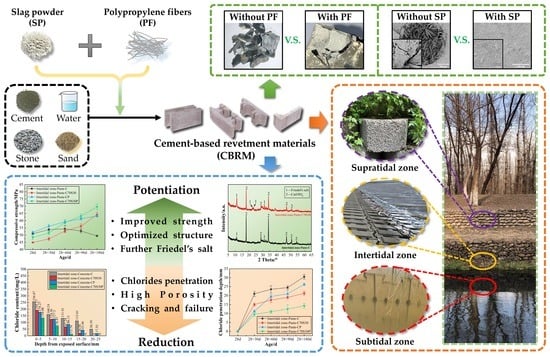
Durability and Improvement of Cement-Based Revetment Materials Serving in Subtidal, Intertidal, and Supratidal Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Methods
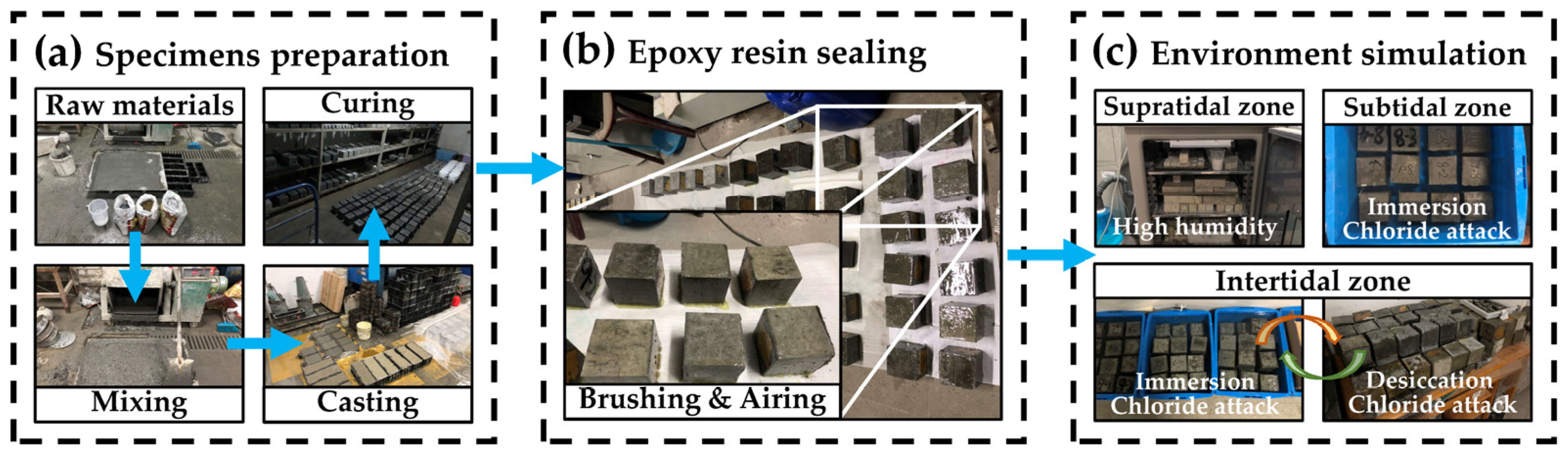
2.2.1. Specimen Preparation
2.2.2. Environment Simulation Procedure
2.2.3. Measurement Methods
- Mechanical properties: the compressive strength and flexural strength of specimens were tested by an electronic universal testing machine.
- Macroscopic morphology of the exposed surface to chloride: Durability was visually analyzed by the macroscopic morphology comparison. A digital camera was used to take pictures of the exposed surface of the specimens serving in the intertidal zone and the subtidal zone.
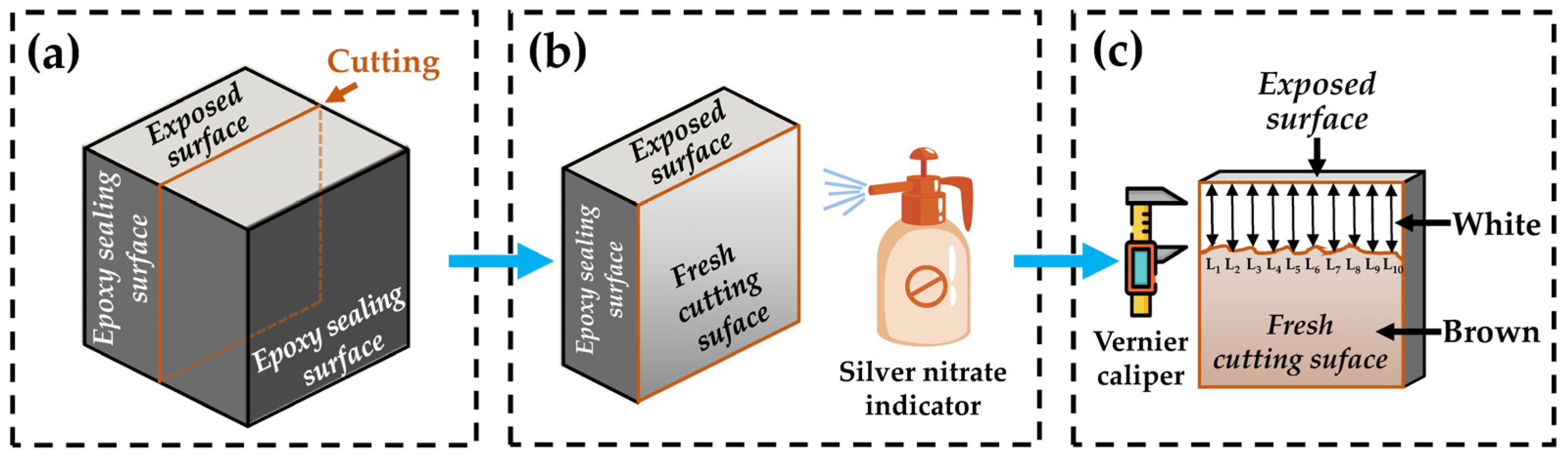
- Chloride ions’ penetration depth: The chloride ion penetration depth was measured by the silver nitrate colorimetry. First, the specimen was cut into two halves along the direction perpendicular to the exposed surface. Second, the fresh cutting surface was immediately sprayed by the silver nitrate indicator with a concentration of 0.1 mol/L. Third, the chloride ion penetration area which was in white was traced by a waterproof pen, 15 min after spraying. Fourth, the depth from the exposed surface to the deep contour line was measured with a Vernier caliper. For one specimen, depth data from ten points equally spaced horizontally were collected to obtain the average value as the chloride ions’ penetration depth. If there was exactly a stone located at the measured point, the data of this point were immediately removed. The measurement process is shown in Figure 3.
