Hybrid Lime–Pozzolan Geopolymer Systems: Microstructural, Mechanical and Durability Studies
Abstract
:1. Introduction
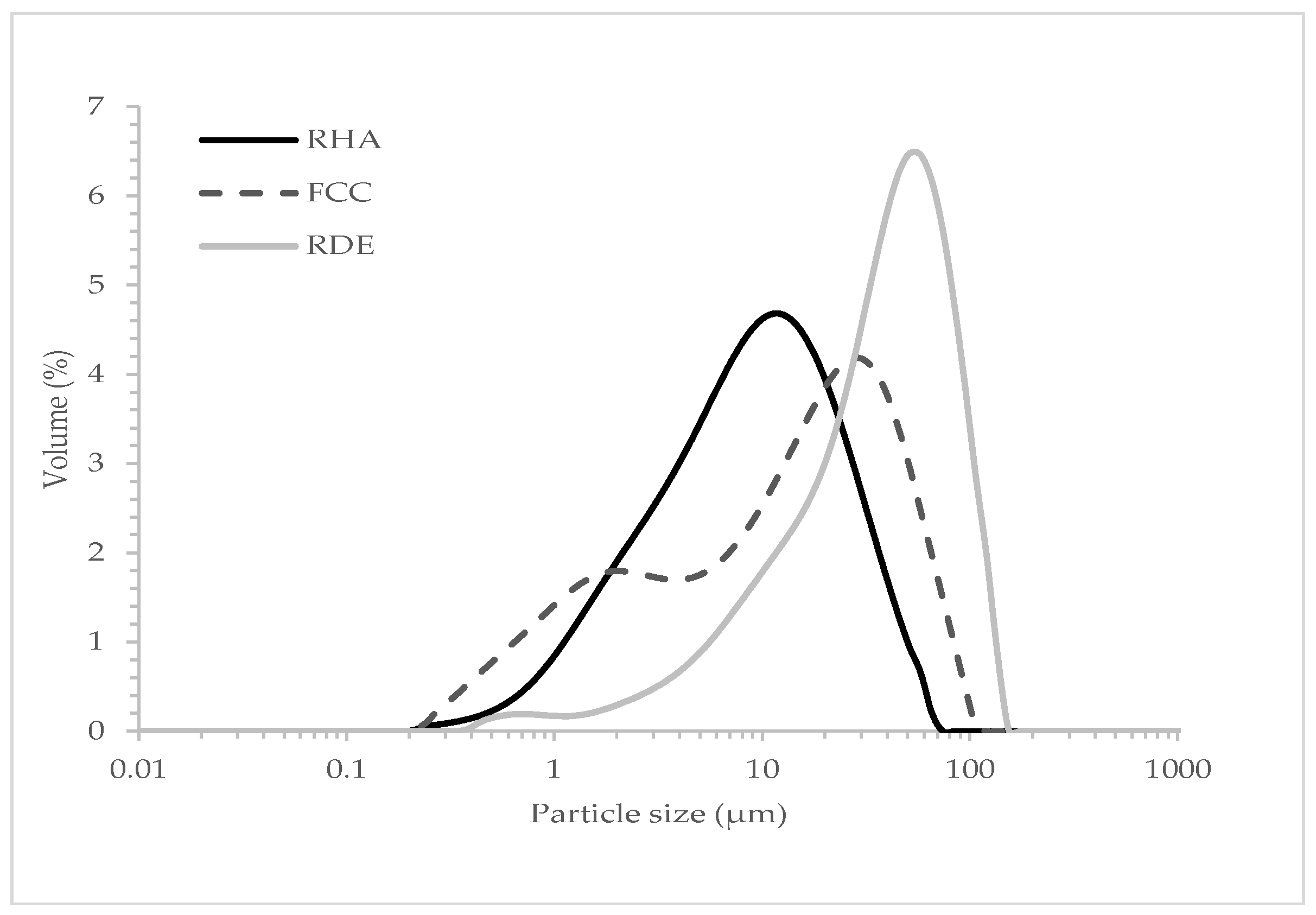
2. Materials and Methods
Experimental Procedure
3. Results
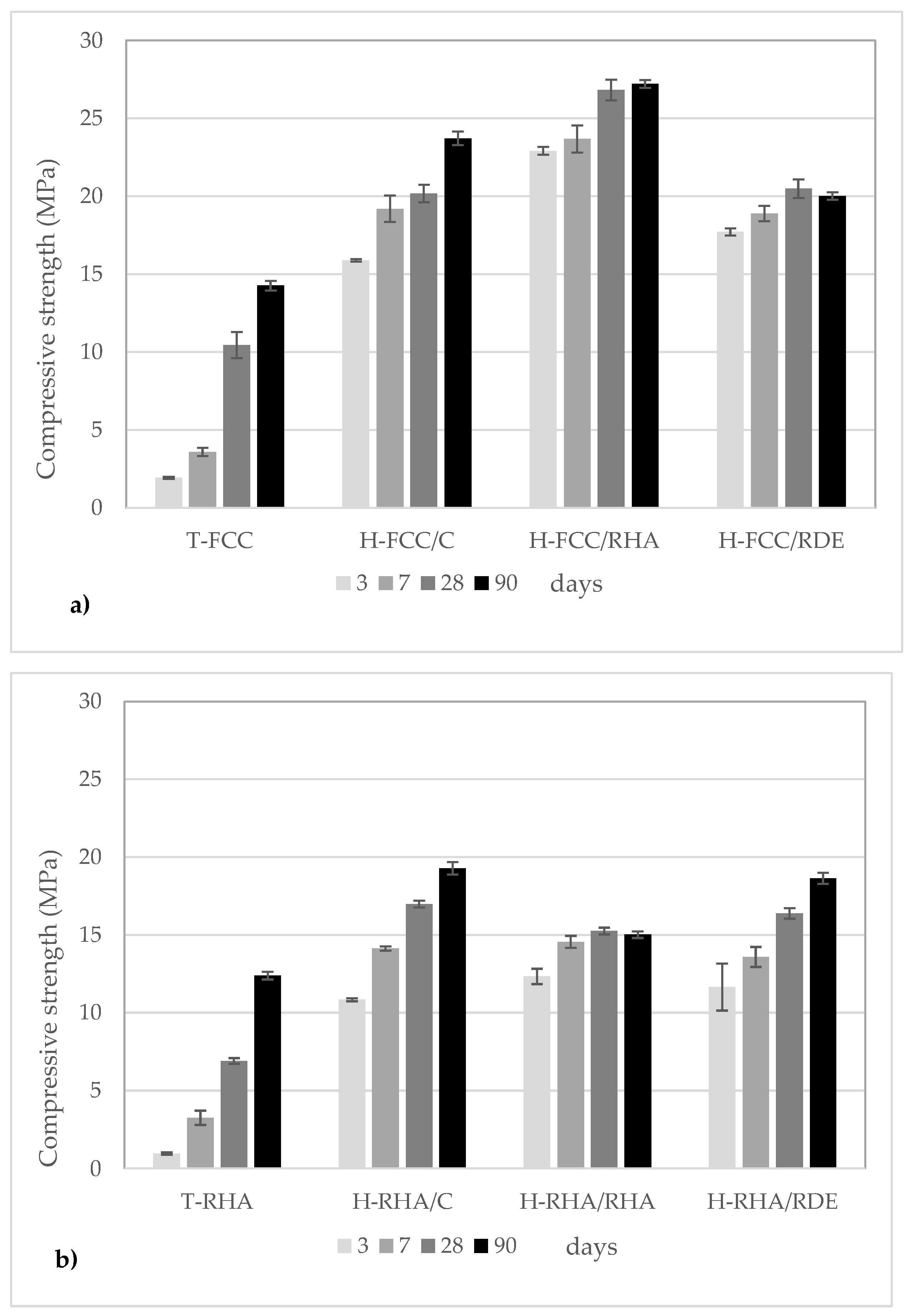
3.1. Compressive Strength
3.2. Durability Studies in Mortars
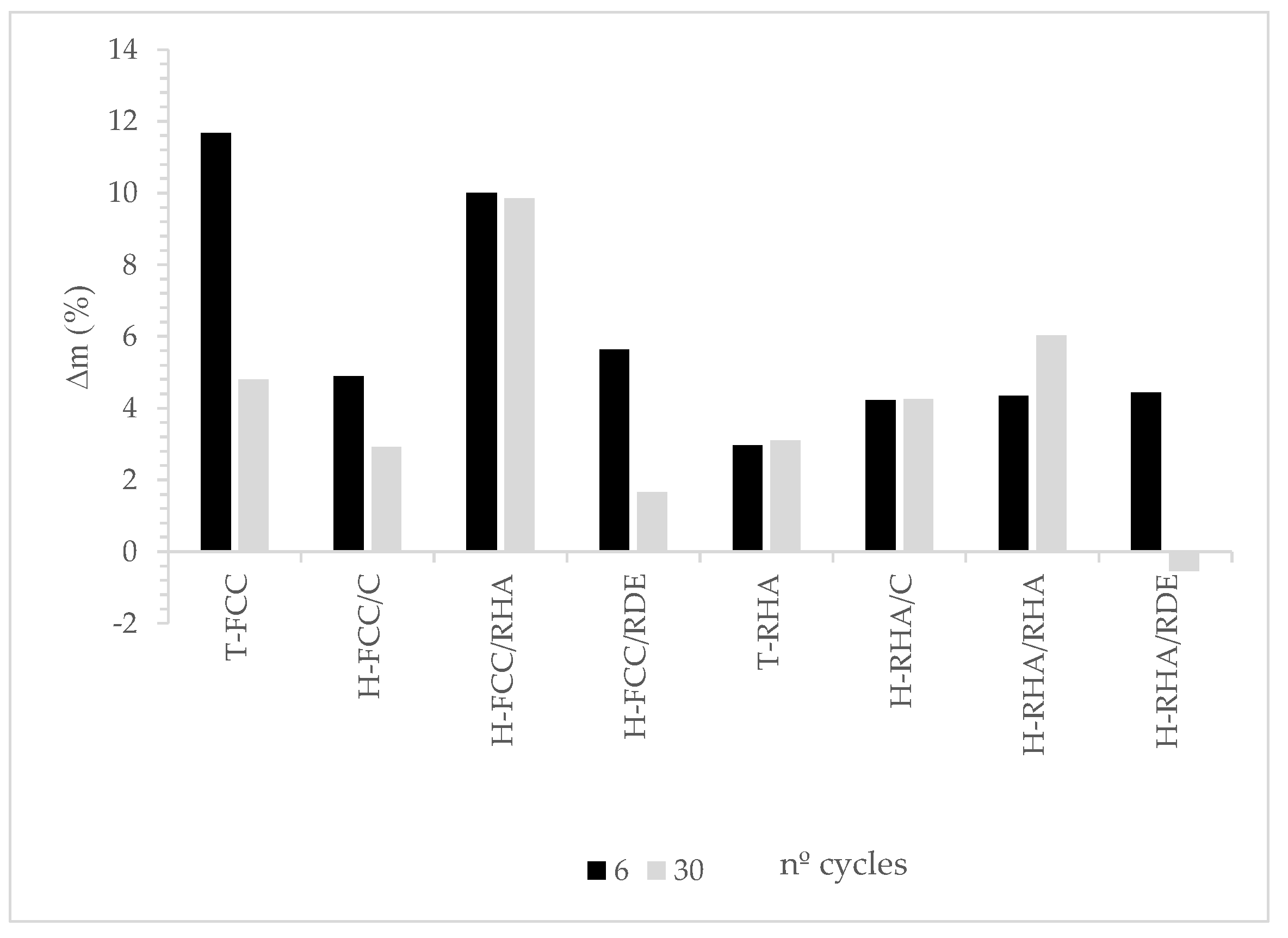
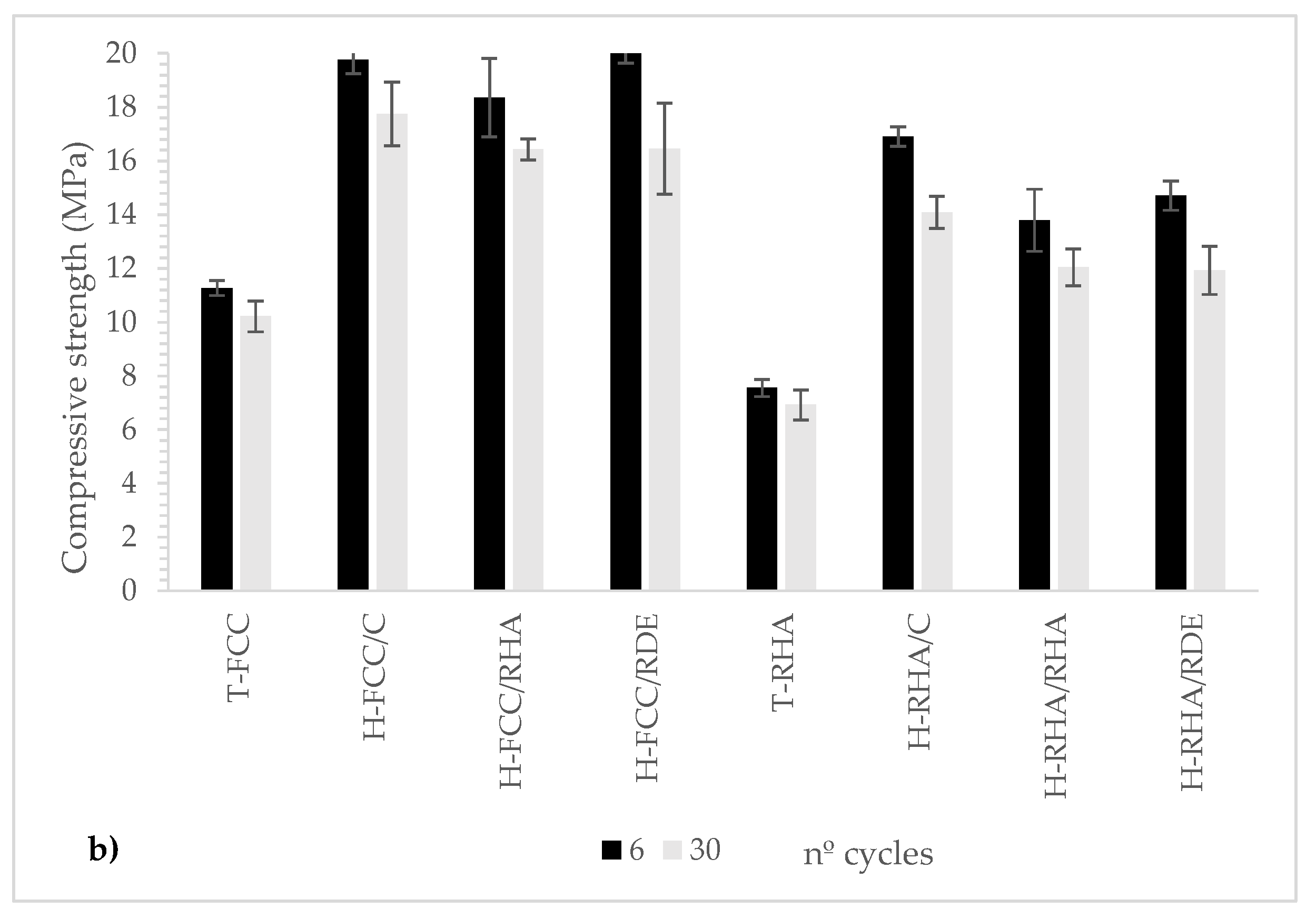
3.2.1. Freeze-Thaw Cycles
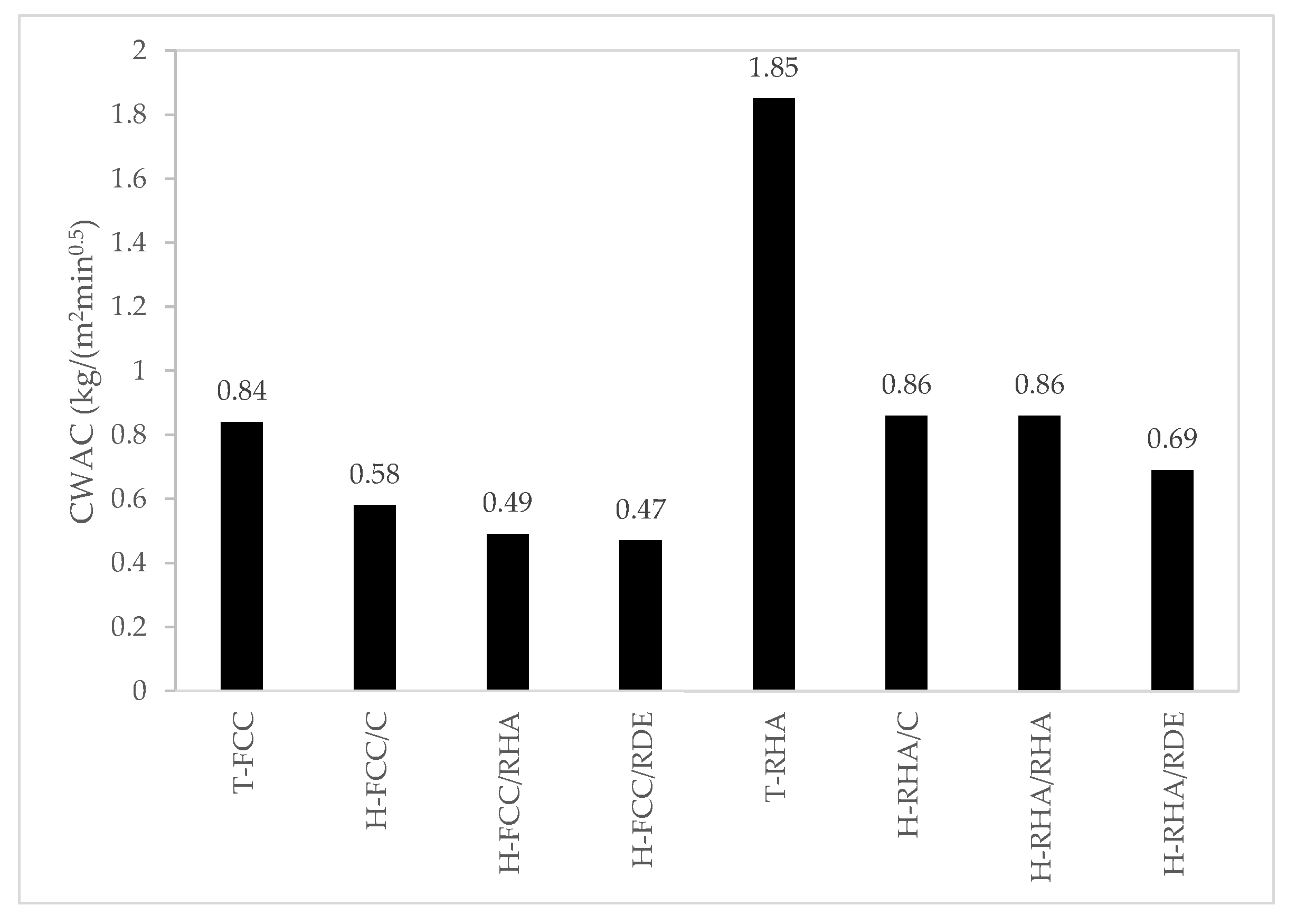
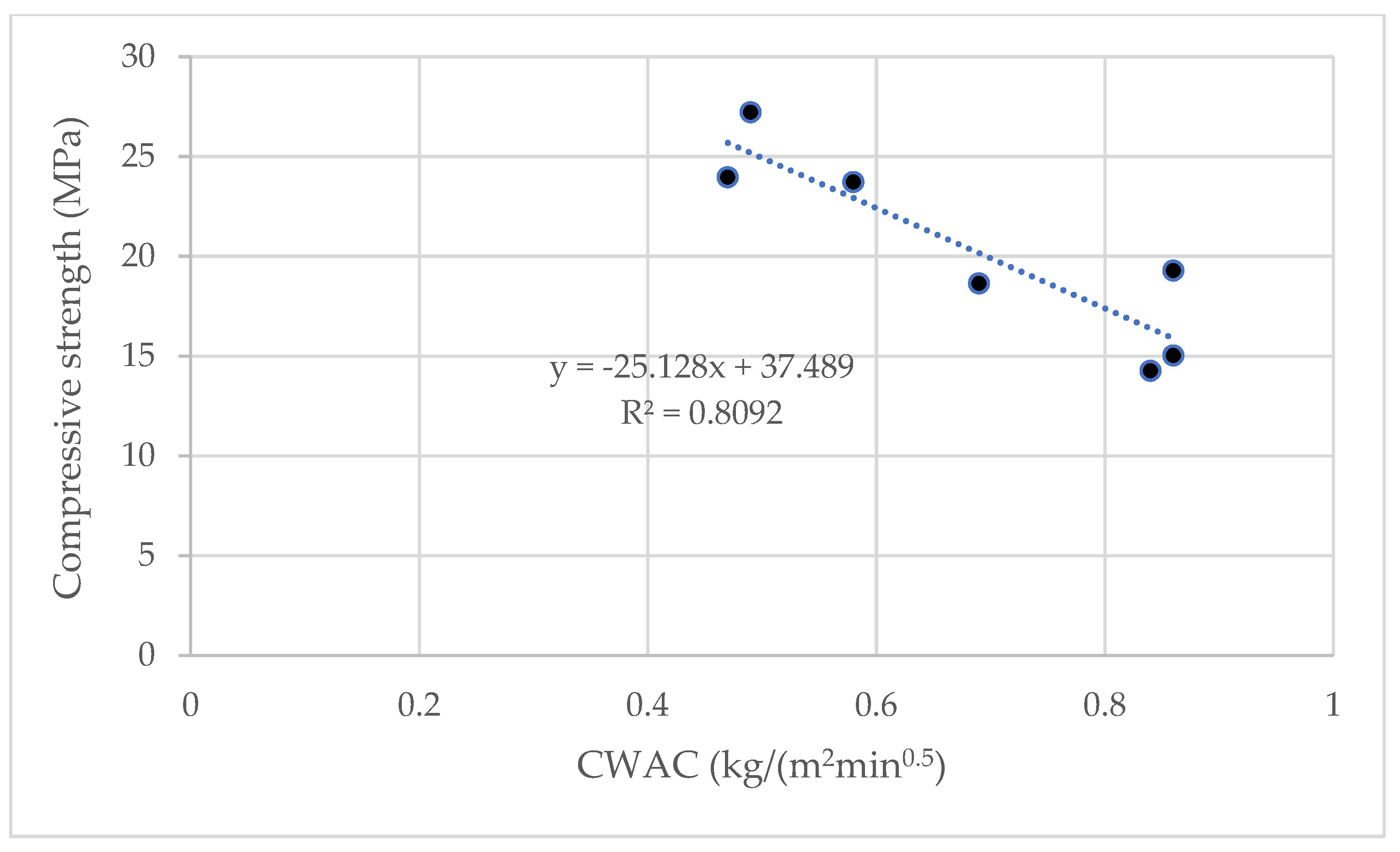
3.2.2. Capillary Water Absorption
3.3. Studies in Pastes
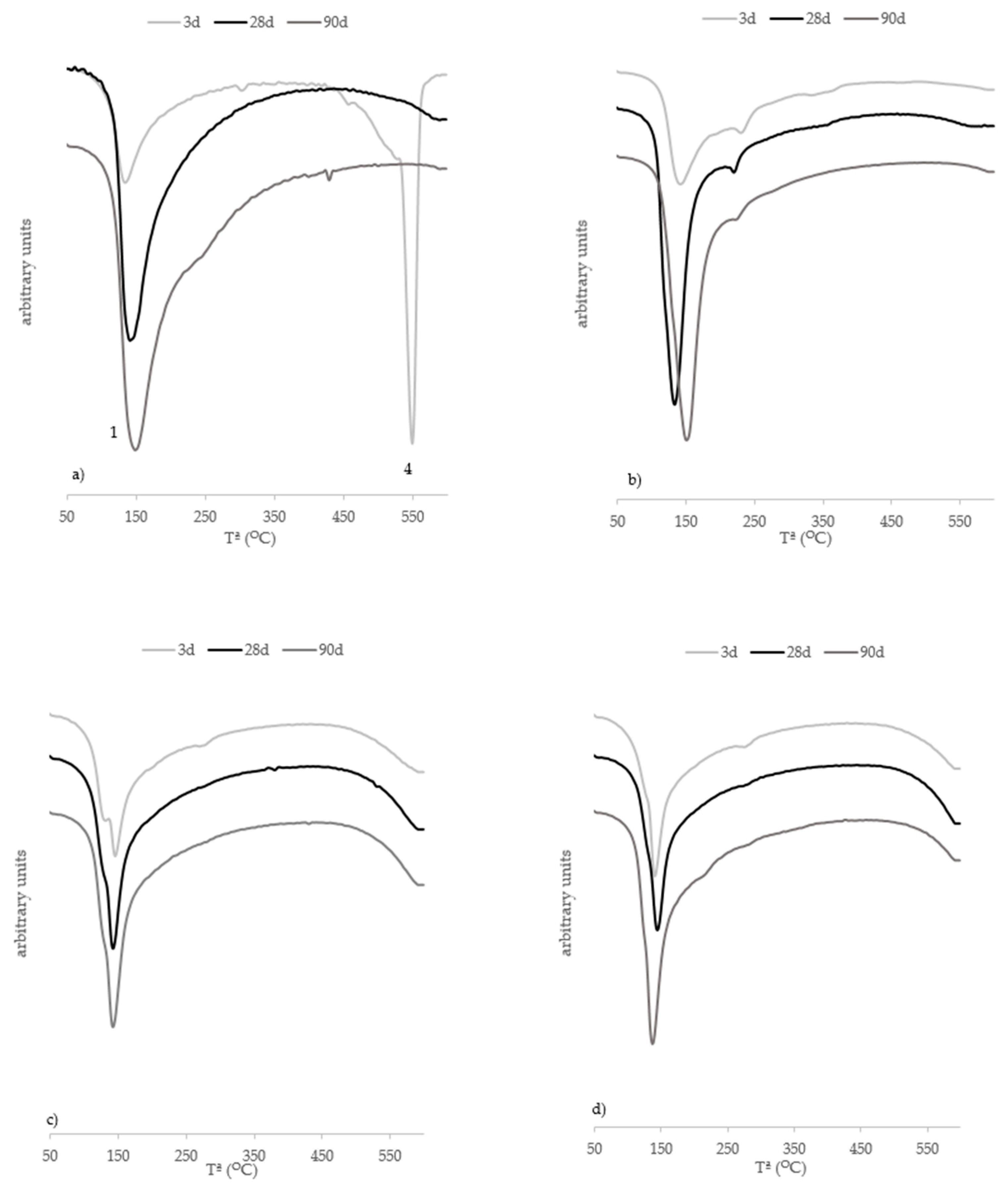
Thermogravimetric Analysis
4. Conclusions
- -
- The hybrid binder improves compressive strength and yields good values at 3–7 curing days.
- -
- The systems with alternative silica sources obtain similar results to commercial reagents. This is a very important goal for achieving more environmentally friendly systems.
- -
- The CWAC values of the hybrid systems are lower than those of their respective traditional systems. This behaviour is positive in durability terms.
