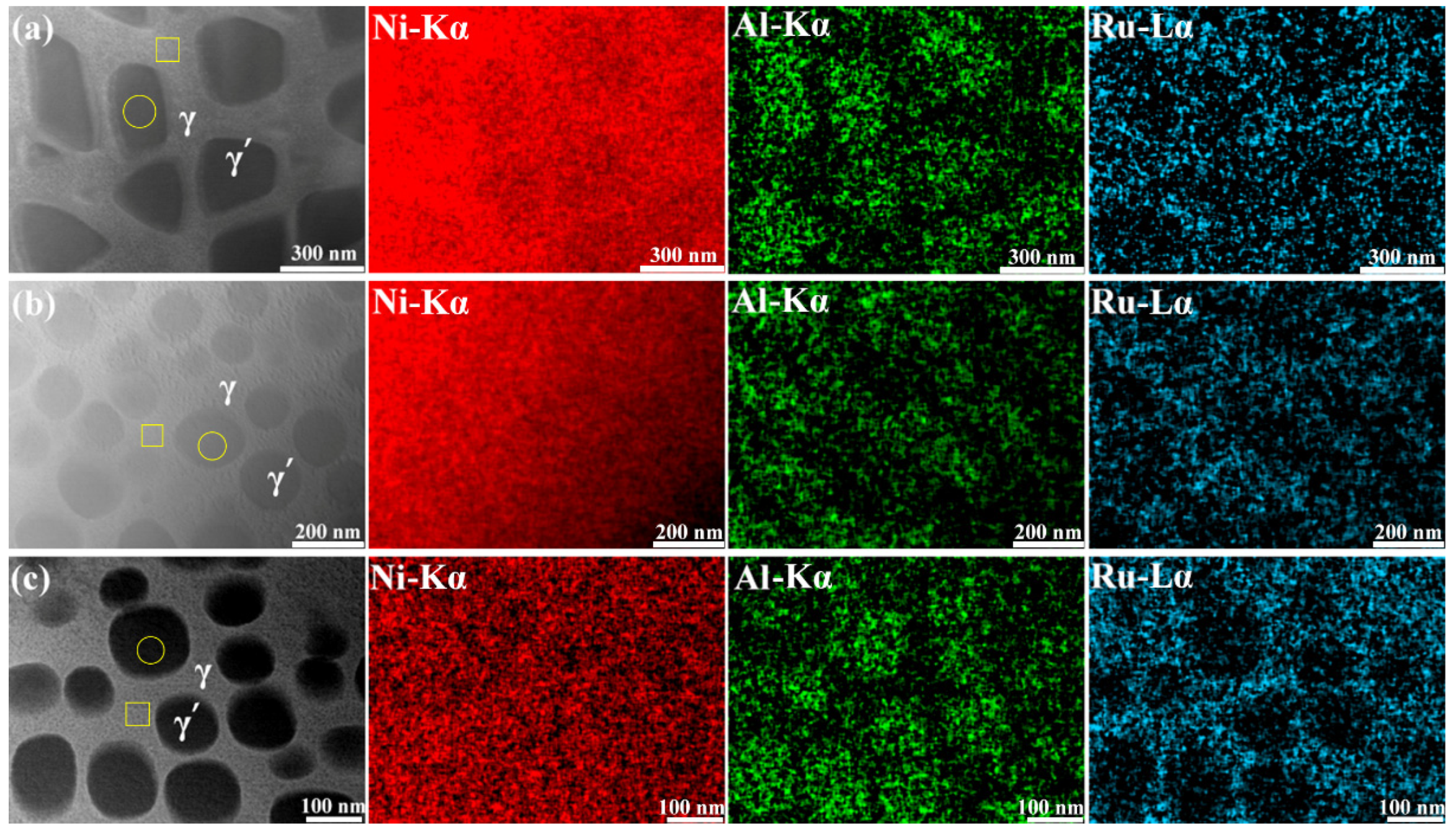
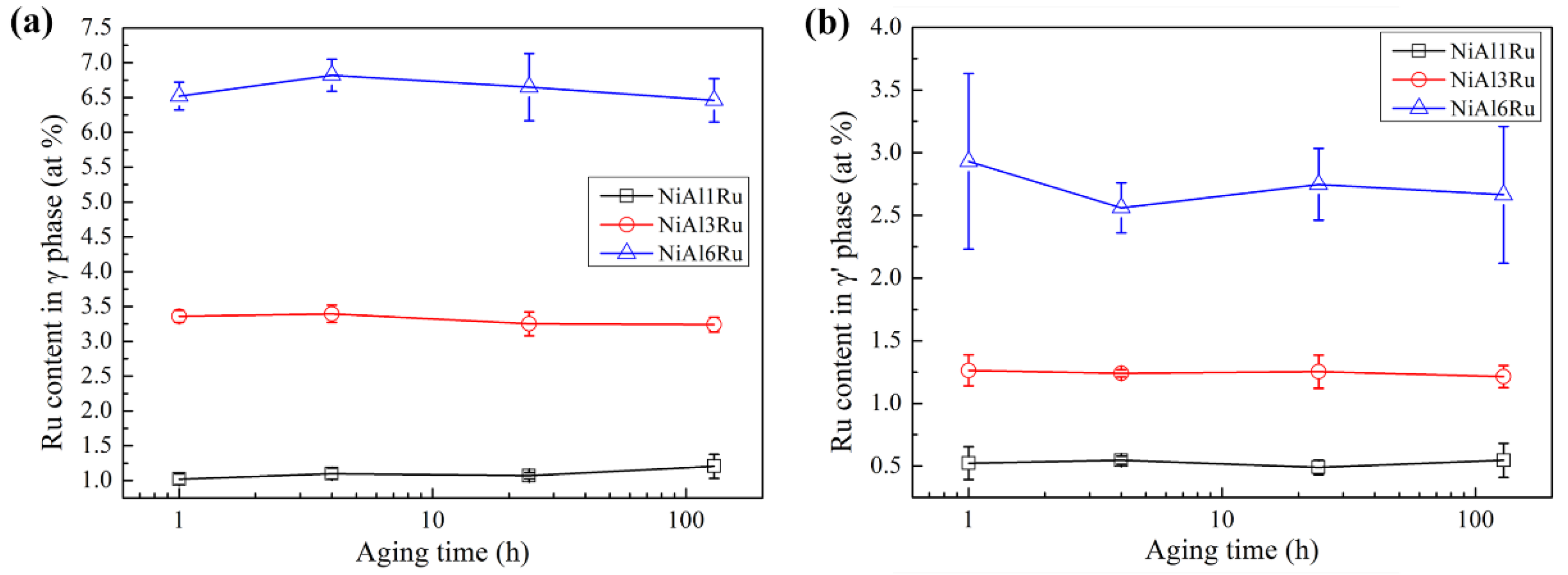
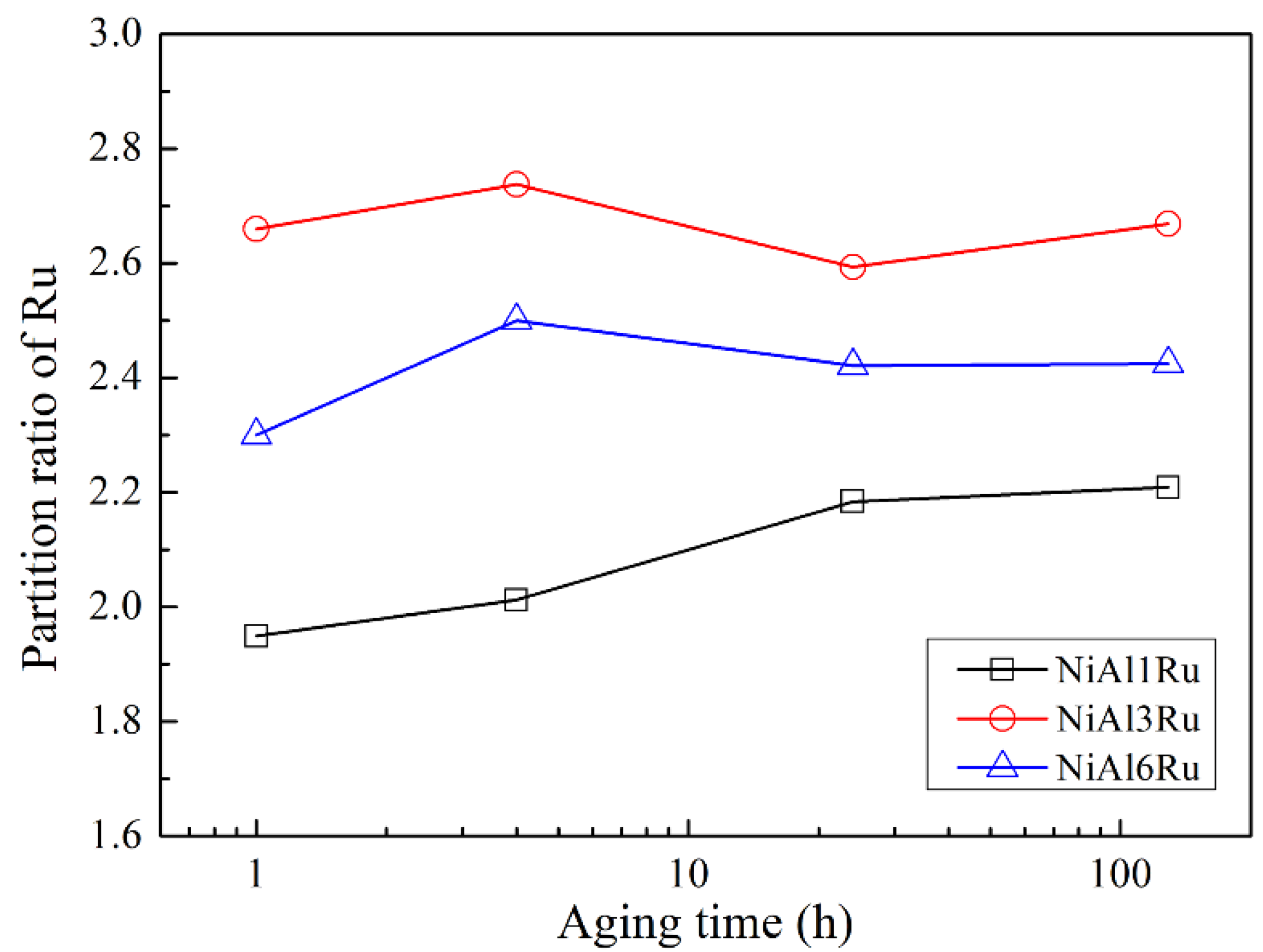
3.2. The Partitioning Behavior of Ru
4. Conclusions
- The addition of Ru can hinder the coarsening and rafting process of γ′ precipitates during aging treatment, but the effect of Ru on hindering γ′ phase growth reduced when the Ru content was over 3 at%;
- Ru mainly existed in the γ phase, and its partition ratio to the γ phase increased with the variation in Ru content from 1 at% to 3 at% and decreased for the NiAl6Ru alloy;
- The lattice misfit of the alloys was positive and was reduced with the increase in Ru content, which hindered the Ru atoms to diffuse into the γ phase and promoted the shape of γ′ precipitates to change from cubic to spherical.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shang, S.L.; Kim, D.E.; Zacherl, C.L.; Wang, Y.; Du, Y.; Liu, Z.K. Effects of Alloying Elements and Temperature on the Elastic Properties of Dilute Ni-Base Superalloys from First-Principles Calculations. J. Appl. Phys. 2012, 112, 053515. [Google Scholar] [CrossRef]
- Sato, A.; Harada, H.; Yokokawa, T.; Murakumo, T.; Koizumi, Y.; Kobayashi, T.; Imai, H. The Effects of Ruthenium on the Phase Stability of Fourth Generation Ni-Base Single Crystal Superalloys. Scr. Mater. 2006, 54, 1679–1684. [Google Scholar] [CrossRef]
- Pandey, P.; Sawant, A.; Baler, N.; Makineni, S.K.; Chattopadhyay, K. Effect of Ru Addition on γ/γ′ Microstructural Stability in a Low-Density CoNi Based Superalloy. Scr. Mater. 2022, 208, 11431. [Google Scholar] [CrossRef]
- Shi, Q.; Huo, J.; Zheng, Y.; Feng, Q. Influence of Mo and Ru Additions on the Creep Behavior of Ni-Based Single Crystal Superalloys at 1100 °C. Mater. Sci. Eng. A 2018, 725, 148–159. [Google Scholar] [CrossRef]
