SiC/MoSi2-SiC-Si Oxidation Protective Coatings for HTR Graphite Spheres with Residual Si Optimized
Abstract
:1. Introduction
2. Experiment
3. Results and Discussion
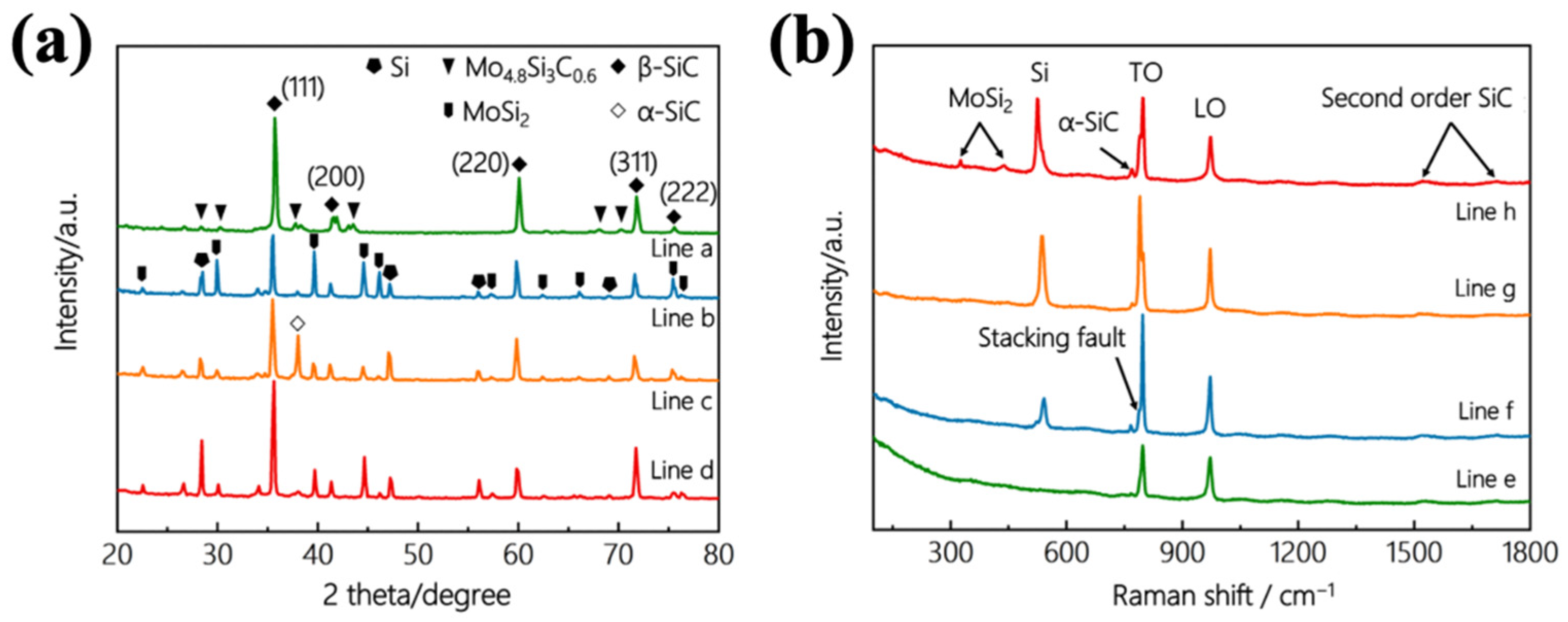
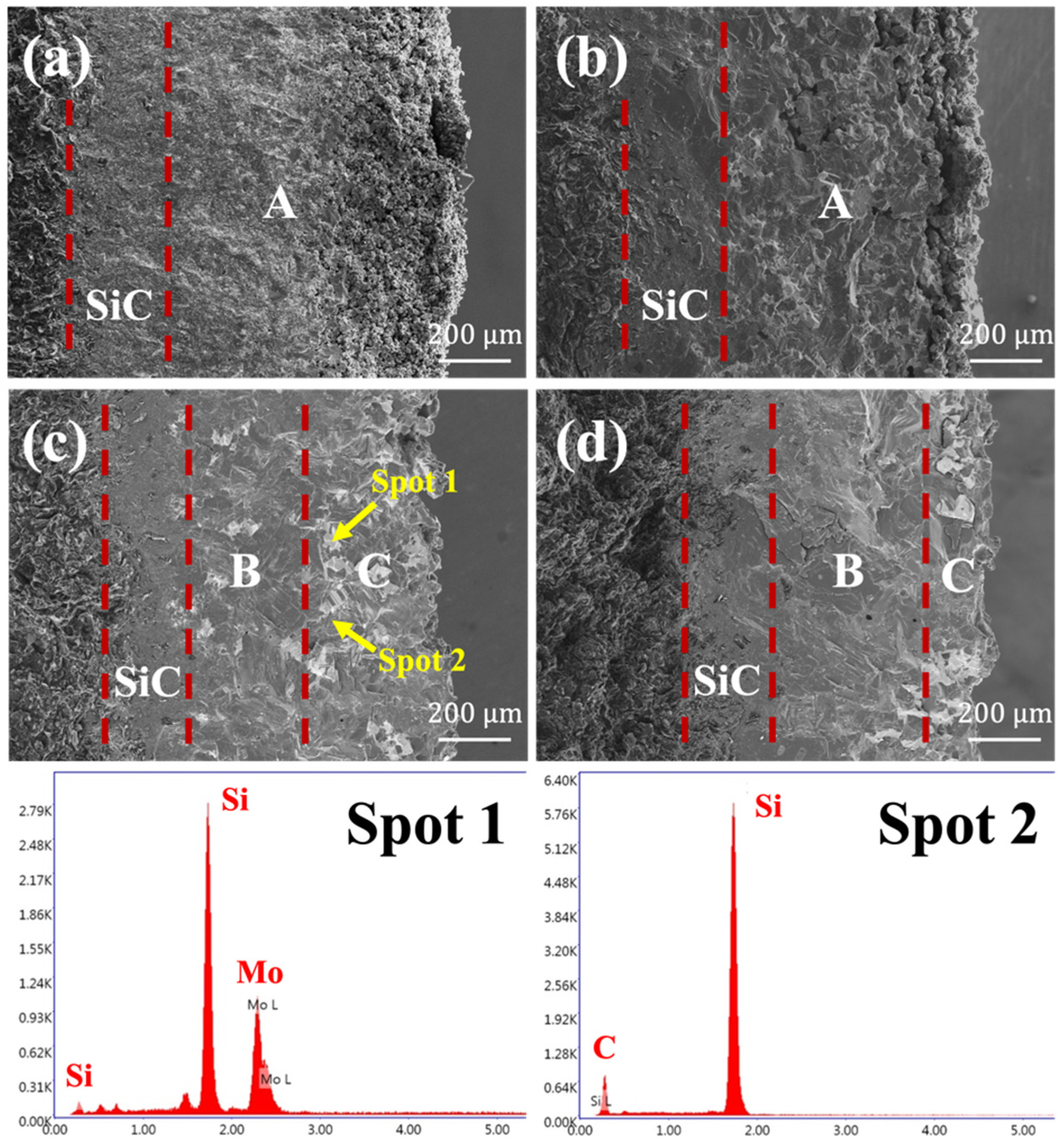
3.1. Microstructure
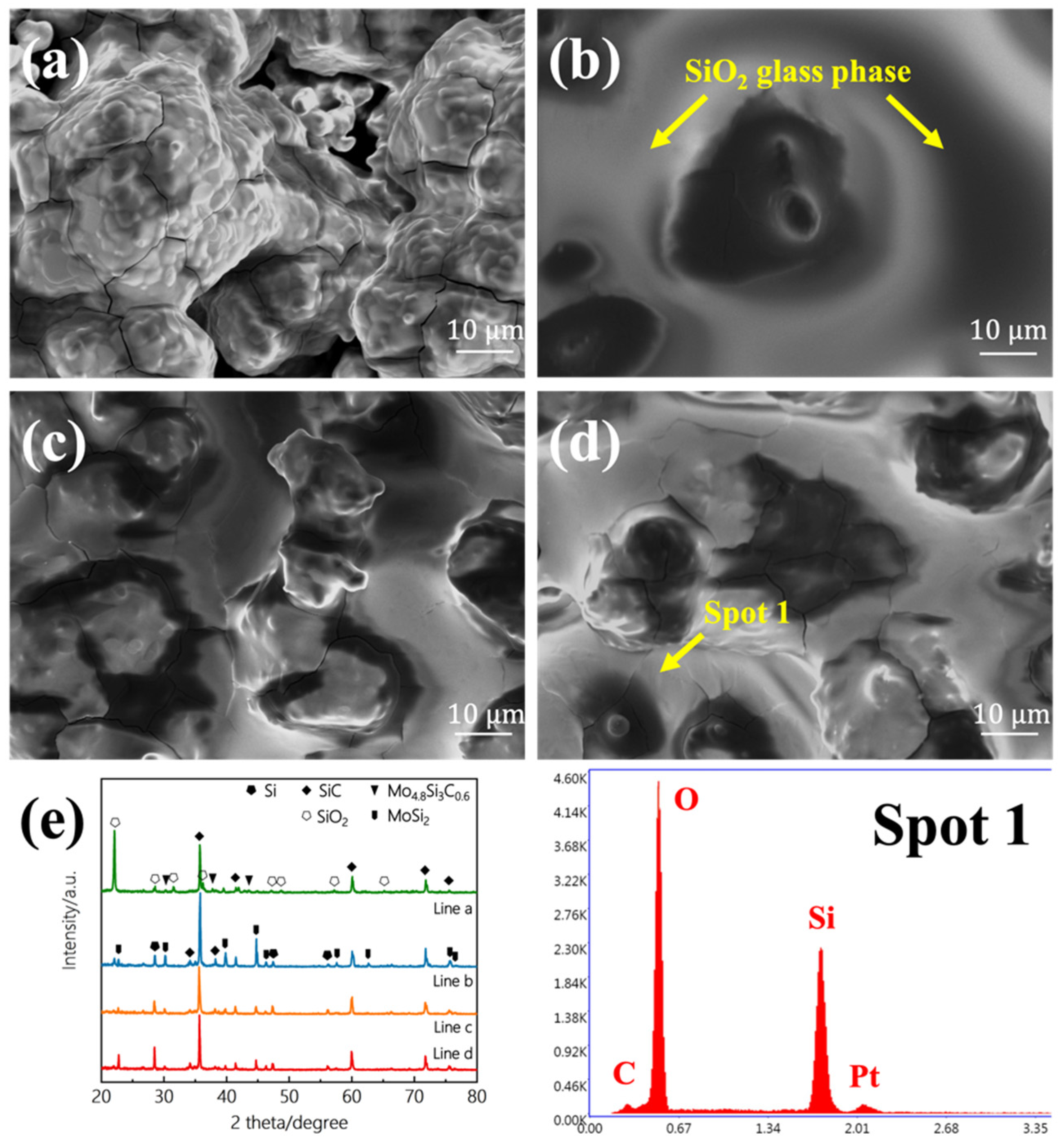
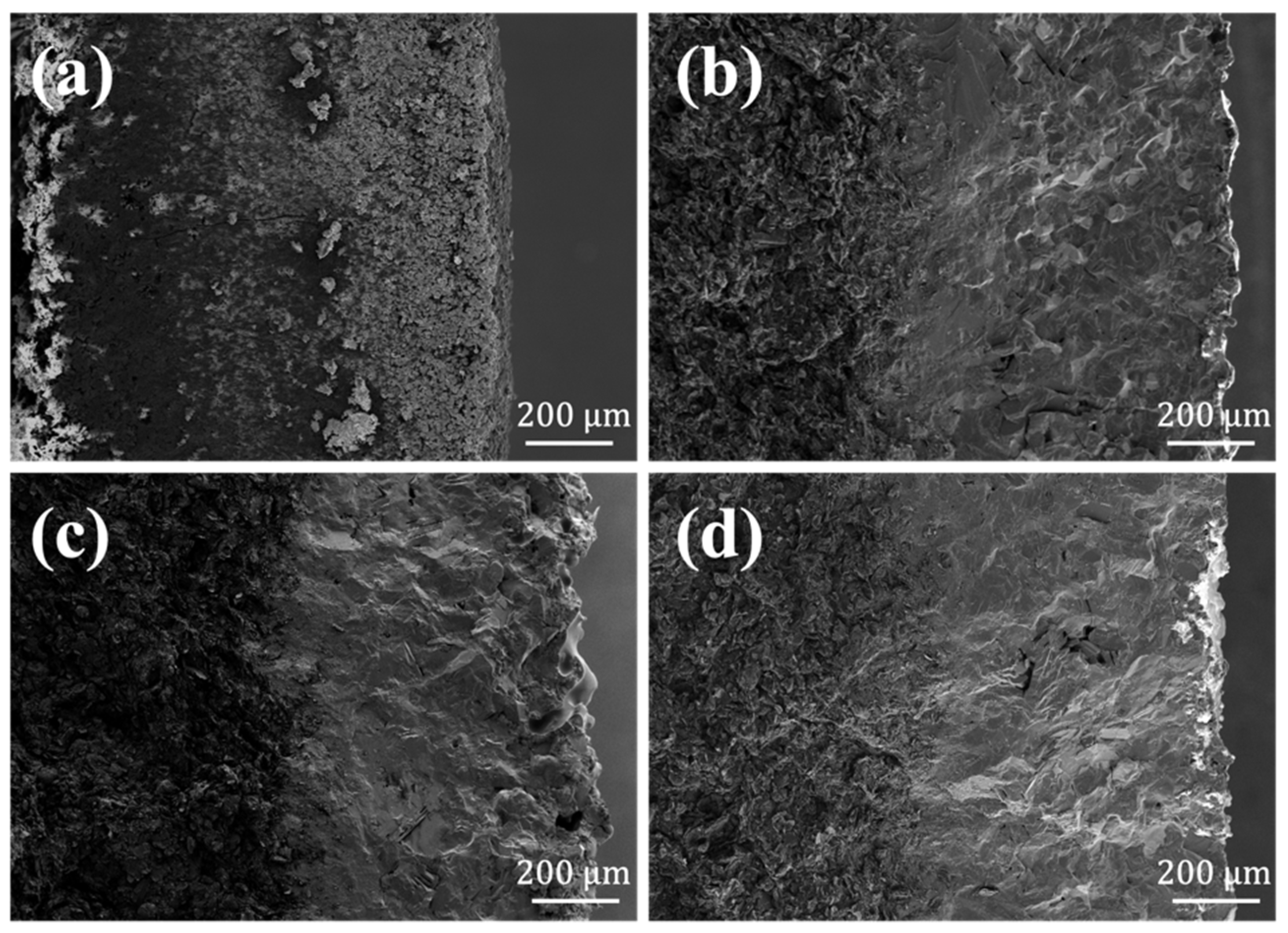
3.2. Oxidation Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bao, W.; Deng, Z.; Zhang, S.; Ji, Z.; Zhang, H. Next-Generation Composite Coating System: Nanocoating. Front. Mater. 2019, 72, 1–6. [Google Scholar] [CrossRef]
- Chen, S.; Qiu, X.; Zhang, B.; Xu, J.; Zhong, F.; Zhu, B.; Zhang, Y.; Ou-Yang, J.; Yang, X. Advances in antioxidation coating materials for carbon/carbon composites. J. Alloys Compd. 2021, 886, 161143. [Google Scholar] [CrossRef]
- Jin, X.; Fan, X.; Lu, C.; Wang, T. Advances in oxidation and ablation resistance of high and ultra-high temperature ceramics modified or coated carbon/carbon composites. J. Eur. Ceram. Soc. 2018, 38, 1–28. [Google Scholar] [CrossRef]
- Jara, A.D.; Betemariam, A.; Woldetinsae, G.; Kim, J.Y. Purification, application and current market trend of natural graphite: A review. Int. J. Min. Sci. Technol. 2019, 29, 671–689. [Google Scholar] [CrossRef]
- Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K. A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci. 2011, 36, 638–670. [Google Scholar] [CrossRef]
