Effect of Parylene C on the Corrosion Resistance of Bioresorbable Cardiovascular Stents Made of Magnesium Alloy ‘Original ZM10’
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Stents
2.3. Parylene C Coating
2.4. Polymer Coating
2.5. Contraction and Expansion of the Stent
2.6. Surface Observation
2.7. Evaluation of Corrosion Behavior
2.8. SRL Elution from the Polymer Layer
3. Results and Discussion
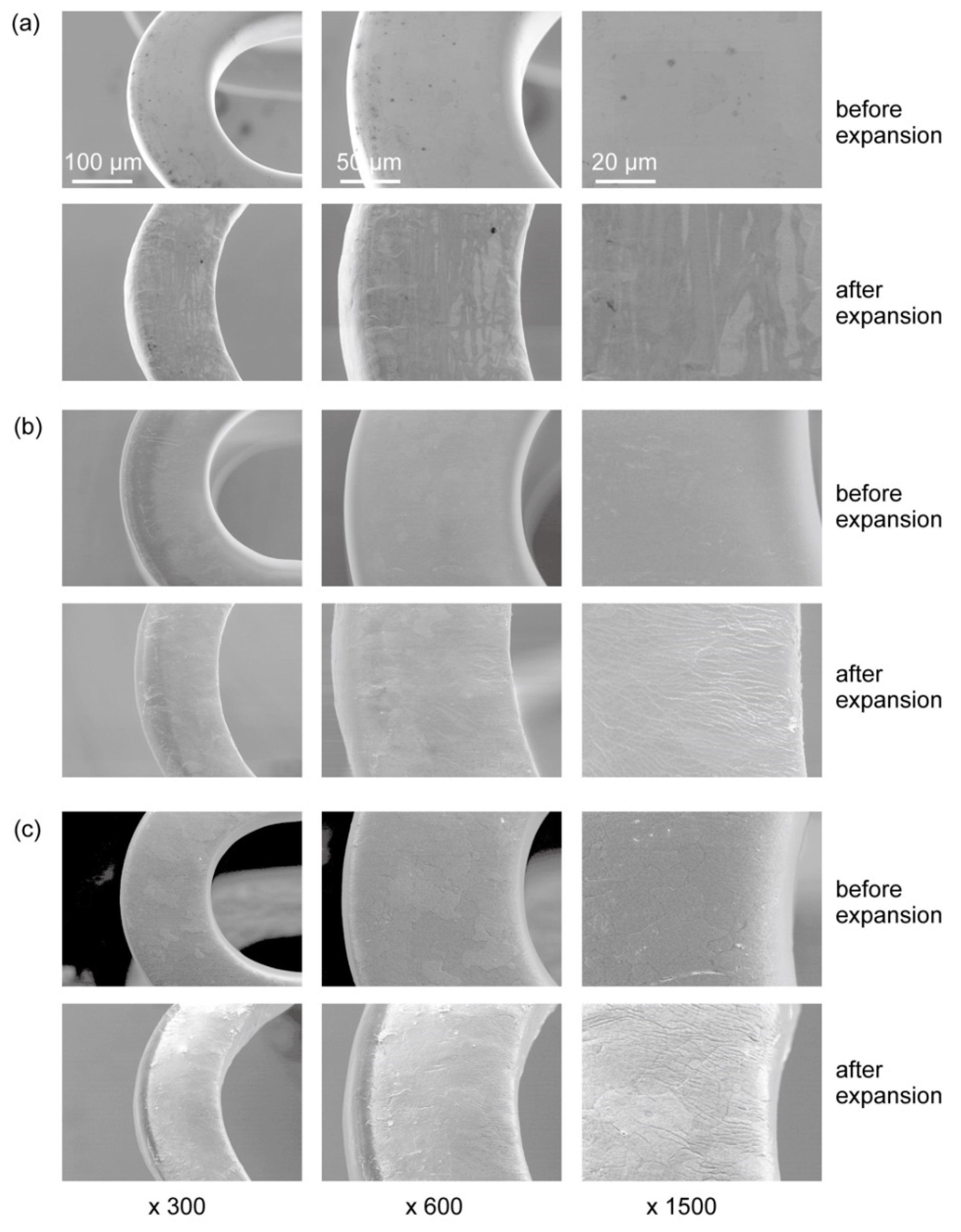
3.1. Parylene C Coating of Mg Alloy Stents
