Effect of Different Root Canal Irrigant Solutions on the Release of Dentin-Growth Factors: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process and Data Items
2.6. Studies Risk of Bias Assessment
2.7. Synthesis Methods and Effect Measures
2.8. Certainty of Evidence Assessment
3. Results
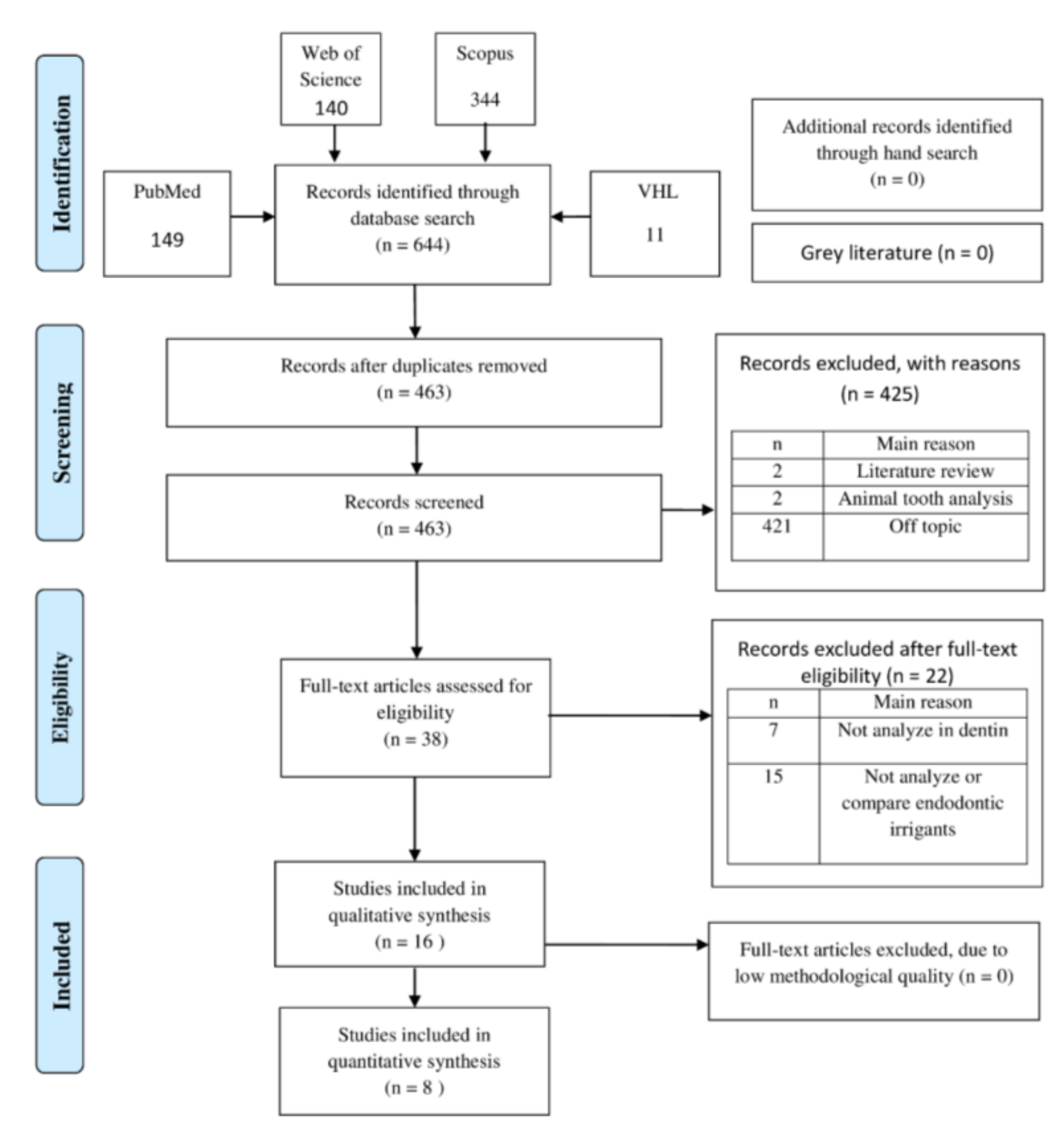
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Risk of Bias in Studies
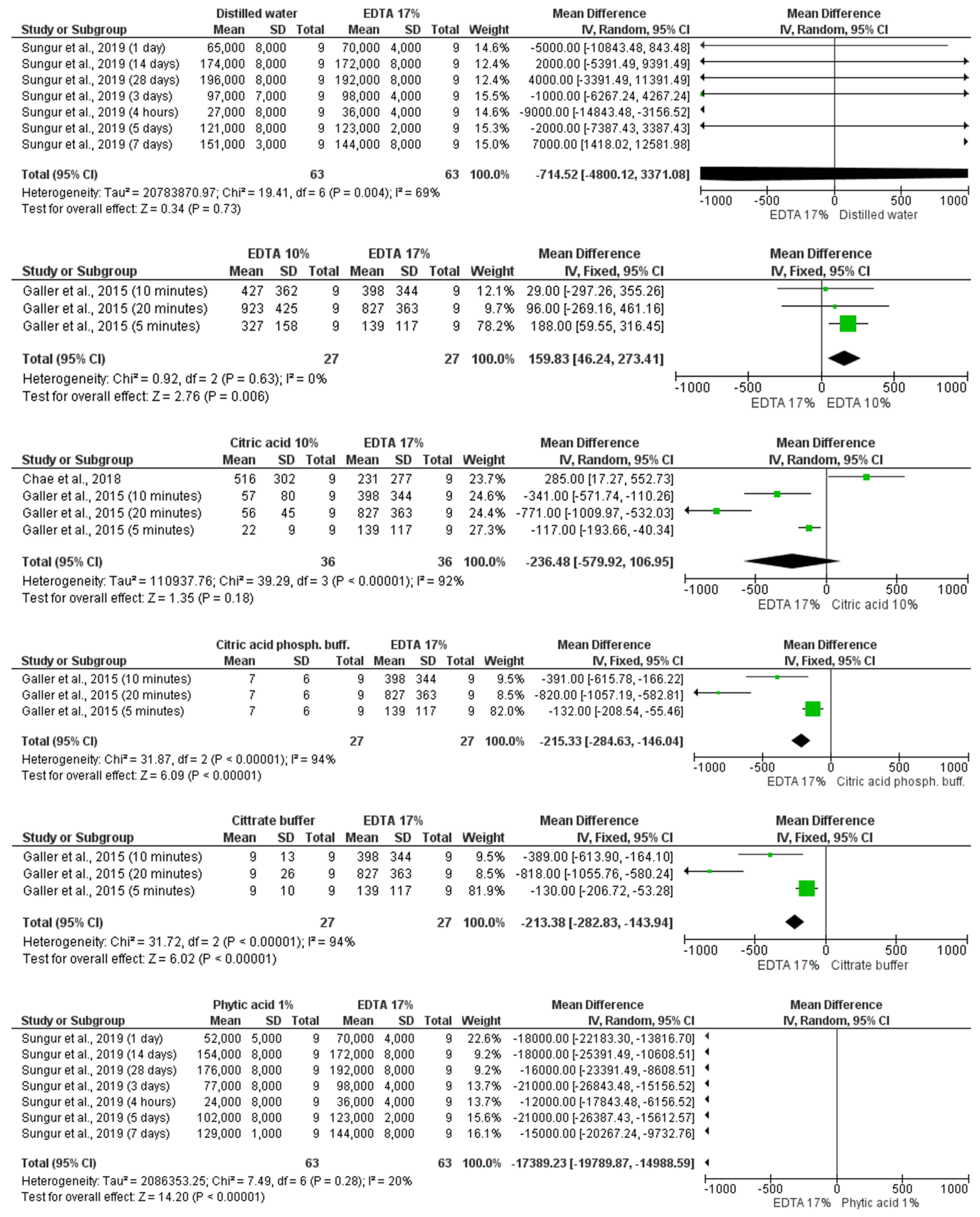
3.4. Results of Synthesis, Meta-Analyses, and Certainty of Evidence
3.4.1. 10% EDTA Versus 10% Citric Acid
3.4.2. 17% EDTA Versus Other Irrigants
3.4.3. Detection of TGF-β1 by the Immunogold Method
4. Discussion
4.1. Regenerative Endodontics
4.2. Methodological Issues
4.3. The Action of Irrigants in the Release of Dentin-Growth Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nazzal, H.; Duggal, M.S. Regenerative endodontics: A true paradigm shift or a bandwagon about to be derailed? Eur. Arch. Paediatr. Dent. Off. J. Eur. Acad. Paediatr. Dent. 2017, 18, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, K.M.; Diogenes, A.; Teixeira, F.B. Treatment options: Biological basis of regenerative endodontic procedures. Pediatr. Dent. 2013, 35, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Scelza, P.; Gonçalves, F.; Caldas, I.; Nunes, F.; Lourenço, E.S.; Tavares, S.; Magno, M.; Pintor, A.; Montemezzi, P.; Di Edoardo, E.; et al. Prognosis of regenerative endodontic procedures in mature teeth: A systematic review and meta-analysis of clinical and radiographic parameters. Materials 2021, 14, 4418. [Google Scholar] [CrossRef]
