Collagen Cross-Linking Lignin Improves the Bonding Performance of Etch-and-Rinse Adhesives to Dentin
Abstract
1. Introduction
2. Materials and Methods
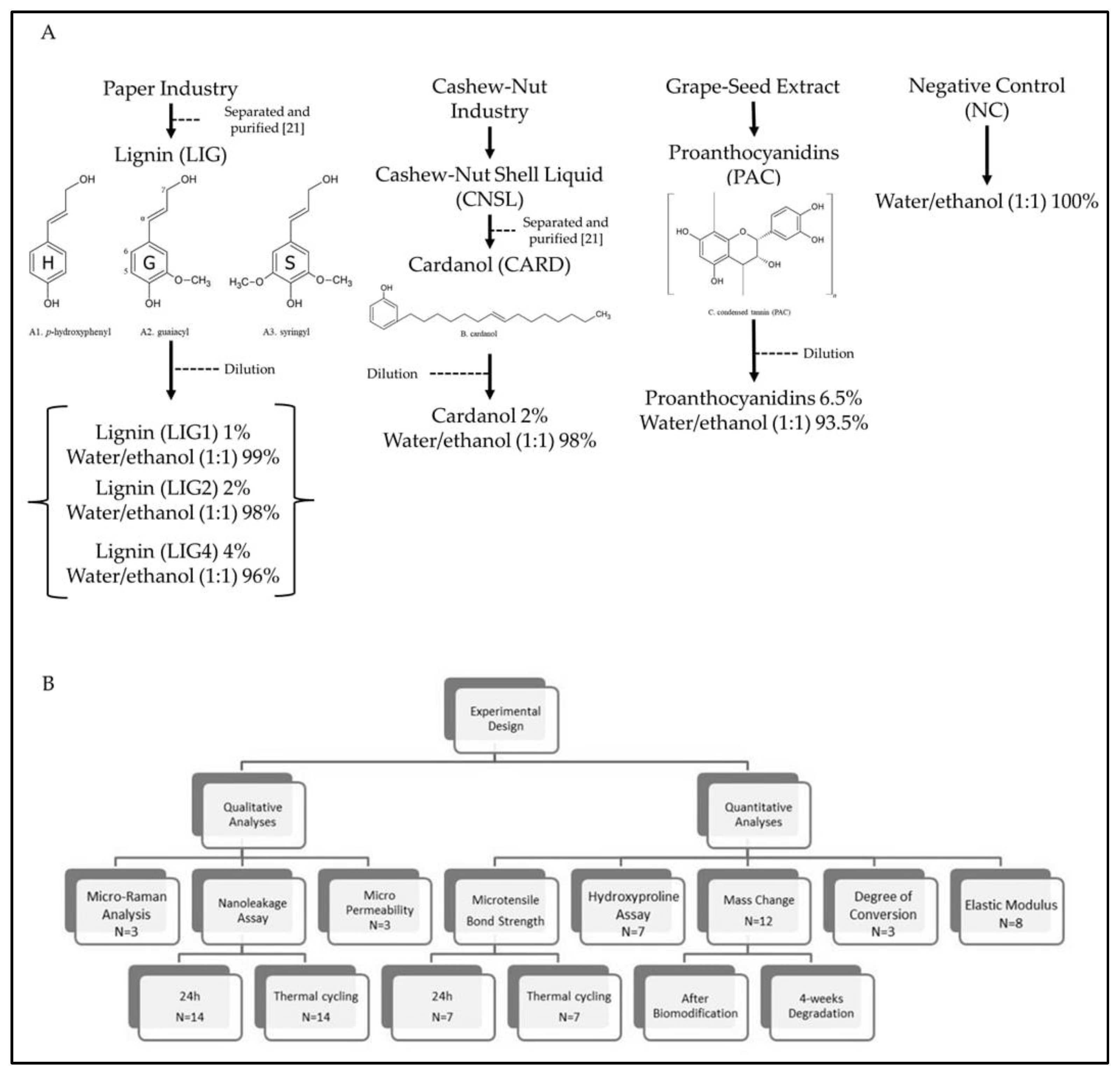
2.1. Preparation of Biomodification Solutions
2.2. Bonding Procedures
2.3. Microtensile Bond Strength (µTBS) and Failure Pattern
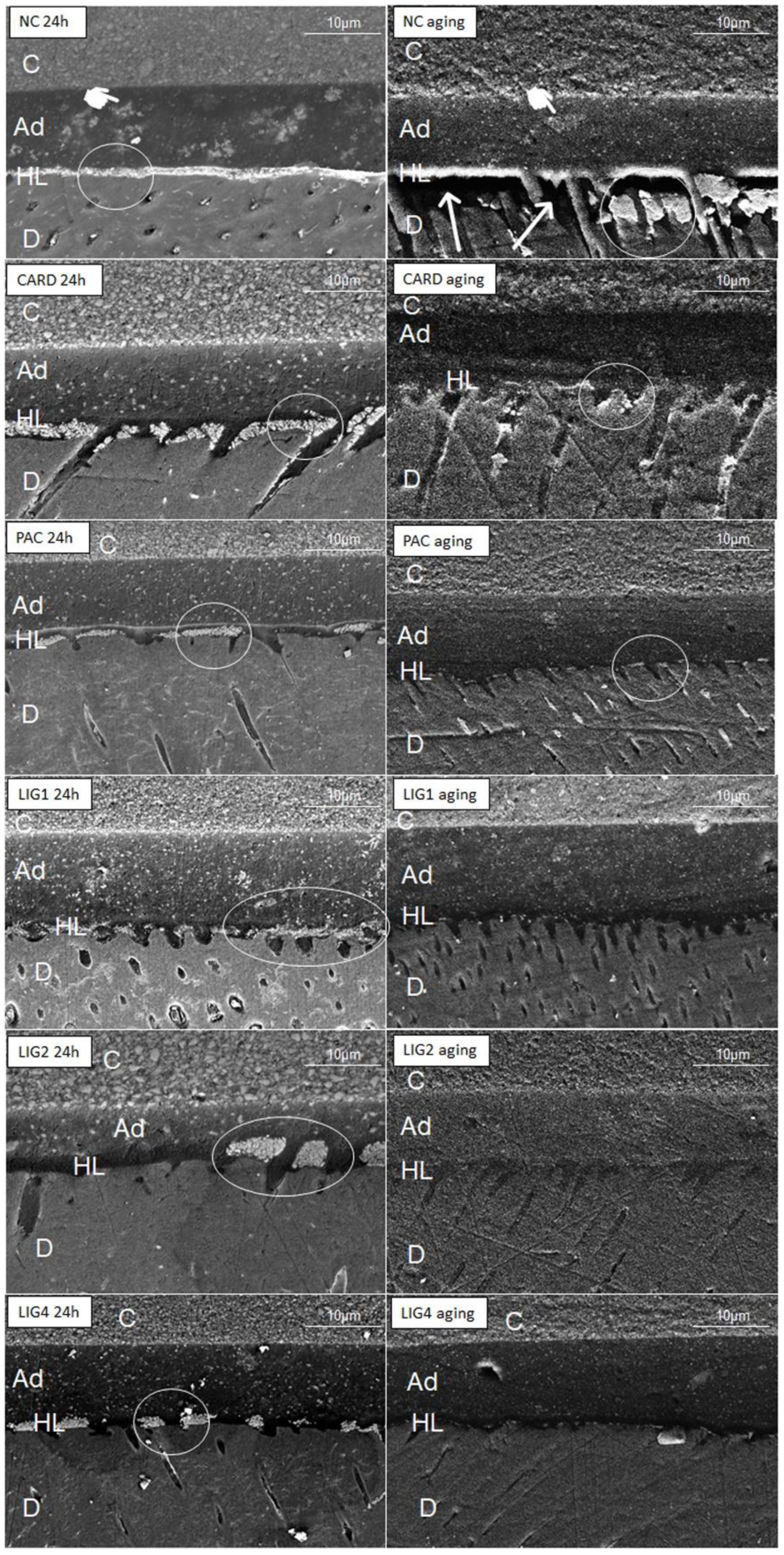
2.4. Nanoleakage Survey
2.5. Micropermeability Assay
2.6. In Situ Degree of Conversion (DC)
2.7. Elastic Modulus (E)
2.8. Mass Change (Wmc) and Biodegradation Rate (R)
2.9. Hydroxyproline Assay (HYP)
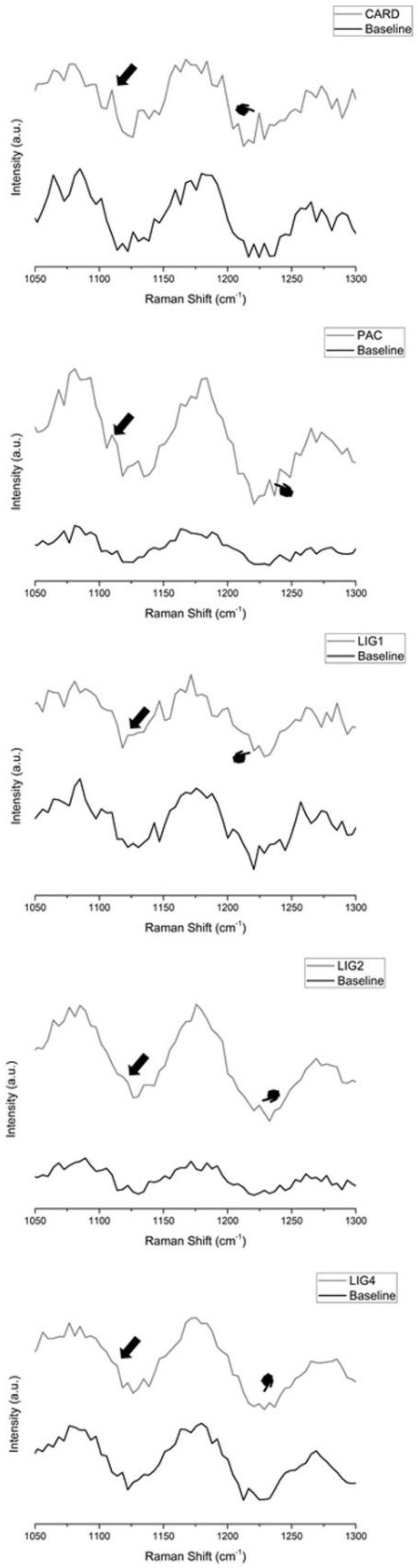
2.10. Micro-Raman Cross-Linking Identification
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakabayashi, N.; Kojima, K.; Masuhara, E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J. Biomed. Mater. Res. 1982, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, composition and mineralization. Front. Biosci. Elite Ed. 2011, 3, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Pashley, D.H. Strategies to stabilise dentine-bonded interfaces through remineralising operative approaches: State of the art. Int. J. Adhes. Adhes. 2016, 69, 39–57. [Google Scholar] [CrossRef]
- Al-Ammar, A.; Drummond, J.L.; Bedran-Russo, A.K. The use of collagen cross-linking agents to enhance dentin bond strength. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Shan, T.; Ma, Y.X.; Tay, F.R.; Niu, L. Novel Biomedical Applications of Crosslinked Collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef]
- Maravic, T.; Mancuso, E.; Comba, A.; Checchi, V.; Generali, L.; Mazzitelli, C.; Josic, U.; Hass, V.; Reis, A.; Loguercio, A.D.; et al. Dentin Cross-linking Effect of Carbodiimide after 5 Years. J. Dent. Res. 2021, 100, 1090–1098. [Google Scholar] [CrossRef]