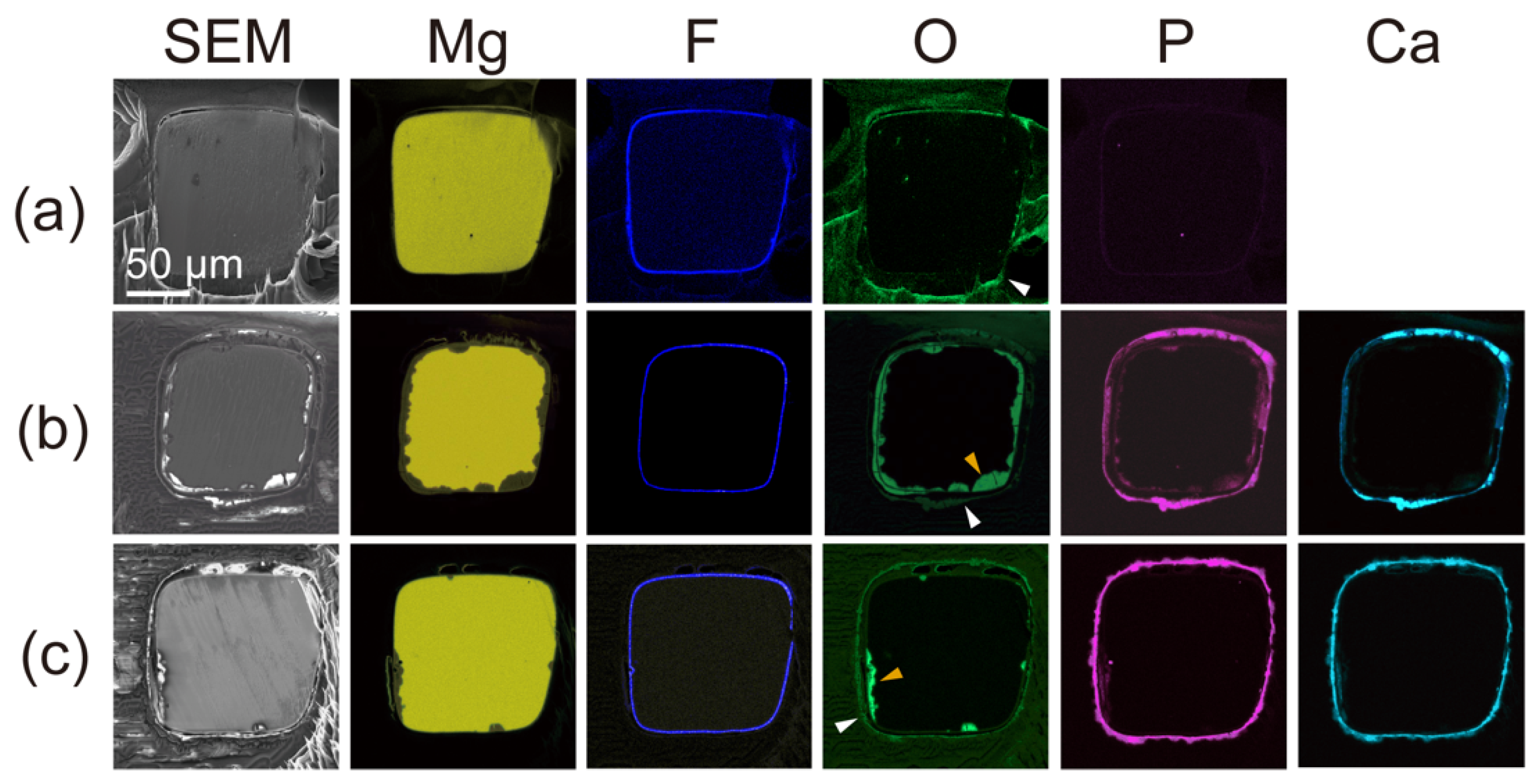
3.2. Corrosion Behavior of the Parylene-Coated Mg Alloy Stents after Expansion
3.3. SRL Release from the Polymer Layer
4. Conclusions
- (1)
- Parylene C coating on a HF-treated Mg alloy stent exhibited a smooth surface without cracks after balloon expansion. Because the parylene C coating was very thin (~0.5 µm) and flexible, it was able to expand and follow the structural changes of the HF-treated Mg stent without peeling off.
