1. Introduction
The use of rare-earth elements (REEs) has been essential in the technological transition toward a sustainable economy. In various fields, especially, the demands of rare-earth-elements-based permanent magnets, which involve the high consumption of REEs for production, have increased with the advancement of technology [
1,
2,
3,
4]. Dysprosium (Dy), which is included in permanent magnets with neodymium (Nd), plays an indispensable role in permanent magnets for preventing the loss of magnetic properties in high-temperature environments [
5,
6,
7]. However, heavy rare-earth elements (HREEs), such as Dy, are more limited resources than light rare-earth elements (LREEs), due to their relatively low composition in ore [
8]. These limitations and biases of resources can result in serious shortages in REE supplies. Therefore, the recycling of REEs is rising as an alternative for the achievement of a circular economy.
The recycling processes of REEs are generally divided into hydrometallurgical and pyrometallurgical recycling processes [
9]. Even though the high purification of REEs and their recovery efficiency can be obtained by hydrometallurgical recycling, chemical wastes can be generated and lead to serious environmental destruction [
10,
11,
12]. In contrast, the pyrometallurgical process involves the electrical or thermal recovery of REEs as an environmentally friendly alternative without emitting waste, despite a lower recovery efficiency than hydrometallurgy.
In the liquid metal extraction (LME) process, as one of the pyrometallurgical recycling methods, the selective extraction of REEs from
RFeB magnets (
R = Nd and Dy) can be induced by the thermodynamically high affinity between REEs and Mg, the extraction agent, compared to other elements, such as Fe and B [
13,
14,
15,
16,
17]. However, the difference in the extraction efficiency between Nd and Dy is notable. While the extraction of Nd can be completed within 6 h, only 68% of Dy is extracted at 12 h [
18,
19,
20]. The reason for this extractive bottleneck is that Dy–Fe intermetallic compounds and Dy–oxide are generated in the matrix and grain boundary during the reaction between Dy and Mg [
18]. In particular, decomposing Dy–Fe intermetallic compounds have been attributed to their dominant role in the initial recovery of Dy, due to the different stabilities of Dy–oxide and Dy–Fe intermetallic compounds [
18,
19,
20]. Even if the control of various process parameters was observed to partially improve the extraction efficiency of Dy, such as adjusting the reaction time, input Mg, and scrap sizes in previous studies [
18,
19,
20], this issue still remains unsettled.
The boro-additive effect (BAE) can serve as a fundamental breakthrough to suppress the generation of Dy–Fe intermetallic compounds by disturbing the reaction between Dy and Fe. B is known as a minor element that is strongly combined with Fe to form Fe
2B [
18,
19,
20]. It has been indicated that the addition of B disturbs the formation of Dy–Fe intermetallic compounds because of its higher affinity with Fe than any other element in a permanent magnet. However, the pure B metals cannot be decomposed by Mg during the LME process, due to its strong bonding between B atoms. Thus, FeB, iron boride, can be a good catalyst to supply B, instead of pure B metals. Due to its relatively lower stability than Fe
2B, FeB is decomposed and released with excess B to become Fe
2B [
21,
22]. In the LME process, the effect of adding FeB can play a crucial role in releasing excess B in Mg and enhancing the extraction efficiency by suppressing the byproducts.
In the present study, the boro-additive effect (BAE) is thermodynamically described and experimentally verified in ternary and quinary systems. Due to the phase stability, excess B can be produced from the relatively unstable FeB by Mg, and the infiltrated Mg with excess B can be extracted with REEs and the suppression of the Dy–Fe intermetallic phase, which is the extractive hindrance on the LME process. However, it has been found that the growth of the Fe
2B layer limits the Mg reaction zone and results in the formation of
RB
4 as a side effect [
23]. By reducing the ratio of FeB, the extraction efficiency of Dy over 12 h with the addition of FeB was improved to 80%, which is almost the same extraction efficiency as the conventional LME process over 24 h.