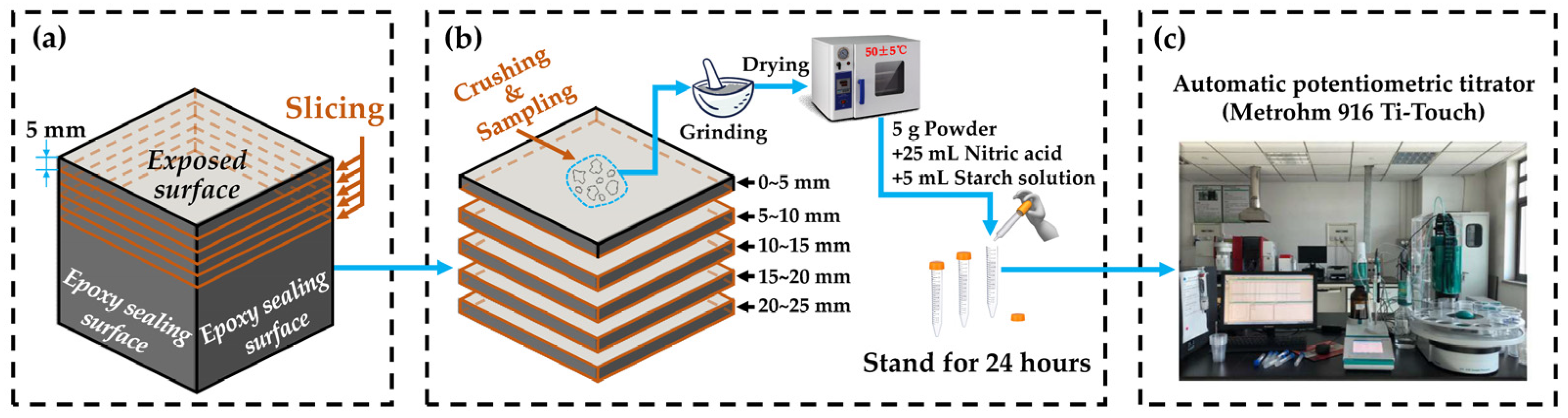
- Chloride ions’ profile: As shown in Figure 4, the part of the specimen near the exposed surface was sliced into 5 pieces with a thickness of 5 mm. Samples from each piece were taken for grinding and drying before chloride ions’ content measurement, and the sampling point should be avoided where there are coarse aggregates. Then, 5 g of the dried powder obtained above was added into 25 mL of nitric acid solution (prepared with nitric acid and distilled water in a volume ratio of 1:7) and 5 mL of starch solution with a concentration of 10 g/L. Standing for 24 h would help the powder to fully dissolve. Then, chloride ions’ content was measured using a Metrohm 916 Ti-Touch automatic potentiometric titrator.
- Composition, microstructure, and morphology of hydration products: CBRM-Paste after 28 + 180 days of the simulation experiment was used to investigate the composition, microstructure, and morphology of hydration products under the chloride ion erosion. Powder samples and thin-sliced samples taken from the 0~5 mm zone of CBRM-Paste were dried in the same drying procedure as in Chloride ions’ profile test. Powder samples were scanned with a Bruker D8 Advance X-ray diffractometer (XRD) to explore the composition of the hydration products. The microstructure and morphology of thin-sliced samples with a thickness of 3~5 mm were observed using a JEOL JSM-6700F and a Phenom Pharos G1 scanning electron microscope (SEM).
3. Results and Discussion
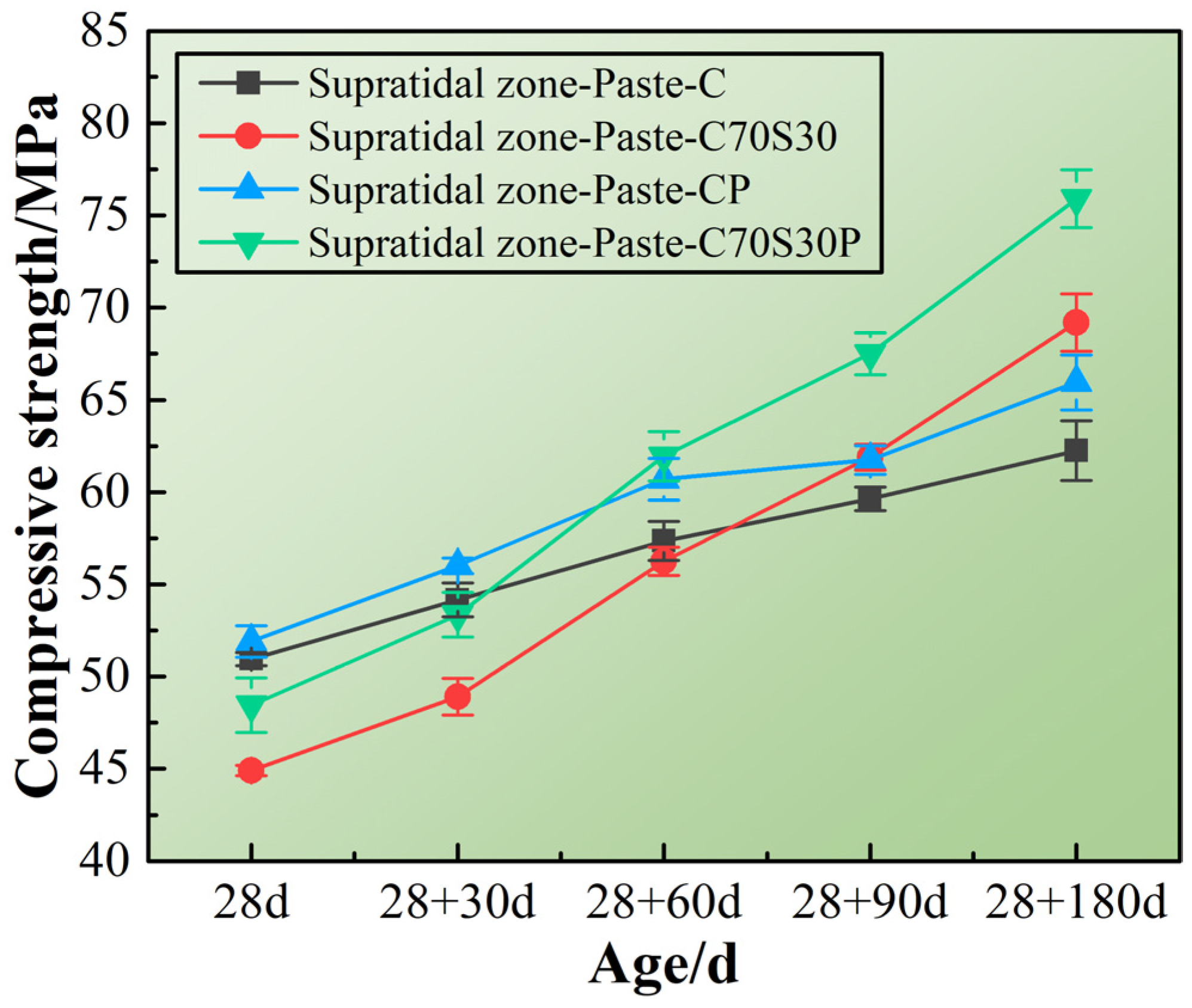
3.1. Durability of CBRM Serving in a Supratidal Environment
3.1.1. Mechanical Properties
3.1.2. Hydration Products
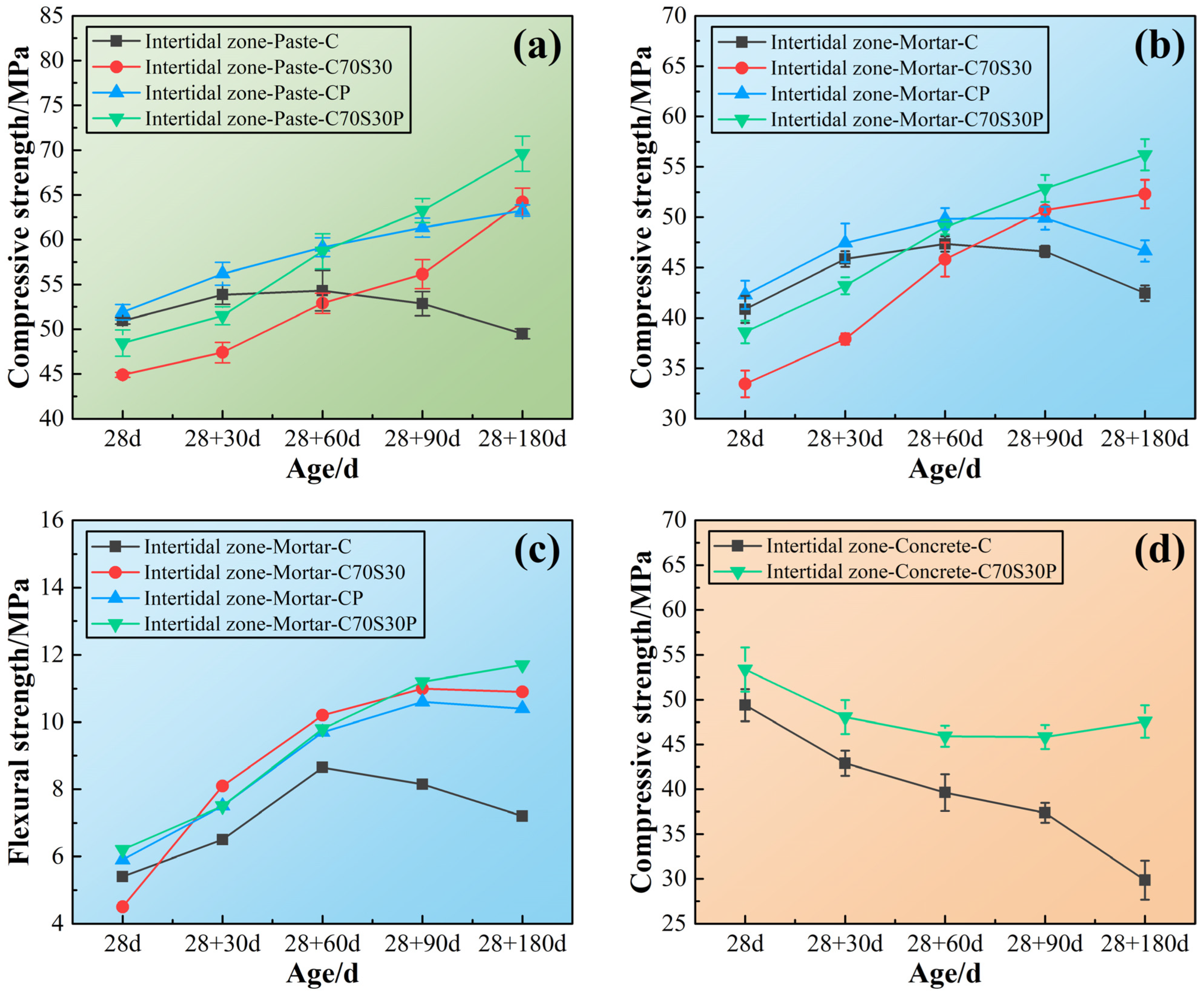
3.2. Durability of CBRM Serving in the Intertidal Environment
3.2.1. Mechanical Properties
3.2.2. Macroscopic Morphology of the Exposed Surface
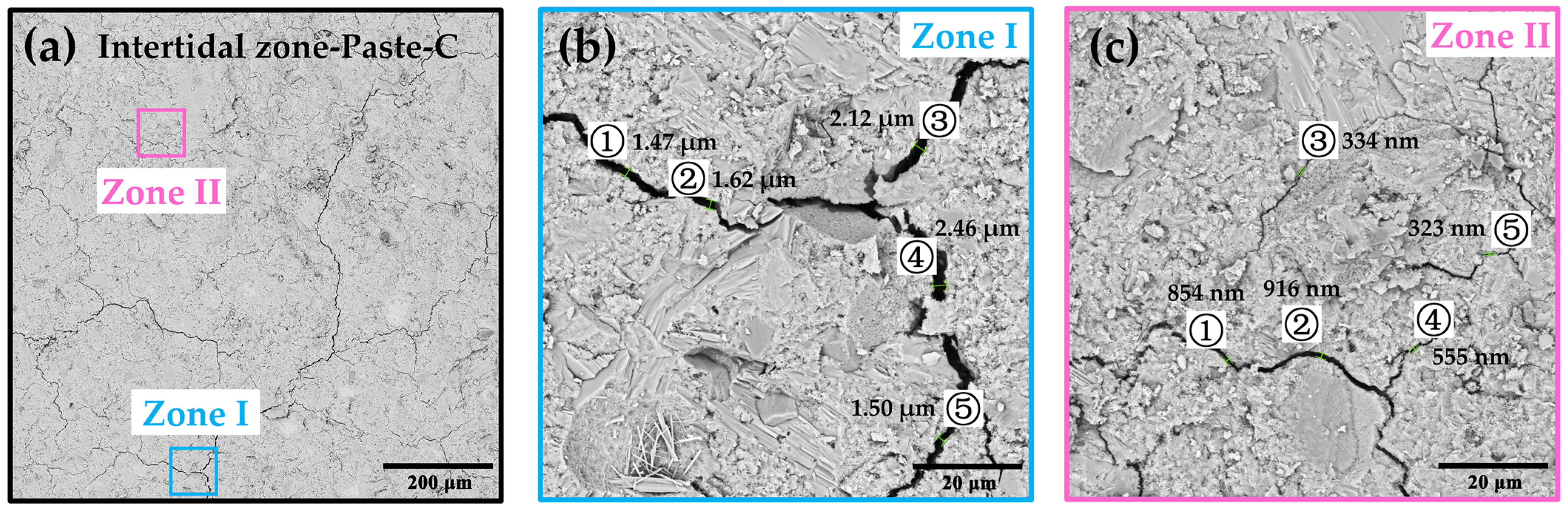
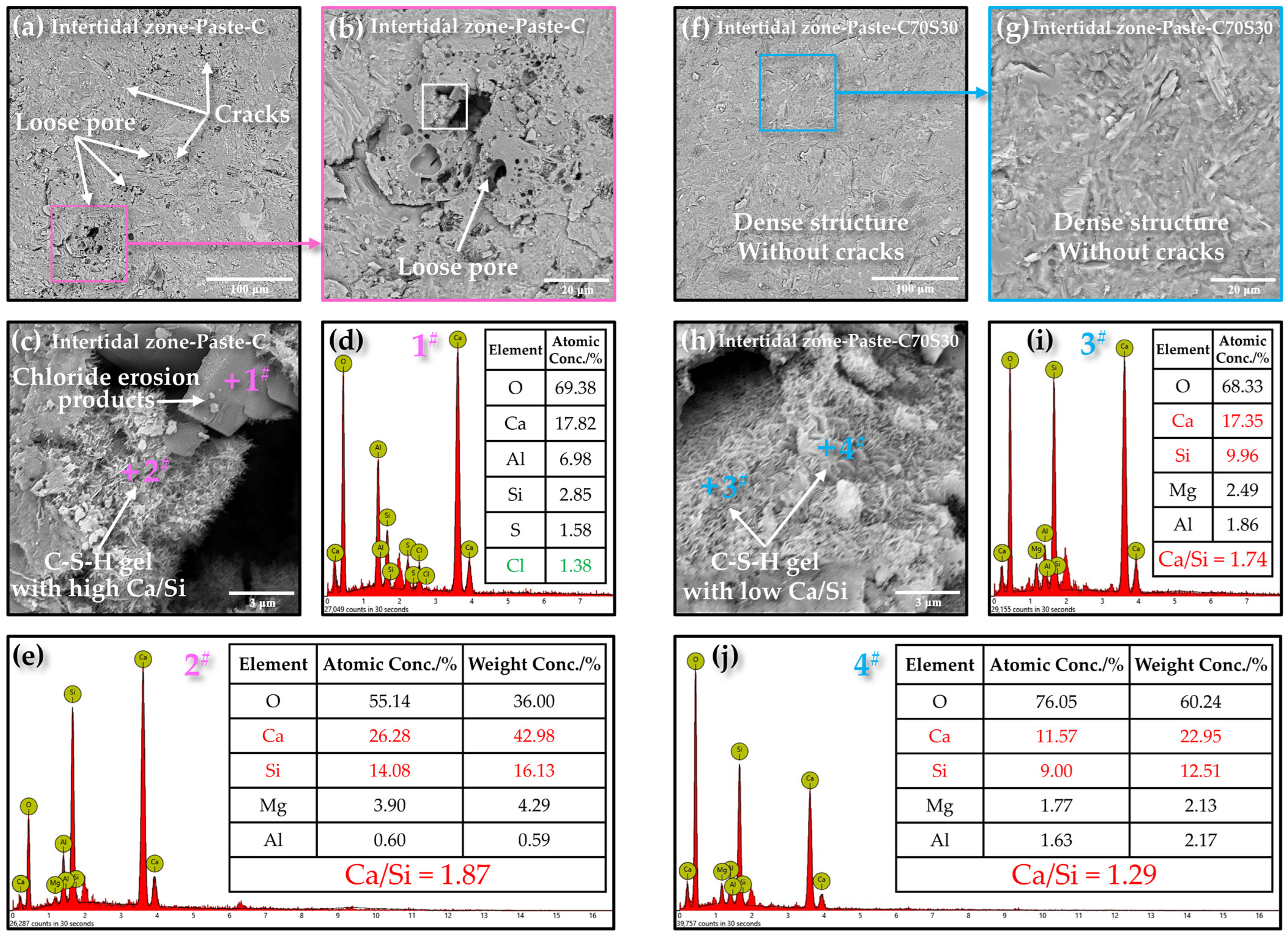
3.2.3. Internal Microstructure and Morphology
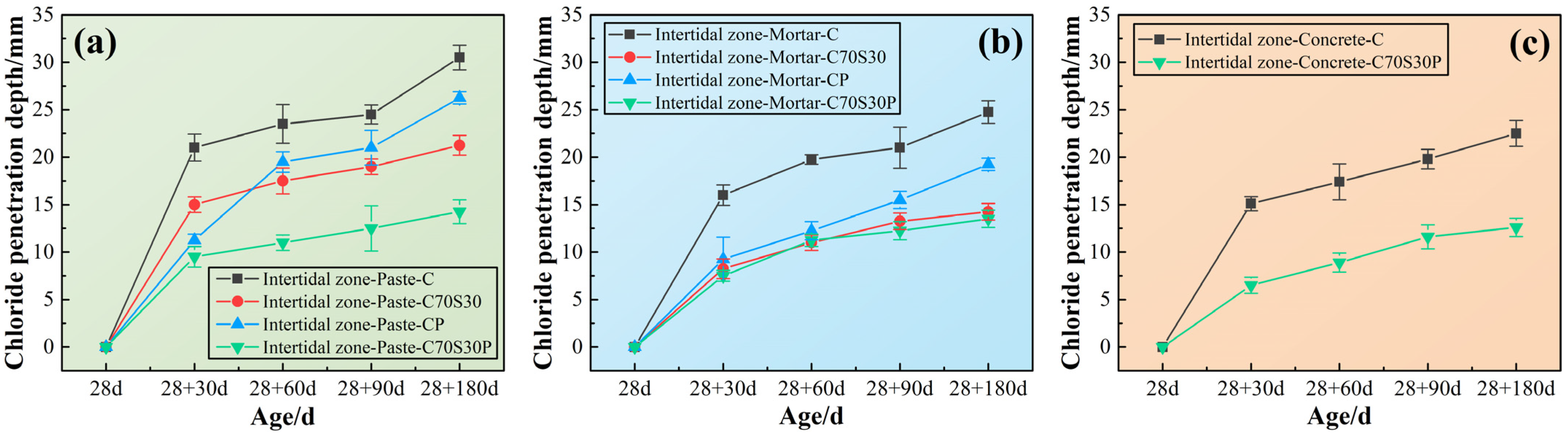
3.2.4. Chloride Ions’ Penetration Depth
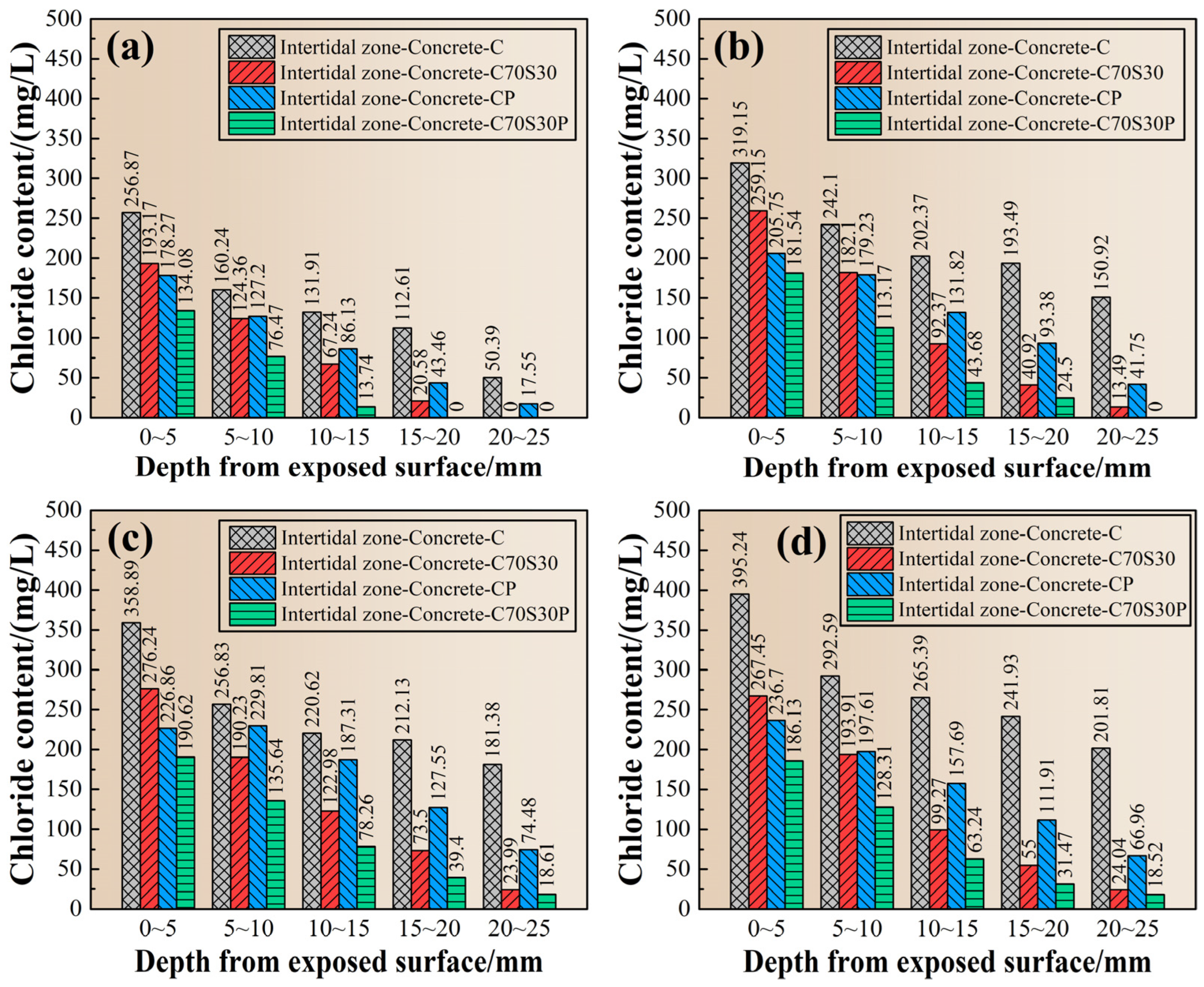
3.2.5. Chloride Ions’ Profile
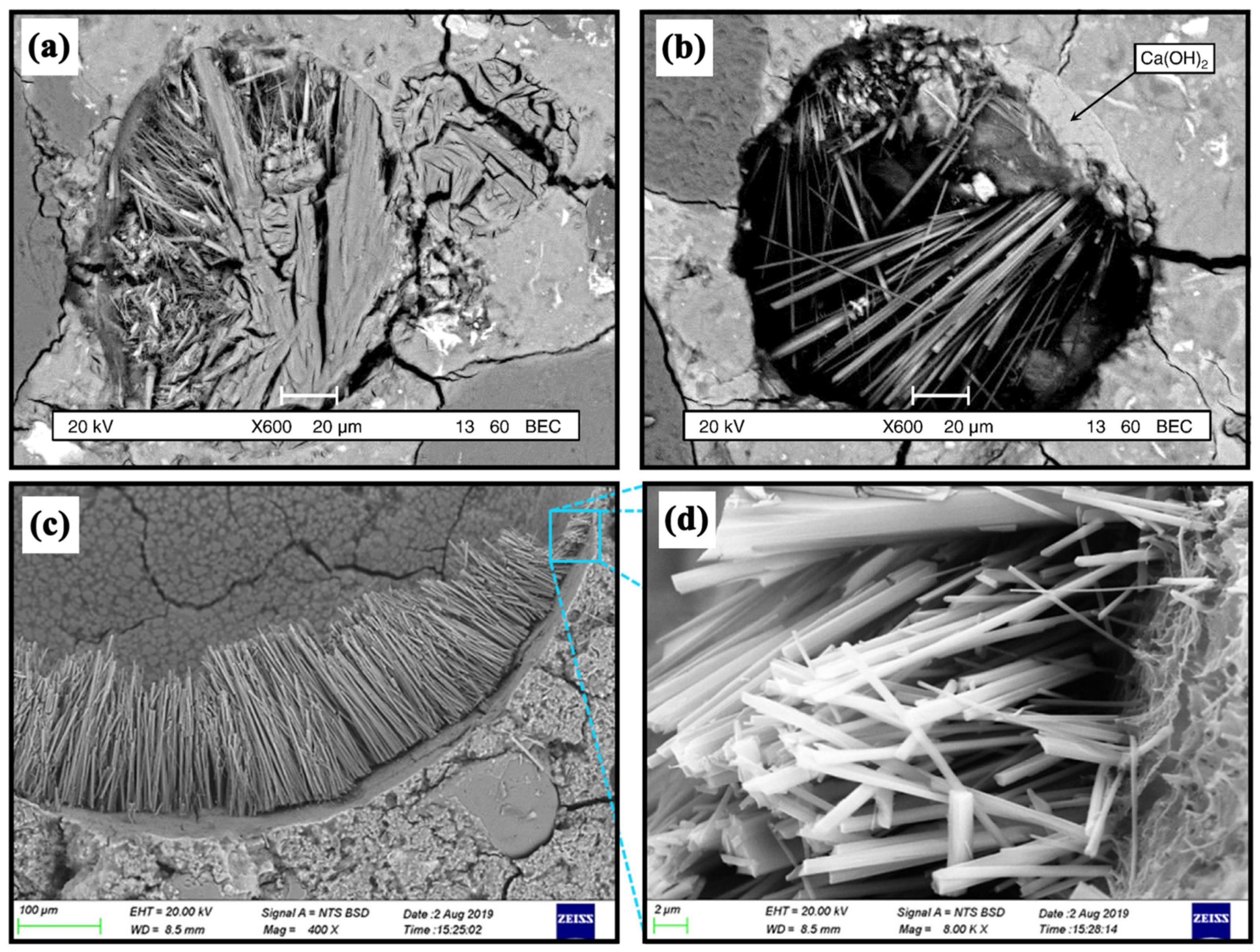
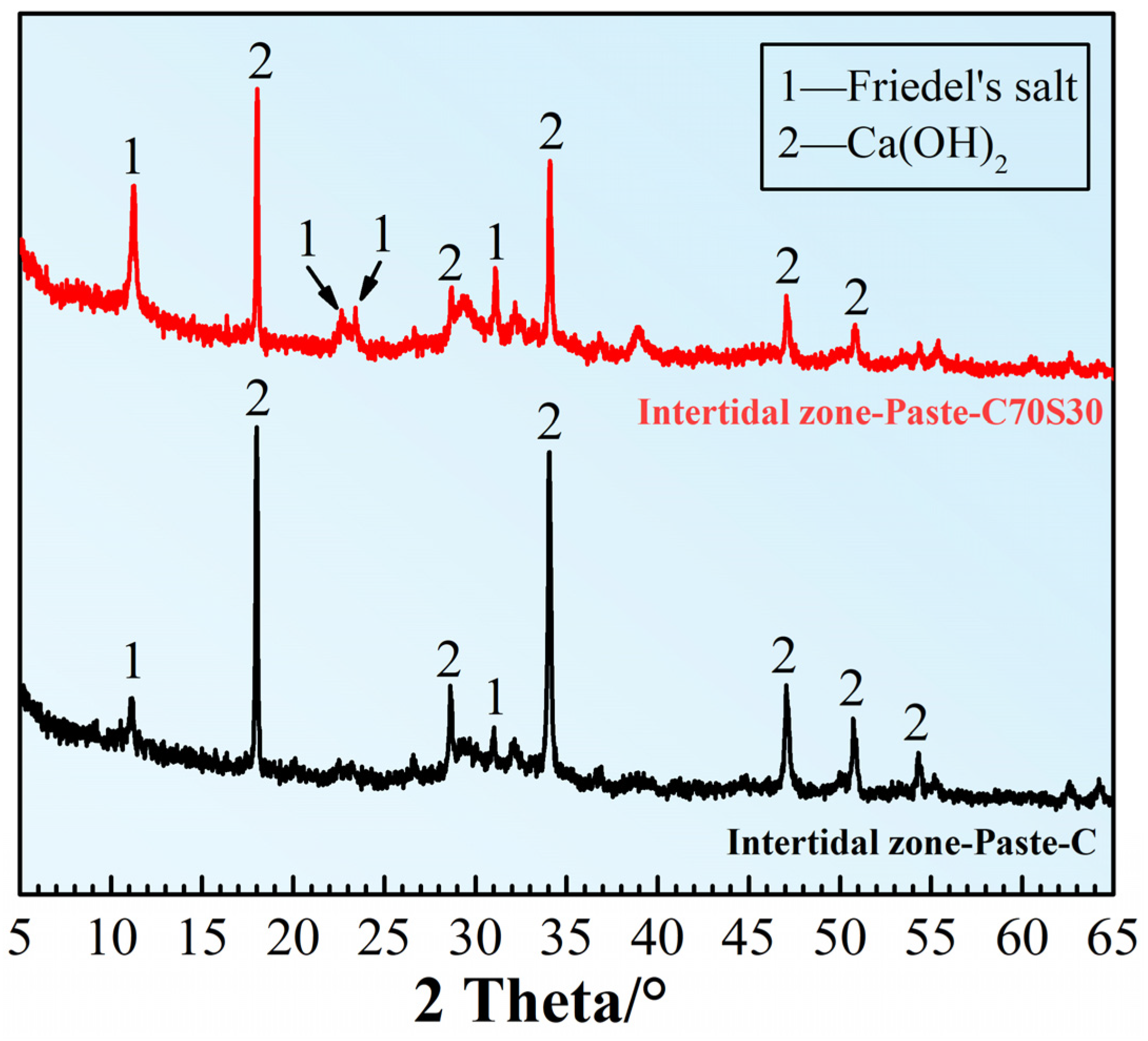
3.2.6. Hydration and Erosion Products
3.3. Durability of CBRM Serving in the Subtidal Environment