- Feng, Q.; Nandy, T.K.; Tin, S.; Pollock, T.M. Solidification of High-Refractory Ruthenium-Containing Superalloys. Acta Mater. 2003, 51, 269–284. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, Y.; Zhang, M.; Gan, B.; Yuan, K.; Wu, X. On the Effect of Ru upon Creep Behaviour and Dislocation Evolution in Ni-Based Single Crystal Superalloys. Mater. Today Commun. 2022, 30, 103220. [Google Scholar] [CrossRef]
- Chen, J.; Feng, Q.; Cao, L.; Sun, Z. Influence of Ru Addition on Microstructure and Stress-Rupture Property of Ni-Based Single Crystal Superalloys. Prog. Nat. Sci. Mater. Int. 2010, 20, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Giese, S.; Bezold, A.; Pröbstle, M.; Heckl, A.; Neumeier, S.; Göken, M. The Importance of Diffusivity and Partitioning Behavior of Solid Solution Strengthening Elements for the High Temperature Creep Strength of Ni-Base Superalloys. Metall. Mater. Trans. A 2020, 51, 6195–6206. [Google Scholar] [CrossRef]
- Neumeier, S.; Pyczak, F.; Göken, M. The Temperature Dependent Lattice Misfit of Rhenium and Ruthenium Containing Nickel-Base Superalloys—Experiment and Modelling. Mater. Des. 2021, 198, 109362. [Google Scholar] [CrossRef]
- Ali, M.A.; Görler, J.V.; Steinbach, I. Role of Coherency Loss on Rafting Behavior of Ni-Based Superalloys. Comput. Mater. Sci. 2020, 171, 109279. [Google Scholar] [CrossRef]
- Song, W.; Wang, X.G.; Li, J.G.; Huang, Y.S.; Meng, J.; Yang, Y.H.; Liu, J.L.; Liu, J.D.; Zhou, Y.Z.; Sun, X.F. Role of Ru on the Microstructural Evolution During Long-Term Aging of Ni-Based Single Crystal Superalloys. Acta Metall. Sin. Engl. Lett. 2020, 33, 1689. [Google Scholar] [CrossRef]
