The Corrosion Susceptibility of 304L Stainless Steel Exposed to Crevice Environments
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
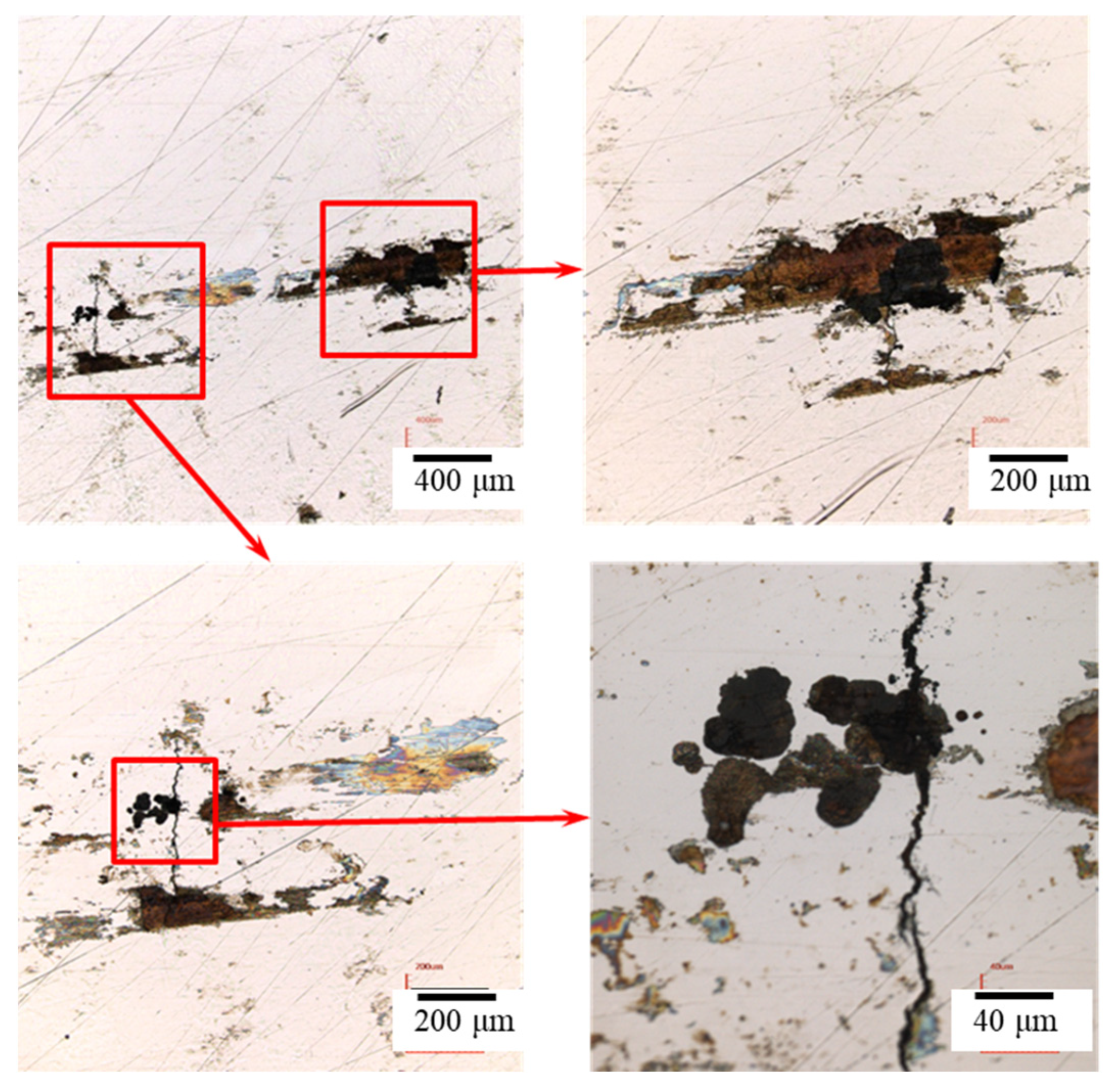
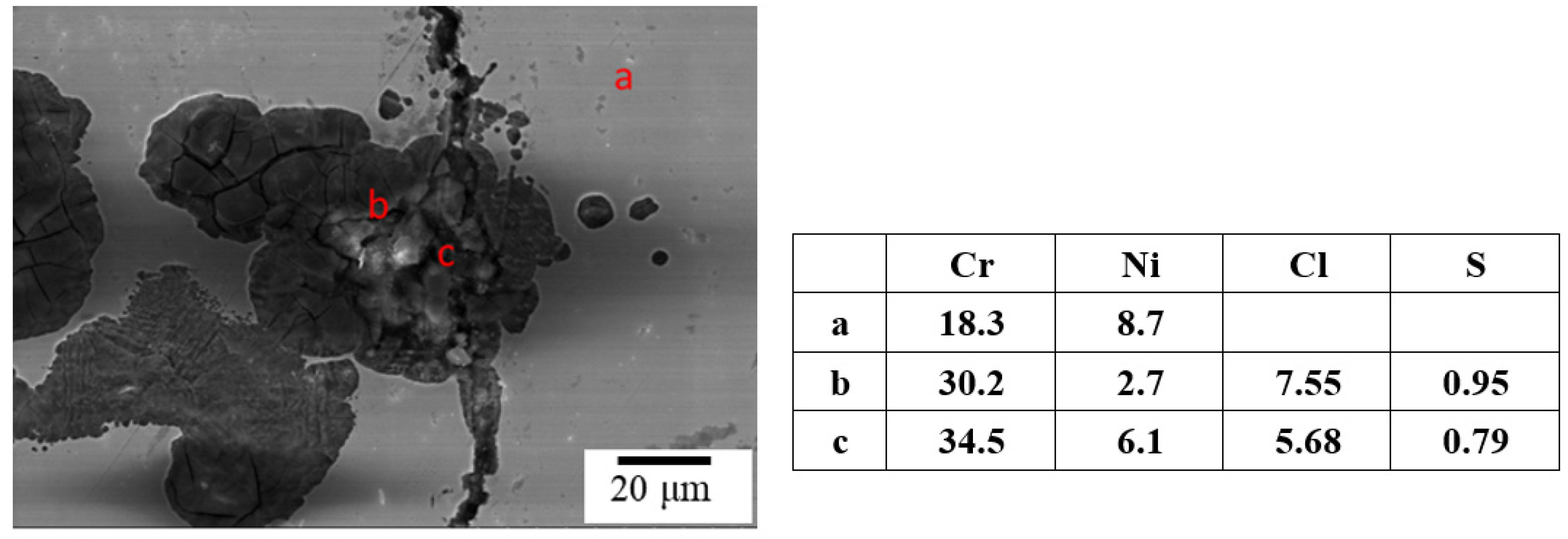
3.1. The Crevice Corrosion Test
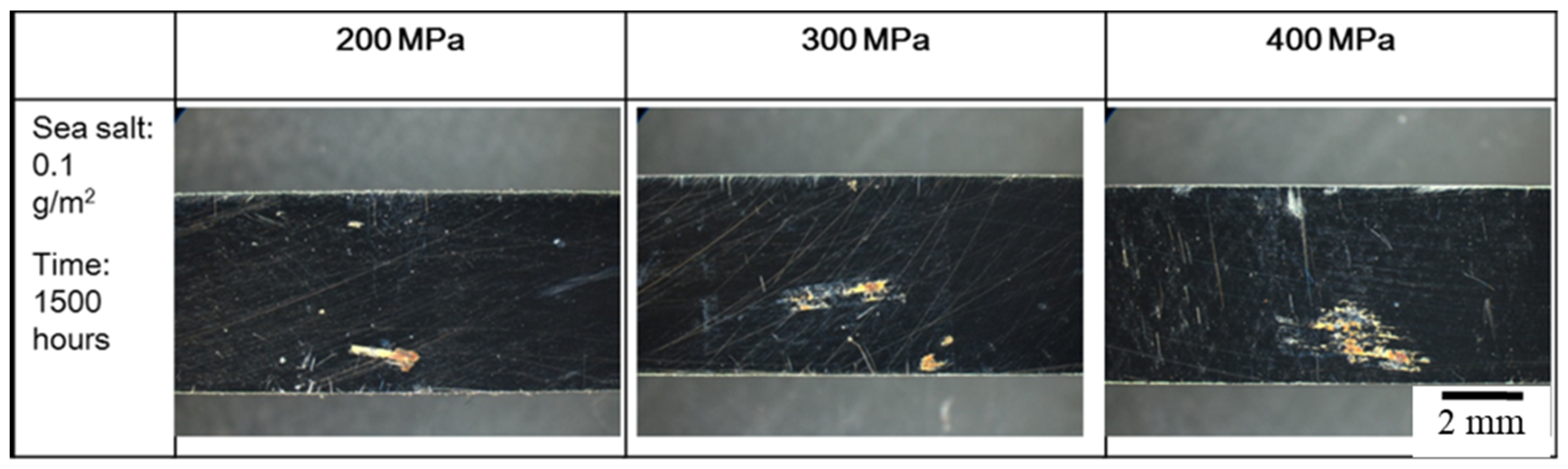
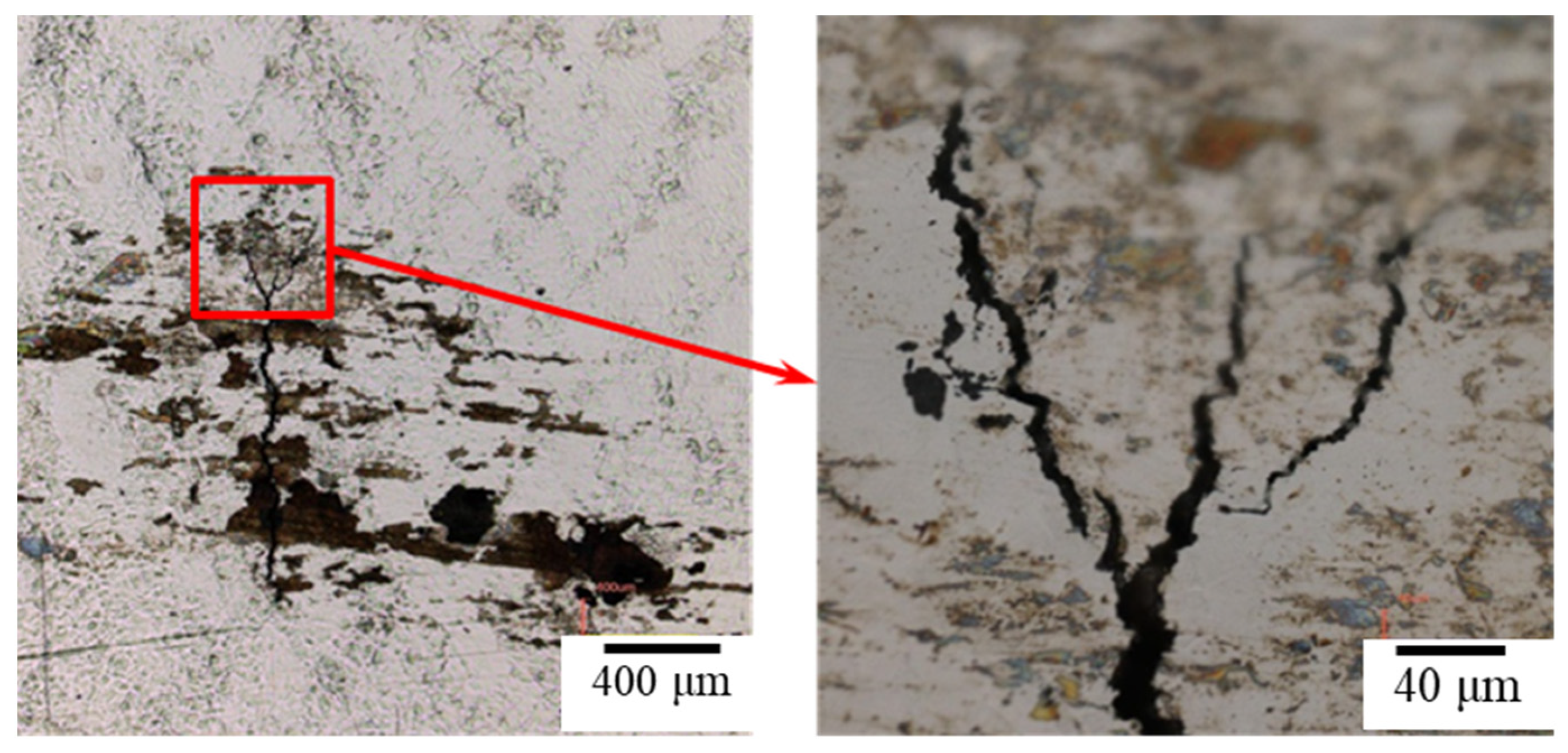
