Effects of 5 wt.% Polycaprolactone, Polyhydroxybutyrate and Polyvinyltrimethoxysilane on the Properties of Ag/Zn/Mg Alloy
Abstract
:1. Introduction
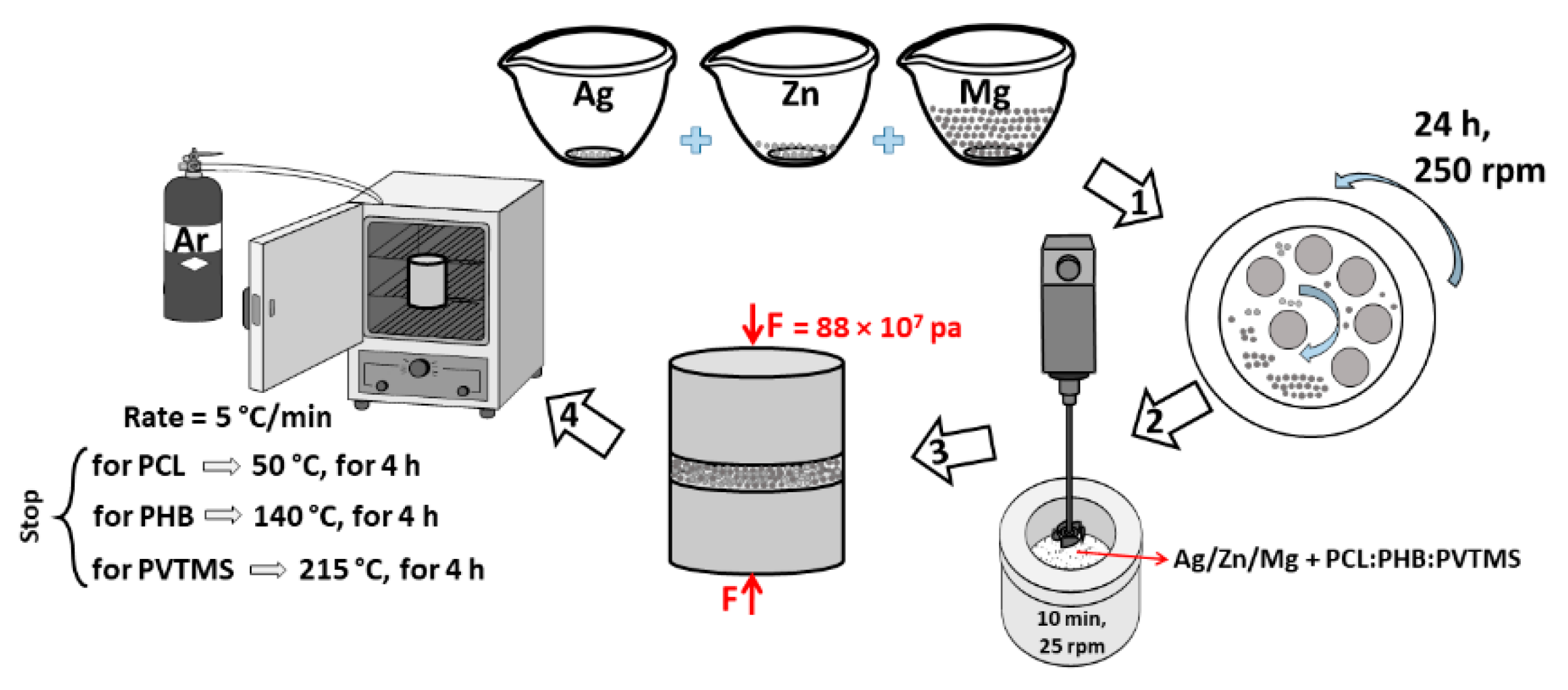
2. Experimental Methods
Preparation of Composite Ag/Zn/Mg + PCL:PHB:PVTMS
3. Results and Discussion
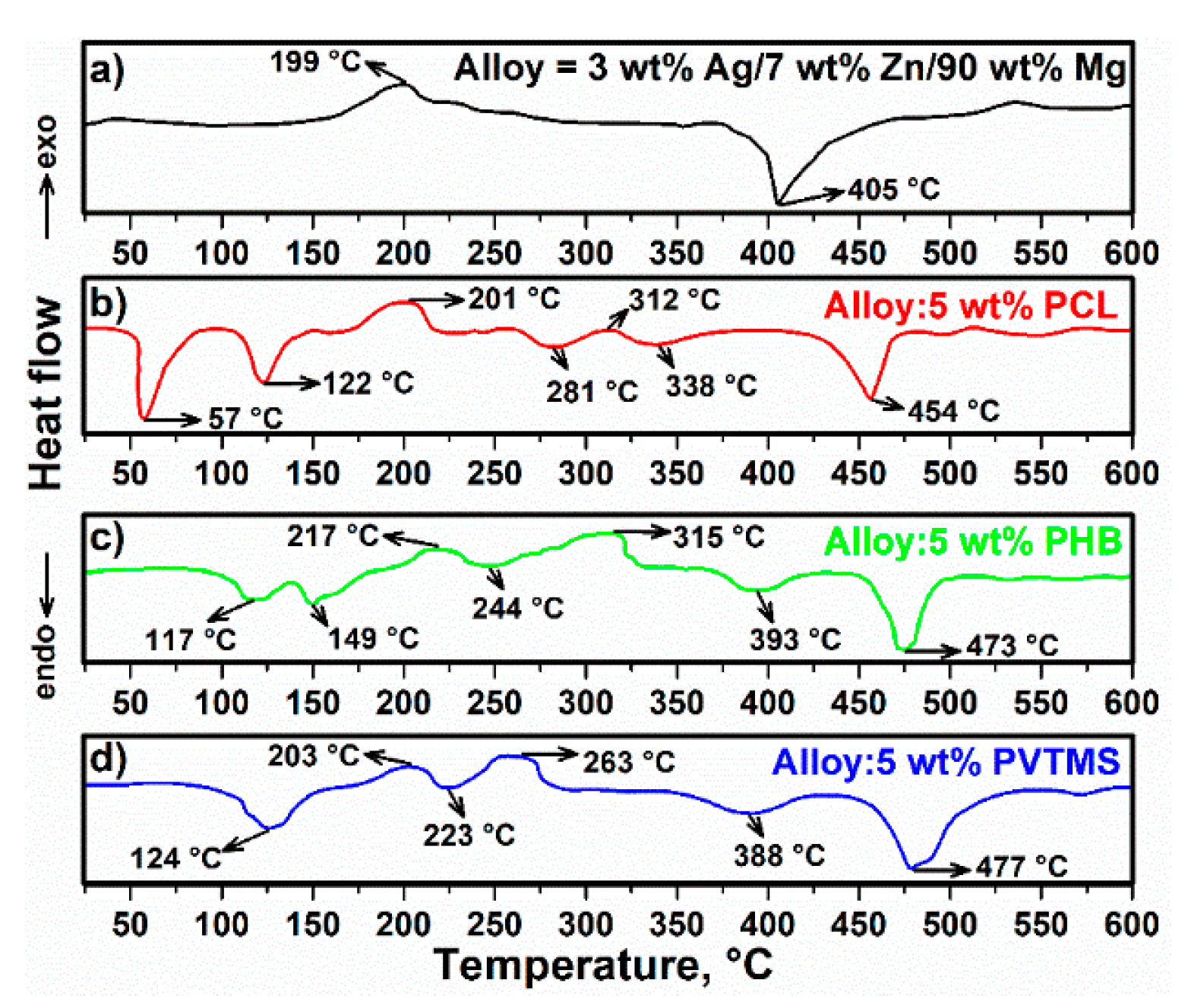
3.1. Thermal Analysis of Alloy and Composites
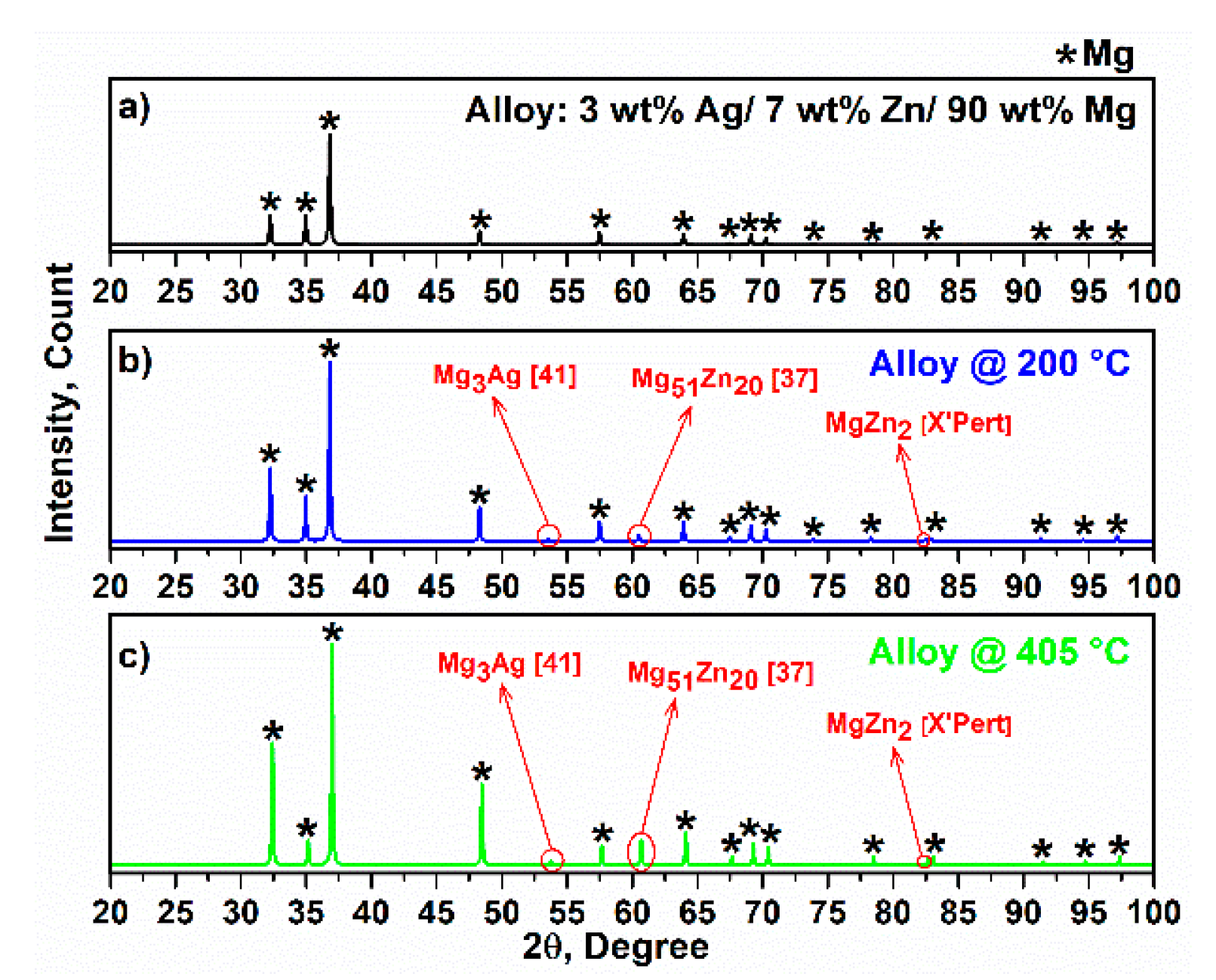
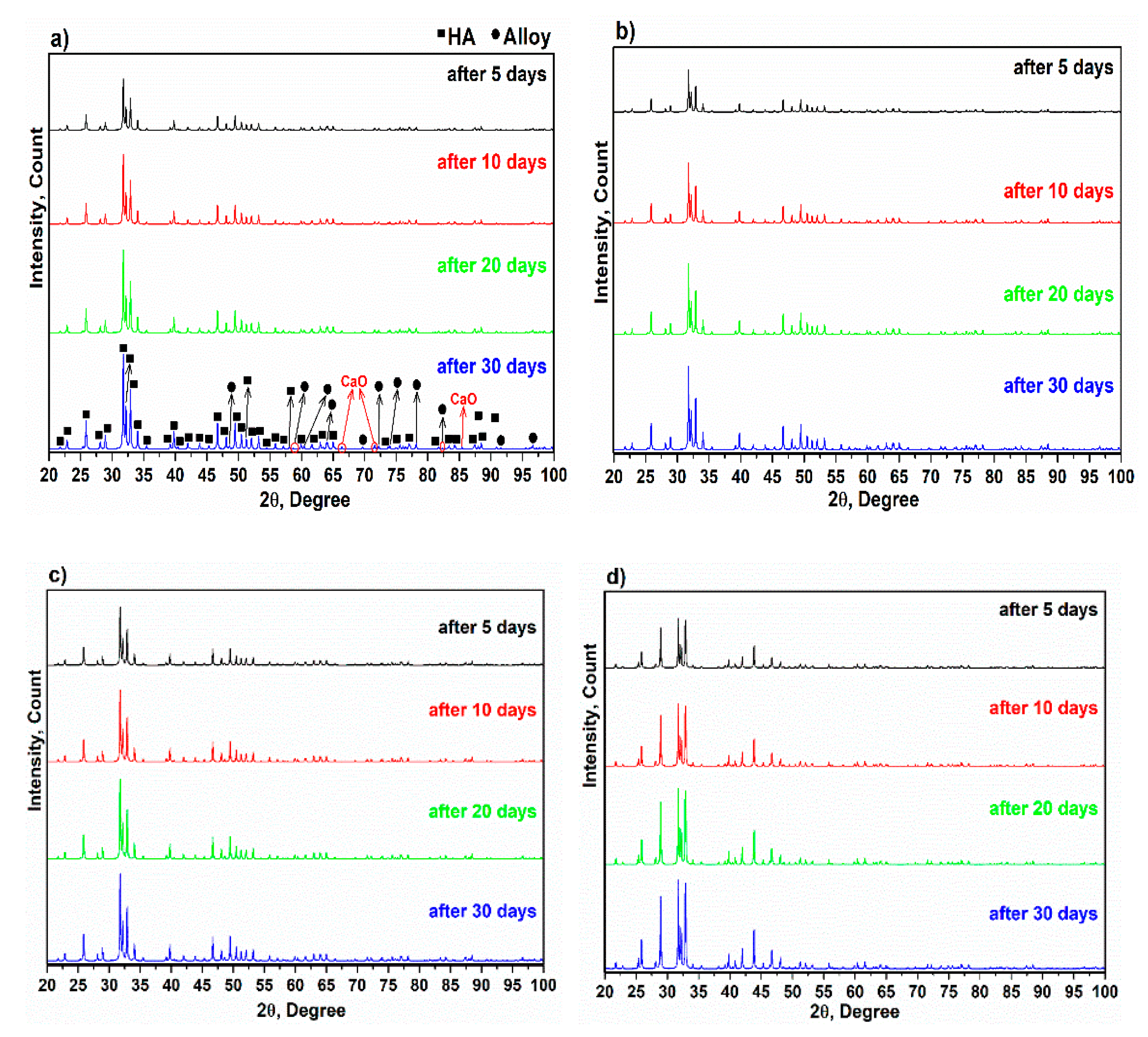
3.2. X-ray Diffraction of Alloy and Composites
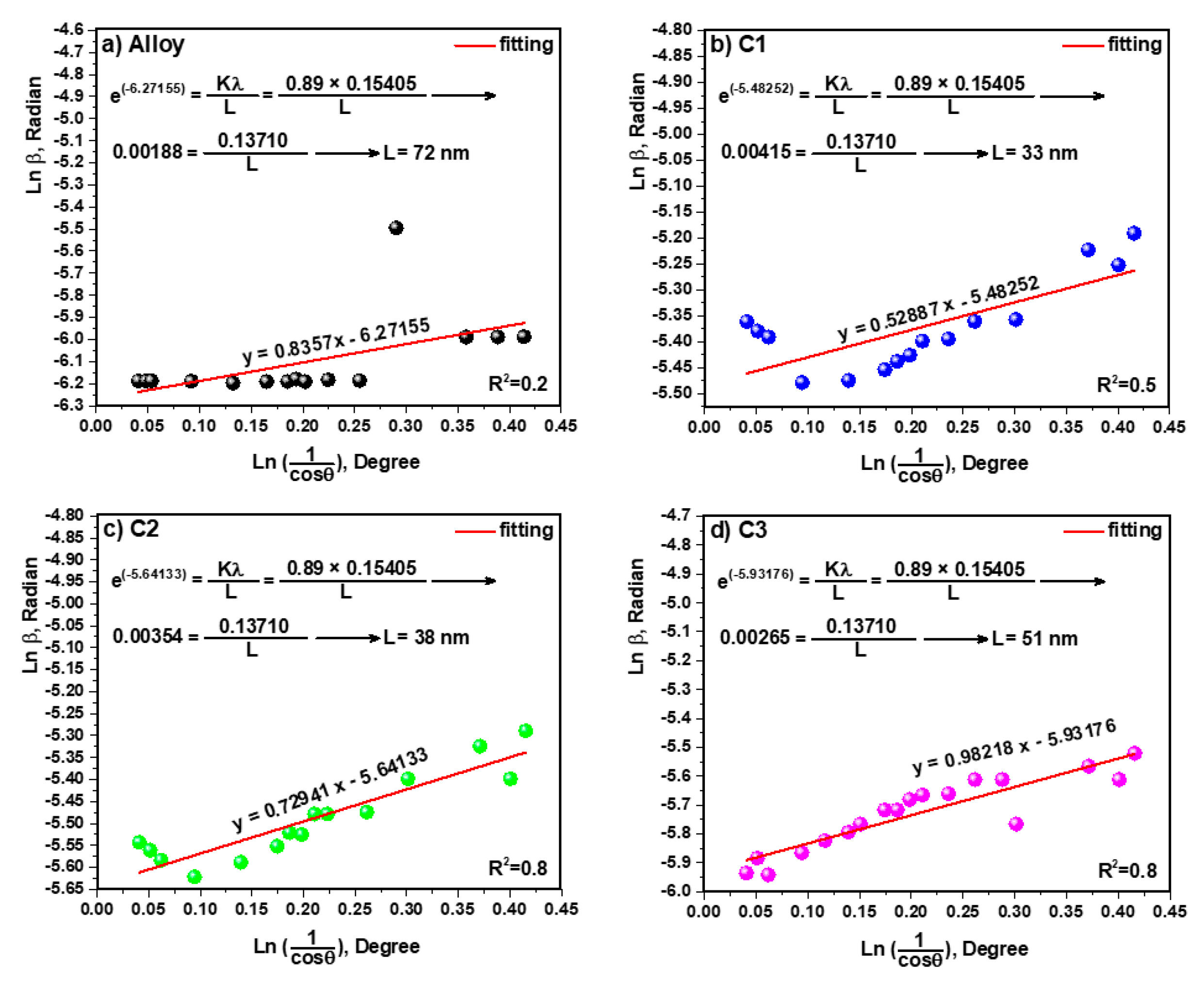
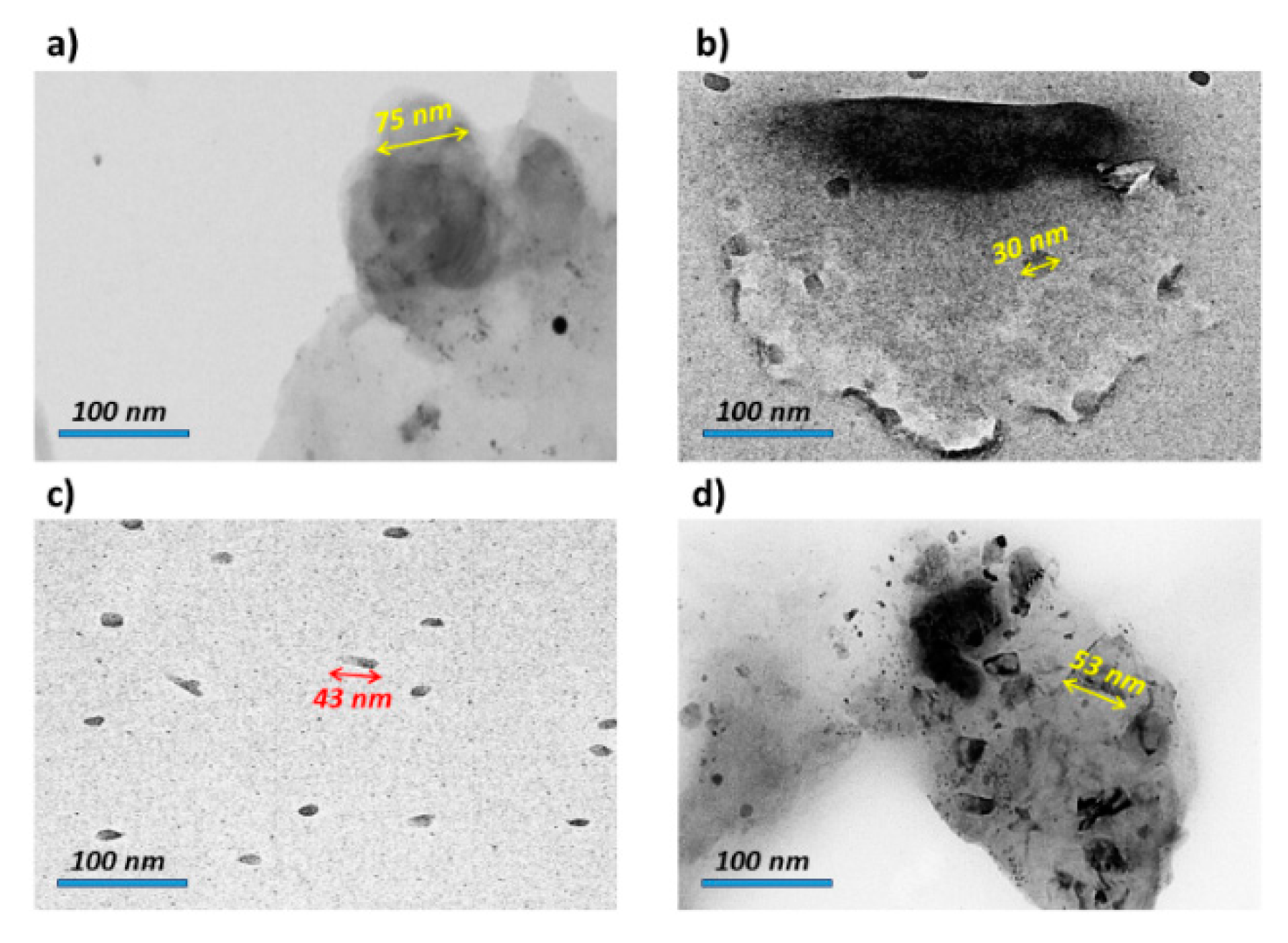
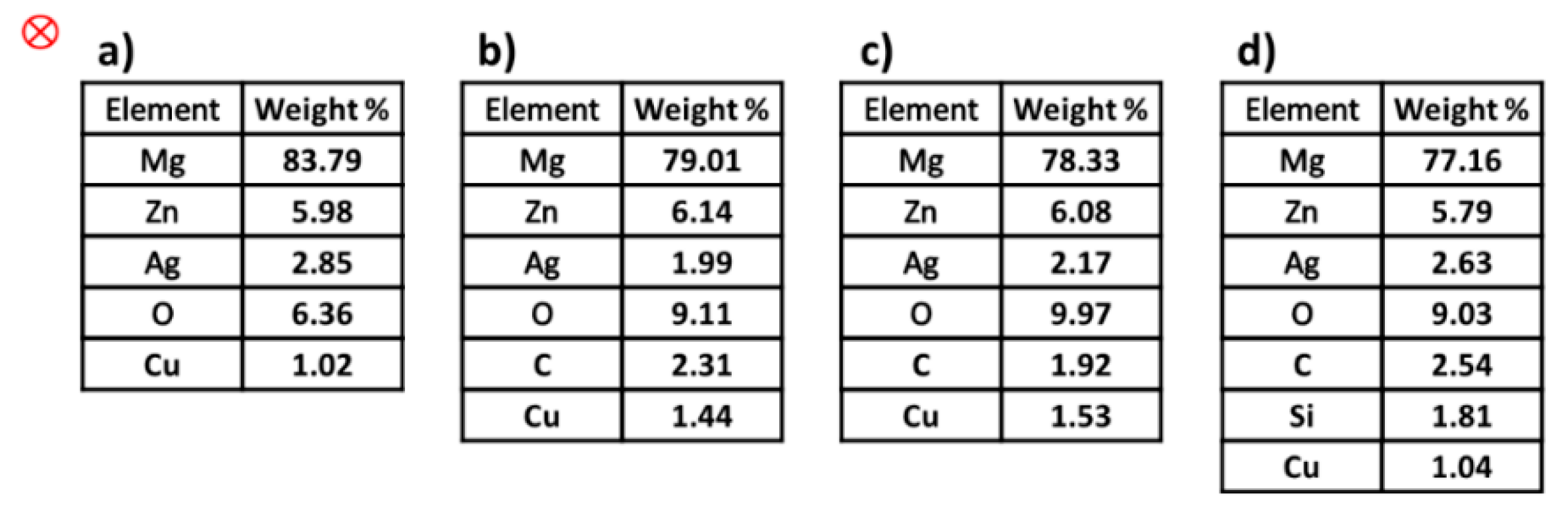
3.3. Study of Crystallite Size and TEM
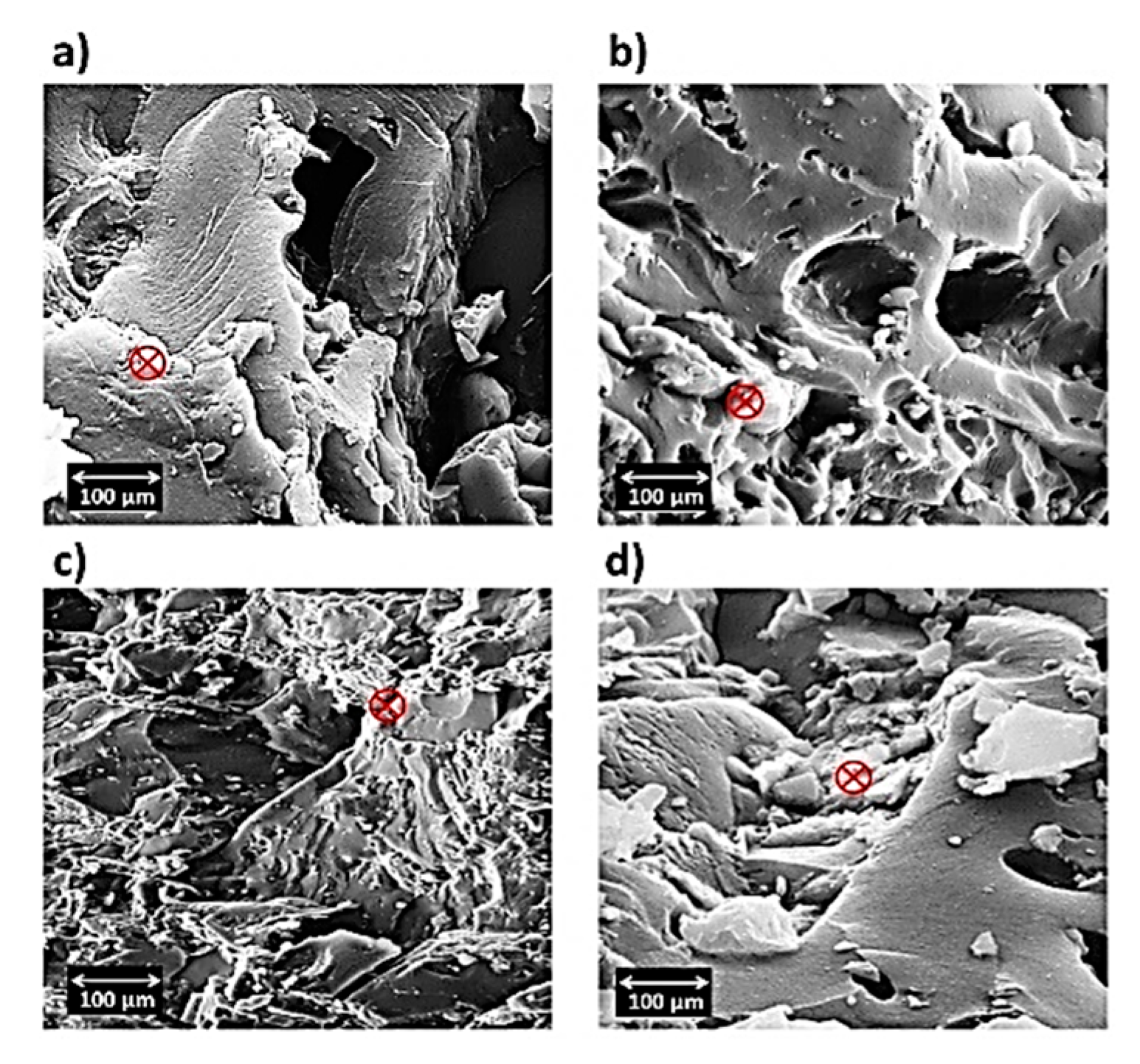