- Bedran-Russo, A.K.; Vidal, C.M.; Dos Santos, P.H.; Castellan, C.S. Long-term effect of carbodiimide on dentin matrix and resin-dentin bonds. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 250–255. [Google Scholar] [CrossRef]
- Bedran-Russo, A.K.; Castellan, C.S.; Shinohara, M.S.; Hassan, L.; Antunes, A. Characterization of biomodified dentin matrices for potential preventive and reparative therapies. Acta Biomater. 2011, 7, 1735–1741. [Google Scholar] [CrossRef]
- Bedran-Russo, A.K.; Pauli, G.F.; Chen, S.N.; McAlpine, J.; Castellan, C.S.; Phansalkar, R.S.; Aguiar, T.R.; Vidal, C.M.; Napotilano, J.G.; Nam, J.W.; et al. Dentin biomodification: Strategies, renewable resources and clinical applications. Dent. Mater. 2014, 30, 62–76. [Google Scholar] [CrossRef]
- Castellan, C.S.; Pereira, P.N.; Grande, R.H.; Bedran-Russo, A.K. Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent. Mater. 2010, 26, 968–973. [Google Scholar] [CrossRef]
- Hass, V.; de Paula, A.M.; Parreiras, S.; Gutiérrez, M.F.; Luque-Martinez, I.; de Paris Matos, T.; Bandeca, M.C.; Loguercio, A.D.; Yao, X.; Wang, Y.; et al. Degradation of dentin-bonded interfaces treated with collagen cross-linking agents in a cariogenic oral environment: An in situ study. J. Dent. 2016, 49, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, T.R.; Vidal, C.M.; Phansalkar, R.S.; Todorova, I.; Napolitano, J.G.; McAlpine, J.B.; Chen, S.N.; Pauli, G.F.; Bedran-Russo, A.K. Dentin biomodification potential depends on polyphenol source. J. Dent. Res. 2014, 93, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.A.; Souza, N.O.; Sousa, R.S.; Freitas, D.Q.; Lemos, M.V.; De Paula, D.M.; Maia, F.J.N.; Lomonaco, D.; Mazzetto, S.E.; Feitosa, V.P. Efficacy of new natural biomodification agents from Anacardiaceae extracts on dentin collagen cross-linking. Dent. Mater. 2017, 33, 1103–1109. [Google Scholar] [CrossRef]