3.2. The Crevice Corrosion Test under Tensile Stress
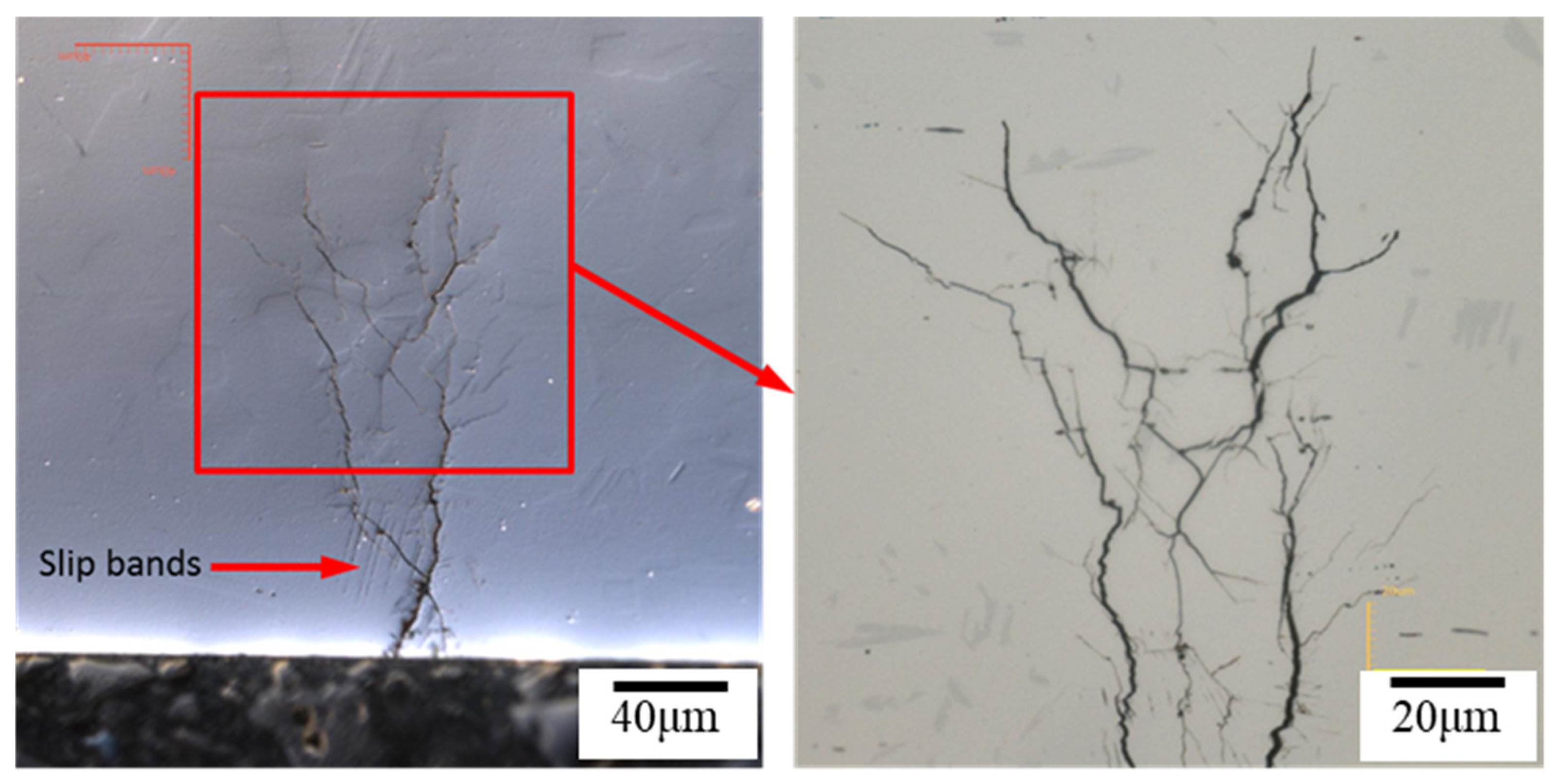
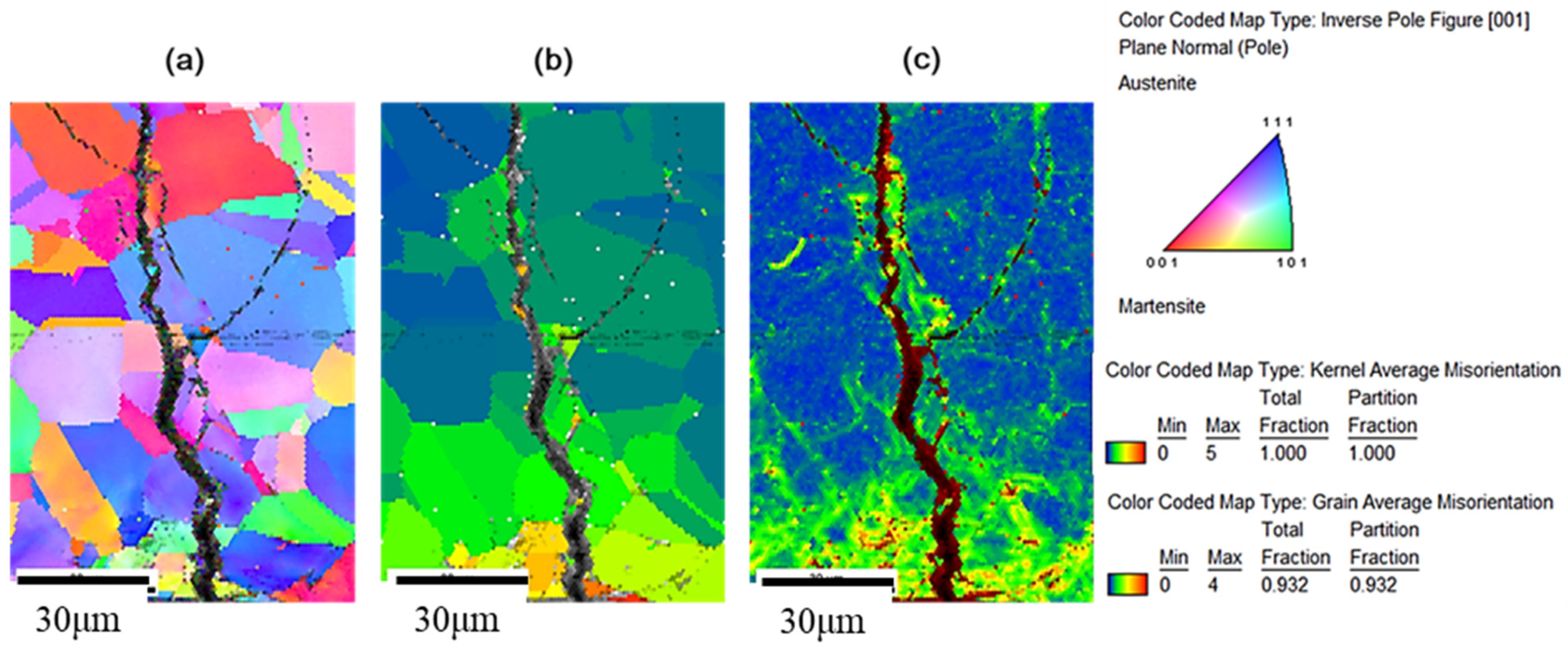
3.3. The EBSD Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tsai, K.C. Study of Trans-granular Stress Corrosion Cracking of 304L Stainless Steel under Saline Crevice Environment. J. Chin. Corros. Eng. 2016, 30, 1. [Google Scholar]
- Marcus, P. Corrosion Mechanisms in Theory and Practice, 3rd ed.; CRC Press: New York, NY, USA; London, UK, 2012; pp. 188, 462, 472. [Google Scholar]
- Chen, D.X.; Wu, X.Q.; Han, E.-H. Research Progress of Crevice Corrosion and Crevice Corrosion Issues of Nuclear-grade Materials. J. Chin. Soc. Corros. Prot. 2014, 34, 295. [Google Scholar]
- Ma, F.Y. (Ed.) Corrosive Effects of Chlorides on Metals; Pitting Corrosion; Shanghai, China. Available online: http://cdn.intechopen.com/pdfs/33625/intech-corrosive_effects_of_chlorides_on_metals.pdf (accessed on 7 January 2020).