- Zhou, P.; Zheng, Z.; Liu, R.; Liu, X.; Liu, M.; Wang, T.; Li, Z.; Shao, Y.; Zhao, H.; Liu, B. Dual layer SiC coating on matrix graphite sphere prepared by pack cementation and fluidized-bed chemical vapor deposition. J. Am. Ceram. Soc. 2017, 100, 3415–3424. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, X.; Zhao, H.; Li, Z.; Zheng, Z.; Zhang, K.; Liu, B. Micro Four-Layer SiC Coating on Matrix Graphite Spheres of HTR Fuel Elements by Two-Step Pack Cementation. J. Am. Ceram. Soc. 2016, 99, 3525–3532. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, P.; Li, Z.; Liu, X.; Zhang, K.; Wang, T.; Wu, B.; Liu, B. Effect of AL2O3 and sic content in pack cementation powders on the microstructure of sic coatings on htr graphite spheres. In Proceedings of the 26th International Conference on Nuclear Engineering (ICONE-26), London, UK, 22–26 July 2018. [Google Scholar]
- Zheng, Z.; Zhao, H.; Li, Z.; Liu, X.; Wu, B.; Liu, B. Research on microstructure and oxidation resistant property of ZrSi2-SiC/SiC coating on HTR graphite spheres. Ceram. Int. 2018, 44, 4795–4800. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, P.; Zhao, H.; Li, Z.; Liu, X.; Zhang, K.; Liu, B. SiC coating on HTR graphite spheres prepared by fluidized-bed chemical vapor deposition. Ann. Nucl. Energy 2019, 134, 11–19. