Effect of Polymer Drag Reducer on Rheological Properties of Rocket Kerosene Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. System Model
2.2. TraPPE-UA Force Field
2.3. Simulation Details
3. Results and Discussion
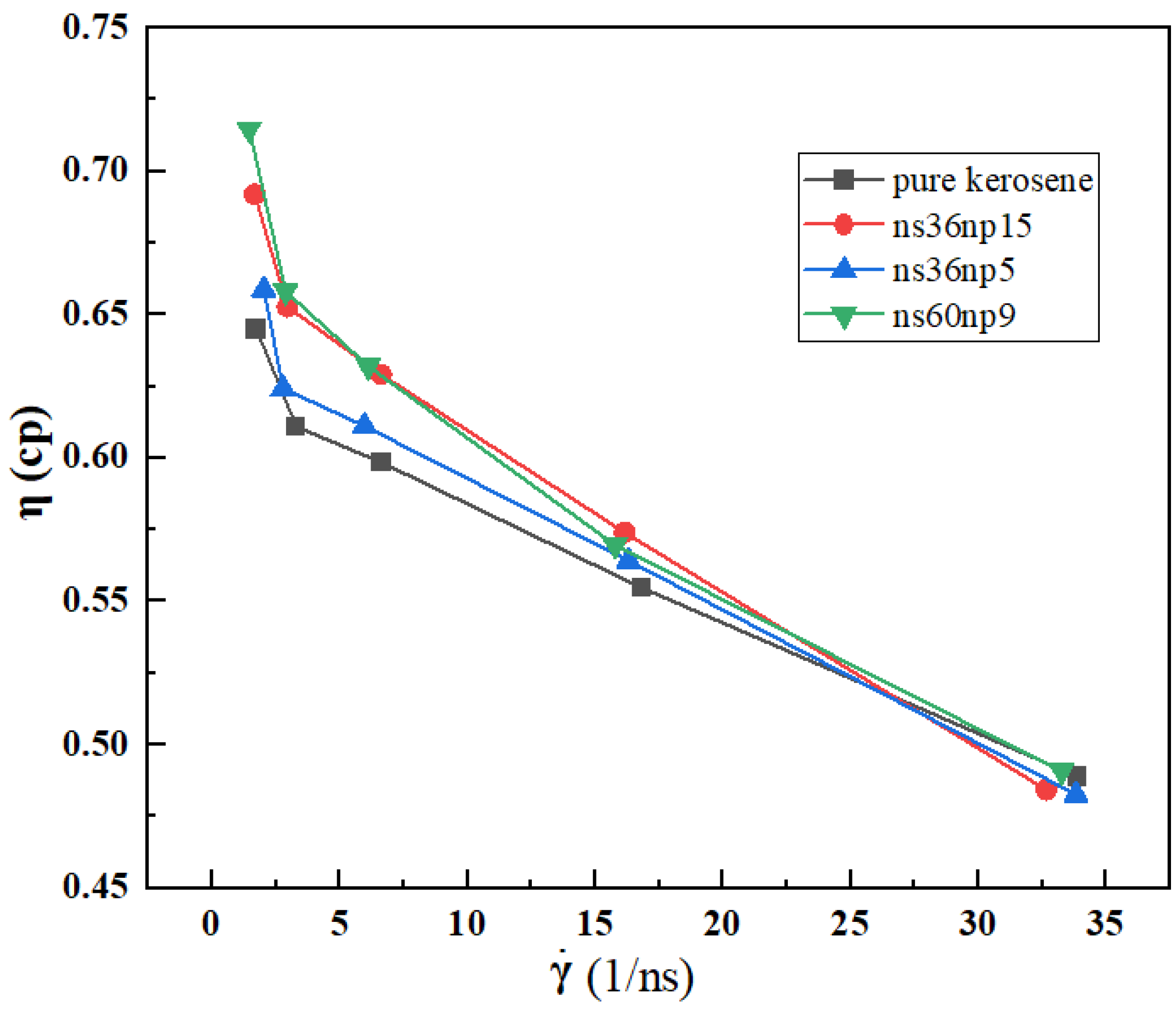
3.1. Shear Viscosity of Different Solutions
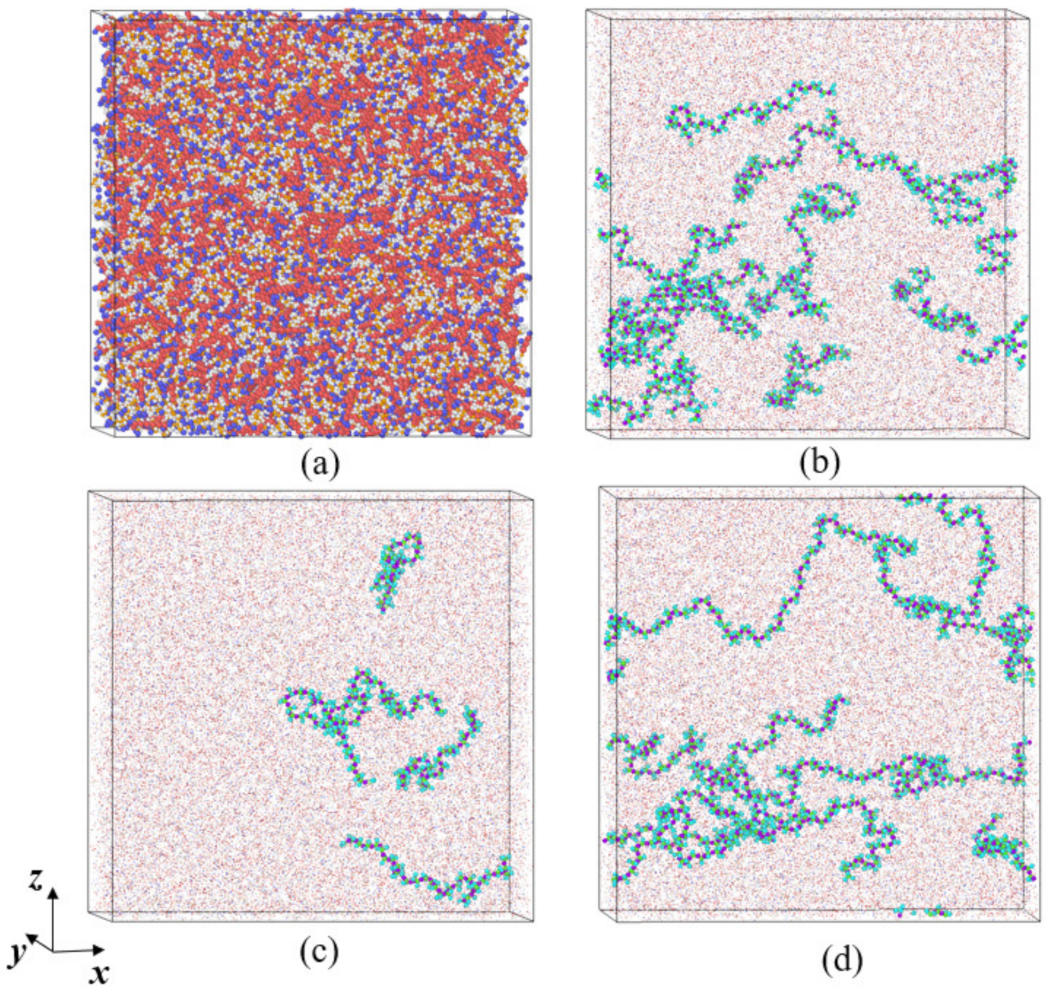
3.2. Microstructure Evolution of Polymer Chains in DRA—Kerosene Solutions
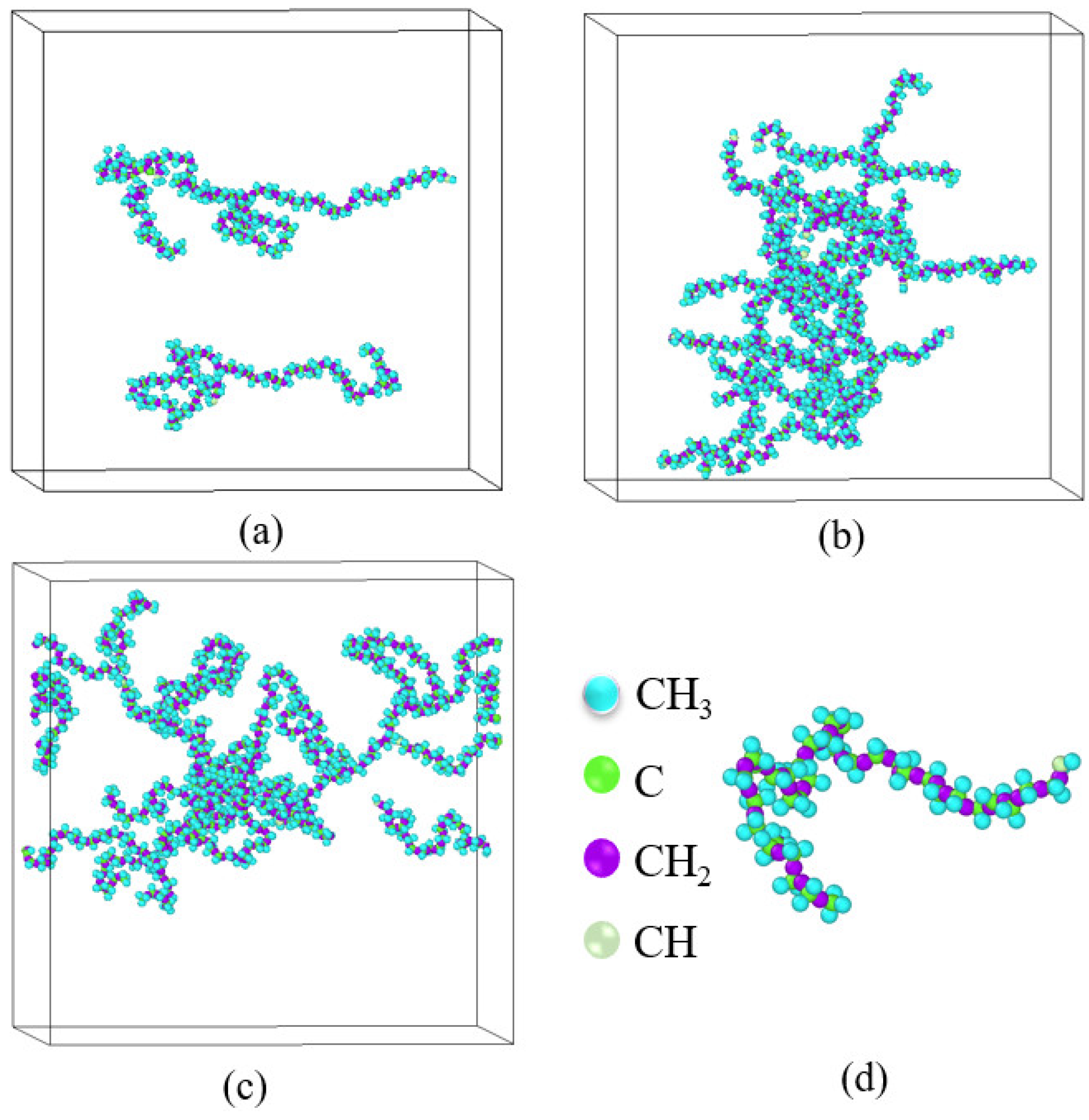
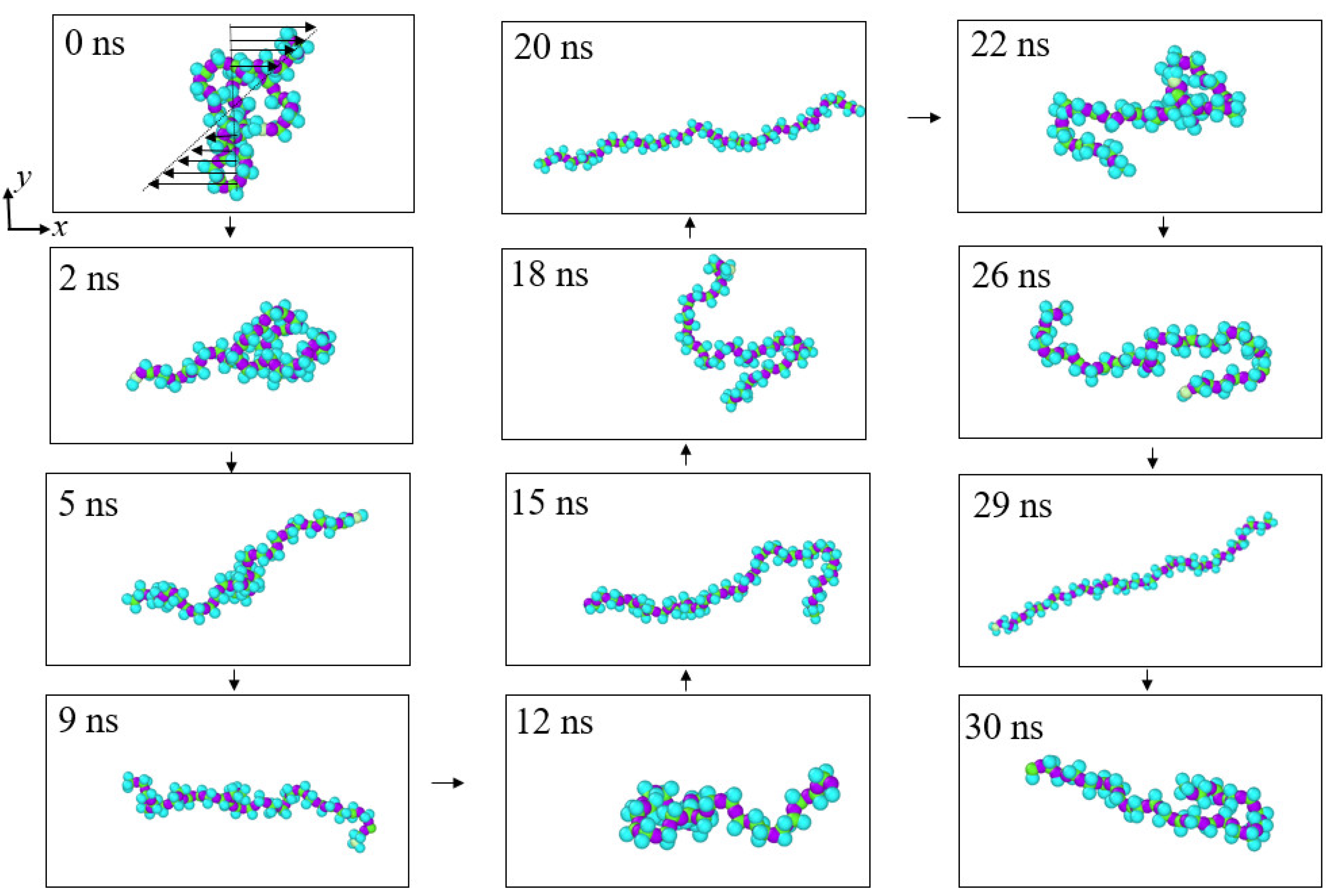
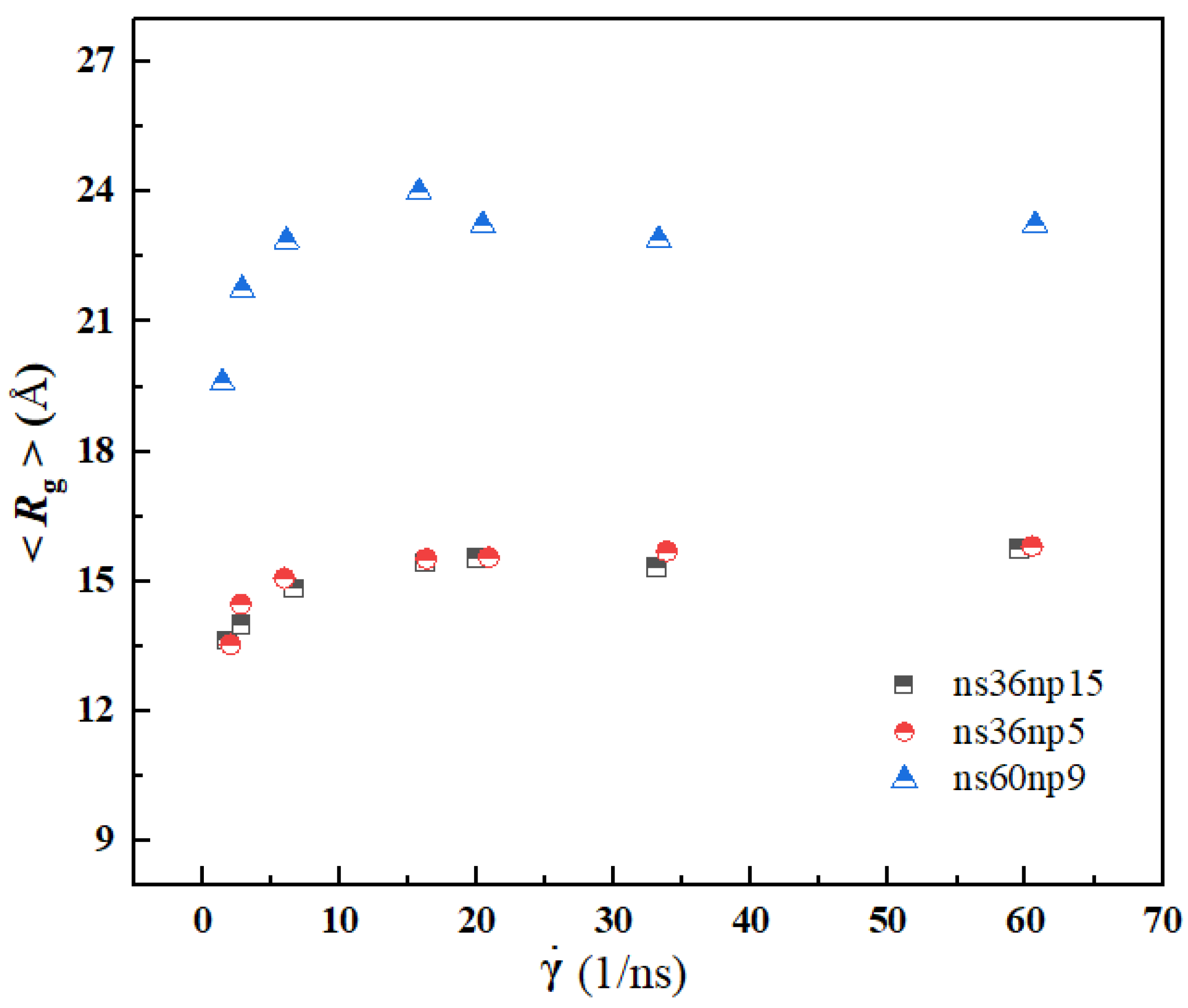
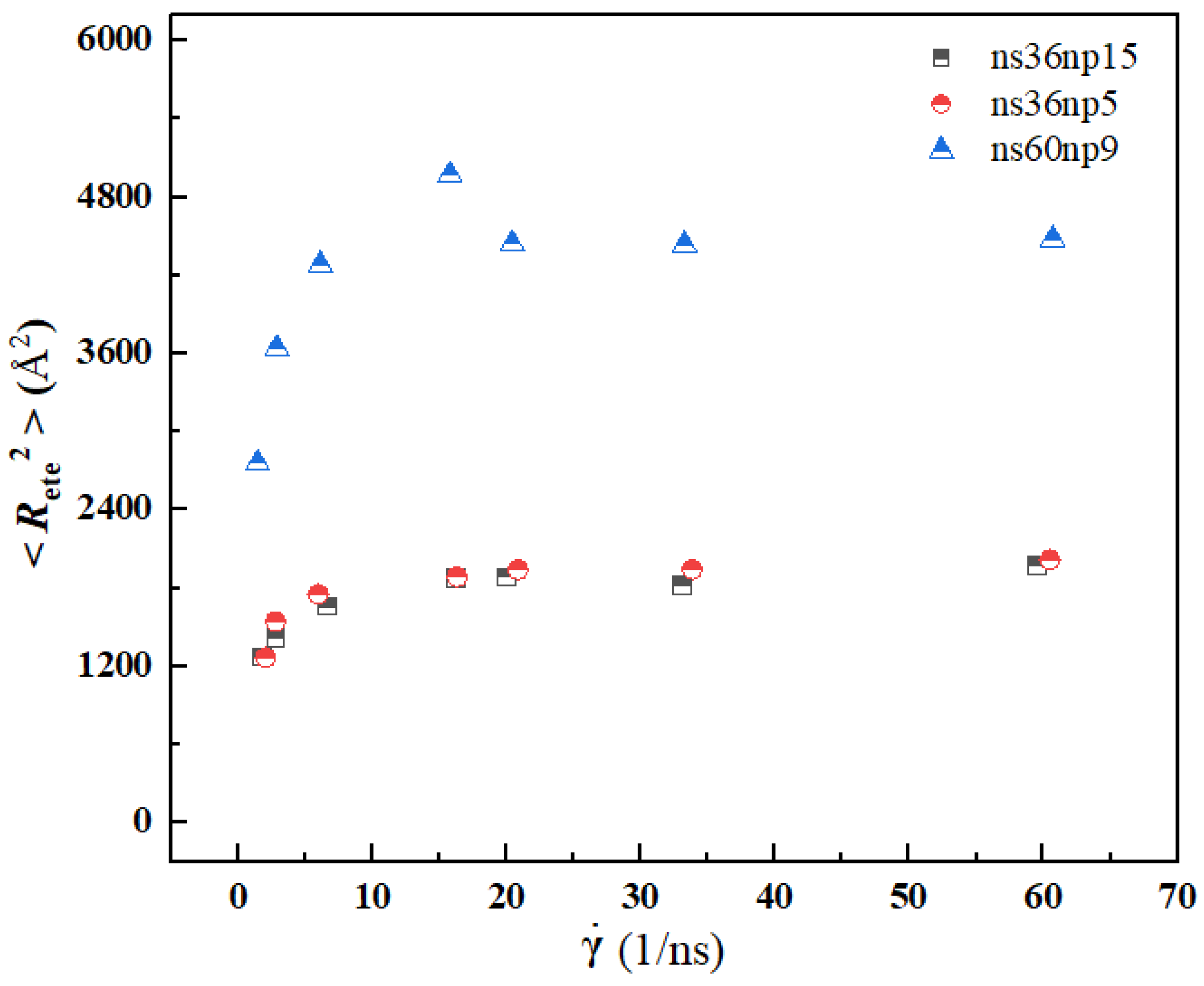
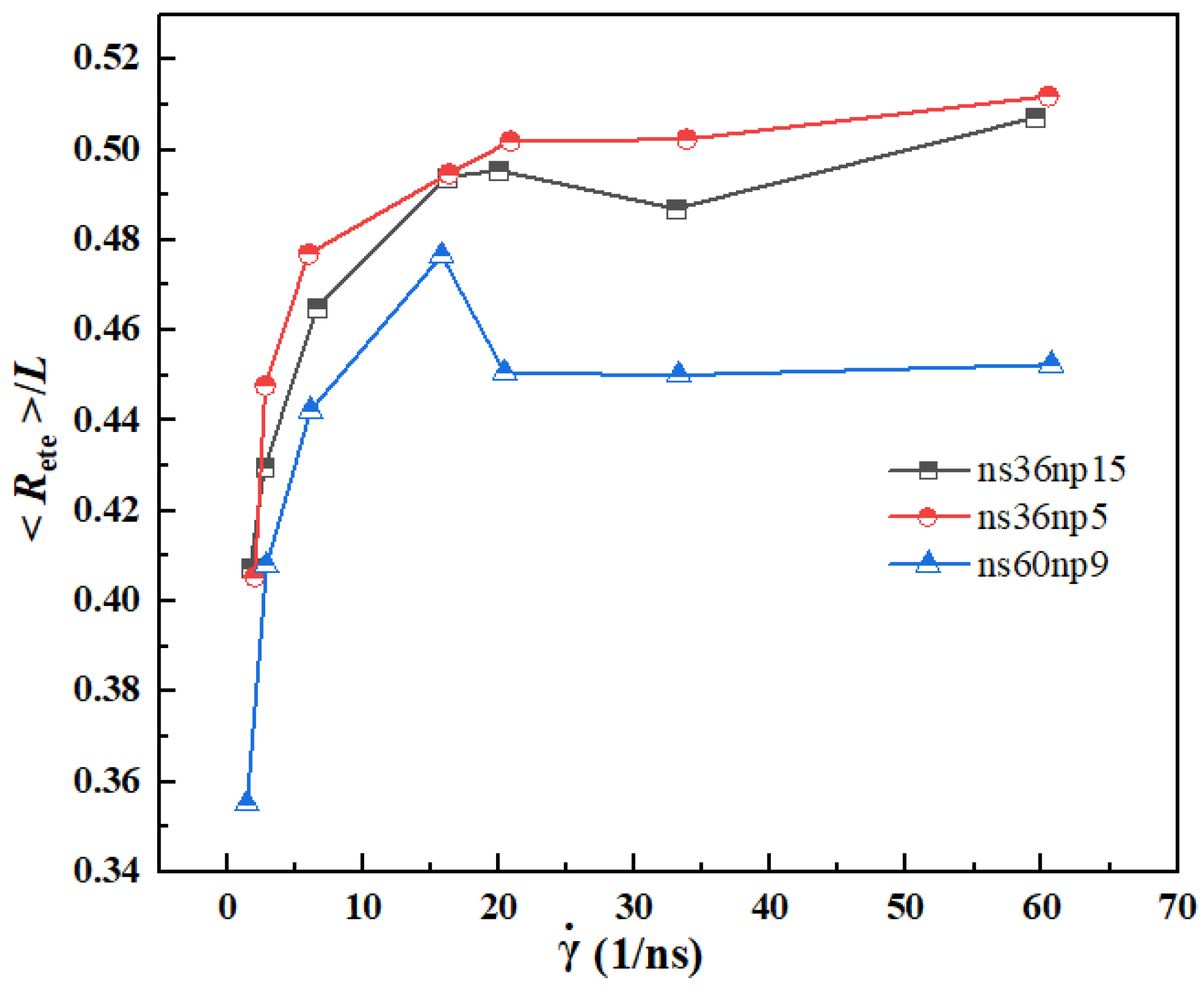
3.3. Quantitative Description of the Morphologic Change of Polymer Chains
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, H.H.; Nie, W.S.; Guo, K.K.; Zhou, S.Y. Influence of deflector on impact properties of multi-nozzle LOX/kerosene engine exhaust plume. In Proceedings of the 2017 8th International Conference on Mechanical and Aerospace Engineering (ICMAE), Prague, Czech Republic, 22–25 July 2017; pp. 296–300. [Google Scholar]
- Qin, T.; Rong, Y.; Qin, X.D.; Zhang, Z. The development characteristics and trends of heavy launch vehicles. Aerosp. China 2018, 19, 29–37. [Google Scholar]
- Sutton, G.P. History of liquid propellant rocket engines in the United States. J. Propuls. Power 2003, 19, 978–1007. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Liu, C.H.; Pan, H. Flow and heat transfer characteriatics of low-flow resistance rocket kerosene under supercritical pressure. J. Xi’an Jiaotong Univ. 2019, 1, 129–134. [Google Scholar]