2. Experimental Details
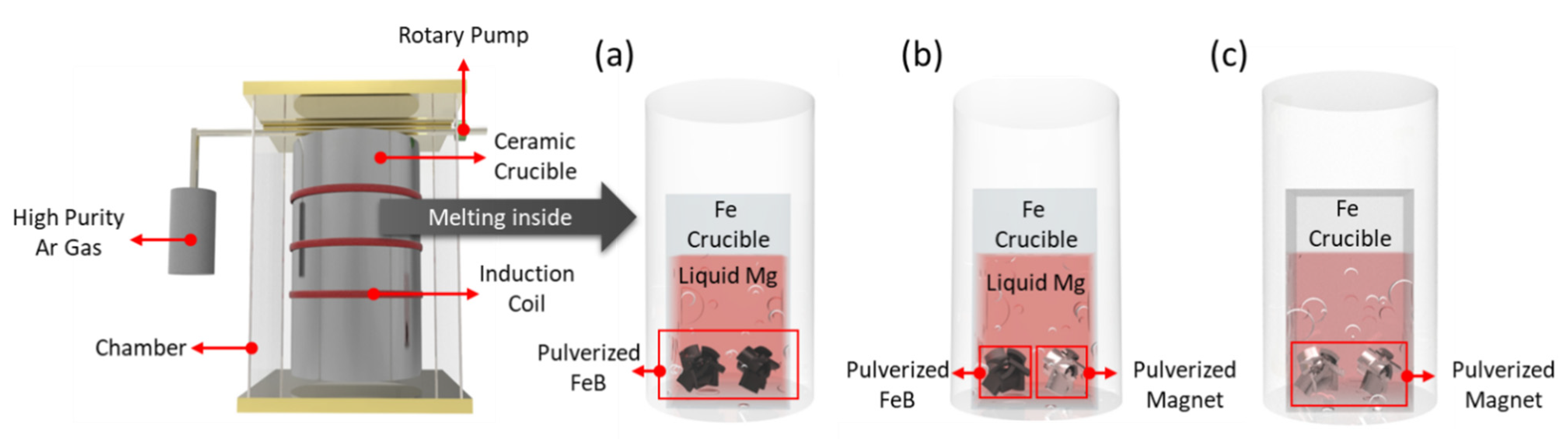
To verify the release of excess B and the BAE by comparing conventional processes, the thermodynamic calculations of ternary and quinary phase diagrams and the sample preparation were carried out at 900 °C. The thermodynamic calculations were conducted by using Factsage 7.1 with FTlite databases and MAGN with private databases. The ternary, quinary, and conventional specimens were prepared at 900 °C for 12 h in a high-frequency induction furnace, as shown in
Figure 1a–c, respectively. To control the Dy–Fe intermetallic phase, the optimal temperature and dwelling time at which the Dy–Fe intermetallic compounds started decompose were presented [
18,
19,
20].
Pulverized FeB and magnet scraps were located at the bottom of the crucible, and pure Mg was placed on the FeB and magnet uni-axially to confirm the reaction behavior. The permanent magnets (Jahwa Electronics Co., Ltd., Seoul, Korea) included 25.8 wt.% Nd and 3.5 wt.% Dy as raw materials. Homogeneous FeB (RND Korea), composed of 88.58 wt.