- Dhillon, H.; Kaushik, M.; Sharma, R. Regenerative endodontics—Creating new horizons. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Mitsiadis, T.A.; Feki, A.; Papaccio, G.; Catón, J. Dental pulp stem cells, niches, and notch signaling in tooth injury. J. Adv. Dent. Res. 2011, 23, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Martins, J.; Diogo, P.; Sequeira, D.; Ramos, J.C.; Diogenes, A.; Santos, J.M. Does apical papilla survive and develop in apical periodontitis presence after regenerative endodontic procedures? Appl. Sci. 2019, 9, 3942. [Google Scholar] [CrossRef] [Green Version]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic evaluation of regenerative endodontic procedures with the use of chitosan scaffolds in immature dog teeth with apical periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef]
- Kim, S.G.; Zhou, J.; Solomon, C.; Zheng, Y.; Suzuki, T.; Chen, M.; Song, S.; Jiang, N.; Cho, S.; Mao, J.J. Effects of growth factors on dental stem/progenitor cells. Dent. Clin. N. Am. 2012, 56, 563–575. [Google Scholar] [CrossRef] [Green Version]
- Diogenes, A.; Simon, S.; Law, A.S. Regenerative Endodontics. In Pathways of the Pulp, 11th ed.; Berman, L.H., Hargreaves, K.M., Eds.; Elsevier Health Sciences: St. Louis, MO, USA, 2011; pp. 447–473. [Google Scholar]
- Galler, K.M.; Buchalla, W.; Hiller, K.A.; Federlin, M.; Eidt, A.; Schiefersteiner, M.; Schmalz, G. Influence of root canal disinfectants on growth factor release from dentin. J. Endod. 2015, 41, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Widbiller, M.; Buchalla, W.; Eidt, A.; Hiller, K.A.; Hoffer, P.C.; Schmalz, G. EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int. Endod. J. 2016, 49, 581–590. [Google Scholar] [CrossRef]