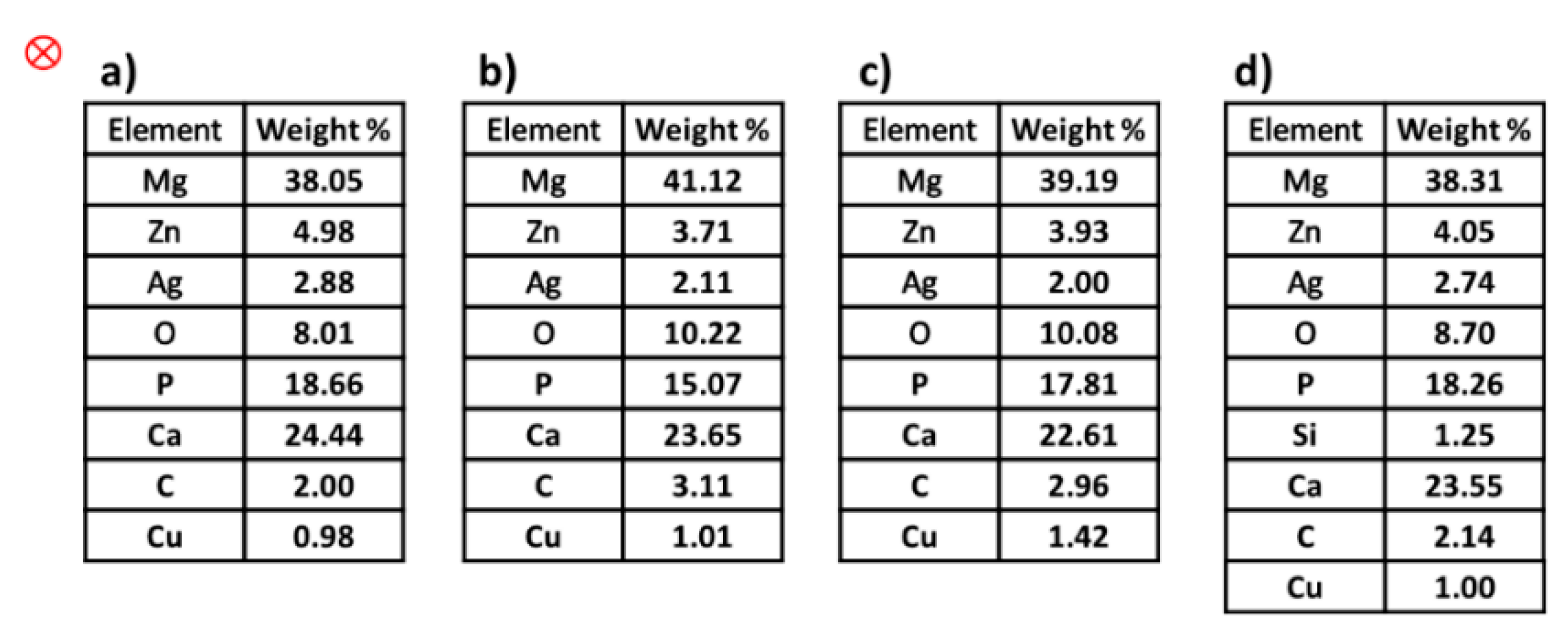
3.4. Study of SEM
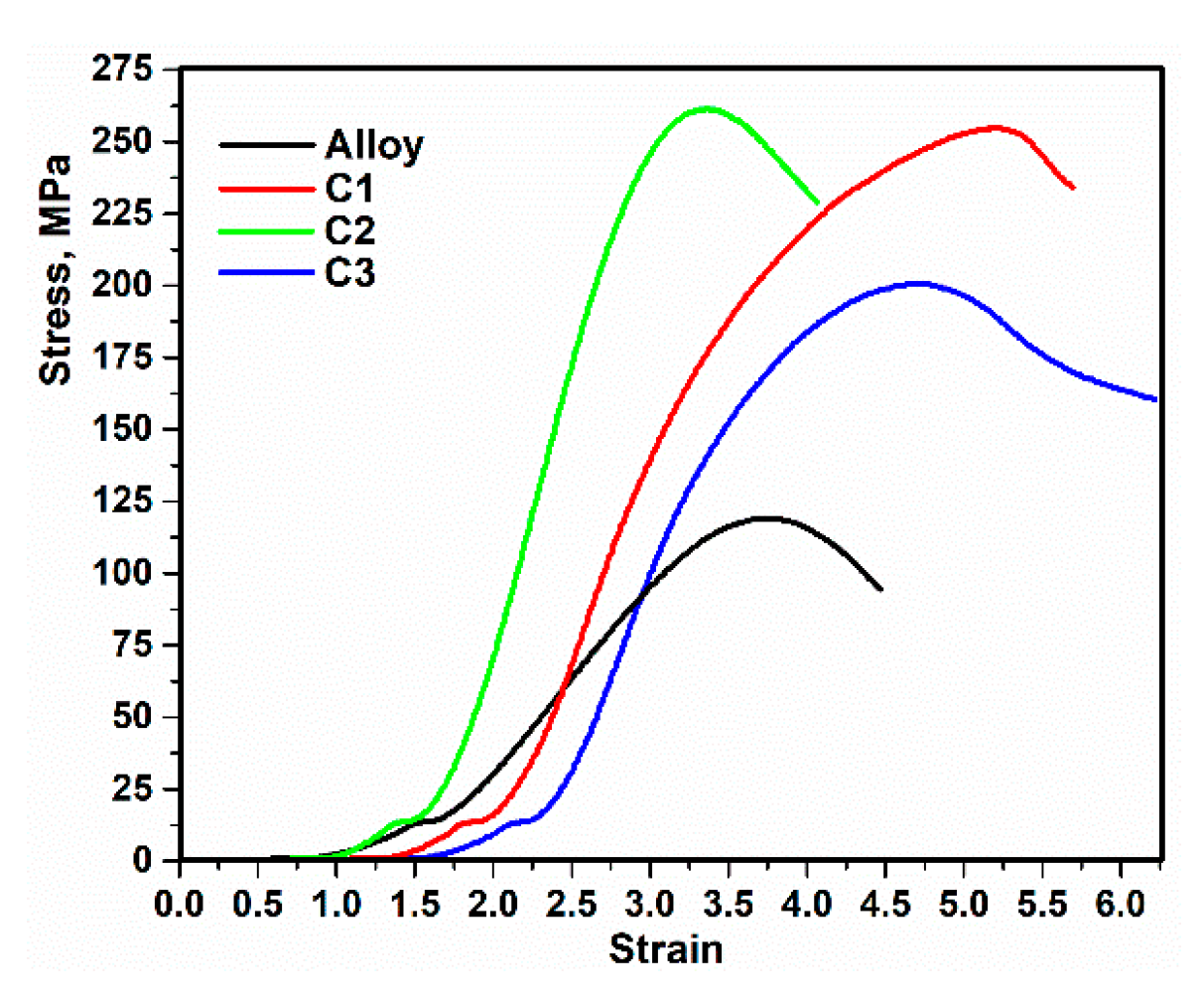
3.5. Mechanical Properties
3.6. Hybrid Bonding between Alloy and Polymers
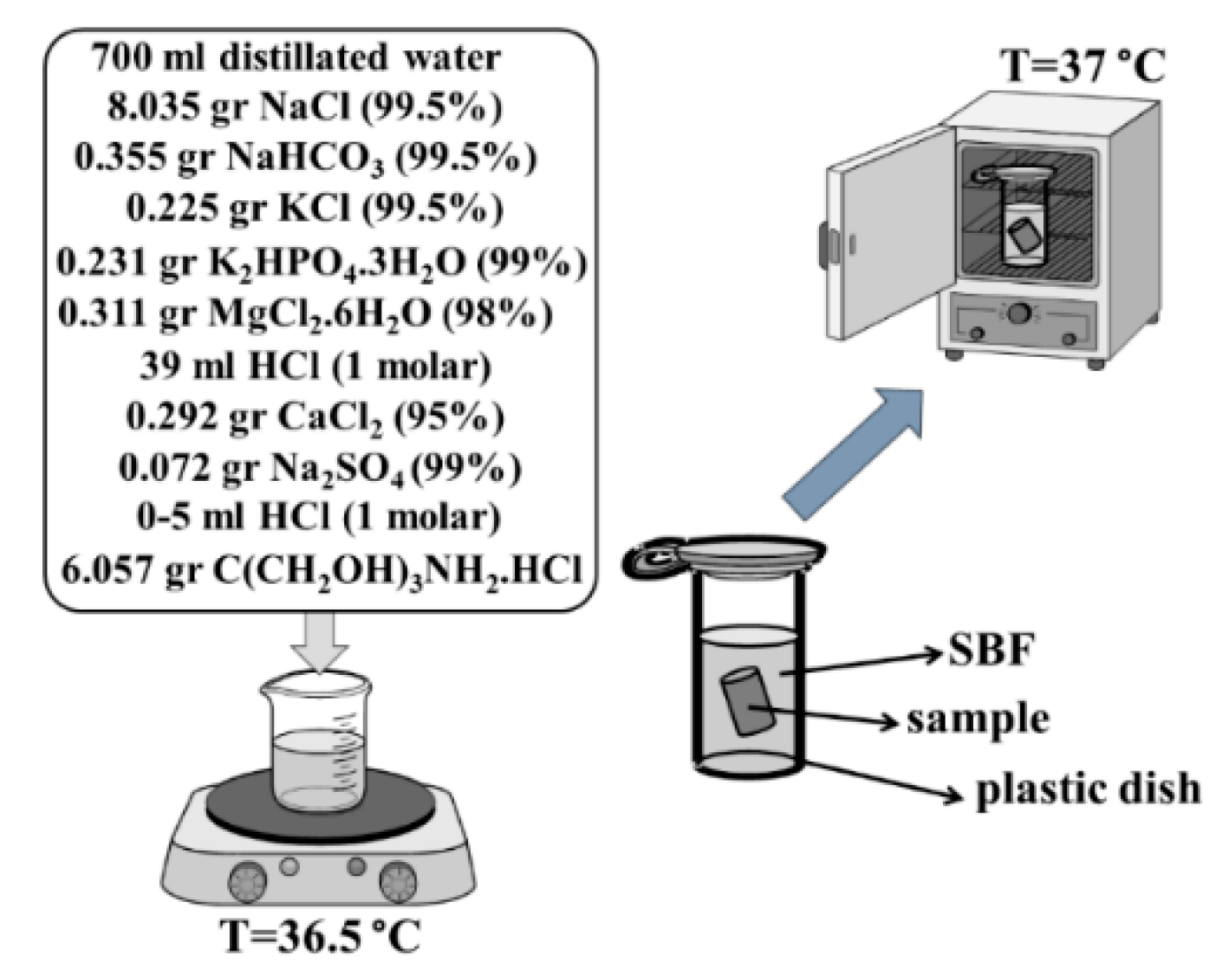
3.7. Investigation of Bioactivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knight, M.; Curliss, D. Composite Materials. Encycl. Phys. Sci. Technol. 2003, 14, 455–468. [Google Scholar]
- Nygren, H.; Bigdeli, N.; Ilver, L.; Malmberg, P. Mg-corrosion, hydroxyapatite, and bone healing. Biointerphases 2017, 12, 02C407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyedraoufi, Z.S.; Mirdamadi, S. Produce of Biodegradable Porous Mg-Zn Scaffold via Powder Metallurgy and Coated with Nano HAP Synthesized by Electrodeposition Process. J. Environ. Friendly Mater. 2017, 1, 40–45. [Google Scholar]