% Fe and 16.22 wt.% B, was prepared. Based on a thermodynamic calculation, the ratios of FeB, Mg, and magnet were estimated to be 0.28~0.83, 10, and 1, respectively. The samples were cut in a vertical direction using a wire saw and polished with 400~2000 grid silicon carbide papers and 0.3 micron alumina suspension for sample preparation, to observe the microstructural and phase analysis. For the microstructural and phase analysis, scanning electron microscopy (FE-SEM, Model JSM-100F) with energy dispersive X-ray spectroscopy (EDS) and X-ray diffraction (XRD, Model D/Max 2500PC) were used in point mode, using a 1 mm slit with a Cu target with a wavelength of 1.5406 Å. The complex phase was analyzed by using FULLPROF software with the Le Bail method. The extraction efficiency was estimated by measuring the composition of the REEs in the Mg zone using X-ray fluorescence spectrometry (XRF, Model ARL PERFORM’X).
3. Results and Discussion
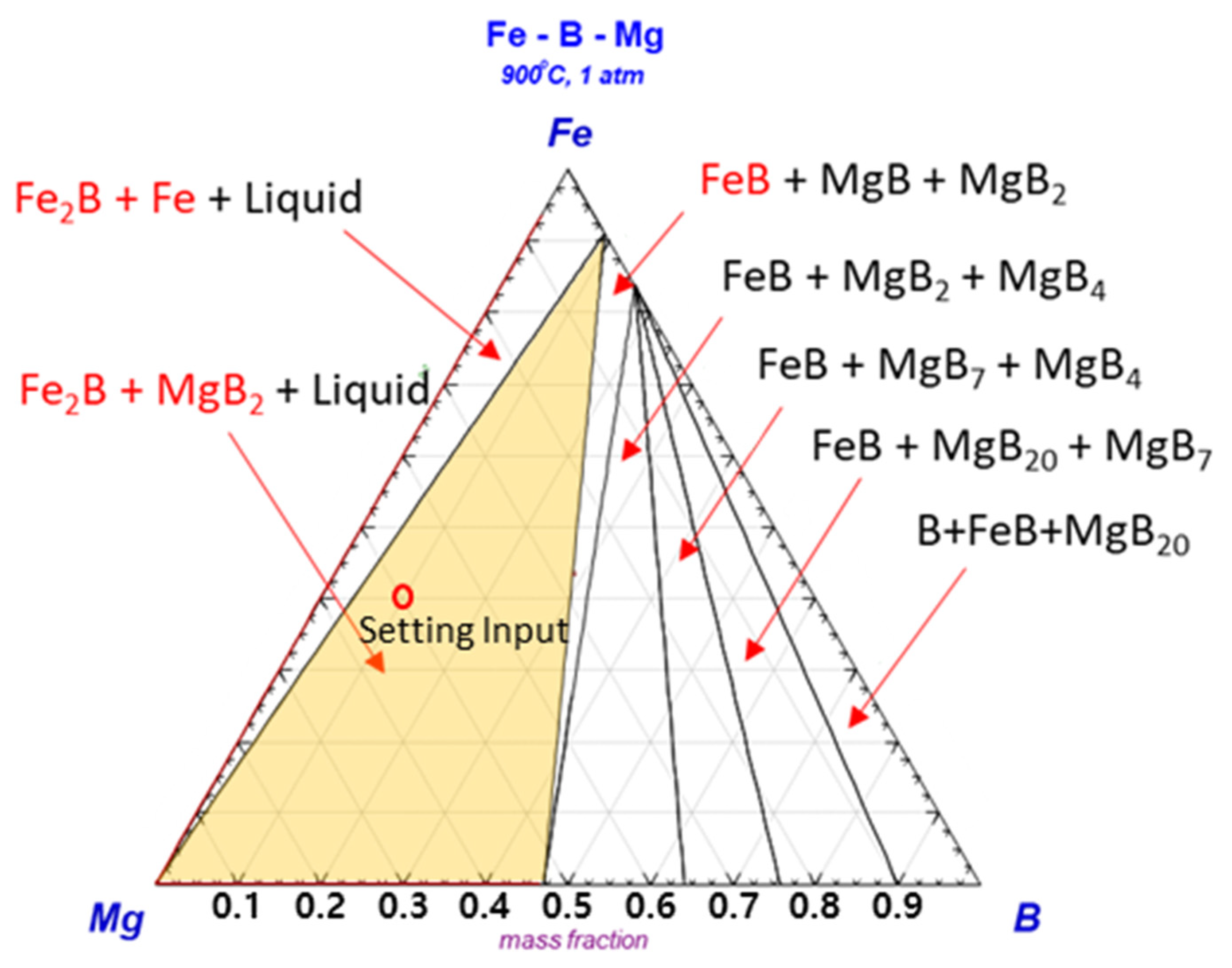