- Dung, S.Z.; Gregory, R.L.; Li, Y.; Stookey, G.K. Effect of lactic acid and proteolytic enzymes on the release of organic matrix components from human root dentin. Caries Res. 1995, 29, 483–489. [Google Scholar] [CrossRef]
- Chae, Y.; Yang, M.; Kim, J. Release of TGF-beta1 into root canals with various final irrigants in regenerative endodontics: An in vitro analysis. Int. Endod. J. 2018, 51, 1389–1397. [Google Scholar] [CrossRef]
- Sloan, A.J.; Smith, A.J. Stem cells and the dental pulp: Potential roles in dentine regeneration and repair. Oral. Dis. 2007, 13, 151–157. [Google Scholar] [CrossRef]
- Arslan, H.; Ahmed, H.M.A.; Şahin, Y.; Doğanay Yıldız, E.; Gündoğdu, E.C.; Güven, Y.; Khalilov, R. Regenerative endodontic procedures in necrotic mature teeth with periapical radiolucencies: A preliminary randomized clinical study. J. Endod. 2019, 45, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Zehnder, M. Root canal irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Beronius, A.; Molander, L.; Zilliacus, J.; Rudén, C.; Hanberg, A. Testing and refining the Science in Risk Assessment and Policy (SciRAP) web-based platform for evaluating the reliability and relevance of in vivo toxicity studies. J. Appl. Toxicol. 2018, 38, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M. Common Mistakes in Meta-Analysis And How to Avoid Them; Biostat Inc.: Tampa, FL, USA, 2019. [Google Scholar]
- Ryan, R.; Hills, S. How to Grade the Quality of the Evidence. Available online: http://cccrgcochraneorg/author-resources (accessed on 20 September 2021).