- (2)
- Combining the parylene C coating with the HF treatment of the Mg alloy stent showed complete corrosion resistance for at least 1 month in physiological conditions. The tight adhesion between the MgF2 and parylene C layers was thought to form a strong and stable water barrier even when the structure of the struts was deformed.
- (3)
- The parylene C coating did not affect subsequent layers of biodegradable polymer coating applied to a long-lasting release of antiproliferative drug for 64 days to suppress restenosis.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gersh, B.J.; Sliwa, K.; Mayosi, B.M.; Yusuf, S. Novel therapeutic concepts: The epidemic of cardiovascular disease in the developing world: Global implications. Eur. Heart J. 2010, 31, 642–648. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Dawkins, K.D.; Mohr, F.W.; Morice, M.C.; Mack, M.J.; Russell, M.E.; Pomar, J.; Serruys, P.W.J.C. Current percutaneous coronary intervention and coronary artery bypass grafting practices for three-vessel and left main coronary artery disease. Insights from the SYNTAX run-in phase. Eur. J. Cardiothorac. Surg. 2006, 29, 486–491. [Google Scholar] [CrossRef]
- Valgimigli, M.; Tebaldi, M.; Borghesi, M.; Vranckx, P.; Campo, G.; Tumscitz, C.; Cangiano, E.; Minarelli, M.; Scalone, A.; Cavazza, C.; et al. Two-year outcomes after first- or second-generation drug-eluting or bare-metal stent implantation in all-comer patients undergoing percutaneous coronary intervention. JACC Cardiovasc. Interv. 2014, 7, 20–28. [Google Scholar] [CrossRef]
- Abraham, R.T.; Wiederrecht, G.J. Immunopharmacology of rapamycin. Annu. Rev. Immunol. 1996, 14, 483–510. [Google Scholar] [CrossRef]
- Gomez-Lara, J.; Brugaletta, S.; Jacobi, F.; Ortega-Paz, L.; Nato, M.; Roura, G.; Romaguera, R.; Ferreiro, J.L.; Teruel, L.; Gracida, M.; et al. Five-year optical coherence tomography in patients with ST-segment-elevation myocardial infarction treated with bare-metal versus everolimus-eluting stents. Circ. Cardiovasc. Interv. 2016, 9, e003670. [Google Scholar] [CrossRef]
- Xu, W.; Sasaki, M.; Niidome, T. Sirolimus release from biodegradable polymers for coronary stent application: A review. Pharmaceutics 2022, 14, 492. [Google Scholar] [CrossRef]
- Omar, W.A.; Kumbhani, D.J. The current literature on bioabsorbable stents: A review. Curr. Atheroscler. Rep. 2019, 21, 54. [Google Scholar] [CrossRef]
- Aljihmani, L.; Alic, L.; Boudjemline, Y.; Hijazi, Z.M.; Mansoor, B.; Serpedin, E.; Qaraqe, K. Magnesium-based bioresorbable stent materials: Review of reviews. J. Bio-Tribo-Corros. 2019, 5, 26. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, T.; Chu, P.K. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef]
- Gastaldi, D.; Sassi, V.; Petrini, L.; Vedani, M.; Trasatti, S.; Migliavacca, F. Continuum damage model for bioresorbable magnesium alloy devices—Application to coronary stents. J. Mech. Behav. Biomed. Mater. 2011, 4, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Etave, F.; Finet, G.; Boivin, M.; Boyer, J.C.; Rioufol, G.; Thollet, G. Mechanical properties of coronary stents determined by using finite element analysis. J. Biomech. 2001, 34, 1065–1075. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; You, Z.; Liu, S.; Guan, K.; Wu, R.; Wang, J.; Feng, J. Towards developing Mg alloys with simultaneously improved strength and corrosion resistance via RE alloying. J. Magnes. Alloys 2021, 9, 41–56. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Lin, G.; Huang, J. The role of rare earth elements in biodegradable metals: A review. Acta Biomater. 2021, 129, 33–42. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Wang, L.; Zeng, M.Q.; Zeng, R.C.; Lin, C.G.; Wang, Z.L.; Chen, D.C.; Zhang, Q. Corrosion resistance and superhydrophobicity of one-step polypropylene coating on anodized AZ31 Mg alloy. J. Magnes. Alloys 2021, 9, 1443–1457. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Abazari, S.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Daroonparvar, M.; Berto, F. A comprehensive review on surface modifications of biodegradable magnesium-based implant alloy: Polymer coatings opportunities and challenges. Coatings 2021, 11, 747. [Google Scholar] [CrossRef]
- Guo, Y.; Li, G.; Xu, Z.; Xu, Y.; Yin, L.; Yu, Z.; Zhang, Z.; Lian, J.; Ren, L. Corrosion resistance and biocompatibility of calcium phosphate coatings with a micro-nanofibrous porous structure on biodegradable magnesium alloys. ACS Appl. Bio Mater. 2022, 5, 1528–1537. [Google Scholar] [CrossRef]
- da Conceicao, T.F.; Scharnagl, N.; Blawert, C.; Dietzel, W.; Kainer, K.U. Surface modification of magnesium alloy AZ31 by hydrofluoric acid treatment and its effect on the corrosion behavior. Thin Solid Films 2010, 518, 5209–5218. [Google Scholar] [CrossRef]
- Weng, W.; Biesiekierski, A.; Li, Y.; Dargusch, M.; Wen, C. A review of the physiological impact of rare earth elements and their uses in biomedical Mg alloys. Acta Biomater. 2021, 130, 80–97. [Google Scholar] [CrossRef]
- Dargusch, M.S.; Balasubramani, N.; Yang, N.; Johnston, S.; Ali, Y.; Venezuela, J.; Carluccio, J.; Lau, C.; Allavena, R.; Liang, D.; et al. In vivo performance of a rare earth free Mg-Zn-Ca alloy manufactured using twin roll casting for potential applications in the cranial and maxillofacial fixation devices. Bioact. Mater. 2022, 12, 85–96. [Google Scholar] [CrossRef]
- Rondeau, V.; Commenges, D.; Jacqmin-Gadda, H.; Dartigues, J.F. Relation between aluminum concentrations in drinking water and Alzheimer’s disease: An 8-year follow-up study. Am. J. Epidemiol. 2000, 152, 59–66. [Google Scholar] [CrossRef]
- Xu, W.; Yagoshi, K.; Koga, Y.; Sasaki, M.; Niidome, T. Optimized polymer coating for magnesium alloy-based bioresorbable scaffolds for long-lasting drug release and corrosion resistance. Colloids Surf. B 2018, 163, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Sato, K.; Koga, Y.; Sasaki, M.; Niidome, T. Corrosion resistance of HF-treated Mg alloy stents following balloon-expansion and its improvement through biodegradable polymer coating. J. Coat. Technol. 2020, 17, 1023–1032. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhen, Z.; Liu, J.; Xi, T.F.; Zheng, Y.D.; Guan, S.K.; Zheng, Y.F.; Cheng, Y. Multifunctional MgF2/polydopamine coating on Mg alloy for vascular stent application. J. Mater. Sci. Technol. 2015, 31, 733–743. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Vladescu, A.; Cotrut, C.M.; Tyurin, A.I.; Pirozhkova, T.S.; Shuvarin, I.A.; Elkin, B.; Surmenev, R.A. Effect of parylene C coating on the antibiocorrosive and mechanical properties of different magnesium alloys. Appl. Surf. Sci. 2018, 427, 617–627. [Google Scholar] [CrossRef]
- Wolf, K.V.; Zong, Z.; Meng, J.; Orana, A.; Rahbar, N.; Balss, K.M.; Papandreou, G.; Maryanoff, C.A.; Soboyejo, W. An investigation of adhesion in drug-eluting stent layers. J. Biomed. Mater. Res. A 2008, 87, 272–281. [Google Scholar] [CrossRef]