- -
- The mechanical properties after the freeze–thaw cycles for the hybrid systems are significantly better than for the traditional hydrated lime-based system.
- -
- The TGA performed with the cured pastes shows that the nature of cementing gels changes with the presence of geopolymeric binders, and the consumption of hydrated lime is completed at early curing ages because of C(N)-A-S-H gel formation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monaco, M.; Aurilio, M.; Tafuro, A.; Guadagnoulo, M. Sustainable mortars for application in the cultural heritage field. Materials 2021, 14, 598. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.A.; Wenk, H.R.; Monteiro, P.J. Comparative investigation of mortars from Roman Colosseum and cistern. Thermochim. Acta 2005, 438, 35–40. [Google Scholar] [CrossRef]
- Izzo, F.; Arizzi, A.; Cappelletti, P.; Cultrone, G.; De Bonis, A.; Geminario, C.; Graziano, S.F.; Grifa, C.; Guarino, V.; Mercurio, M.; et al. The art of building in the Roman period (89 B.C-79 A.D): Mortars plasters and mosaic floors from ancient Stabiae (Naples, Italy). Constr. Build. Mater. 2016, 117, 129–143. [Google Scholar] [CrossRef]
- Matias, G.; Faria, P.; Torres, I. Lime mortars with ceramic wastes: Characterization of components and their influence on the mechanical behaviour. Constr. Build. Mater. 2014, 73, 523–534. [Google Scholar] [CrossRef]
- Vejmelková, E.; Keppert, M.; Rovnanìková, P.; Keršner, Z.; Černý, R. Application of burnt clay shale as pozzolan addition to lime mortar. Cem. Concr. Compos. 2014, 73, 523–534. [Google Scholar] [CrossRef]
- Fernández, F.; Germinario, S.; Basile, R.; Kapetanaki, K.; Gobakis, K.; Lolokotsa, D.; Lagou, A.M.; Dania, P.; Enna, M.T.; Mangiapane, M.; et al. Development of eco-friendly and self-cleaning lime-pozzolan plasters for bio-construction and cultural heritage. Buildings 2020, 10, 172. [Google Scholar] [CrossRef]
- Aggelakopoulou, E.; Bakolas, A.; Moropoulou, A. Design and evaluation of concrete for restoration interventions on Byzantine monuments. J. Cult. Herit. 2014, 73, 523–534. [Google Scholar] [CrossRef]