To confirm the generation of excess B in Mg, the phase stability among Mg, Fe, and B was estimated by thermodynamic calculations.
Figure 2 shows the ternary phase diagram for the assessment of the reaction behavior with phase stability at
T = 900 °C and
P = 1 atm. This shows that the Fe
2B phase and Mg boride compounds are the most stable phases, with an adjusted ratio between Mg and FeB among the intermetallic compounds. This indicates that the formation of the Fe
2B and Mg–B phases is possible by excess B through the mediator Mg, because the FeB phase can be easily decomposed in Mg due to its relatively weak stability.
Based on the possibility of generating excess B from the Mg–FeB reaction on the equilibrium status in a thermodynamic calculation, the experimental model for the ternary phase was investigated in the condition of ratio of 0.83:10 = W
FeB:W
Mg,
T = 900 °C, and
t = 12 h.
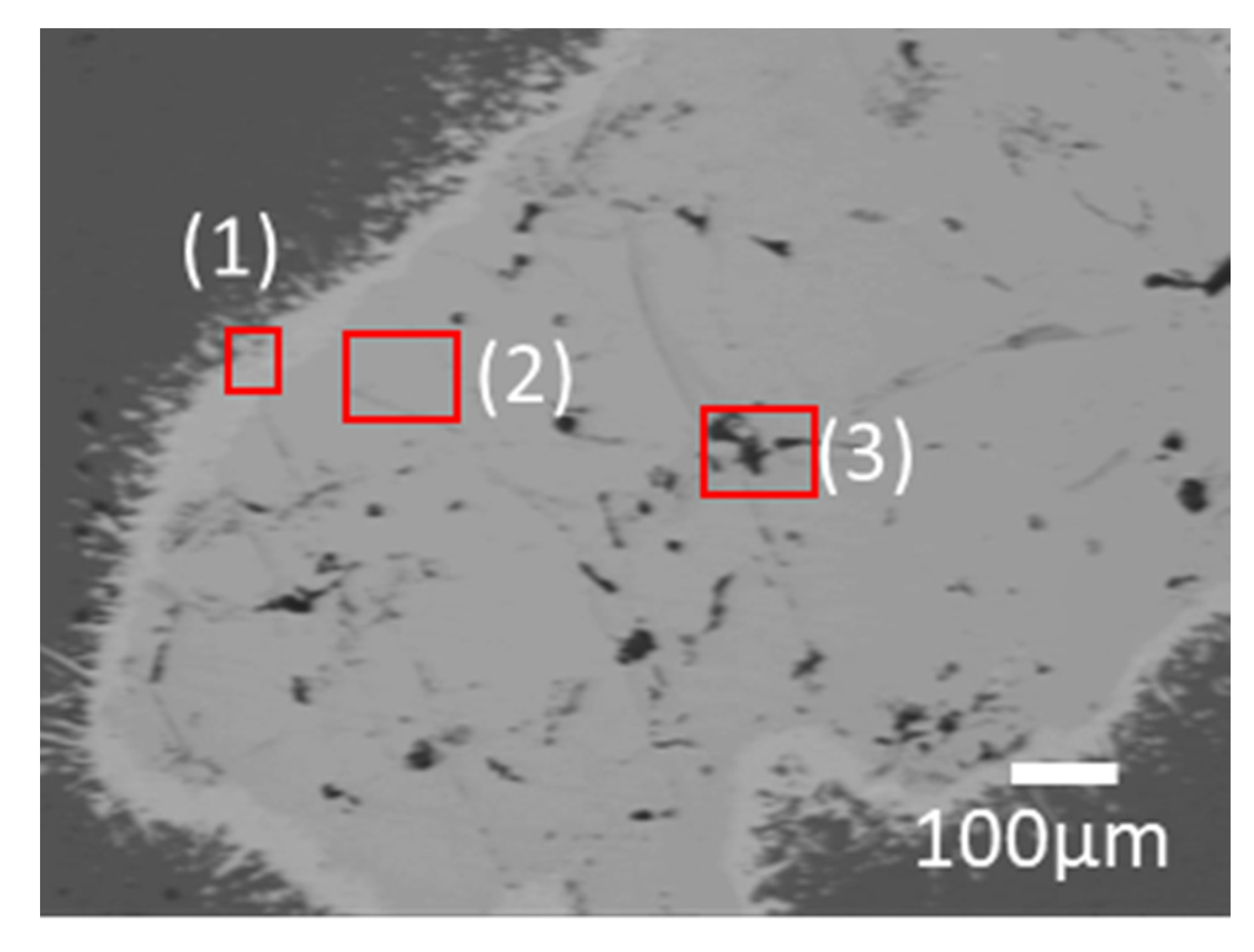
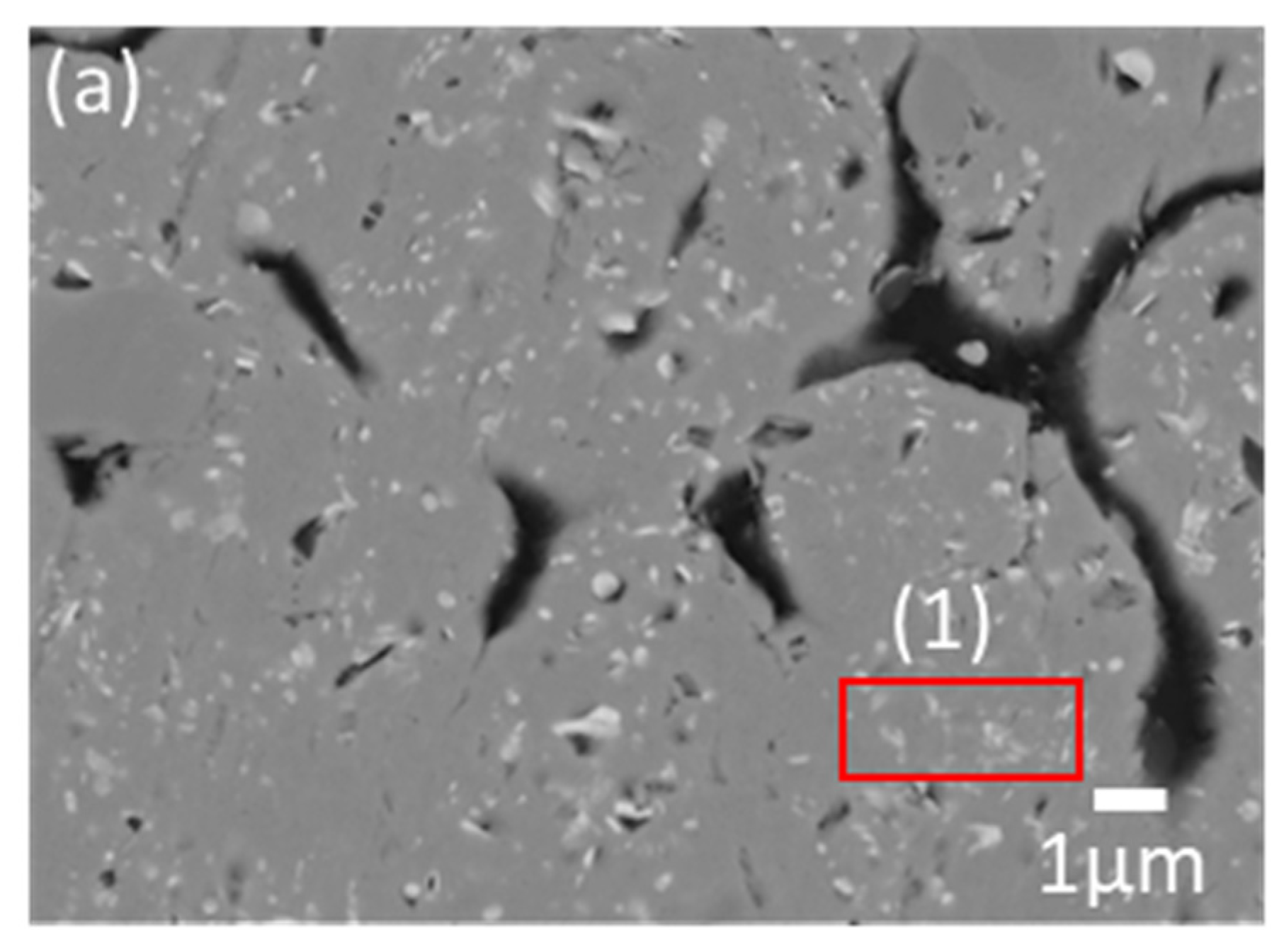