- Stierstorfer, J.; Klapötke, T.M. High Energy Materials. Propellants, Explosives and Pyrotechnics. By Jai Prakash Agrawal. Angew. Chemie Int. Ed. 2010, 49, 6253. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2017, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Wang, M. Developing bioactive composite materials for tissue replacement. Biomaterials 2003, 24, 2133–2151. [Google Scholar] [CrossRef]
- Zhang, L.N.; Hou, Z.T.; Ye, X.; Xu, Z.B.; Bai, X.L.; Shang, P. The effect of selected alloying element additions on properties of Mg-based alloy as bioimplants: A literature review. Front. Mater. Sci. 2013, 7, 227–236. [Google Scholar] [CrossRef]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning 2018, 2018, 9216314. [Google Scholar] [CrossRef] [Green Version]
- Rabiei, M.; Palevicius, A.; Ebrahimi-Kahrizsangi, R.; Nasiri, S.; Vilkauskas, A.; Janusas, G. New Approach for Preparing In Vitro Bioactive Scaffold Consisted of Ag-Doped Hydroxyapatite + Polyvinyltrimethoxysilane. Polymers 2021, 13, 1695. [Google Scholar] [CrossRef]
- David, R.; Lide, J. CRC Handbook of Chemistry and Physics, 2000–2001; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Sun, Y.; Zhang, B.; Wang, Y.; Geng, L.; Jiao, X. Preparation characterization of a new biomedical Mg–Zn–Ca alloy. Mater. Des. 2012, 34, 58–64. [Google Scholar] [CrossRef]