- Moosbrugger, C. (Ed.) ASM Metals Handbook; ASM International: Materials Park, OH, USA, 2002; Volume 13, p. 685. [Google Scholar]
- Tokiwai, M.; Kimura, H.; Kusanagi, H. The Amount of Chlorine Contamination for Prevention of Stress Corrosion Cracking in Sensitized Type 304 Stainless Steel. Corros. Sci. 1985, 25, 837. [Google Scholar] [CrossRef]
- He, X.; Mintz, T.S.; Pabalan, R.; Miller, L.; Oberson, G. Assessment of Stress Corrosion Cracking Susceptibility for Austenitic Stainless Steels Exposed to Atmospheric Chloride and Non-Chloride Salts; US Nuclear Regulatory Commission: Washington DC, USA, 2014; NUREG/CR-7170.
- Guo, L.; Street, S.R.; Mohammed-Ali, H.B.; Ghahari, M.; Mi, N.; Glanvill, S.; Plessis, A.D.; Reinhard, C.; Rayment, T.; Davenport, A.J. The effect of relative humidity change on atmospheric pitting corrosion of stainless steel 304L. Corros. Sci. 2019, 150, 110. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Guo, X.Z.; Chu, W.Y.; Gao, K.W.; Wang, Y.B.; Su, Y.J.; Qiao, L.J. Martensite caused by passive film-induced stress during stress corrosion cracking in type 304 stainless steel. Mater. Sci. Eng. A 2003, 358, 122. [Google Scholar] [CrossRef]
- Jani, S.; Marek, M.; Hochman, R.F.; Meletis, E.I. A Mechanistic Study of Transgranular Stress Corrosion Cracking of Type 304 Stainless Steel. Metall. Trans. A 1991, 22, 1453. [Google Scholar] [CrossRef]