- Vidal, C.M.; Aguiar, T.R.; Phansalkar, R.; McAlpine, J.B.; Napolitano, J.G.; Chen, S.N.; Araújo, L.S.; Pauli, G.F.; Bedran-Russo, A. Galloyl moieties enhance the dentin biomodification potential of plant-derived catechins. Acta Biomater. 2014, 10, 3288–3294. [Google Scholar] [CrossRef] [PubMed]
- Leme-Kraus, A.A.; Aydin, B.; Vidal, C.M.; Phansalkar, R.M.; Nam, J.W.; McAlpine, J.; Pauli, G.F.; Chen, S.; Bedran-Russo, A.K. Biostability of the proanthocyanidins-dentin complex and adhesion studies. J. Dent. Res. 2017, 96, 406–412. [Google Scholar] [CrossRef]
- Liu, R.R.; Fang, M.; Zhang, L.; Tang, C.F.; Dou, Q.; Chen, J.H. Anti-proteolytic capacity and bonding durability of proanthocyanidin-biomodified demineralized dentin matrix. Int. J. Oral Sci. 2014, 6, 168–174. [Google Scholar] [CrossRef]
- Moreira, M.M.; da Silva, L.R.R.; Mendes, T.A.D.; Santiago, S.L.; Mazzetto, S.E.; Lomonaco, D.; Feitosa, V.P. Synthesis and characterization of a new methacrylate monomer derived from the cashew nut shell liquid (CNSL) and its effect on dentinal tubular occlusion. Dent. Mater. 2018, 34, 1144–1153. [Google Scholar] [CrossRef]
- Sakagami, H. Biological activities and possible dental application of three major groups of polyphenols. J. Pharmacol. Sci. 2014, 126, 92–106. [Google Scholar] [CrossRef]
- Yoon, J.; Choi, H.; An, G. Roles of lignin biosynthesis and regulatory genes in plant development. J. Integr. Plant Biol. 2015, 57, 902–912. [Google Scholar] [CrossRef]
- Sakagami, H.; Tomomura, M. Dental Application of Natural Products. Medicines 2018, 5, 21. [Google Scholar] [CrossRef]
- Lomonaco, D.; Maia, F.J.N.; Mazzetto, S.E. Thermal evaluation of cashew nutshell liquid as new bioadditives for poly(methyl methacrylate). J. Therm. Anal. Calorim. 2013, 111, 619–626. [Google Scholar] [CrossRef]