- Toms, B.A. Some observations on the flow of linear polymer solutions through straight tubes at large Reynolds numbers. In Proceedings of the 1st International Congress on Rheology, Hague, The Netherlands, 21–24 September 1948; Volume 2, pp. 135–141. [Google Scholar]
- Virk, P.S. Drag reduction fundamentals. AIChE J. 1975, 21, 625–656. [Google Scholar] [CrossRef]
- Lee, K.H.; Zhang, K.; Choi, H.J. Time dependence of turbulent drag reduction efficiency of polyisobutylene in kerosene. J. Ind. Eng. Chem. 2010, 16, 499–502. [Google Scholar] [CrossRef]
- Du, Z.G.; Zhu, C.C.; Wu, J.; Shan, S.Q.; Yu, X.L.; Shi, X.M.; Fu, Q.J.; Han, W. investigation on drag-reduction technology of rocket kerosene. J. Rocket. Propuls. 2017, 43, 32–37. [Google Scholar]
- Li, B.; Li, W.X.; Zheng, X.; Wang, Y.; Tang, M.M.; Cai, W.H. Numerical study on influences of drag reducing additive in supercritical flow of kerosene in a millichannel. Energies 2021, 14, 6758. [Google Scholar] [CrossRef]
- Aust, C.; Kröger, M.; Hess, S. Structure and dynamics of dilute polymer solutions under shear flow via nonequilibrium molecular dynamics. Macromolecules 1999, 32, 5660–5672. [Google Scholar] [CrossRef]
- Huang, C.C.; Gompper, G.; Winkler, R.G. Non-equilibrium properties of semidilute polymer solutions under shear flow. J. Phys. Conf. Ser. 2012, 392, 012003. [Google Scholar] [CrossRef] [Green Version]