- Kucukkaya Eren, S.; Bahador Zırh, E.; Zeybek, N.D.; Askerbeyli Örs, S.; Aksel, H.; Parashos, P. Effect of benzalkonium chloride addition to EDTA on attachment and proliferation of dental pulp stem cells on dentin and on transforming growth factor-β1 release. Odontology 2021, 109, 313–320. [Google Scholar] [CrossRef]
- Khan, J.A.; Hasan, A.; Dossa, S.; Ali, B. Effect of natural and artificial dentin conditioners on the release of vascular endothelial growth factor. J. Endod. 2021, 47, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Hancerliogullari, D.; Erdemir, A.; Kisa, U. The effect of different irrigation solutions and activation techniques on the expression of growth factors from dentine of extracted premolar teeth. Int. Endod. J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.N.; Puppin-Rontani, R.M.; Pascon, F.M. Effect of intracanal medicaments and irrigants on the release of transforming growth factor beta 1 and vascular endothelial growth factor from cervical root dentin. J. Endod. 2020. [Google Scholar] [CrossRef] [PubMed]
- Aksel, H.; Albanyan, H.; Bosaid, F.; Azim, A.A. Dentin conditioning protocol for regenerative endodontic procedures. J. Endod. 2020, 46, 1099–1104. [Google Scholar] [CrossRef]
- Atesci, A.A.; Avci, C.B.; Tuglu, M.I.; Ozates Ay, N.P.; Eronat, A.C. Effect of different dentin conditioning agents on growth factor release, mesenchymal stem cell attachment and morphology. J. Endod. 2020, 46, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Ivica, A.; Zehnder, M.; Mateos, J.M.; Ghayor, C.; Weber, F.E. Biomimetic conditioning of human dentin using citric acid. J. Endod. 2019, 45, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Deniz Sungur, D.; Aksel, H.; Ozturk, S.; Yılmaz, Z.; Ulubayram, K. Effect of dentine conditioning with phytic acid or etidronic acid on growth factor release, dental pulp stem cell migration and viability. Int. Endod. J. 2019, 52, 838–846. [Google Scholar] [CrossRef]
- Duncan, H.F.; Smith, A.J.; Fleming, G.J.; Reid, C.; Smith, G.; Cooper, P.R. Release of bio-active dentine extracellular matrix components by histone deacetylase inhibitors (HDACi). Int. Endod. J. 2017, 50, 24–38. [Google Scholar] [CrossRef]
- Goncalves, L.F.; Fernandes, A.P.; Cosme-Silva, L.; Colombo, F.A.; Martins, N.S.; Oliveira, T.M.; Araujo, T.H.; Sakai, V.T. Effect of EDTA on TGF-β1 released from the dentin matrix and its influence on dental pulp stem cell migration. Braz. Oral. Res. 2016, 30, e131. [Google Scholar] [CrossRef] [Green Version]
- Sadaghiani, L.; Gleeson, H.B.; Youde, S.; Waddington, R.J.; Lynch, C.D.; Sloan, A.J. Growth factor liberation and DPSC response following dentine conditioning. J. Dent. Res. 2016, 95, 1298–1307. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Nguyen, S.; Zhang, H.; Chebrolu, H.P.; Alzebdeh, D.; Badi, M.A.; Kim, J.R.; Ling, J.; Yang, M. Release of growth factors into root canal by irrigations in regenerative endodontics. J. Endod. 2016, 42, 1760–1766. [Google Scholar] [CrossRef]
- Graham, L.; Cooper, P.R.; Cassidy, N.; Nor, J.E.; Sloan, A.J.; Smith, A.J. The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials 2006, 27, 2865–2873. [Google Scholar] [CrossRef]
- Zhao, S.; Sloan, A.J.; Murray, P.E.; Lumley, P.J.; Smith, A.J. Ultrastructural localisation of TGF-β exposure in dentine by chemical treatment. Histochem. J. 2000, 32, 489–494. [Google Scholar] [CrossRef]