- Spencer, D.T.; Ewards, M.R.; Wenman, M.R.; Tsitsion, C.; Scatingo, G.G.; Chard-Tuckey, P.R. The Initiation and Propagation of Chloride-induced Transgranular Stress-Corrosion Cracking (TGSCC) of 304L Austenitic Stainless Steel under Atmospheric Conditions. Corros. Sci. 2014, 88, 76. [Google Scholar] [CrossRef]
- Alyousif, O.M.; Nishimura, R. On the Stress Corrosion Cracking and Hydrogen Embrittlement Behavior of Austenitic Stainless Steels in Boiling Saturated Magnesium Chloride Solutions. Int. J. Corros. 2008, 50, 2353. [Google Scholar] [CrossRef]
- Zhu, L.; Yan, Y.; Li, J.; Qiao, L.; Li, Z.; Volinsky, A.A. Stress corrosion cracking at low loads: Surface slip and crystallographic analysis. Corros. Sci. 2015, 100, 619. [Google Scholar] [CrossRef] [Green Version]
- Alyousif, O.M.; Nishimura, R. The stress corrosion cracking behavior of austenitic stainless steels in boiling magnesium chloride solutions. Corros. Sci. 2007, 49, 3040. [Google Scholar] [CrossRef]
- Raja, V.S.; Shoji, T. Stress Corrosion Cracking: Theory and Practice, 1st ed.; Woodhead Pub: Oxford, PA, USA, 2011; pp. 20, 25, 2972. [Google Scholar]
- Turnbull, A.; Mingard, K.; Lord, J.D.; Roebuck, B.; Tice, D.R.; Mottershead, K.J.; Fairweather, N.D.; Bradbury, A.K. Sensitivity of Stress Corrosion Cracking of Stainless Steel to Surface Machining and Grinding Procedure. Corros. Sci. 2011, 53, 3398. [Google Scholar] [CrossRef]