Figure 3 shows the microstructure of the results of the reaction between Mg and pulverized FeB to observe the phase transformation. It is divided into three zones, which are colored as light gray (1), dark gray (2), and black (3) in the reacted FeB alloy. The light gray zone (1) is the region in which it is possible to mainly distribute Fe
2B as the interfacial zone between Mg and FeB, the dark gray zone (2) is the remaining region that cannot react as much with Mg as the original bulk FeB, and the black zone (3) is the infiltrated trace of Mg through the cracks caused by the initial FeB sample preparation. While the composition in zone (2) is consistent with the initial FeB composition in
Supplementary Table S1, the Fe contents in zone (1) are definitely increased to 90.66 wt.% from 82.66 wt.% in
Table 1. This result is in good agreement with the converted value of the atomic weight of Fe
2B with Fe:B = 65.58:34.42. The phase analysis in zone (1) and zone (2) is exhibited in
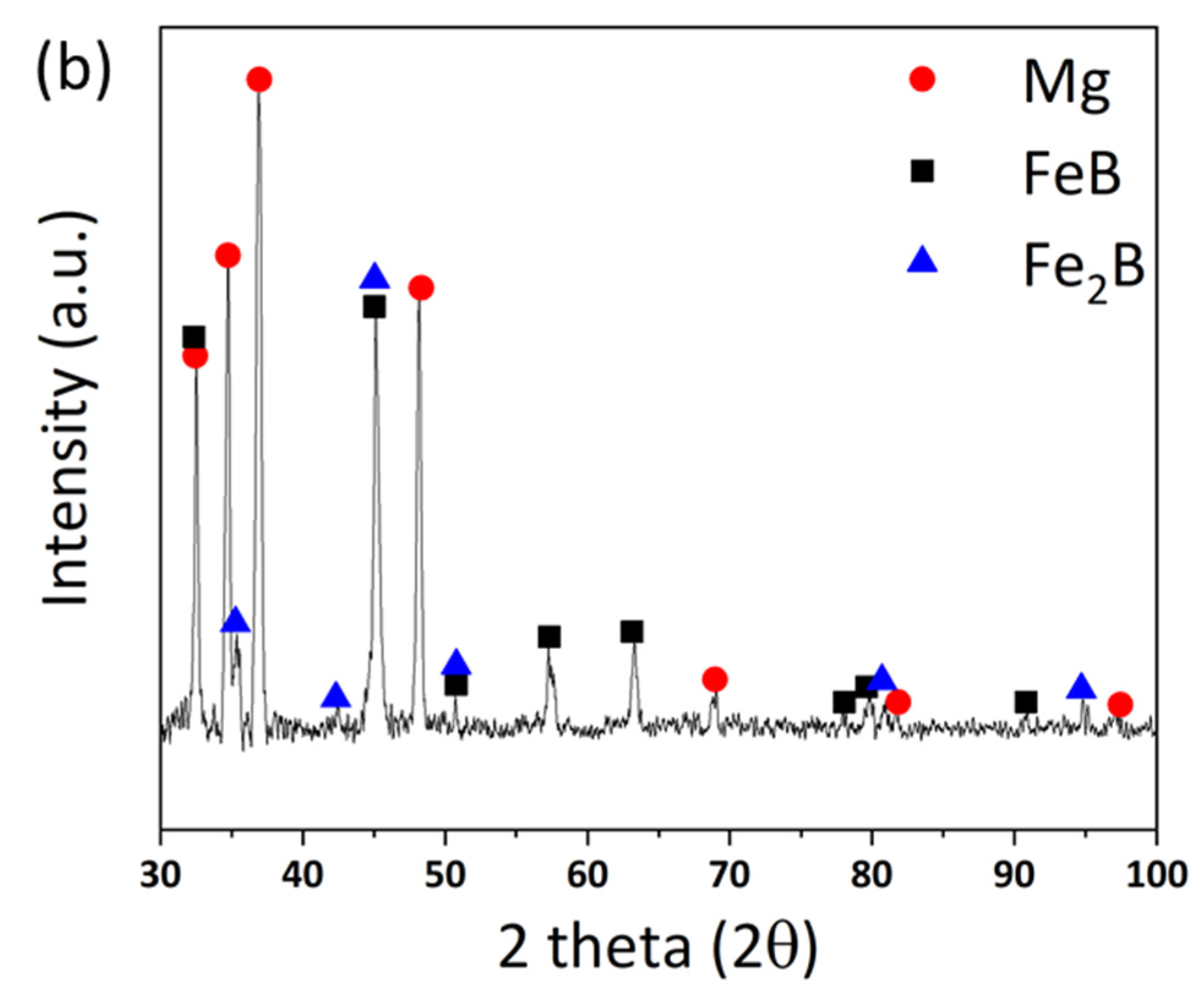
Figure 4a,b, respectively. Even though the XRD measurement cannot be perfectly separated between zones (1) and (2) due to the limit of the slit size, it is inferred that the Fe