- Huang, G.T. A paradigm shift in endodontic management of immature teeth: Conservation of stem cells for regeneration. J. Dent. 2008, 36, 379–386. [Google Scholar] [CrossRef]
- Diogenes, A.; Henry, M.A.; Teixeira, F.B.; Hargreaves, K.M. An update on clinical regenerative endodontics. Endod. Top. 2013, 28, 2–23. [Google Scholar] [CrossRef]
- Topbas, C.; Adiguzel, O. Endodontic irrigation solutions: A review. Int. Dent. Res. 2017, 7, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Cameron, R.; Claudia, E.; Ping, W.; Erin, S.; Ruparel, N.B. Effect of a residual biofilm on release of transforming growth factor β1 from dentin. J. Endod. 2019, 45, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Jain, A. Current overview on dental stem cells applications in regenerative dentistry. J. Nat. Sci. Biol. Med. 2015, 6, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Stone, W.; Grabias, B.; Lanke, K.; Zheng, H.; Locke, E.; Diallo, D.; Birkett, A.; Morin, M.; Bousema, T.; Kumar, S. A comparison of Plasmodium falciparum circumsporozoite protein-based slot blot and ELISA immuno-assays for oocyst detection in mosquito homogenates. Malar. J. 2015, 14, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doucet, J.; Zhao, A.; Fu, J.; Avrameas, A. Development and validation of an ELISA at acidic pH for the quantitative determination of IL-13 in human plasma and serum. Dis. Markers 2013, 35, 465–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scelza, M.F.; Pierro, V.; Scelza, P.; Pereira, M. Effect of three different time periods of irrigation with EDTA-T, EDTA, and citric acid on smear layer removal. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Bhandary, S.; Kakamari, S.; Srinivasan, R.; Chandrappa, M.M.; Nasreen, F.; Junjanna, P. A comparative evaluation of the effect of 8% and 17% ethylenediaminetetraacetic acid exposure for 1 min and 10 min on the fracture resistance of endodontically treated roots: An in vitro study. J. Conserv. Dent. 2017, 20, 21–24. [Google Scholar] [CrossRef]
- Martin, D.E.; De Almeida, J.F.; Henry, M.A.; Khaing, Z.Z.; Schmidt, C.E.; Teixeira, F.B.; Diogenes, A. concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J. Endod. 2014, 40, 51–55. [Google Scholar] [CrossRef] [PubMed]
- American Association of Endodontists—AAE. Clinical Considerations for a Regenerative Procedure; American Association of Endodontists: Chicago, IL, USA, 2018; pp. 1–6. [Google Scholar]
- Trevino, E.G.; Patwardhan, A.N.; Henry, M.A.; Perry, G.; Dybdal-Hargreaves, N.; Hargreaves, K.M.; Diogenes, A. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J. Endod. 2011, 37, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Kawashima, N.; Ichinose, S.; Nara, K.; Noda, S.; Okiji, T. EDTA Treatment for sodium hypochlorite-treated dentin recovers disturbed attachment and induces differentiation of mouse dental papilla cells. J. Endod. 2018, 44, 256–262. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Pondrelli, A.; Zamparini, F.; Prati, C.; Spagnuolo, G. Demineralization, collagen modification and remineralization degree of human dentin after EDTA and Citric Acid treatments. Materials 2018, 12, 25. [Google Scholar] [CrossRef] [Green Version]
- Lyons, R.M.; Keski-Oja, J.; Moses, H.L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J. Cell. Biol. 1988, 106, 1659–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts-Clark, D.J.; Smith, A.J. Angiogenic growth factors in human dentine matrix. Arch. Ooral Biol. 2000, 45, 1013–1016. [Google Scholar] [CrossRef]