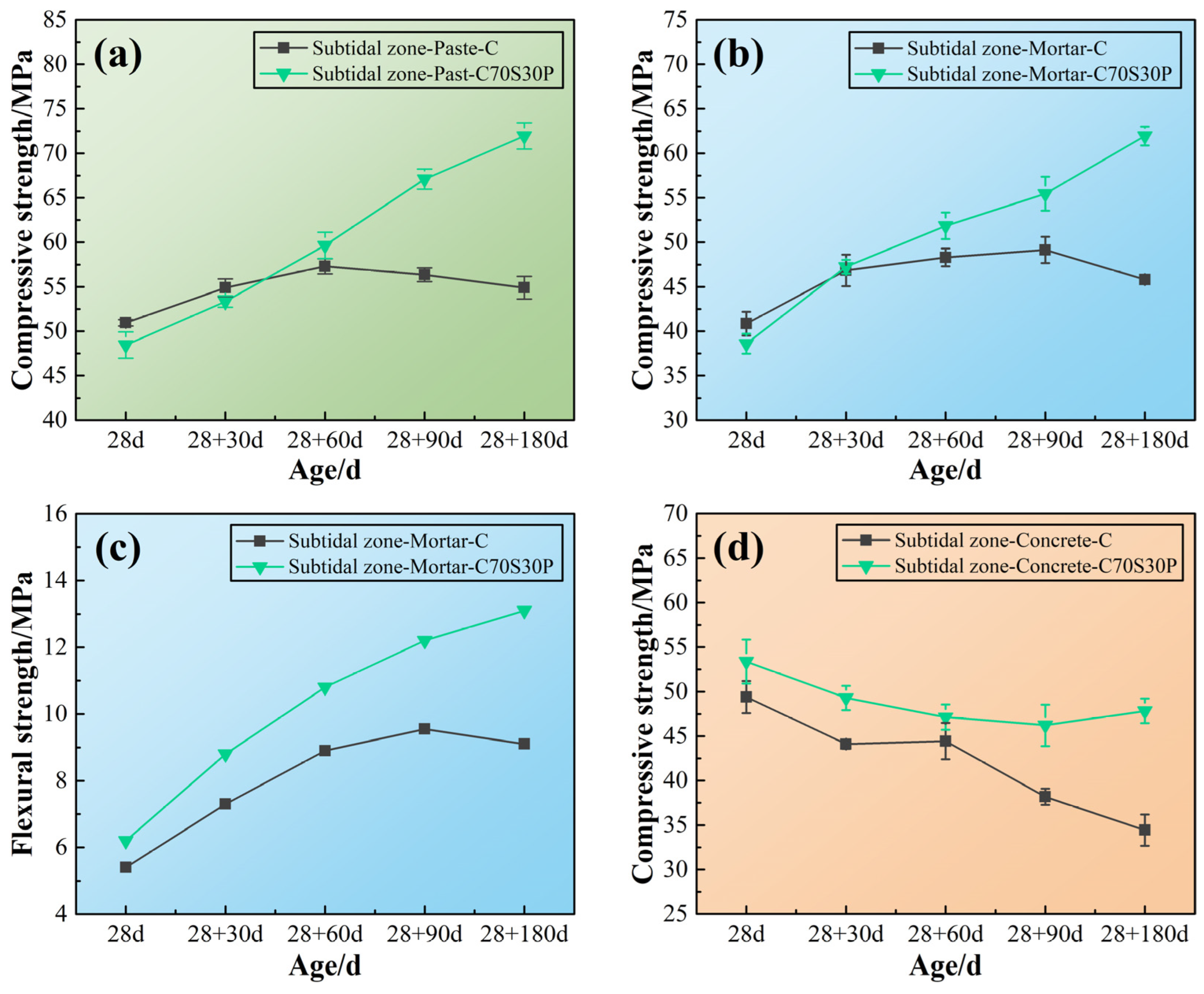
3.3.1. Mechanical Properties
3.3.2. Macroscopic Morphology of the Exposed Surface
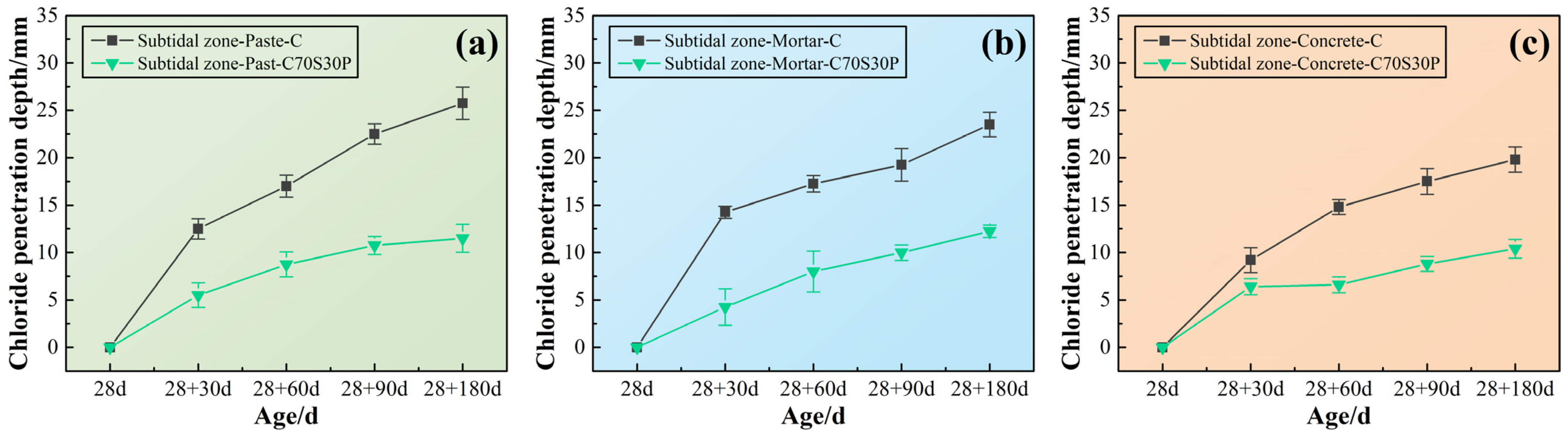
3.3.3. Chloride Ions’ Penetration Depth
3.3.4. Chloride Ions’ Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meira, G.R.; Andrade, C.; Padaratz, I.J.; Alonso, C.; Borba, J.C. Chloride penetration into concrete structures in the marine atmosphere zone—Relationship between deposition of chlorides on the wet candle and chlorides accumulated into concrete. Cem. Concr. Compos. 2007, 29, 667–676. [Google Scholar] [CrossRef]
- Wattanapornprom, R.; Ishida, T. Modeling of Chloride Penetration into Concrete under Airborne Chloride Environmental Conditions Combined with Washout Effects. J. Adv. Concr. Technol. 2017, 15, 126–142. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Cai, G.; Gao, D.; Zhao, S. Influences of freeze–thaw cycle and curing time on chloride ion penetration resistance of Sulphoaluminate cement concrete. Constr. Build. Mater. 2014, 53, 305–311. [Google Scholar] [CrossRef]
- Mu, S.; De Schutter, G.; Ma, B.-G. Non-steady state chloride diffusion in concrete with different crack densities. Mater. Struct. 2013, 46, 123–133. [Google Scholar] [CrossRef]
- Yin, S.; Jing, L.; Yin, M.; Wang, B. Mechanical properties of textile reinforced concrete under chloride wet-dry and freeze-thaw cycle environments. Cem. Concr. Compos. 2019, 96, 118–127. [Google Scholar] [CrossRef]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. NPJ Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Sun, R.; Wang, D.; Cao, H.; Wang, Y.; Lu, Z.; Xia, J. Ecological pervious concrete in revetment and restoration of coastal Wetlands: A review. Constr. Build. Mater. 2021, 303, 124590. [Google Scholar] [CrossRef]
- Guo, J.J.; Wang, K.; Qi, C.G. Determining the Mineral Admixture and Fiber on Mechanics and Fracture Properties of Concrete under Sulfate Attack. J. Mar. Sci. Eng. 2021, 9, 251. [Google Scholar] [CrossRef]
- Honglei, C.; Zuquan, J.; Penggang, W.; Jianhong, W.; Jian, L. Comprehensive resistance of fair-faced concrete suffering from sulfate attack under marine environments. Constr. Build. Mater. 2021, 277, 122312. [Google Scholar] [CrossRef]