- Kong, Y.; Manke, C.W.; Madden, W.G.; Schlijper, A.G. Modeling the rheology of polymer solutions by dissipative particle dynamics. Tribol. Lett. 1997, 3, 133–138. [Google Scholar] [CrossRef]
- Prabhakar, R.; Prakash, J.R. Multiplicative separation of the influences of excluded volume, hydrodynamic interactions and finite extensibility on the rheological properties of dilute polymer solutions. J. Non-Newton. Fluid Mech. 2004, 116, 163–182. [Google Scholar] [CrossRef]
- Schlijper, A.G.; Hoogerbrugge, P.J.; Manke, C.W. Computer simulation of dilute polymer solutions with the dissipative particle dynamics method. J. Rheol. 1995, 39, 567–579. [Google Scholar] [CrossRef] [Green Version]
- Stoltz, C.; de Pablo, J.J.; Graham, M.D. Concentration dependence of shear and extensional rheology of polymer solutions: Brownian dynamics simulations. J. Rheol. 2006, 50, 137–167. [Google Scholar] [CrossRef]
- Edwards, T. “Kerosene” fuels for aerospace propulsion-composition and properties. In Proceedings of the 38th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Indianapolis, Indiana, 7–10 July 2002; p. 3874. [Google Scholar]
- Zheng, D.; Yu, W.M.; Zhong, B.J. RP-3 aviation kerosene surrogate fuel and the chemical reaction kinetic model. Acta Phys. Chim. Sin. 2015, 31, 636–642. [Google Scholar]
- Farmer, R.C.; Anderson, P.G.; Cheng, G.C. Propellant Chemistry for CFD Applications; NASA Technical Report, Document ID 19960029262; SECA Inc.: Huntsville, AL, USA, 1996. [Google Scholar]