- Weirich, T.D.; Srinivasan, J.; Taylor, J.M.; Melia, M.A.; Noell, P.J.; Bryan, C.R.; Frankel, G.S.; Locke, J.S.; Schindelholz, E.J. Humidity Effects on Pitting of Ground Stainless Steel Exposed to Sea Salt Particles. J. Electrochem. Soc. 2019, 166, C3477. [Google Scholar] [CrossRef]
- Xie, Y.; Zhan, J. Chloride-induced stress corrosion cracking of used nuclear fuel welded stainless steel canisters: A review. J. Nucl. Mater. 2015, 466, 85. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Nishikata, A.; Tsuru, T. Pitting Corrosion Mechanism of Type 304 Stainless Steel under a Droplet of Chloride Solutions. Corros. Sci. 2007, 49, 1394. [Google Scholar] [CrossRef]
- Chiba, A.; Muto, I.; Sugawara, Y.; Hara, N. Pit Initiation Mechanism at MnS Inclusions in Stainless Steel: Synergistic Effect of Elemental Sulfur and Chloride Ions. J. Electrochem. Soc. 2013, 160, C511. [Google Scholar] [CrossRef]
- Betts, A.J.; Boulton, L.H. Crevice corrosion: Review of mechanisms, modelling, and mitigation. Br. Corros. J. 1993, 28, 279. [Google Scholar] [CrossRef]
- Qiao, L.J.; Gao, K.W.; Volinsky, A.A.; Li, X.Y. Discontinuous Surface Cracks During Stress Corrosion Cracking of Stainless Steel Single Crystal. Corros. Sci. 2011, 53, 3509. [Google Scholar] [CrossRef]