- Guo, A.; Li, H.; Ba, X.; Guan, X.; Li, H. Experimental investigation on the cyclic performance of reinforced concrete piers with chloride-induced corrosion in marine environment. Eng. Struct. 2015, 105, 1–11. [Google Scholar] [CrossRef]
- Li, C.; Zhu, L.; Dai, Z.; Wu, Z. Study on Spatiotemporal Evolution of the Yellow River Delta Coastline from 1976 to 2020. Remote Sens. 2021, 13, 4789. [Google Scholar] [CrossRef]
- Li, X.N.; Zuo, X.B.; Zou, Y.X. Modeling and simulation on coupled chloride and calcium diffusion in concrete. Constr. Build. Mater. 2021, 271, 121557. [Google Scholar] [CrossRef]
- Yu, L.; Xu, F. Bilateral chloride diffusion model of nanocomposite concrete in marine engineering. Constr. Build. Mater. 2020, 263, 120634. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Shi, M.; Cui, J.; Xie, F. Degradation of cast-in-situ concrete subjected to sulphate-chloride combined attack. Constr. Build. Mater. 2020, 241, 117995. [Google Scholar] [CrossRef]
- Cao, C.; Cheung, M.M.S. Non-uniform rust expansion for chloride-induced pitting corrosion in RC structures. Constr. Build. Mater. 2014, 51, 75–81. [Google Scholar] [CrossRef]
- Li, D.; Li, L.-y.; Wang, X. Chloride diffusion model for concrete in marine environment with considering binding effect. Mar. Struct. 2019, 66, 44–51. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Huang, Z.; Zhu, J.; Liu, W.; Zhang, W. Effect of Fly Ash as Cement Replacement on Chloride Diffusion, Chloride Binding Capacity, and Micro-Properties of Concrete in a Water Soaking Environment. Appl. Sci. 2020, 10, 6271. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Tang, L.; Xing, F. Modelling analysis of chloride redistribution in sea-sand concrete exposed to atmospheric environment. Constr. Build. Mater. 2021, 274, 121962. [Google Scholar] [CrossRef]
- Andrade, C.; Buják, R. Effects of some mineral additions to Portland cement on reinforcement corrosion. Cem. Concr. Res. 2013, 53, 59–67. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chotithanorm, C.; Cao, H.T.; Sirivivatnanon, V. Influence of fly ash fineness on the chloride penetration of concrete. Constr. Build. Mater. 2007, 21, 356–361. [Google Scholar] [CrossRef]
- Jung, M.S.; Kim, K.B.; Lee, S.A.; Ann, K.Y. Risk of chloride-induced corrosion of steel in SF concrete exposed to a chloride-bearing environment. Constr. Build. Mater. 2018, 166, 413–422. [Google Scholar] [CrossRef]