- Huber, M.L.; Lemmon, E.W.; Bruno, T.J. Effect of RP-1 compositional variability on thermophysical properties. Energy Fuels 2009, 23, 5550–5555. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Peng, Q.T.; Sheng, T. GC-MS determination of components in rocket kerosene and rocket kerosene vapour. Missiles Space Veh. 2011, 4, 59–62. [Google Scholar]
- Kehimkar, B.; Hoggard, J.C.; Marney, L.C.; Billingsley, M.C.; Fraga, C.G.; Bruno, T.J.; Synovec, R.E. Correlation of rocket propulsion fuel properties with chemical composition using comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry followed by partial least squares regression analysis. J. Chromatogr. A 2014, 1327, 132–140. [Google Scholar] [CrossRef]
- Martin, M.G.; Siepmann, J.I. Transferable potentials for phase equilibria. 1. United-atom description of n-alkanes. J. Phys. Chem. B 1998, 102, 2569–2577. [Google Scholar] [CrossRef]
- Martin, M.G. Comparison of the AMBER, CHARMM, COMPASS, GROMOS, OPLS, TraPPE and UFF force fields for prediction of vapor–liquid coexistence curves and liquid densities. Fluid Phase Equilibria 2006, 248, 50–55. [Google Scholar] [CrossRef]
- Müller, E.A.; Mejia, A. Comparison of united-atom potentials for the simulation of vapor–liquid equilibria and interfacial properties of long-chain n-alkanes up to n-c100. J. Phys. Chem. B 2011, 115, 12822–12834. [Google Scholar]
- Papavasileiou, K.D.; Peristeras, L.D.; Bick, A.; Economou, I.G. Molecular dynamics simulation of pure n-alkanes and their mixtures at elevated temperatures using atomistic and coarse-grained force fields. J. Phys. Chem. B 2019, 123, 6229–6243. [Google Scholar] [CrossRef] [PubMed]