- Pavía, S.; Walker, R.; Veale, P.; Wood, A. Impact of the properties and reactivity of rice husk as on lime mortar properties. J. Mater. Civ. Eng. 2014, 26, 04014066. [Google Scholar] [CrossRef]
- Méndez, R.; Borrachero, M.V.; Payá, J.; Monzó, J. Mechanical strength of lime-rice husk ash mortars: A preliminary study. Key Eng. Mater. 2012, 517, 495–499. [Google Scholar] [CrossRef]
- Billong, N.; Melo, U.C.; Kamseu, E.; Kinuthia, J.M.; Njopwouo, D. Improving hydraulic properties of lime-rice hush ash (RHA) binders with metakaolin (MK). Constr. Build. Mater. 2011, 25, 2157–2161. [Google Scholar] [CrossRef]
- Arizzi, A.; Cultrone, G. Comparing the pozzolanic activity of aerial lime mortars made with metakaolin and fluid catalytic cracking catalyst residue: A petrographic and physical-mechanical study. Constr. Build. Mater. 2018, 184, 382–390. [Google Scholar] [CrossRef]
- García, J.; Borrachero, M.V.; Payá, J.; Monzó, J. Reutilización de ceniza de cascarilla de arroz y residuo de catalizador de craqueo catalítico de bajo coste económico y