- Schurtz, G.; Delhaye, C.; Hurt, C.; Thieuleux, H.; Lemesle, G. Biodegradable polymer Biolimus-eluting stent (Nobori®) for the treatment of coronary artery lesions: Review of concept and clinical results. Med. Devices 2014, 7, 35–43. [Google Scholar] [CrossRef][Green Version]
- Chang, T.Y.; Yadav, V.G.; Leo, S.D.; Mohedas, A.; Rajalingam, B.; Chen, C.L.; Selvarasah, S.; Dokmeci, M.R.; Khademhosseini, A. Cell and protein compatibility of parylene-C surfaces. Langmuir 2007, 23, 11718–11725. [Google Scholar] [CrossRef]
- Tan, C.P.; Craighead, H.G. Surface engineering and patterning using parylene for biological applications. Materials 2010, 3, 1803–1832. [Google Scholar] [CrossRef]
- Xu, L.; Yamamoto, A. Characteristics and cytocompatibility of biodegradable polymer film on magnesium by spin coating. Colloids Surf. B 2012, 93, 67–74. [Google Scholar] [CrossRef]
- Pan, C.; Han, Y.; Lu, J. Structural design of vascular stents: A review. Micromachines 2021, 12, 770. [Google Scholar] [CrossRef]
- Zieliński, M.; Czajka, B.; Pietrowski, M.; Tomska-Foralewska, I.; Alwin, E.; Kot, M.; Wojciechowska, M. MgO-MgF2 system obtained by sol–gel method as an immobilizing agent of the electrolyte applied in the high temperature cells. J. Sol-Gel Sci. Technol. 2017, 84, 368–374. [Google Scholar] [CrossRef]
- Kandala, B.S.P.K.; Zhang, G.; An, X.; Pixley, S.; Shanov, V. Effect of surface-modification on in vitro corrosion of biodegradable magnesium-based helical stent fabricated by photo-chemical etching. Med. Res. Arch. 2020, 8, 3. [Google Scholar] [CrossRef][Green Version]
- Kandala, B.S.P.K.; Zhang, G.; Hopkins, T.M.; An, X.; Pixley, S.; Shanov, V. In vitro and in vivo testing of zinc as a biodegradadble material for stents fabricated by photo-chemical etching. Appl. Sci. 2019, 9, 4503. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Egorkin, V.S.; Ustinov, A.Y.; Sergienko, V.I.; Gnedenkov, S.V. The detailed corrosion performance of bioresorbable Mg-0.8Ca alloy in physiological solutions. J. Magnes. Alloys 2022, 1, 51. [Google Scholar] [CrossRef]
- Rizas, K.D.; Mehilli, J. Stent polymers Do they make a difference? Circ. Cardiovasc. Interv. 2016, 9, e002943. [Google Scholar] [CrossRef]
- Stefanini, G.G.; Taniwaki, M.; Windecker, S. Coronary stents: Novel developments. Heart 2012, 100, 989–990. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, M.; Xu, W.; Koga, Y.; Okazawa, Y.; Wada, A.; Shimizu, I.; Niidome, T. Effect of Parylene C on the Corrosion Resistance of Bioresorbable Cardiovascular Stents Made of Magnesium Alloy ‘Original ZM10’. Materials 2022, 15, 3132. https://doi.org/10.3390/ma15093132
Sasaki M, Xu W, Koga Y, Okazawa Y, Wada A, Shimizu I, Niidome T. Effect of Parylene C on the Corrosion Resistance of Bioresorbable Cardiovascular Stents Made of Magnesium Alloy ‘Original ZM10’. Materials. 2022; 15(9):3132. https://doi.org/10.3390/ma15093132
Chicago/Turabian StyleSasaki, Makoto, Wei Xu, Yuki Koga, Yuki Okazawa, Akira Wada, Ichiro Shimizu, and Takuro Niidome. 2022. "Effect of Parylene C on the Corrosion Resistance of Bioresorbable Cardiovascular Stents Made of Magnesium Alloy ‘Original ZM10’" Materials 15, no. 9: 3132. https://doi.org/10.3390/ma15093132
APA StyleSasaki, M., Xu, W., Koga, Y., Okazawa, Y., Wada, A., Shimizu, I., & Niidome, T. (2022). Effect of Parylene C on the Corrosion Resistance of Bioresorbable Cardiovascular Stents Made of Magnesium Alloy ‘Original ZM10’. Materials, 15(9), 3132. https://doi.org/10.3390/ma15093132