- Borovik, I.; Strokach, E.; Kozlov, A.; Gaponov, V.; Chvanov, V.; Levochkin, P.; Yoon, Y. Influence of polyisobutylene kerosene additive on combustion efficiency in a liquid propellant rocket engine. Aerospace 2019, 6, 129. [Google Scholar] [CrossRef] [Green Version]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Jewett, A.I.; Stelter, D.; Lambert, J.; Saladi, S.M.; Roscioni, O.M.; Ricci, M.; Autin, L.; Maritan, M.; Bashusqeh, S.M.; Keyes, T.; et al. Moltemplate: A tool for coarse-grained modeling of complex biological matter and soft condensed matter physics. J. Mol. Biol. 2021, 433, 166841. [Google Scholar] [CrossRef]
- Yiannourakou, M.; Ungerer, P.; Lachet, V.; Rousseau, B.; Teuler, J.M. United atom forcefield for vapor-liquid equilibrium (VLE) properties of cyclic and polycyclic compounds from Monte Carlo simulations. Fluid Phase Equilibria 2019, 481, 28–43. [Google Scholar] [CrossRef]
- Martin, M.G.; Siepmann, J.I. Novel configurational-bias Monte Carlo method for branched molecules. Transferable potentials for phase equilibria. 2. United-atom description of branched alkanes. J. Phys. Chem. B 1999, 103, 4508–4517. [Google Scholar] [CrossRef]
- Keasler, S.J.; Charan, S.M.; Wick, C.D.; Economou, I.G.; Siepmann, J.I. Transferable potentials for phase equilibria-united atom description of five-and six-membered cyclic alkanes and ethers. J. Phys. Chem. B 2012, 116, 11234–11246. [Google Scholar] [CrossRef]