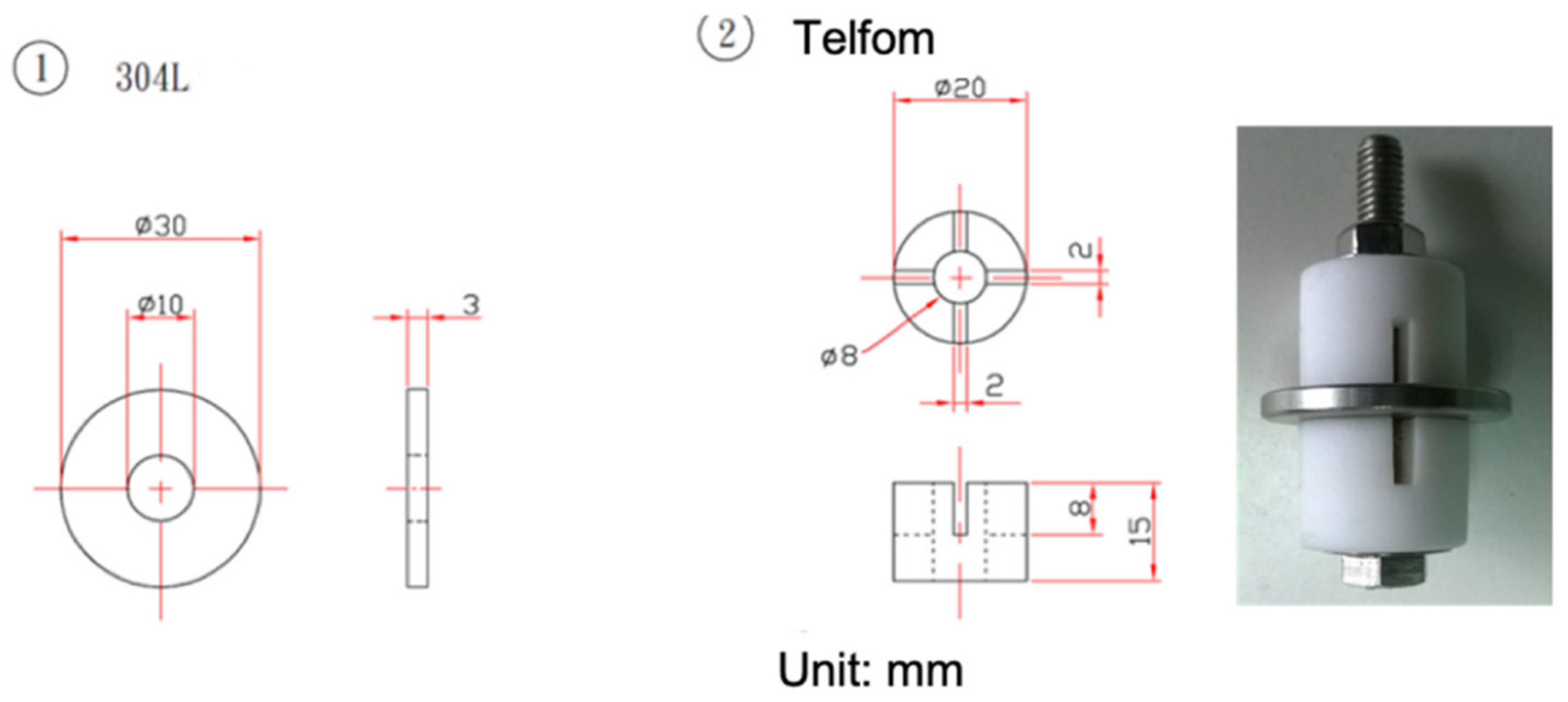
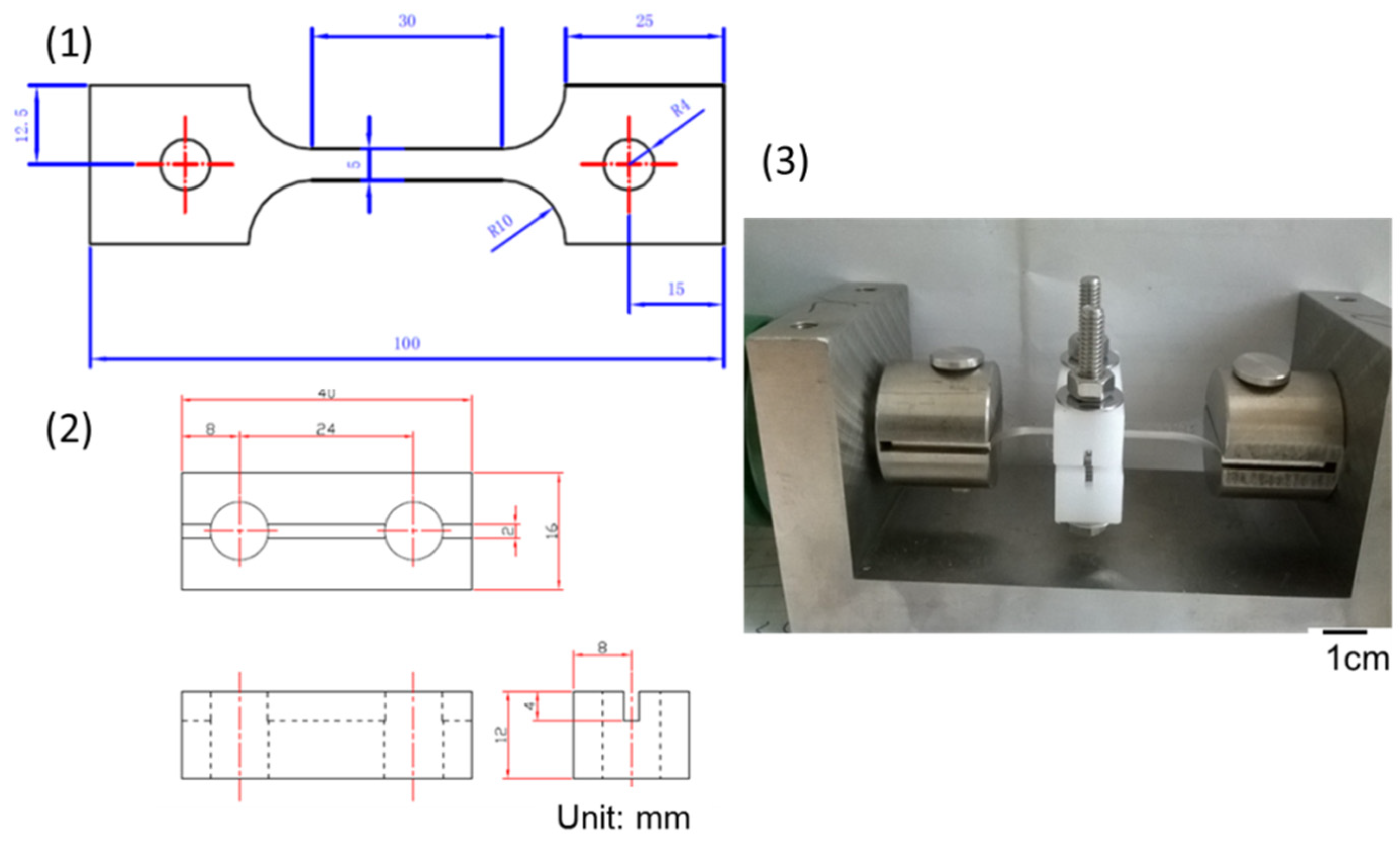

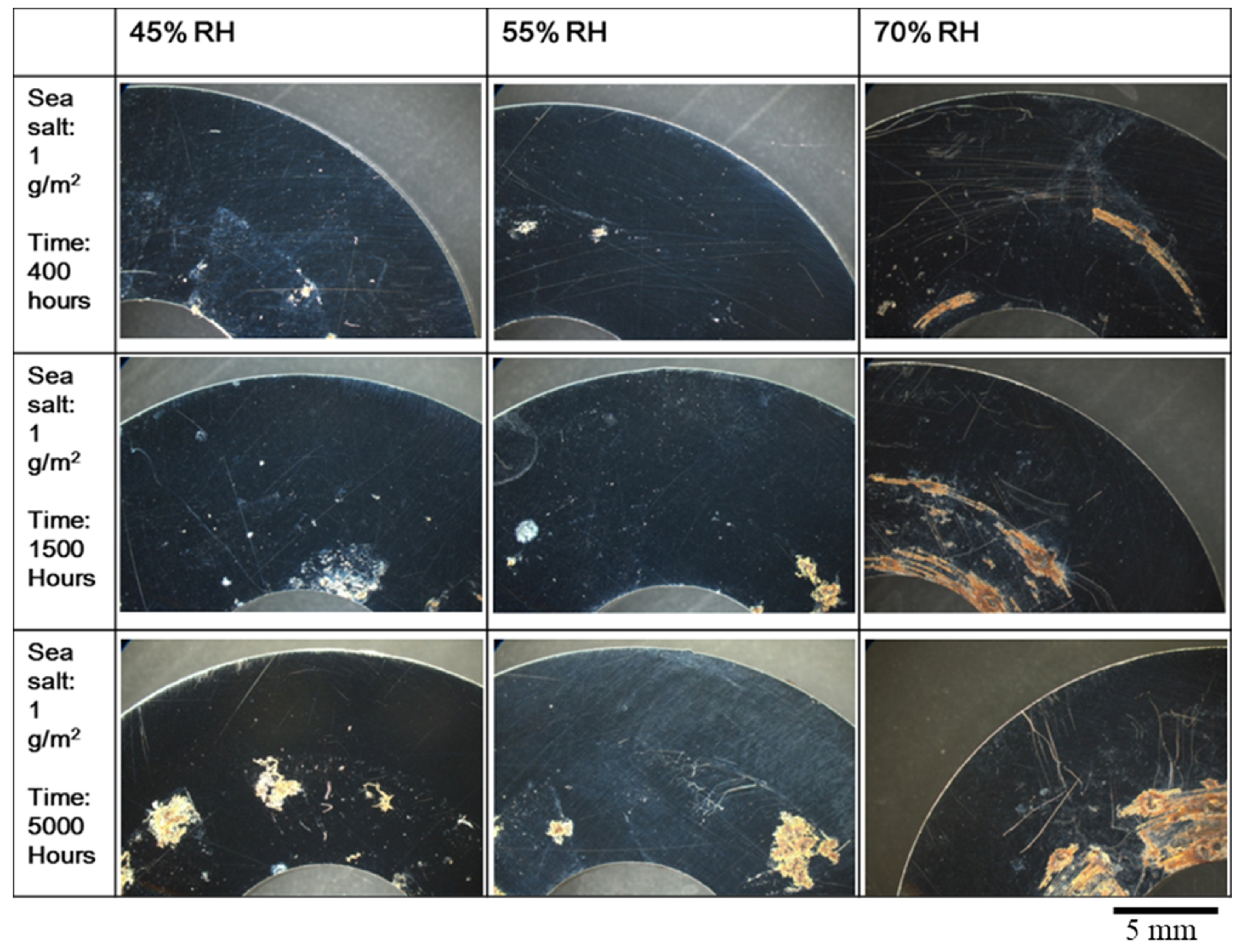
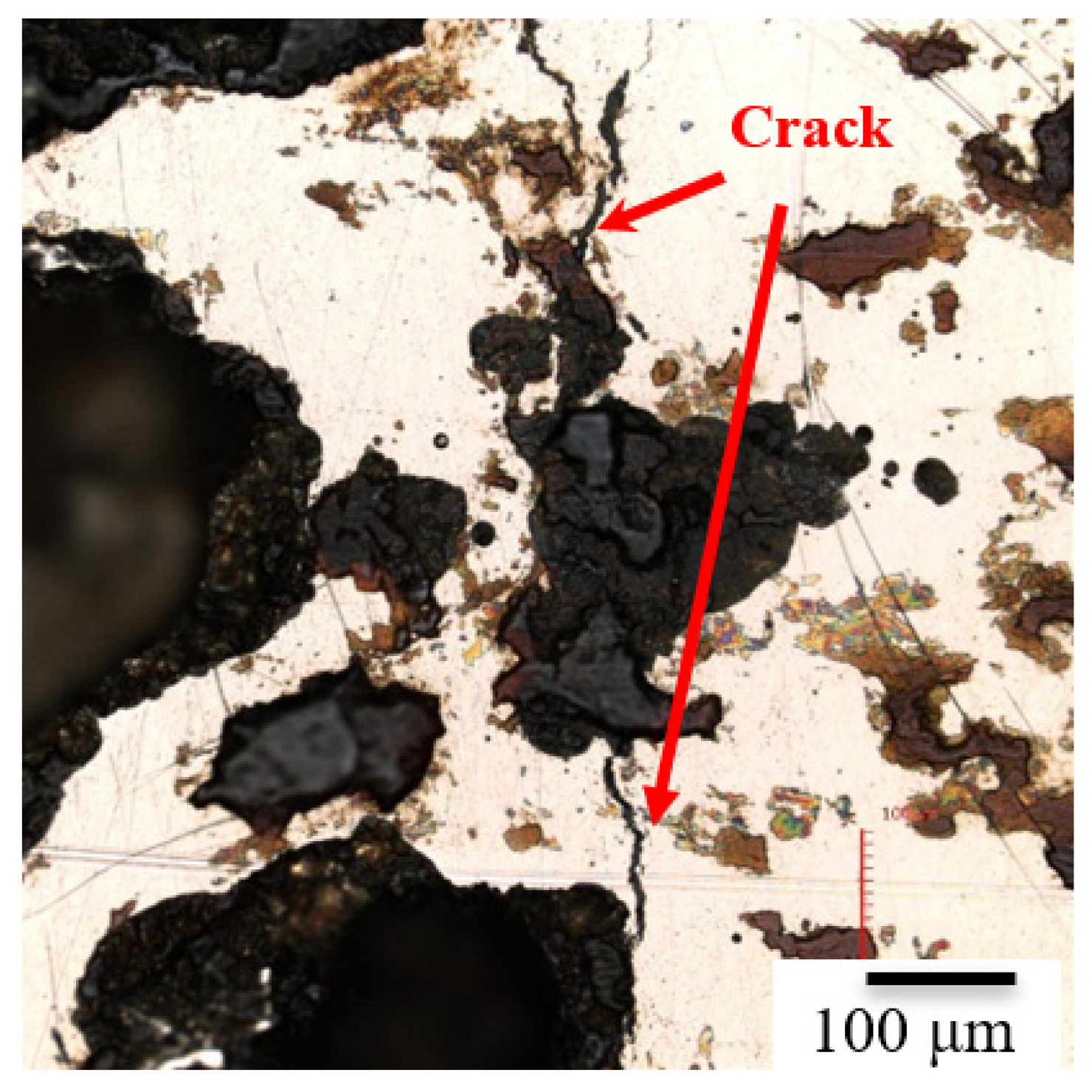



| Element | C | Si | Cr | Ni | Mn | S | Fe |
|---|---|---|---|---|---|---|---|
| wt% | 0.017 | 0.45 | 18 | 9 | 1.54 | 0.029 | Bal |
| Composition | NaCl2 | MgCl2·6H2O | Na2SO4 | CaCl2 | KCl | NaHCO3 | KBr | H3BO3 | SrCl2·6H2O | NaF |
|---|---|---|---|---|---|---|---|---|---|---|
| % | 58.49 | 26.46 | 9.75 | 2.765 | 1.645 | 0.477 | 0.238 | 0.071 | 0.095 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, K.-C.; Yeh, C.-P. The Corrosion Susceptibility of 304L Stainless Steel Exposed to Crevice Environments. Materials 2022, 15, 3055. https://doi.org/10.3390/ma15093055
Tsai K-C, Yeh C-P. The Corrosion Susceptibility of 304L Stainless Steel Exposed to Crevice Environments. Materials. 2022; 15(9):3055. https://doi.org/10.3390/ma15093055
Chicago/Turabian StyleTsai, Kun-Chao, and Chun-Ping Yeh. 2022. "The Corrosion Susceptibility of 304L Stainless Steel Exposed to Crevice Environments" Materials 15, no. 9: 3055. https://doi.org/10.3390/ma15093055
APA StyleTsai, K.-C., & Yeh, C.-P. (2022). The Corrosion Susceptibility of 304L Stainless Steel Exposed to Crevice Environments. Materials, 15(9), 3055. https://doi.org/10.3390/ma15093055