medioambiental. In Proceedings of the 6th Amazon & Pacific Green Materials Congress and Sustainable Construction Materials, Cali, Colombia, 27–29 April 2016; ISBN 9585954400/9789585954403. (In Spanish). [Google Scholar]
- Davidovits, J.; Morris, M. Why the Pharaohs Built the Pyramids with Fake Stones; Geopolymer Institute: Saint-Quentin, France, 2009; ISBN 9782951482043. [Google Scholar]
- Arcones, G.; Hernández, F.; Sepulcre, A. Comparative properties of a lime mortar with different metakaolin and natron additions. Constr. Build. Mater. 2016, 114, 747–754. [Google Scholar] [CrossRef]
- Chakraborty, S.; Wan, B.; Ho, J.; Baloch, Z. Effectiveness of sewage sludge ash combined with waste pozzolanic minerals in developing sustainable construction material: An alternative approach for waste management. J. Clean. Prod. 2017, 153, 253–263. [Google Scholar] [CrossRef]
- Aziz, A.; El Hassani, I.; El Khadiri, A.; Sadik, C.; El Bouari, A.; Ballil, A.; El Haddar, A. Effect of slaked lime on geopolymers synthesis of natural pozzolan from Moroccan middle atlas. J. Aust. Ceram. 2020, 56, 67–78. [Google Scholar] [CrossRef]