2B phase is dominant in zone (1) rather than (2), by the difference in the intensity of Fe
2B. The peaks of Fe
2B, FeB, and Mg are specified as blue triangles, black squares, and red circles in
Figure 4. The original peaks of Fe
2B and FeB mainly appear in
Figure 4a,b, respectively, even if some peaks overlap with each other.
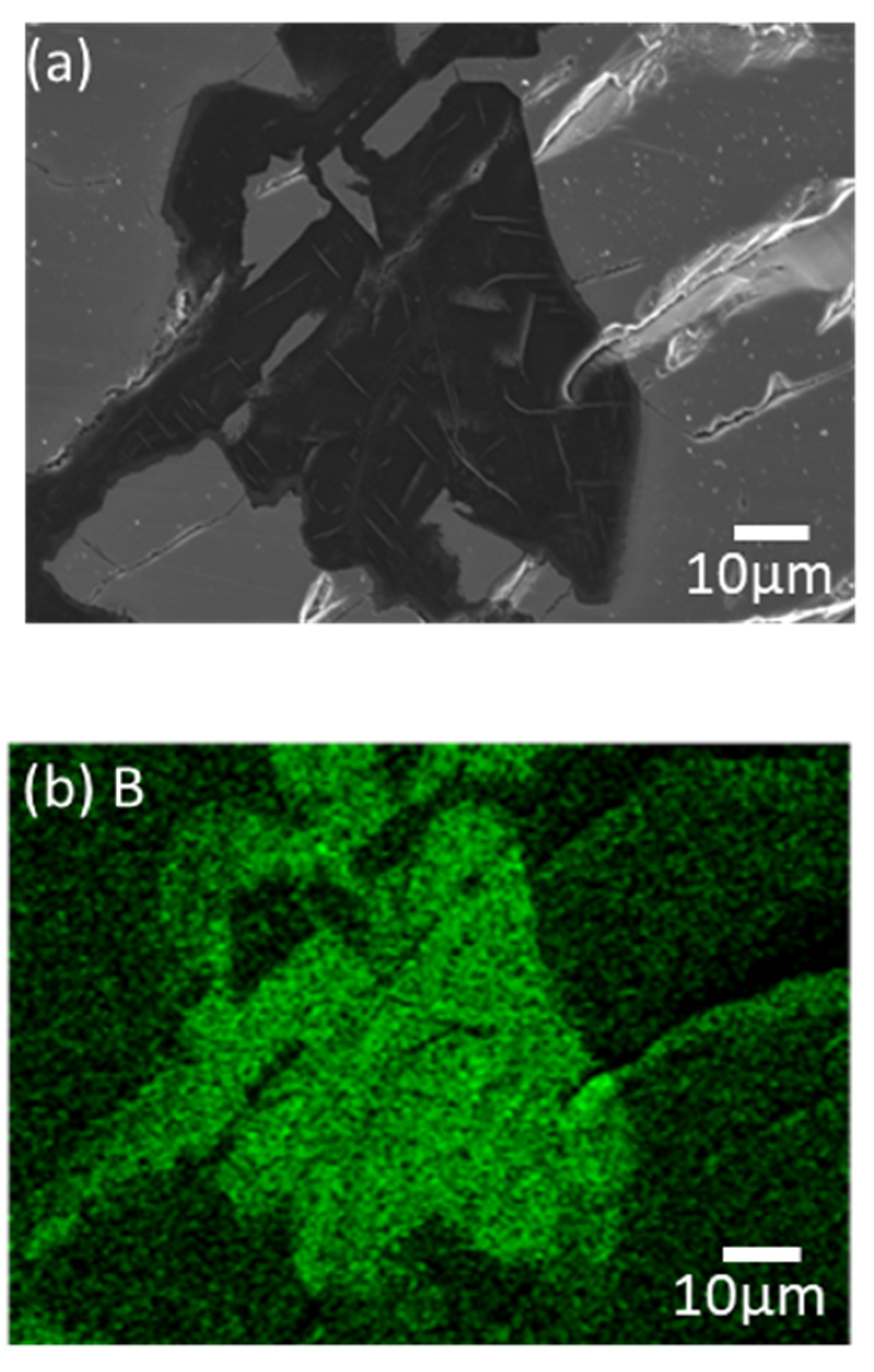
Figure 5 shows the magnified microstructure of zone (3) with colored EDS mapping to distinguish components, which are B, Mg, and Fe, in green, red, and sky blue, respectively. The cracks caused by the pulverized FeB definitely offer the diffused route for Mg. However, the B contents and Mg contents are dominant in zone (3), as shown in
Table 1. Thermodynamically, the excess B from the FeB can be diffused to the liquid Mg as an intermediate state, because MgB
2 exhibits a stable phase in the equilibrium state. It is indicated that the liquid state of Mg leads not only to the depletion of B in the relatively unstable FeB phase, but also to the conveying role.