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, H.; Wang, T.; Zhang, K.; Li, Z.; Liu, X.; Liu, B. SiC/YSiC composite coating on matrix graphite sphere prepared by pack cementation and molten salt. Ceram. Int. 2019, 45, 21917–21922. [Google Scholar] [CrossRef]
- Yan, Z.-Q.; Xiong, X.; Xiao, P.; Chen, F.; Zhang, H.-B.; Huang, B.-Y. A multilayer coating of dense SiC alternated with porous Si-Mo for the oxidation protection of carbon/carbon silicon carbide composites. Carbon 2008, 46, 149–153. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Li, H.-J.; Fu, Q.-G.; Li, K.-Z.; Hou, D.-S.; Fei, J. A Si-Mo oxidation protective coating for C/SiC coated carbon/carbon composites. Carbon 2007, 45, 1130–1133. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Li, H.-J.; Hu, Z.-X.; Li, K.-Z.; Zhang, L.-L. C/SiC/MoSi2-SiC-Si multilayer coating for oxidation protection of carbon/carbon composites. Trans. Nonferrous Met. Soc. China 2013, 23, 2118–2122. [Google Scholar] [CrossRef]
- Yan, Z.Q.; Xiong, X.; Xiao, P.; Chen, F.; Zhang, H.B.; Huang, B.Y. Si-Mo-SiO2 oxidation protective coatings prepared by slurry painting for C/C-SiC composites. Surf. Coat. Technol. 2008, 202, 4734–4740. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, Q.; Shi, J.; Zhai, G.; Liu, L. SiC/Si-MoSi2 oxidation protective coatings for carbon materials. Surf. Coat. Technol. 