- Koç, E.; Kannan, M.B.; Ünal, M.; Candan, E. Influence of zinc on the microstructure, mechanical properties and in vitro corrosion behavior of magnesium–zinc binary alloys. J. Alloys Compd. 2015, 648, 291–296. [Google Scholar] [CrossRef]
- Gao, C.; Li, C.; Peng, S.; Shuai, C. Spiral-eutectic-reinforced Biodegradable Zn–Mg–Ag Alloy Prepared via Selective Laser Melting. Chin. J. Mech. Eng. Addit. Manuf. Front. 2022, 1, 100022. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, H.; Li, J. Influence of Zn and/or Ag additions on microstructure and properties of Al-Mg based alloys. J. Alloys Compd. 2022, 904, 163998. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Zhang, D.; Zhang, J. The effect of Ag on the tensile strength and fracture toughness of novel Al-Mg-Zn alloys. J. Alloys Compd. 2022, 908, 164640. [Google Scholar] [CrossRef]
- Marzieh, R.; Sohrab, N.; Arvydas, P.; Giedrius, J. Preparation and investigation of bioactive organic-inorganic nano-composite derived from PVB-co-VA-co-VAc/HA. In Proceedings of the 15th International Conference Mechatronic Systems Materials (MSM), Bialystok, Poland, 1–3 July 2020. [Google Scholar]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys composites for orthopedic implant applications—A review. J. Magnes. Alloy. 2017, 5, 286–312. [Google Scholar] [CrossRef]
- The Effect of Long Term Thermal Exposure on Plastics and Elastomers; Elsevier Science: New York, NY, USA, 2021.