- Liu, L.; Jin, T.; Liu, J.; Sun, X.; Hu, Z. Effect of Ruthenium on γ′ Precipitation Behavior and Evolution in Single Crystal Superalloys. Trans. Nonferrous Met. Soc. China 2013, 23, 14–22. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, E.; Guan, X.; Zheng, Z. Influence of Ru on Solidification Behavior, Microstructure and Hardness of Re-Free Ni-Based Equiaxed Superalloys with High Cr Content. J. Mater. Sci. Technol. 2016, 32, 271–281. [Google Scholar] [CrossRef]
- Rettig, R.; Heckl, A.; Singer, R.F. Modeling of Precipitation Kinetics of TCP-Phases in Single Crystal Nickel-Base Superalloys. Adv. Mater. Res. 2011, 278, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Matuszewski, K.; Rettig, R.; Matysiak, H.; Peng, Z.; Povstugar, I.; Choi, P.; Müller, J.; Raabe, D.; Spiecker, E.; Kurzydłowski, K.J.; et al. Effect of Ruthenium on the Precipitation of Topologically Close Packed Phases in Ni-Based Superalloys of 3rd and 4th Generation. Acta Mater. 2015, 95, 274–283. [Google Scholar] [CrossRef]
- Wilson, A.S. Formation and Effect of Topologically Close-Packed Phases in Nickel-Base Superalloys. Mater. Sci. Technol. 2017, 33, 1108–1118. [Google Scholar] [CrossRef]
- Song, W.; Wang, X.G.; Li, J.G.; Ye, L.H.; Hou, G.C.; Yang, Y.H.; Liu, J.L.; Liu, J.D.; Pei, W.L.; Zhou, Y.Z.; et al. Effect of Ruthenium on Microstructure and High-Temperature Creep Properties of Fourth Generation Ni-Based Single-Crystal Superalloys. Mater. Sci. Eng. A 2020, 772, 138646. [Google Scholar] [CrossRef]
- Heckl, A.; Neumeier, S.; Cenanovic, S.; Göken, M.; Singer, R.F. Reasons for the Enhanced Phase Stability of Ru-Containing Nickel-Based Superalloys. Acta Mater. 2011, 59, 6563–6573. [Google Scholar] [CrossRef]
- Hobbs, R.A.; Zhang, L.; Rae, C.M.F.; Tin, S. The Effect of Ruthenium on the Intermediate to High Temperature Creep Response of High Refractory Content Single Crystal Nickel-Base Superalloys. Mater. Sci. Eng. A 2008, 489, 65–76. [Google Scholar] [CrossRef]