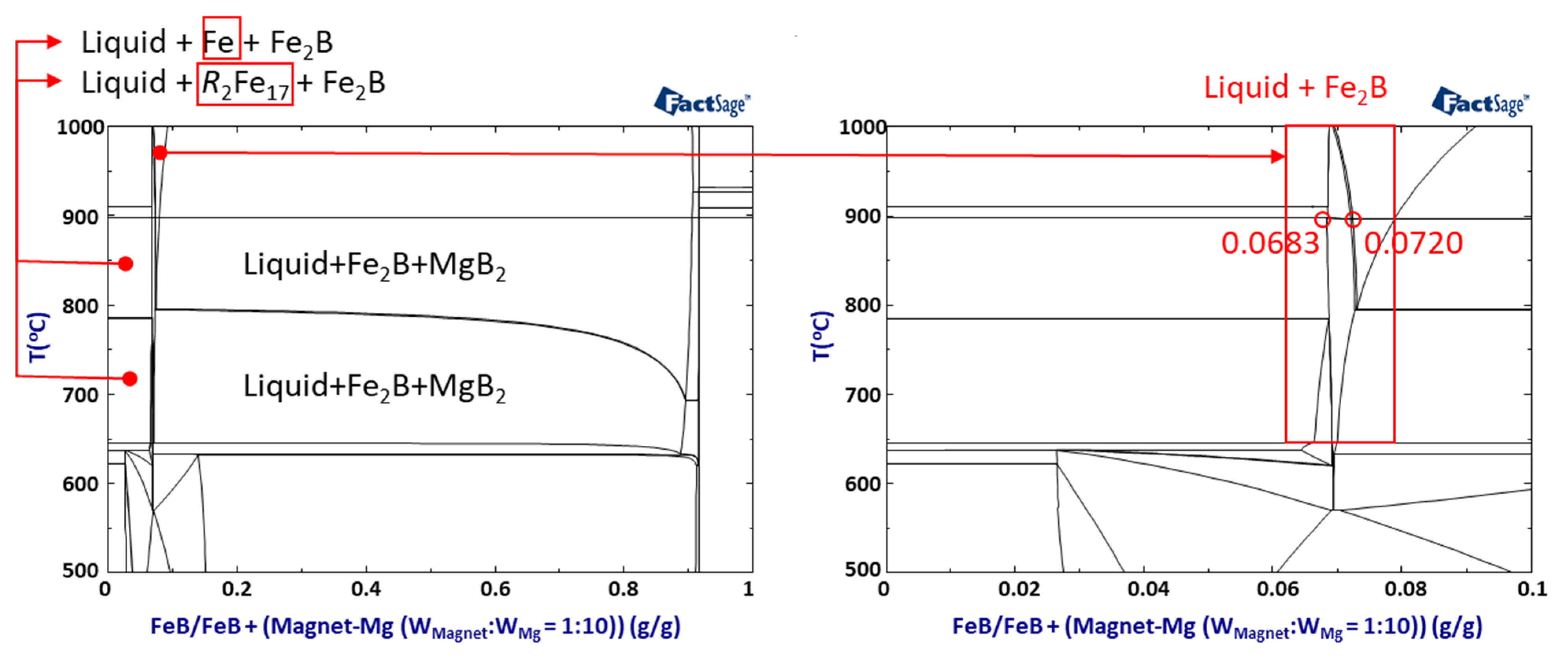
To verify the B additive effect based on the release of excess B, thermodynamic calculations on the quinary system are conducted to simulate the reaction by FeB addition under conventional conditions of 1:10 (W
Magnet:W
Mg), as shown in
Figure 6. The quinary phase diagram shows that the phase of
R–Fe intermetallic compounds can be suppressed by BAE, only in the range of 0.0683 < FeB < 0.0720. The adequate range of FeB addition can be supported to release excess B to Mg, and the reaction between REEs and Fe is disturbed due to the high reactivity between Fe and B in the LME process.