- Eppler, S.M.; Combs, D.L.; Henry, T.D.; Lopez, J.J.; Ellis, S.G.; Yi, J.H.; Annex, B.H.; McCluskey, E.R.; Zioncheck, T.F. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin. Pharmacol. Ther. 2002, 72, 20–32. [Google Scholar] [CrossRef]
- Widbiller, M.; Eidt, A.; Hiller, K.A.; Buchalla, W.; Schmalz, G.; Galler, K.M. Ultrasonic activation of irrigants increases growth factor release from human dentine. Clin. Oral Investig. 2017, 21, 879–888. [Google Scholar] [CrossRef]
- Uzunoglu, E.; Aktemur, S.; Uyanik, M.O.; Durmaz, V.; Nagas, E. Effect of ethylenediaminetetraacetic acid on root fracture with respect to concentration at different time exposures. J. Endod. 2012, 38, 1110–1113. [Google Scholar] [CrossRef]
- Toyota, Y.; Yoshihara, T.; Hisada, A.; Yawaka, Y. Removal of smear layer by various root canal irrigations in primary teeth. Pediatr. Dent. J. 2017, 27, 8–13. [Google Scholar] [CrossRef]
- Demirel, A.; Yüksel, B.N.; Ziya, M.; Gümüş, H.; Doğan, S.; Sari, Ş. The effect of different irrigation protocols on smear layer removal in root canals of primary teeth: A SEM study. Acta. Odontol. Scand. 2019, 77, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Tang, K.; Wang, Q.; Estill, J.; Yao, L.; Wang, X.; Chen, Y.; Yang, K. The use of GRADE approach in systematic reviews of animal studies. J. Evid. Based Med. 2016, 9, 98–104. [Google Scholar] [CrossRef] [PubMed]



| Study (Year) | Type of Sample | Root Canal Irrigant | Growth Factors | Evaluation Method |
|---|---|---|---|---|
| Kucukkaya Eren et al., 2021 [22] | Dentin slices | 17% EDTA and 17% EDTA + 0.008% benzalkonium chloride | TGF-β1 | ELISA |
| Khan et al., 2021 [23] | Dentin slices and Root canal segment | 17% EDTA, 9% etidronic acid, and 1% phytic acid | VEGF | ELISA |
| Hancerliogullari et al., 2021 [24] | Root canal segment | 17% EDTA, 10% citric acid (break)Both irrigants was tested with follow irrigation activation technique (conventional syringe irrigation, passive ultrasonic irrigation, PUI, and Er:YAG laser activation | TGF-β1, IGF-I, BMP-7 and VEGF-A | ELISA |
| Ferreira et al., 2020 [25] | Root canal segment | 2.5% NaOCl, 2% chlorhexidine, and 10% EDTA | TGF-β1 and VEGF | ELISA |
| Aksel et al., 2020 [26] | Dentin slices | 1.5% NaOCl + PBS + 17% EDTA + PBS, 17% EDTA with Nanobubble water, 17% EDTA activated with ultrasonic, 17% EDTA with Nanobubble water activated with ultrasonic, and phosphate-buffered saline (PBS) | TGF-β | ELISA |
| Atesci et al., 2020 [27] | Dentin slices and Powdered dentine * | 17% EDTA, 10% citric acid, 1% phytic acid, 37% phosphoric acid, distilled water | TGF-β1, BMP-2, FGF-2 and VEGF | ELISA |
| Ivica et al., 2019 [28] | Dentin slices | 10% Citric Acid, 17% EDTA and phosphate-buffered saline | TGF-β1 | Slot blot |
| Deniz Sungur et al., 2019 [29] | Dentin slices | 17% EDTA, 1% phytic acid, 9% etidronic acid, and distilled water | TGF-β | ELISA |
| Chae et al., 2018 [13] | Root canal segment | Saline, 17% EDTA, 10% citric acid, 10% phosphoric acid, and 37% phosphoric acid | TGF-β1 | ELISA |
| Duncan et al., 2017 [30] | Powdered dentine | 10% EDTA, valproic acid, trichostatin A and suberoylanilide hydroxamic acid | TGF-β1 | ELISA |
| Gonçalves et al., 2016 [31] | Dentin slices | EDTA 10%, NaOCl 2,5% and phosphate-buffered saline | TGF-β1 | ELISA |