- Kioupis, L.I.; Maginn, E.J. Rheology, dynamics, and structure of hydrocarbon blends: A molecular dynamics study of n-hexane/n-hexadecane mixtures. Chem. Eng. J. 1999, 74, 129–146. [Google Scholar] [CrossRef]
- Müller-Plathe, F. Reversing the perturbation in nonequilibrium molecular dynamics: An easy way to calculate the shear viscosity of fluids. Phys. Rev. E 1999, 59, 4894. [Google Scholar] [CrossRef] [Green Version]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695. [Google Scholar] [CrossRef] [Green Version]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Modell. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.L.; Chen, J.Z.; An, L.J. Shear thinning behavior of linear polymer melts under shear flow via nonequilibrium molecular dynamics. J. Chem. Phys. 2014, 140, 174902. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.S.; Chen, J.S.; Ettelaie, R.; Law, Z.; Alevisopoulos, S.; Day, E.; Smith, S. Study of the shear and extensional rheology of casein, waxy maize starch and their mixtures. Food Hydrocoll. 2007, 21, 716–725. [Google Scholar] [CrossRef]
- Nickzare, M.; Zohuriaan-Mehr, M.J.; Yousefi, A.A.; Ershad-Langroudi, A. Novel acrylic-modified acacia gum thickener: Preparation, characterization and rheological properties. Starch-Stärke 2009, 61, 188–198. [Google Scholar] [CrossRef]
- Wang, L.C.; Wu, Y.J.; Li, J.S.; Qiao, H.Z.; Di, L.Q. Rheological and mucoadhesive properties of polysaccharide from Bletilla striata with potential use in pharmaceutics as bio-adhesive excipient. Int. J. Biol. Macromol. 2018, 120, 529–536. [Google Scholar] [CrossRef]