- Han, Y.; Ma, W.; Dong, Z.; Li, S.; Gong, S. Effect of Ruthenium on Microstructure and Stress Rupture Properties of a Single Crystal Nickel-Base Superalloy. In Superalloys 2008, Proceedings of the 11th International Symposium on Superalloys, Warrendale, PA, USA, 14–18 September 2008; Reed, R.C., Green, K.A., Caron, P., Gabb, T.P., Fahrmann, M.G., Huron, E.S., Woodard, S.A., Eds.; Minerals, Metals & Materials Society: Warrendale, PA, USA, 2008. [Google Scholar]
- Song, W.; Wang, X.G.; Li, J.G.; Meng, J.; Yang, Y.H.; Liu, J.L.; Liu, J.D.; Zhou, Y.Z.; Sun, X.F. Effect of Ru on Tensile Behavior and Deformation Mechanism of a Nickel-Based Single Crystal Superalloy. Mater. Sci. Eng. A 2021, 802, 140430. [Google Scholar] [CrossRef]
- Sudbrack, C.; Yoon, K.; Noebe, R.; Seidman, D. Temporal Evolution of the Nanostructure and Phase Compositions in a Model Ni-Al-Cr Alloy. Acta Mater. 2006, 54, 3199–3210. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.Z.; Jin, T.; Liu, J.L.; Sun, X.F.; Guan, H.R.; Hu, Z.Q. Role of Re and Co on Microstructures and γ′ Coarsening in Single Crystal Superalloys. Mater. Sci. Eng. A 2008, 479, 148–156. [Google Scholar] [CrossRef]
- Giamei, A.F.; Anton, D.L. Rhenium Additions to a Ni-Base Superalloy: Effects on Microstructure. Metall. Trans. A 1985, 16A, 1985–1997. [Google Scholar] [CrossRef]
- Ofori, A.P.; Humpherys, C.J.; Tin, S.; Jones, C.N. A TEM Study of the Effect of Platinum Group Metals in Advanced Single Crystal Nickel-Base Superalloys. In Superalloys 2004, Proceedings of the Tenth International Symposium on Superalloys, Warrendale, PA, USA, 19–23 September 2004; Green, K.A., Pollock, T.M., Harada, H., Howson, T.E., Reed, R.C., Schirra, J.J., Walston, S., Eds.; Minerals, Metals & Materials Society: Warrendale, PA, USA, 2004. [Google Scholar]