| Sadaghiani et al., 2016 [32] | Dentin slices | 10% EDTA, 37% phosphoric acid, 10% citric acid, 25% polyacrylic acid, buffered saline, and calcium hydroxide | TGF-β1, BMP-2 and VEGF | ELISA and Immunogold method |
| Zeng et al., 2016 [33] | Root canal segment | 1.5% NaOCl + 17% EDTA, 2.5% NaOCl + 17% EDTA, 17% EDTA, and deionized water | TGF-β1 and bFGF | ELISA |
| Galler et al., 2015 [10] | Dentin slices | 10% EDTA, 17% EDTA, 10% citric acid, citrate buffer, and citric acid phosphate buffer | TGF-β1 | ELISA |
| Graham et al., 2006 [34] | Powdered dentine | 10% EDTA and calcium hydroxide | TGF-β1 | ELISA |
| Zhao et al., 2000 [35] | Dentin slices | 3% NaOCl, 17% EDTA, 10% citric acid, and phosphate-buffered saline | TGF-β1 TGF-β2 TGF-β3 | Immunogold method |
| Study (Year) | Main Conclusions |
|---|---|
| Kucukkaya Eren et al., 2021 [22] | Both 17% EDTA and 17% EDTA + 0.008% benzalkonium chloride were similar in the amount of TGF-β1 released by dentin. |
| Khan et al., 2021 [23] | VEGF release by 9% etidronic acid was greater in dentin cylinders than 17% EDTA and 1% phytic acid, however similar between irrigants groups.in the dentin discs analysis. |
| Hancerliogullari et al., 2021 [24] | The 17% EDTA caused significantly more IGF-I release than 10% citric acid, while for TGF-β1, BMP-7, and VEGF-A, both irrigants were equally effective. |
| Ferreira et al., 2020 [25] | The 2% chlorhexidine and 10% EDTA irrigants released significantly more TGF- β1 than 2.5% NaOCl. No VEGF release was detected for any group. |
| Aksel et al., 2020 [26] | Although there is no significant difference between the groups of irrigants used, the ultrasonic activation enhanced the TGF- β release. |
| Atesci et al., 2020 [27] | For TGF- β1, 10% citric acid was responsible for releasing significantly more than EDTA, IP6, and with no statistically significant difference when compared to 37% phosphoric acid. For VEGF, there was a very minor release with no significant difference, while for BMP-2 and FGF-2, the release was similar to all irrigants. |
| Ivica et al., 2019 [28] | The 17% EDTA released a 5-fold higher concentration of TGF- β1 than 10% citric acid. |
| Deniz Sungur et al., 2019 [29] | The greatest release of TGF-β1 was obtained in the 9% etidronic acid group, whilethe lowest in the 1% phytic acid group, with no significant difference between groups. |
| Chae et al., 2018 [13] | The use of 10% citric acid released significantly more TGF- β1 than 17% EDTA and 10% phosphoric acid, while 37% phosphoric acid and saline released the least amount. |
| Ducan et al., 2017 [30] | The 10% EDTA promoted significantly greater release of TGF- β1 than valproic acid, trichostatin A, and suberoylanilide hydroxamic acid. |
| Gonçalves et al., 2016 [31] | The 10% EDTA released significantly more TGF- β1of dentin matrix than 2.5% NaOCl or PBS. |
| Sadaghiani et al., 2016 [32] | Under the immunogold method, calcium hydroxide significantly increased the release of TGF-β1 within 5 min of conditioning, BMP-2 and VEGF within 10 min. The 10% EDTA, 10% citric acid, and 37% phosphoric acid showed intermediate values for TGF-β1 release, while for BMP-2 and VEGF, the phosphoric acid was lower in the time of 10 min. In the ELISA method, only 17% EDTA detected TGF-β1 release, whereas for BMP-2 and VEGF the most effective irrigant was 10% citric acid. |
| Zeng et al., 2016 [33] | The groups with 1.5% NaOCl + 17% EDTA and 2.5% NaOCl + 17% EDTA had significantly higher release of TGF- β1 than 17% EDTA, with a peak release at day 1. The release of bFGF was detected at a low level in all irrigants. |