- Wang, F.; Ma, Z.L.; Du, B.; Li, W.; Wang, D.P.; Chen, S.T.; Wang, Y.F. Study on tensile rheological properties of polyethylene melt. China Plast. 2021, 35, 13. [Google Scholar]
- Hou, C.; Wang, C.G.; Ying, L. Effect of inorganic salts on viscosity of acrylonitrile-N-vinylpyrrolidone copolymer solutions. J. Appl. Polym. Sci. 2003, 89, 3492–3495. [Google Scholar] [CrossRef]
- Devasia, R.; Nair, C.P.R.; Ninan, K.N. Temperature and shear dependencies of rheology of poly (acrylonitrile-co-itaconic acid) dope in DMF. Polym. Adv. Technol. 2008, 19, 1771–1778. [Google Scholar] [CrossRef]



| Species | Components | Molecular Formula | Molecular Number | Atomic Number |
|---|---|---|---|---|
| Paraffins | n-tridecane | C13H28 | 1210 | 15,730 |
| Monocycloalkanes | n-heptylcyclohexane | C13H26 | 2244 | 29,172 |
| Dicycloalkanes | Naphthalene, decahydro-2,6-dimethyl | C12H22 | 2827 | 33,924 |
| sum | - | 6281 | 78,826 |
| United Atom Type | Molecule | σ (Å) | ε/kB (K) |
|---|---|---|---|
| CH3 [22] | ALL | 3.75 | 98 |
| CH2 [22] | n-alkane/DRA | 3.95 | 46 |
| CH [30] | DRA | 4.68 | 10 |
| C [30] | DRA | 6.4 | 0.5 |
| CH2(cyc) [31] | Monocylic and dicycloalkanes | 3.91 | 52.5 |
| CH(cyc) [29] | Monocylic and dicycloalkanes | 4.68 | 12 |
| Bond Type | Molecule | R0 (Å) | K (kcal/mol/Å2) |
|---|---|---|---|
| CHx-CHy [32] | n-alkane/DRA | 1.54 | 899.52 |
| CHx(cyc)-CHy(cyc) [29] | Monocylic and dicycloalkanes | 1.54 | 536 |
| Bend Type | Molecule | θ (°) | Kθ/kB (K·rad−2) |
|---|---|---|---|