- Cotes, C.; Cardoso, M.; Melo, R.M.; Valandro, L.F.; Bottino, M.A. Effect of composite surface treatment and aging on the bond strength between a core build-up composite and a luting agent. J. Appl. Oral Sci. 2015, 23, 71–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Deeb, H.A.; Daifalla, L.E.; Badran, O.I.; Mobarak, E.H. Bond Strength Durability of Different Adhesives to Dentin after Aging in Two Different Solutions. J. Adhes. Dent. 2016, 18, 303–309. [Google Scholar] [CrossRef]
- Castellan, C.S.; Bedran-Russo, A.K.; Antunes, A.; Pereira, P.N. Effect of dentin biomodification using naturally derived collagen cross-linkers: One-year bond strength study. Int. J. Dent. 2013, 2013, 918010. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H.; Yoshiyama, M. Two modes of nanoleakage expression in single-step adhesives. J. Dent. Res. 2002, 81, 472–476. [Google Scholar] [CrossRef]
- Feitosa, V.P.; Sauro, S.; Ogliari, F.A.; Ogliari, A.O.; Yoshihara, K.; Zanchi, C.H.; Correr-Sobrinho, L.; Sinhoreti, M.A.; Correr, A.B.; Watson, T.F.; et al. Impact of hydrophilicity and length of spacer chains on the bonding of functional monomers. Dent. Mater. 2014, 30, e317–e323. [Google Scholar] [CrossRef]
- Feitosa, V.P.; Sauro, S.; Ogliari, F.A.; Stansbury, J.W.; Carpenter, G.H.; Watson, T.F.; Sinhoreti, M.A.; Correr, A.B. The role of spacer carbon chain in acidic functional monomers on the physicochemical properties of self-etch dental adhesives. J. Dent. 2014, 42, 565–574. [Google Scholar] [CrossRef]
- Mattinen, M.L.; Riviere, G.; Henn, A.; Nugroho, R.W.N.; Leskinen, T.; Nivala, O.; Valle-Delgado, J.J.; Kostiainen, M.A.; Österberg, M. Colloidal Lignin Particles as Adhesives for Soft Materials. Nanomaterials 2018, 8, 1001. [Google Scholar] [CrossRef]
- Castilho-Almeida, E.W.; De Almeida, W.B.; Dos Santos, H.F. Conformational analysis of lignin models: A chemometric approach. J. Mol. Model. 2013, 19, 2149–2163. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H.; et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Abuna, G.; Feitosa, V.P.; Correr, A.B.; Cama, G.; Giannini, M.; Sinhoreti, M.A.; Pashley, D.H.; Sauro, S. Bonding performance of experimental bioactive/biomimetic self-etch adhesives doped with calcium-phosphate fillers and biomimetic analogs of phosphoproteins. J. Dent. 2016, 52, 79–86. [Google Scholar] [CrossRef]
- He, L.; Mu, C.; Shi, J.; Zhang, Q.; Shi, B.; Lin, W. Modification of collagen with a natural cross-linker, procyanidin. Int. J. Biol. Macromol. 2011, 48, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Barreto, A.C.H.; Maia, F.J.N.; Santiago, V.R.; Ribeiro, V.G.P.; Denardin, J.C.; Mele, G.; Carbone, L.; Lomonaco, D.; Mazzetto, S.E.; Fechine, P.B.A. Novel ferrofluids coated a renewable material obtained from cashew nut shell liquid. Microfluid. Nanofluid. 2012, 12, 677–686. [Google Scholar] [CrossRef]
- Lomonaco, D.; Mele, G.; Mazzetto, S.E. Cashew Nutshell Liquid (CNSL): From an Agro-industrial Waste to a Sustainable Alternative to Petrochemical Resources; Anilkumar, P., Ed.; Cashew Nut Shell Liquid; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Agache, C.; Popa, V. Ab initio studies on the molecular conformation of lignin model compounds I. Conformational preferences of the phenolic hydroxyl and methoxy groups in guaiacol. Monatsh. Chem. 2006, 137, 137–155. [Google Scholar] [CrossRef]
- Perote, L.C.; Kamozaki, M.B.; Gutierrez, N.C.; Tay, F.R.; Pucci, C.R. Effect of Matrix Metalloproteinase-inhibiting Solutions and Aging Methods on Dentin Bond Strength. J. Adhes. Dent. 2015, 17, 347–352. [Google Scholar] [CrossRef]
- Seseogullari-Dirihan, R.; Mutluay, M.M.; Pashley, D.H.; Tezvergil-Mutluay, A. Is the inactivation of dentin proteases by crosslinkers reversible? Dent. Mater. 2017, 33, e62–e68. [Google Scholar] [CrossRef]