- Nasiri, S.; Dashti, A.; Hosseinnezhad, M.; Rabiei, M.; Palevicius, A.; Doustmohammadi, A.; Janusas, G. Mechanochromic and thermally activated delayed fluorescence dyes obtained from D–A–D′ type, consisted of xanthen and carbazole derivatives as an emitter layer in organic light emitting diodes. Chem. Eng. J. 2022, 430, 131877. [Google Scholar] [CrossRef]
- Nasiri, S.; Hosseinnezhad, M.; Rabiei, M.; Palevicius, A.; Janusas, G. The effect of calcination temperature on the photophysical and mechanical properties of copper iodide (5 mol%)–doped hydroxyapatite. Opt. Mater. 2021, 121, 111559. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.N.; Hudon, P.; Jung, I.H.; Medraj, M.; Chartrand, P. Experimental study of the phase equilibria in the Mg–Zn–Ag ternary system at 300 °C. J. Alloys Compd. 2015, 639, 593–601. [Google Scholar] [CrossRef]
- Chemingui, M.; Ameur, R.; Optasanu, V.; Khitouni, M. DSC analysis of phase transformations during precipitation hardening in Al–Zn–Mg alloy (7020). J. Therm. Anal. Calorim. 2019, 136, 1887–1894. [Google Scholar] [CrossRef]
- Tsai, A.P.; Murakami, Y.; Niikura, A. The Zn-Mg-Y phase diagram involving quasicrystals. Philos. Mag. A Phys. Condens. Matter. Struct. Defects Mech. Prop. 2000, 80, 1043–1054. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Guo, P.T.; Yu, W.B.; Wang, F.; Pan, Z.Y. Microstructure Evolution of the Mg-5.8 Zn-0.5 Zr-1.0 Yb Alloy During Homogenization. Mater. Res. 2017, 20, 1063–1071. [Google Scholar] [CrossRef] [Green Version]
- Materials Data on MgZn2 by Materials Project. 2020. Available online: https://www.osti.gov/dataexplorer/biblio/dataset/1187663 (accessed on 16 July 2020).
- Deng, C.J.; Wong, M.L.; Ho, M.W.; Yu, P.; Ng, D.H.L. Formation of MgO and Mg–Zn intermetallics in an Mg-based composite by in situ reactions. Compos. Part. A Appl. Sci. Manuf. 2005, 36, 551–557. [Google Scholar] [CrossRef]
- Miller, N.D.; Williams, D.F. On the biodegradation of poly-β-hydroxybutyrate (PHB) homopolymer and poly-β-hydroxybutyrate-hydroxyvalerate copolymers. Biomaterials 1987, 8, 129–137. [Google Scholar] [CrossRef]
- Barham, P.J.; Barker, P.; Organ, S.J. Physical properties of poly(hydroxybutyrate) and copolymers of hydroxybutyrate and hydroxyvalerate. FEMS Microbiol. Rev. 1992, 9, 289–298. [Google Scholar] [CrossRef]
- Bauer, H.; Owen, A.J. Some structural and mechanical properties of bacterially produced poly-β-hydroxybutyrate-co-β-hydroxyvalerate. Colloid Polym. Sci. 1988, 266, 241–247. [Google Scholar] [CrossRef]
- Karabulut, A.; Baştan, F.E.; Erdoʇan, G.; Üstel, F. Heat treatment’s effects on hydroxyapatite powders in water vapor and air atmosphere. AIP Conf. Proc. 2015, 1653, 020053. [Google Scholar]
- Wang, S.; Fu, L.; Nai, Z.; Liang, J.; Cao, B. Comparison of corrosion resistance and cytocompatibility of MgO and ZrO2 coatings on AZ31 magnesium alloy formed via plasma electrolytic oxidation. Coatings 2018, 8, 441. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.L.; Mukherjee, S.; Nisbet, D.R.; Birbilis, N.; Chen, X.B. In vitro evaluation of biodegradable magnesium alloys containing micro-alloying additions of strontium, with and without zinc. J. Mater. Chem. B 2015, 3, 8874–8883. [Google Scholar] [CrossRef]
- Němec, M.; Gärtnerová, V.; Klementová, M.; Jäger, A. Analysis of intermetallic particles in Mg–12 wt.%Zn binary alloy using transmission electron microscopy. Mater. Charact. 2015, 106, 428–436. [Google Scholar] [CrossRef]
- Seyedraoufi, Z.-S. Effect of Zn and porosity on the biodegradability and Mechanical Properties of Mg-Zn Scaffolds ARTICLE INFO ABSTRACT. J. Adv. Mater. Process. 2018, 6, 42–52. [Google Scholar]
- Mei, L.; Lu, Z.; Zhang, X.; Li, C.; Jia, Y. Polymer-Ag nanocomposites with enhanced antimicrobial activity against bacterial infection. ACS Appl. Mater. Interfaces 2014, 6, 15813–15821. [Google Scholar] [CrossRef] [PubMed]
- Hanemann, T.; Szabó, D.V. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials 2010, 3, 3468. [Google Scholar] [CrossRef]
- Hassan, A.; Abd El-Hamid, M.; Wagih, A.; Fathy, A. Effect of Mechanical Milling on the Morphologyand Structural Evaluation of Al-Al2O3 Nanocomposite Powders. Int. J. Eng. 2014, 27, 625–632. [Google Scholar]
- Park, S.J.; Seo, M.K. Composite Characterization. Interface Sci. Technol. 2011, 18, 631–738. [Google Scholar]
- Baker, H. ASM Handbook, Volume 3 Alloy Phase Diagrams, 10th ed.; ASM International: Almere, The Netherlands, 2016. [Google Scholar]
- Schlichting, J.; Riley, F.L. Silicon Carbide. In Concise Encyclopaedia of Advanced Ceramic Materials; Pergamon: Oxford, UK, 1991; pp. 426–429. [Google Scholar]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing methods for calculating nano crystal size of natural hydroxyapatite using X-ray diffraction. Nanomaterials 2020, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, M.; Palevicius, A.; Nasiri, S.; Dashti, A.; Vilkauskas, A.; Janusas, G. Relationship between Young’s Modulus and Planar Density of Unit Cell, Super Cells (2 × 2 × 2), Symmetry Cells of Perovskite (CaTiO3) Lattice. Materials 2021, 14, 1258. [Google Scholar] [CrossRef]
- Salleh, E.M.; Ramakrishnan, S.; Hussain, Z. Synthesis of Biodegradable Mg-Zn Alloy by Mechanical Alloying: Effect of Milling Time. Procedia Chem. 2016, 19, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Lotnyk, A.; Bauer, J.; Breitenstein, O.; Blumtritt, H. A TEM study of SiC particles and filaments precipitated in multicrystalline Si for solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 1236–1240. [Google Scholar] [CrossRef]
- Zhang, X.; Ba, Z.; Wang, Z.; He, X.; Shen, C.; Wang, Q. Influence of silver addition on microstructure and corrosion behavior of Mg–Nd–Zn–Zr alloys for biomedical application. Mater. Lett. 2013, 100, 188–191. [Google Scholar] [CrossRef]
- ASTM International-ASTM E9-09; Standard Test Methods of Compression Testing of Metallic Materials at Room Temperature. ASTM International: West Conshohocken, PA, USA, 2013.