2006, 201, 1861–1865. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, P.; Zhao, H.; Wang, T.; Li, Z.; Liu, X.; Liu, B. Corrosion of SiC layers on coated zirconia particles in wet atmosphere. Ceram. Int. 2018, 44, 12797–12804. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, H.J.; Yao, X.Y.; Li, K.Z.; Qiang, X.F. Oxidation resistant Si-Mo-Al coating for C/SiC coated carbon/carbon composites at high temperature. Surf. Eng. 2012, 28, 257–260. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Li, H.-J.; Yao, X.-Y.; Li, K.-Z.; Qiang, X.-F. Oxidation protection of C/SiC coated carbon/carbon composites with Si-Mo coating at high temperature. Corros. Sci. 2011, 53, 2075–2079. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Li, H.-J.; Hu, Z.-X.; Ren, J.-C.; Li, K.-Z. Microstructure and oxidation resistance of Si-Mo-B coating for C/SiC coated carbon/carbon composites. Corros. Sci. 2013, 72, 150–155. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Fu, Q.; Li, K.; Ouyang, H. Effect of Mo/Si Ratio on the Microstructure and Oxidation Resistance of Si-Mo Coating for C/C Composites. Rare Met. Mater. Eng. 2009, 38, 109–112. [Google Scholar]