- Das, S.K.; Mustakim, S.M.; Adesina, A.; Mishra, J.; Alomayri, T.S.; Assaedi, H.S.; Kaze, C.R. Fresh, strength and microstructure properties of geopolymer concrete incorporating lime and silica fume as replacement. J. Build. Eng. 2020, 32, 101780. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Dong, Z. Improving strength of calcinated coal gangue geopolymer mortars via increasing calcium content. Constr. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Arham, A.; Hayati, N.; Wayan, I.; Rupang, N. The effect of lime addition on the setting time and strength of ambient cured fly ash based geopolymer binder. MATEC Web Conf. 2016, 47, 01015. [Google Scholar] [CrossRef]
- Villaquirán-Caicedo, M.A. Studying different silica sources for preparation of alternative waterglass used in preparation of binary geopolymer binders from metakaolin/boiler slag. Constr. Build. Mat. 2019, 227, 116621. [Google Scholar] [CrossRef]
- Font, A.; Soriano, L.; Reig, L.; Tashima, M.M.; Borrachero, M.V.; Monzó, J.; Payá, J. Use of residual diatomaceous earth as a silica source in geopolymer production. Mater. Lett. 2018, 223, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Bernal, S.A.; Rodríguez, E.D.; Mejía de Gutiérrez, R.; Provis, J. Performance at high temperature of alkali-activated slag pastes produced with silica fume and rice husk ash based activators. Mater. Construcc. 2015, 65, e049. [Google Scholar] [CrossRef]
- UNE-EN 459-1; Building Lime. Part I: Definitions, Specifications and Conformity Criteria. AENOR: Madrid, Spain, 2016.
- Bouzón, N.; Payá, J.; Borrachero, M.V.; Soriano, L.; Tashima, M.M.; Monzó, J. Refluxed rice husk ash/NaOH suspension for preparing alkali activated binders. Mater. Lett. 2014, 115, 72–74. [Google Scholar] [CrossRef]
- UNE-EN 1015-18; Methods of Test for Mortar for Masonry. Part 18: Determination of Water Absorption Coefficient Due to Capillary Action of Hardened Mortar. AENOR: Madrid, Spain, 2003.
- UNE-EN 12371; Natural Stone Test Methods. Determination of Frost Resistance. AENOR: Madrid, Spain, 2011.