The LME experiment of the quinary system is designed based on the conventional conditions of
T = 900 °C,
t = 12 h, and 1:0.83:10 (W
Magnet:W
FeB:W
Mg), which is approximately 0.07 of the FeB composition in the phase diagram. Generally, in the conventional LME process, the magnet scrap keeps its shape as an input raw material with Mg ligaments after the reaction, because REEs selectively diffuse into liquid Mg, and Fe remains in
Figure 7a [
13,
14,
15,
16,
17,
18,
19,
20]. However, the microstructure of the magnet scraps with the addition of FeB shows two zones, which are composed of the Fe–B zone and the reacted zone in the magnet scraps in
Figure 7b. To analyze those phases,
Figure 8 and
Table 2 show the magnified microstructure and the investigated composition in each zone.
Figure 8b shows that the scattered particles in the reacted zone are simply characterized as the Fe
2B phase. Mg, including excess B, can infiltrate the magnet, and Fe
2B can be generated because the remaining Fe in the magnet is combined with B before forming Dy–Fe bonds. Thus, this suggests that the Dy–Fe intermetallic phase, which is the reason for the extractive bottleneck, can be controlled by the supported BAE on the conventional LME process. On the other hand, there is a decisive shortcoming, which is the phase of the Fe–B zone in
Figure 8c. The characterized composition in the Fe–B zone is quite different from that in the reacted zone in
Table 2. The complex phases are analyzed by the refinement of the XRD pattern in
Figure 9. While the reacted zone is composed of only Fe
2B and Mg, as shown in
Figure 9a, the phase analysis of the Fe–B zone shows Fe
2B phases, and REEs–B intermetallic compounds are detected as
RB
4 phases, as shown in
Figure 9b. Interestingly, even though REEs–B intermetallic compounds cannot be fundamentally formed at 900 °C due to the high affinity between Fe and B, as shown in
Figure 6, Fe
2B undergoes a growth of the boride layer at 900 °C because of the abundant FeB environment during the phase transformation from FeB to Fe
2B [
21,
22]. The REE extraction efficiency is inevitably suppressed because the growth of the layered Fe
2B results in the formation of
RB
4, which is confirmed as the most stable phase in the
R–B system [
23] and blocks Mg infiltration. Thus, FeB not only plays a crucial role in removing extractive hindrance, but also blocks the diffusion route of Mg by simultaneously producing other byproducts.
Even though there is compensation for REEs–Fe intermetallic compounds, the amount of FeB is reduced to avoid the growth of the Fe
2B layer in
Supplementary Figure S2. To observe the distribution of the intermetallic phases inside the magnet, the microstructures of the specimen of the conventional LME process and the specimen of the optimized FeB ratio are compared in
Figure 10a,b, respectively. The Dy–Fe intermetallic components in the matrix of magnets are predominant in the microstructure of the conventional process in
Table 3 [
18,
19,
20]. On the other hand, in the case of the FeB addition process, the magnet turned to Fe
2B with partially removed Dy–Fe intermetallic compounds in the reacted zone, due to the reduction of the FeB ratio. Thus, it is found that the Dy contents significantly decreased by 21.42 wt.% using the supported BAE in
Table 3.
To observe the catalytic effect of the BAE on the extraction efficiency of REEs by removing Dy–Fe intermetallic compounds, the extraction efficiency was calculated with Equations (1)–(4). The extraction efficiency of REEs, which includes all the REEs in the magnet, was calculated by Equations (1) and (2), and the extraction efficiency of Dy was calculated individually by Equations (3) and (4). By calculating the ratio between the total weight of the REEs (W
REEs) and the total weight of the Mg–REEs alloy (W
Mg + W
REEs), the composition of Mg when the REEs were totally extracted (C
100%) can be calculated using Equation (1). The extraction efficiency of the REEs can be estimated as the ratio between the composition of REEs in Mg (C
REEs) and C
100%, as shown in Equation (2). C
REEs is the XRF result of the Mg zone after the reaction experiment. The calculation of the extraction efficiency of Dy is equally estimated as follows:
C
100% of REEs, C
100% of Dy, C
REEs in Mg, C
Dy in Mg, and the extraction efficiency are indicated in
Table 4. The extraction efficiency of Dy was approximately 17% higher in the FeB addition process than in the conventional process. In addition, the extraction efficiency of Dy in the FeB addition process over 12 h was similar to that of the conventional LME process over 24 h. It is suggested that the LME process with an FeB addition can be a decisive parameter in improving the energy consumption for processing and enhancing the extraction efficiency of REEs.