- Aydin, B.; Leme-Kraus, A.A.; Vidal, C.M.P.; Aguiar, T.R.; Phansalkar, R.S.; Nam, J.W.; McAlpine, J.B.; Chen, S.N.; Pauli, G.F.; Bedran-Russo, A.K. Evidence to the role of interflavan linkages and galloylation of proanthocyanidins at sustaining long-term dentin biomodification. Dent. Mater. 2019, 35, 328–334. [Google Scholar] [CrossRef]
- Phansalkar, R.S.; Nam, J.W.; Chen, S.N.; McAlpine, J.B.; Napolitano, J.G.; Leme, A.; Vidal, C.M.; Aguiar, T.; Bedran-Russo, A.K.; Pauli, G.F. A galloylated dimeric proanthocyanidin from grape seed exhibits dentin biomodification potential. Fitoterapia 2015, 101, 169–178. [Google Scholar] [CrossRef]
- Xu, Z.; Wei, L.; Ge, Z.; Zhu, W.; Li, C. Comparison of the degradation kinetics of A-type and B-type proanthocyanidins dimers as a function of pH and temperature. Eur. Food Res. Technol. 2015, 240, 707–714. [Google Scholar] [CrossRef]
- Boteon, A.P.; Kato, M.T.; Buzalaf, M.A.R.; Prakki, A.; Wang, L.; Rios, D.; Honório, H.M. Effect of proanthocyanidin-enriched extracts on the inhibition of wear and degradation of dentin demineralized organic matrix. Arch. Oral Biol. 2017, 84, 118–124. [Google Scholar] [CrossRef]

| Groups | µTBS (MPa) [Fracture Mode A/CD/CC/M] | DC | Modulus of Elasticity | Mass Change (%) | HYP (µg/mL) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Immediate | Aging | (%) | Baseline (MPa) | Treated (MPa) | Variation (%) | After Biomodif | 4-Weeks Degradation | Not-Demineralized 0.02 (0.01) D | |
| NC | 51.2 (4.9) Aa [90/2/3/5] | 36.8 (7.2) ABb [97/0/0/3] | 75.7 (4.5) A | 10.6 (1.9) a | 4.1 (7.2) b | −60.4 (23.2) C | 13.5 (6.7) C | −34.4 (29.8) C | 0.96 (0.12) A |
| LIG1 | 39.6 (3.3) ABa [98/0/0/2] | 35.7 (7.4) Ba [92/0/0/8] | 71.1 (4.3) AB | 3.6 (1.1) a | 4.3 (1.9) a | 22.8 (35.7) BC | 30.7 (8.6) A | 45.2 (13.2) A | 0.33 (0.01) C |
| LIG2 | 38.1 (6.2) BCa [98/0/0/2] | 37.7 (2.6) ABa [95/0/0/5] | 64.1 (0.2) BC | 2.7 (0.8) b | 5.6 (2.2) a | 116.5 (74.5) A | 25.7 (8.7) AB | 42.9 (19.1) A | 0.53 (0.01) B |