- Liu, J.; Ou, G.; Qiu, Q.; Chen, X.; Hong, J.; Xing, F. Chloride transport and microstructure of concrete with/without fly ash under atmospheric chloride condition. Constr. Build. Mater. 2017, 146, 493–501. [Google Scholar] [CrossRef]
- Kim, S.; kim, Y.; Usman, M.; Park, C.; Hanif, A. Durability of slag waste incorporated steel fiber-reinforced concrete in marine environment. J. Build. Eng. 2021, 33, 101641. [Google Scholar] [CrossRef]
- Otieno, M.; Beushausen, H.; Alexander, M. Effect of chemical composition of slag on chloride penetration resistance of concrete. Cem. Concr. Compos. 2014, 46, 56–64. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.A.; Glasser, F.P. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Hooton, R.D.; Scott, A.; Zibara, H. The effect of supplementary cementitious materials on chloride binding in hardened cement paste. Cem. Concr. Res. 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Sun, R.; Wang, D.; Wang, Y.; Zhang, L.; Gu, Y. Study on Durability against Dry-Wet Cycles and Chloride Ion Erosion of Concrete Revetment Materials at the Water-Level-Fluctuations Zone in Yellow River Delta Wetlands. Wetlands 2020, 40, 2713–2727. [Google Scholar] [CrossRef]
- Berrocal, C.G.; Lundgren, K.; Löfgren, I. Corrosion of steel bars embedded in fibre reinforced concrete under chloride attack: State of the art. Cem. Concr. Res. 2016, 80, 69–85. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, H.; He, Z.; Yang, L.; Zhao, L.; Wen, C. Experimental investigation on chloride-ion penetration resistance of slag containing fiber-reinforced concrete under drying-wetting cycles. Constr. Build. Mater. 2021, 274, 121829. [Google Scholar] [CrossRef]
- Qureshi, L.A.; Ali, B.; Ali, A. Combined effects of supplementary cementitious materials (silica fume, GGBS, fly ash and rice husk ash) and steel fiber on the hardened properties of recycled aggregate concrete. Constr. Build. Mater. 2020, 263, 120636. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, X.; Lv, J. Experimental study on the resistance of basalt fibre-reinforced concrete to chloride penetration. Constr. Build. Mater. 2019, 223, 142–155. [Google Scholar] [CrossRef]