- Wang, S.; Wang, L.; Meng, F.; Yu, H.; Sun, D. Quantitative Study on Ru Local Atomic Structure in Ni-Al-Ru Ternary Alloys. J. Alloys Compd. 2022, 909, 164766. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Abbasi, S.M.; Madar, K.Z.; Yazdi, H.M.K. The Effect of Boron and Zirconium on Wrought Structure and γ-γ′ Lattice Misfit Characterization in Nickel-Based Superalloy ATI 718Plus. Mater. Chem. Phys. 2018, 211, 302–311. [Google Scholar] [CrossRef]
- Yoon, K.E.; Noebe, R.D.; Seidman, D.N. Effects of Rhenium Addition on the Temporal Evolution of the Nanostructure and Chemistry of a Model Ni-Cr-Al Superalloy. I: Experimental Observations. Acta Mater. 2007, 55, 1145–1157. [Google Scholar] [CrossRef]

| Alloy | Al | Ru | Ni |
|---|---|---|---|
| NiAl1Ru | 17.82 | 1.00 | Balance |
| NiAl3Ru | 17.46 | 3.00 | Balance |
| NiAl6Ru | 16.92 | 6.00 | Balance |
| Aging Time (h) | Mean Particle Size (μm) | ||
|---|---|---|---|
| NiAl1Ru | NiAl3Ru | NiAl6Ru | |
| 1 | 0.18 ± 0.053 | 0.12 ± 0.028 | 0.11 ± 0.024 |
| 4 | 0.17 ± 0.053 | 0.16 ± 0.041 | 0.13 ± 0.035 |
| 24 | 0.30 ± 0.108 | 0.22 ± 0.056 | 0.20 ± 0.051 |
| 129 | 0.50 ± 0.331 | 0.42 ± 0.131 | 0.32 ± 0.095 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Meng, F.; Wang, L.; Yu, H.; Sun, D. The Effect of Ru on the Evolution of the γ′ Phase in Ni-Al-Ru Alloys. Materials 2022, 15, 3344. https://doi.org/10.3390/ma15093344
Wang S, Meng F, Wang L, Yu H, Sun D. The Effect of Ru on the Evolution of the γ′ Phase in Ni-Al-Ru Alloys. Materials. 2022; 15(9):3344. https://doi.org/10.3390/ma15093344
Chicago/Turabian StyleWang, Shaoyang, Fanqiang Meng, Lu Wang, Hongying Yu, and Dongbai Sun. 2022. "The Effect of Ru on the Evolution of the γ′ Phase in Ni-Al-Ru Alloys" Materials 15, no. 9: 3344. https://doi.org/10.3390/ma15093344
APA StyleWang, S., Meng, F., Wang, L., Yu, H., & Sun, D. (2022). The Effect of Ru on the Evolution of the γ′ Phase in Ni-Al-Ru Alloys. Materials, 15(9), 3344. https://doi.org/10.3390/ma15093344