| LIG4 | 37.5 (3.0) BCb [99/0/0/1] | 46.4 (5.6) Aa [92/0/3/5] | 64.1 (6.1) BC | 3.6 (1.2) b | 8.4 (3.7) a | 177.6 (263.8) AB | 16.1 (11.5) BC | 27.3 (16.1) AB | 0.55 (0.09) B |
| CARD | 37.9 (4.0) BCa [100/0/0/0] | 31.2 (5.5) Bb [97/2/0/1] | 57.7 (2.3) C | 4.4 (1.0) b | 7.9 (3.9) a | 85.5 (96.7) AB | 21.4 (9,0) ABC | 20.3 (17.5) AB | 0.50 (0.07) BC |
| PAC | 30.3 (4.9) Ca [100/0/0/0] | 30.2 (4.0) Ba [100/0/0/0] | 56.8 (3.3) C | 7.4 (2.0) a | 8.6 (2.2) a | 17.1 (12.7) BC | 19.2 (5.4) ABC | 4.5 (33.0) B | 0.61 (0.06) B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Paula, D.M.; Lomonaco, D.; Parente da Ponte, A.M.; Cordeiro, K.E.; Magalhães Moreira, M.; Giovarruscio, M.; Sauro, S.; Pinheiro Feitosa, V. Collagen Cross-Linking Lignin Improves the Bonding Performance of Etch-and-Rinse Adhesives to Dentin. Materials 2022, 15, 3218. https://doi.org/10.3390/ma15093218
de Paula DM, Lomonaco D, Parente da Ponte AM, Cordeiro KE, Magalhães Moreira M, Giovarruscio M, Sauro S, Pinheiro Feitosa V. Collagen Cross-Linking Lignin Improves the Bonding Performance of Etch-and-Rinse Adhesives to Dentin. Materials. 2022; 15(9):3218. https://doi.org/10.3390/ma15093218
Chicago/Turabian Stylede Paula, Diego Martins, Diego Lomonaco, Antônio Moisés Parente da Ponte, Karen Evellin Cordeiro, Madiana Magalhães Moreira, Massimo Giovarruscio, Salvatore Sauro, and Victor Pinheiro Feitosa. 2022. "Collagen Cross-Linking Lignin Improves the Bonding Performance of Etch-and-Rinse Adhesives to Dentin" Materials 15, no. 9: 3218. https://doi.org/10.3390/ma15093218
APA Stylede Paula, D. M., Lomonaco, D., Parente da Ponte, A. M., Cordeiro, K. E., Magalhães Moreira, M., Giovarruscio, M., Sauro, S., & Pinheiro Feitosa, V. (2022). Collagen Cross-Linking Lignin Improves the Bonding Performance of Etch-and-Rinse Adhesives to Dentin. Materials, 15(9), 3218. https://doi.org/10.3390/ma15093218