- Afroughsabet, V.; Biolzi, L.; Monteiro, P.J.M. The effect of steel and polypropylene fibers on the chloride diffusivity and drying shrinkage of high-strength concrete. Compos. Part B-Eng. 2018, 139, 84–96. [Google Scholar] [CrossRef]
- Cao, Q.; Gao, Q.; Gao, R.; Jia, J. Chloride penetration resistance and frost resistance of fiber reinforced expansive self-consolidating concrete. Constr. Build. Mater. 2018, 158, 719–727. [Google Scholar] [CrossRef]
- Su, L.; Niu, D.; Huang, D.; Luo, Y.; Qiao, H.; Zhang, Y. Chloride diffusion behavior and microstructure of basalt-polypropylene hybrid fiber reinforced concrete in salt spray environment. Constr. Build. Mater. 2022, 324, 126716. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Liu, Y.; Wang, Y. Comparison of Three Different Methods for Measuring Chloride Transport in Predamaged Concretes. J. Mater. Civ. Eng. 2020, 32, 04020033. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Q.; Jia, Z.; Liu, C.; Deng, Z.; Zhang, Y. Effect of drying environment on mechanical properties, internal RH and pore structure of 3D printed concrete. Constr. Build. Mater. 2022, 315, 125731. [Google Scholar] [CrossRef]
- Shoukry, S.N.; William, G.W.; Downie, B.; Riad, M.Y. Effect of moisture and temperature on the mechanical properties of concrete. Constr. Build. Mater. 2011, 25, 688–696. [Google Scholar] [CrossRef]
- Chang, J.; Fang, Y.F.; Shang, X.P. The role of beta-C2S and gamma-C2S in carbon capture and strength development. Mater. Struct. 2016, 49, 4417–4424. [Google Scholar] [CrossRef]
- Ghebrab, T.T.; Soroushian, P. Mechanical Properties of Hydrated Cement Paste: Development of Structure-property Relationships. Int. J. Concr. Struct. M 2010, 4, 37–43. [Google Scholar] [CrossRef]
- Xue, Y.; Veazie, D.R.; Glinsey, C.; Horstemeyer, M.F.; Rowell, R.M. Environmental effects on the mechanical and thermomechanical properties of aspen fiber-polypropylene composites. Compos. Part B-Eng. 2007, 38, 152–158. [Google Scholar] [CrossRef]
- Vedalakshmi, R.; Rajagopal, K.; Palaniswamy, N. Durability performance of rebar embedded in chloride admixed blended cement concretes. Corros. Eng. Sci. Techn. 2011, 46, 256–270. [Google Scholar] [CrossRef]
- Oh, B.H.; Jang, S.Y. Effects of material and environmental parameters on chloride penetration profiles in concrete structures. Cem. Concr. Res. 2007, 37, 47–53. [Google Scholar] [CrossRef]