- Habert, G.; d´Espinose de Lcaillerie, J.B.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Silva, B.; Ferreira, A.P.; Gomes, A.; Candeias, A. Admixtures potential role on the improvement of the freeze-thaw resistance of lime mortars. J. Build. Eng. 2021, 35, 101977. [Google Scholar] [CrossRef]
- Nunes, C.; Sližková, Z. Freezing and thawing resistance of aerial lime mortar with metakaolin and a traditional water-repellent admixture. Constr. Build. Mater. 2016, 114, 896–905. [Google Scholar] [CrossRef]
- Beck, K.; Al-Mukhtar, M. Formulation and characterization of an appropriate lime-based mortar for use with a porous limestone. Environ. Geol. 2008, 56, 715–725. [Google Scholar] [CrossRef]
- Maras, M.M. Characterization of performable geopolymer mortars for use as repair material. Struct. Concr. 2021, 22, 3173–3188. [Google Scholar] [CrossRef]
- Zhang, P.; Wittmann, F.H.; Vogel, M.; Harald, S.M.; Zhao, T. Influence of freeze-thaw cycles on capillary absorption and chloride penetration into concrete. Cem. Concr. Res. 2017, 100, 60–67. [Google Scholar] [CrossRef]
- Veiga, M.R.; Velosa, A.; Magalhães, A. Experimental applications of mortars with pozzolanic additions: Characterization and performance evaluation. Constr. Build. Mater. 2009, 23, 318–327. [Google Scholar] [CrossRef]
- Payá, J.; Monzó, J.; Borrachero, M.V.; Velázquez, S.; Bonilla, M. Determination of the pozzolanic activity of fluid catalytic cracking residue. Thermogravimetric analysis studies on FC3R-lime pastes. Cem. Concr. Res. 2003, 33, 1085–1091. [Google Scholar] [CrossRef]
- Tashima, M.M.; Soriano, L.; Aksaki, J.L.; Castaldelli, V.N.; Monzó, J.; Payá, J.; Borrachero, M.V. Spent FCC catalyst for preparing alkali-activaded binders: An opportunity for a high-degree valorization. Key Eng. Mater. 2014, 600, 709–716. [Google Scholar] [CrossRef] [Green Version]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Variation in hybrid cements over time. Alkaline activation of fly ash-portland cement blends. Cem. Concr. Res. 2013, 52, 112–122. [Google Scholar] [CrossRef]
- Alonso, S.; Palomo, A. Alkaline activation of metakaolin and calcium hydroxide mixtures: Influence of temperature, activator concentration and solids ratios. Mater. Lett. 2001, 47, 55–62. [Google Scholar] [CrossRef]
- Alonso, S.; Palomo, A. Calorimetric study of alkaline activation of calcium hydroxide-metakaolin solid mixtures. Cem. Concr. Res. 2001, 31, 25–30. [Google Scholar] [CrossRef]






| Al2O3 | SiO2 | CaO | Fe2O3 | K2O | Na2O | P2O5 | MgO | SO3 | Other | LOI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FCC | 49.26 | 47.76 | 0.11 | 0.60 | 0.02 | 0.31 | 0.01 | 0.17 | 0.02 | 1.23 | 0.51 |
| RHA | 0.25 | 85.58 | 1.83 | 0.21 | 3.39 | - | 0.67 | 0.50 | 0.26 | 0.32 | 6.99 |
| RDE | 5.67 | 81.70 | 1.28 | 3.71 | 0.86 | 1.30 | 0.36 | 0.41 | - | 1.37 | 3.34 |
| Lime–Pozzolan Binder | Geopolymeric Binder | Sand | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lime | Pozzolan | H2O | FCC | Alkaline-Activating Solution | |||||
| H2O | NaOH | Na2SiO3 | RHA or RDE | ||||||
| T-FCC | 262.5 | 262.5 | 420.0 | - | - | - | - | - | 1575.0 |
| H-FCC/C | 183.8 | 183.8 | 294.0 | 157.5 | 37.8 | 19.2 | 88.6 | - | 1575.0 |
| H-FCC/RHA | 183.8 | 183.8 | 294.0 | 157.5 | 94.5 | 37.8 | - | 27.6 | 1575.0 |
| H-FCC/RDE | 183.8 | 183.8 | 294.0 | 157.5 | 94.5 | 37.8 | - | 27.6 | 1575.0 |
| T-RHA | 175 | 350.0 | 420.0 | - | - | - | - | - | 1575.0 |
| H-RHA/C | 122.5 | 245.0 | 294.0 | 157.5 | 37.8 | 19.2 | 88.6 | - | 1575.0 |
| H-RHA/RHA | 122.5 | 245.0 | 294.0 | 157.5 | 94.5 | 37.8 | - | 27.6 | 1575.0 |
| H-RHA/RDE | 122.5 | 245.0 | 294.0 | 157.5 | 94.5 | 37.8 | - | 27.6 | 1575.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villca, A.R.; Soriano, L.; Borrachero, M.V.; Payá, J.; Monzó, J.M.; Tashima, M.M. Hybrid Lime–Pozzolan Geopolymer Systems: Microstructural, Mechanical and Durability Studies. Materials 2022, 15, 2736. https://doi.org/10.3390/ma15082736
Villca AR, Soriano L, Borrachero MV, Payá J, Monzó JM, Tashima MM. Hybrid Lime–Pozzolan Geopolymer Systems: Microstructural, Mechanical and Durability Studies. Materials. 2022; 15(8):2736. https://doi.org/10.3390/ma15082736
Chicago/Turabian StyleVillca, Ariel Rey, Lourdes Soriano, María Victoria Borrachero, Jordi Payá, José María Monzó, and Mauro Mitsuuchi Tashima. 2022. "Hybrid Lime–Pozzolan Geopolymer Systems: Microstructural, Mechanical and Durability Studies" Materials 15, no. 8: 2736. https://doi.org/10.3390/ma15082736
APA StyleVillca, A. R., Soriano, L., Borrachero, M. V., Payá, J., Monzó, J. M., & Tashima, M. M. (2022). Hybrid Lime–Pozzolan Geopolymer Systems: Microstructural, Mechanical and Durability Studies. Materials, 15(8), 2736. https://doi.org/10.3390/ma15082736