- Fu, Q.-G.; Li, H.-J.; Wang, Y.-J.; Li, K.-Z.; Shi, X.-H. B2O3 modified SiC-MoSi2 oxidation resistant coating for carbon/carbon composites by a two-step pack cementation. Corros. Sci. 2009, 51, 2450–2454. [Google Scholar] [CrossRef]
- Fu, Q.-G.; Li, H.-J.; Li, K.-Z.; Tong, K. A Si-Mo-W Coating to Protect SiC-Coated Carbon/Carbon Composites Against Oxidation. J. Am. Ceram. Soc. 2009, 92, 2132–2135. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Li, H.-J.; Li, K.-Z.; Fei, J.; Zeng, X.-R. C/SiC/Si-Mo-Cr multilayer coating for carbon/carbon composites for oxidation protection. New Carbon Mater. 2012, 27, 105–110. [Google Scholar] [CrossRef]
- Yang, J.; Ilegbusi, O.J. Kinetics of silicon-metal alloy infiltration into porous carbon. Compos. Part A Appl. Sci. Manuf. 2000, 31, 617–625. [Google Scholar] [CrossRef]
- Grashchenko, A.S.; Kukushkin, S.A.; Osipov, A.V.; Redkov, A.V. Formation of composite SiC-C coatings on graphite via annealing Si-melt in CO. Surf. Coat. Technol. 2021, 423, 127610. [Google Scholar] [CrossRef]
- Lide, D.R. 77th CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]


| Si/wt% | Mo/wt% | C/wt% | Al2O3/wt% | Si/Mo Mass Ratio | Si/C/Mo Molar Ratio |
|---|---|---|---|---|---|
| 42.5 | 42.5 | 13 | 2 | 1:1 | 3.4:2.4:1 |
| 64 | 21 | 3:1 | 10.3:4.9:1 | ||
| 71 | 14 | 5:1 | 17.1:7.4:1 | ||
| 74.4 | 10.6 | 7:1 | 23.9:9.8:1 |
| Reaction No. | Reaction Equation | ∆Gθ/(kJ·mol−1) |
|---|---|---|