- Zhu, F.Z.; Ma, Z.M.; Zhang, M.M. Chloride Penetration into Concrete under the Coupling Effects of Internal and External Relative Humidity. Adv. Civ. Eng. 2020, 2020, 14. [Google Scholar] [CrossRef]
- Elakneswaran, Y.; Nawa, T.; Kurumisawa, K. Electrokinetic potential of hydrated cement in relation to adsorption of chlorides. Cem. Concr. Res. 2009, 39, 340–344. [Google Scholar] [CrossRef]
- Rosenqvist, M.; Bertron, A.; Fridh, K.; Hassanzadeh, M. Concrete alteration due to 55years of exposure to river water: Chemical and mineralogical characterisation. Cem. Concr. Res. 2017, 92, 110–120. [Google Scholar] [CrossRef]
- Neumann, C.; Faria, E.F.; dos Santos, A.C.P. Concrete leaching of a hydroelectric powerhouse due to 40 years of exposure to river water. Constr. Build. Mater. 2021, 302, 124253. [Google Scholar] [CrossRef]
- Kunther, W.; Ferreiro, S.; Skibsted, J. Influence of the Ca/Si ratio on the compressive strength of cementitious calcium-silicate-hydrate binders. J. Mater. Chem. A 2017, 5, 17401–17412. [Google Scholar] [CrossRef]
- Zhang, S.P.; Zhao, B.H. Influence of polypropylene fibre on the mechanical performance and durability of concrete materials. Eur. J. Environ. Civ. Eng. 2012, 16, 1269–1277. [Google Scholar] [CrossRef]
- Petkova, M.; Hylova, M.; Ujhelyiova, A. Physical modification of polypropylene fibers used in construction. J. Text. Inst. 2017, 108, 196–202. [Google Scholar]
- Pakravan, H.R.; Jamshidi, M. Tensile properties of strain-hardening cementitious composites containing polyvinyl-alcohol fibers hybridized with polypropylene fibers. J. Cent. South Univ. 2018, 25, 51–59. [Google Scholar] [CrossRef]
- Xu, F.; Yang, Z.; Liu, W.; Wang, S.; Zhang, H. Experimental investigation on the effect of sulfate attack on chloride diffusivity of cracked concrete subjected to composite solution. Constr. Build. Mater. 2020, 237, 117643. [Google Scholar] [CrossRef]
- Smarzewski, P. Study of Toughness and Macro/Micro-Crack Development of Fibre-Reinforced Ultra-High Performance Concrete After Exposure to Elevated Temperature. Materials 2019, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Mu, S.; Xie, D.; Wang, P. Influence of pore structure and moisture distribution on chloride “maximum phenomenon” in surface layer of specimens exposed to cyclic drying-wetting condition. Constr. Build. Mater. 2017, 131, 16–30. [Google Scholar] [CrossRef]