- Xia, X.; Chen, Q.; Huang, S.; Lin, J.; Hu, C.; Zhao, Z. Hot deformation behavior of extruded Mg–Zn–Y–Zr alloy. J. Alloys Compd. 2015, 644, 308–316. [Google Scholar] [CrossRef]
- Wang, L.X.; Fang, G.; Leeflang, M.A.; Duszczyk, J.; Zhou, J. Constitutive behavior and microstructure evolution of the as-extruded AE21 magnesium alloy during hot compression testing. J. Alloys Compd. 2015, 622, 121–129. [Google Scholar] [CrossRef]
- Khan, A.S.; Pandey, A.; Gnäupel-Herold, T.; Mishra, R.K. Mechanical response and texture evolution of AZ31 alloy at large strains for different strain rates and temperatures. Int. J. Plast. 2011, 27, 688–706. [Google Scholar] [CrossRef]
- ASTM E384; Testing at WMT&R Inc. ASTM International: West Conshohocken, PA, USA, 2016.
- Zhao, J.W.; Zhang, J.L.; Li, Y.Z.; Cao, J.; Chen, L.J. Novel one-dimensional organic-inorganic polyoxometalate hybrids constructed from heteropolymolybdate units and copper-aminoacid complexes. Cryst. Growth Des. 2014, 14, 1467–1475. [Google Scholar] [CrossRef]
- Wei, M.J.; Zang, H.Y.; Zhou, E.L.; Shao, K.Z.; Song, B.Q.; Wang, X.L.; Su, Z.M. Coordination and supramolecular assembly of {Cd2Ge8V12O48} building block and cucurbit[6] to form rotaxane-shaped hybrids. Dalt. Trans. 2016, 45, 4989–4992. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Guo, S.X.; Ding, L.; Ohlin, C.A.; Bond, A.M.; Zhang, J. Lindqvist Polyoxoniobate Ion-Assisted Electrodeposition of Cobalt and Nickel Water Oxidation Catalysts. ACS Appl. Mater. Interfaces 2015, 7, 16632–16644. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Shahrjerdi, A.; Mustapha, F.; Sapuan, S.M.; Bayat, M.; Majid, D.L.A.; Zahari, R. Optimal Sintering Procedure to Fabrication of Functionally Graded Hydroxyapatite-Titanium. Key Eng. Mater. 2011, 471–472, 140–144. [Google Scholar] [CrossRef]
- Kostov-Kytin, V.V.; Dyulgerova, E.; Ilieva, R.; Petkova, V. Powder X-ray diffraction studies of hydroxyapatite and β-TCP mixtures processed by high energy dry milling. Ceram. Int. 2018, 44, 8664–8671. [Google Scholar] [CrossRef]
- Guillen-Romero, L.D.; Oropeza-Guzmán, M.T.; López-Maldonado, E.A.; Iglesias, A.L.; Paz-González, J.A.; Ng, T.; Serena-Gómez, E.; Villarreal-Gómez, L.J. Synthetic hydroxyapatite and its use in bioactive coatings. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800018817463. [Google Scholar] [CrossRef] [Green Version]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A novel hydroxyapatite–Hardystonite nanocomposite ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Rehm, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Legeros, R.Z.; Legeros, J.P. Hydroxyapatite. Bioceram. Their Clin. Appl. 2008, 3, 367–394. [Google Scholar]




| Sample | (gr/cm3) | (gr/cm3) | Porosity, (%) | |
|---|---|---|---|---|
| Alloy | 1.792 ± 0.005 | 1.721 ± 0.003 | 0.96038 | 4.610 ± 0.008 |
| C1 | 1.613 ± 0.007 | 1.608 ± 0.008 | 0.9969 | 5.011 ± 0.006 |
| C2 | 1.779 ± 0.001 | 1.711 ± 0.003 | 0.96178 | 5.063 ± 0.009 |
| C3 | 1.783 ± 0.003 | 1.701 ± 0.004 | 0.95401 | 4.917 ± 0.005 |
| Sample | Maximum Compressive Strength, (MPa) | Elasticity Coefficient, (MPa) | Hardness, (HV) |
|---|---|---|---|
| Alloy | 119.05 ± 1.03 | 65.45 ± 1.01 | 57.39 ± 1.00 |
| C1 | 255.39 ± 1.11 | 134.25 ± 1.00 | 68.71 ± 1.01 |
| C2 | 261.84 ± 1.08 | 228.88 ± 1.03 | 71.05 ± 1.01 |
| C3 | 201.61 ± 1.14 | 134.84 ± 1.00 | 60.26 ± 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabiei, M.; Raziyan, M.S.; Ebrahimi-Kahrizsangi, R.; Nasiri, S.; Palevicius, A.; Janusas, G.; Vilkauskas, A. Effects of 5 wt.% Polycaprolactone, Polyhydroxybutyrate and Polyvinyltrimethoxysilane on the Properties of Ag/Zn/Mg Alloy. Materials 2022, 15, 5421. https://doi.org/10.3390/ma15155421
Rabiei M, Raziyan MS, Ebrahimi-Kahrizsangi R, Nasiri S, Palevicius A, Janusas G, Vilkauskas A. Effects of 5 wt.% Polycaprolactone, Polyhydroxybutyrate and Polyvinyltrimethoxysilane on the Properties of Ag/Zn/Mg Alloy. Materials. 2022; 15(15):5421. https://doi.org/10.3390/ma15155421
Chicago/Turabian StyleRabiei, Marzieh, Motahareh Sadat Raziyan, Reza Ebrahimi-Kahrizsangi, Sohrab Nasiri, Arvydas Palevicius, Giedrius Janusas, and Andrius Vilkauskas. 2022. "Effects of 5 wt.% Polycaprolactone, Polyhydroxybutyrate and Polyvinyltrimethoxysilane on the Properties of Ag/Zn/Mg Alloy" Materials 15, no. 15: 5421. https://doi.org/10.3390/ma15155421
APA StyleRabiei, M., Raziyan, M. S., Ebrahimi-Kahrizsangi, R., Nasiri, S., Palevicius, A., Janusas, G., & Vilkauskas, A. (2022). Effects of 5 wt.% Polycaprolactone, Polyhydroxybutyrate and Polyvinyltrimethoxysilane on the Properties of Ag/Zn/Mg Alloy. Materials, 15(15), 5421. https://doi.org/10.3390/ma15155421