| (1) | 2Si + CO (g) = SiC + SiO (g) | −32.488 |
| (2) | Si + O2 (g) = SiO2 | −595.800 |
| (3) | 2SiC + 3O2 (g) = 2SiO2 + 2CO (g) | −807.984 |
| (4) | SiC + O2 (g) = SiO (g) + CO (g) | −456.856 |
| (5) | 2SiC + 3O2 (g) = SiO (g) + 2CO2 (g) | −1172.173 |
| (6) | 2MoSi2 + 7O2 (g) = 2MoO3 (g) + 4SiO2 | −1283.830 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Yang, H.; Zhao, H.; Liu, X.; Zhang, K.; Li, Z.; Gao, Y.; Liu, B. SiC/MoSi2-SiC-Si Oxidation Protective Coatings for HTR Graphite Spheres with Residual Si Optimized. Materials 2022, 15, 3203. https://doi.org/10.3390/ma15093203
Wei X, Yang H, Zhao H, Liu X, Zhang K, Li Z, Gao Y, Liu B. SiC/MoSi2-SiC-Si Oxidation Protective Coatings for HTR Graphite Spheres with Residual Si Optimized. Materials. 2022; 15(9):3203. https://doi.org/10.3390/ma15093203
Chicago/Turabian StyleWei, Xiaoyu, Hui Yang, Hongsheng Zhao, Xiaoxue Liu, Kaihong Zhang, Ziqiang Li, Yuan Gao, and Bing Liu. 2022. "SiC/MoSi2-SiC-Si Oxidation Protective Coatings for HTR Graphite Spheres with Residual Si Optimized" Materials 15, no. 9: 3203. https://doi.org/10.3390/ma15093203
APA StyleWei, X., Yang, H., Zhao, H., Liu, X., Zhang, K., Li, Z., Gao, Y., & Liu, B. (2022). SiC/MoSi2-SiC-Si Oxidation Protective Coatings for HTR Graphite Spheres with Residual Si Optimized. Materials, 15(9), 3203. https://doi.org/10.3390/ma15093203