| Oxides | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | R2O | LOI |
|---|---|---|---|---|---|---|---|---|
| Cement | 22.73 | 4.34 | 2.75 | 62.96 | 1.81 | 2.55 | 0.59 | 2.27 |
| Slag powder | 38.54 | 6.87 | 0.95 | 43.44 | 6.55 | 0.70 | 0.70 | 2.25 |
| Specimens | C | S | P (vol. %) | W/B Ratio |
|---|---|---|---|---|
| Paste-C | 100% | 0 | 0 | 0.43 |
| Paste-C70S30 | 70% | 30% | 0 | 0.43 |
| Paste-CP | 100% | 0 | 0.10% | 0.43 |
| Paste-C70S30P | 70% | 30% | 0.10% | 0.43 |
| Specimens | C | S | Sand | P (vol. %) | W/B Ratio |
|---|---|---|---|---|---|
| Mortar-C | 100% | 0 | 300% | 0 | 0.43 |
| Mortar-C70S30 | 70% | 30% | 300% | 0 | 0.43 |
| Mortar-CP | 100% | 0 | 300% | 0.10% | 0.43 |
| Mortar-C70S30P | 70% | 30% | 300% | 0.10% | 0.43 |
| Specimens | C | S | Sand | Stone | P (vol. %) | PCE | W/B Ratio |
|---|---|---|---|---|---|---|---|
| Concrete-C | 384 | 0 | 836 | 1065 | 0 | 1.92 | 0.43 |
| Concrete-C70S30 | 268.8 | 115.2 | 836 | 1065 | 0 | 1.92 | 0.43 |
| Concrete-CP | 384 | 0 | 836 | 1065 | 0.91 | 1.92 | 0.43 |
| Concrete-C70S30P | 268.8 | 115.2 | 836 | 1065 | 0.91 | 1.92 | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Wang, D.; Wang, Y.; Zhang, L.; Gu, Y. Durability and Improvement of Cement-Based Revetment Materials Serving in Subtidal, Intertidal, and Supratidal Environments. Materials 2022, 15, 3210. https://doi.org/10.3390/ma15093210
Sun R, Wang D, Wang Y, Zhang L, Gu Y. Durability and Improvement of Cement-Based Revetment Materials Serving in Subtidal, Intertidal, and Supratidal Environments. Materials. 2022; 15(9):3210. https://doi.org/10.3390/ma15093210
Chicago/Turabian StyleSun, Rui, Dongmin Wang, Yiren Wang, Lei Zhang, and Yue Gu. 2022. "Durability and Improvement of Cement-Based Revetment Materials Serving in Subtidal, Intertidal, and Supratidal Environments" Materials 15, no. 9: 3210. https://doi.org/10.3390/ma15093210
APA StyleSun, R., Wang, D., Wang, Y., Zhang, L., & Gu, Y. (2022). Durability and Improvement of Cement-Based Revetment Materials Serving in Subtidal, Intertidal, and Supratidal Environments. Materials, 15(9), 3210. https://doi.org/10.3390/ma15093210