| Galler et al., 2015 [10] | Conditioning with 10% EDTA resulted in the release of the highest amounts of TGF- β1, while 17% EDTA was less effective. The release after treatment with citric acid and its variations was significantly smaller than EDTA. |
| Graham et al., 2006 [34] | The 10% EDTA released higher concentrations of TGF- β1 from dentin than calcium hydroxide. |
| Zhao et al., 2000 [35] | Conditioning with 17% EDTA generated a greater release of TGF-β1 while treatments with 10% citric acid and 3% NaOCl revealed smaller amounts of this isoform. TGF-β2 and -β3 isoforms could not be detected in samples with any of the irrigants. |
| Study (Year) | Reporting Quality | Methodological Quality | Relevance | Quality Rating |
|---|---|---|---|---|
| Kucukkaya Eren et al., 2021 [22] | 97.73 | 100 | Directly relevant | Low risk |
| Khan et al., 2021 [23] | 93.75 | 100 | Directly relevant | Low risk |
| Hancerliogullari et al., 2021 [24] | 90 | 91.67 | Directly relevant | Low risk |
| Ferreira et al., 2020 [25] | 93.75 | 91.67 | Directly relevant | Low risk |
| Aksel et al., 2020 [26] | 90.48 | 96.43 | Directly relevant | Low risk |
| Atesci et al., 2019 [27] | 83.33 | 92.31 | Directly relevant | Low risk |
| Ivica et al., 2019 [28] | 88.1 | 96.43 | Directly relevant | Low risk |
| Sungur et al., 2019 [29] | 97.62 | 100 | Directly relevant | Low risk |
| Chae et al., 2018 [13] | 78.57 | 78.57 | Directly relevant | Low risk |
| Duncan et al., 2016 [30] | 92.86 | 95.83 | Directly relevant | Low risk |
| Gonçalves et al., 2016 [31] | 90.48 | 92.31 | Directly relevant | Low risk |
| Sadaghiani et al., 2016 [32] | 88.1 | 92.31 | Directly relevant | Low risk |
| Zeng et al., 2016 [33] | 92.86 | 92.31 | Directly relevant | Low risk |
| Galler et al., 2015 [10] | 78.13 | 95.83 | Directly relevant | Low risk |
| Graham et al., 2006 [34] | 80.95 | 92.31 | Directly relevant | Low risk |
| Zhao et al., 2000 [35] | 71.88 | 79.17 | Directly relevant | Low risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, S.; Pintor, A.; Mourão, C.F.d.A.B.; Magno, M.; Montemezzi, P.; Sacco, R.; Alves, G.; Scelza, M.Z. Effect of Different Root Canal Irrigant Solutions on the Release of Dentin-Growth Factors: A Systematic Review and Meta-Analysis. Materials 2021, 14, 5829. https://doi.org/10.3390/ma14195829
Tavares S, Pintor A, Mourão CFdAB, Magno M, Montemezzi P, Sacco R, Alves G, Scelza MZ. Effect of Different Root Canal Irrigant Solutions on the Release of Dentin-Growth Factors: A Systematic Review and Meta-Analysis. Materials. 2021; 14(19):5829. https://doi.org/10.3390/ma14195829
Chicago/Turabian StyleTavares, Sandro, Andrea Pintor, Carlos Fernando de Almeida Barros Mourão, Marcela Magno, Pietro Montemezzi, Roberto Sacco, Gutemberg Alves, and Miriam Zaccaro Scelza. 2021. "Effect of Different Root Canal Irrigant Solutions on the Release of Dentin-Growth Factors: A Systematic Review and Meta-Analysis" Materials 14, no. 19: 5829. https://doi.org/10.3390/ma14195829
APA StyleTavares, S., Pintor, A., Mourão, C. F. d. A. B., Magno, M., Montemezzi, P., Sacco, R., Alves, G., & Scelza, M. Z. (2021). Effect of Different Root Canal Irrigant Solutions on the Release of Dentin-Growth Factors: A Systematic Review and Meta-Analysis. Materials, 14(19), 5829. https://doi.org/10.3390/ma14195829