| CHx-CH2-CHy [22] | n-alkane/DRA | 114 | 62,500 |
| CHx(cyc)-CH2(cyc)-CHy(cyc) [31] | Monocylic and dicycloalkanes | 114 | 62,500 |
| CHx(cyc)-CH(cyc)-CH3 (or CH2) [30] | Monocylic and dicycloalkanes | 112 | 62,500 |
| CHx-C-CHy [30] | DRA | 109.47 | 62,500 |
| Torsion | Molecule | C0/kB (K) | C1/kB (K) | C2/kB (K) | C3/kB (K) |
|---|---|---|---|---|---|
| CHx-(CH2)-(CH2)-CHy [22] | n-alkane | 0 | 355.03 | −68.19 | 791.32 |
| CH2(cyc)-CH2(cyc)-CH2(cyc)-CH(cyc) [29] | Monocylic and dicycloalkanes | 0 | 355.03 | −68.19 | 791.32 |
| CHn(cyc)-CH2(cyc)-CH(cyc)-CHn(cyc) (n = 1 or 2) [29] | −251.06 | 428.73 | −111.85 | 441.27 | |
| CHx-(CH2)-(C)-CHy [30] | DRA | 0 | 0 | 0 | 461.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Chen, X.; Zhou, W.; Wei, J. Effect of Polymer Drag Reducer on Rheological Properties of Rocket Kerosene Solutions. Materials 2022, 15, 3343. https://doi.org/10.3390/ma15093343
Guo X, Chen X, Zhou W, Wei J. Effect of Polymer Drag Reducer on Rheological Properties of Rocket Kerosene Solutions. Materials. 2022; 15(9):3343. https://doi.org/10.3390/ma15093343
Chicago/Turabian StyleGuo, Xiaodie, Xuejiao Chen, Wenjing Zhou, and Jinjia Wei. 2022. "Effect of Polymer Drag Reducer on Rheological Properties of Rocket Kerosene Solutions" Materials 15, no. 9: 3343. https://doi.org/10.3390/ma15093343
APA StyleGuo, X., Chen, X., Zhou, W., & Wei, J. (2022). Effect of Polymer Drag Reducer on Rheological Properties of Rocket Kerosene Solutions. Materials, 15(9), 3343. https://doi.org/10.3390/ma15093343





