Atomic Force Microscopy Nanoindentation Method on Collagen Fibrils
Abstract
1. Introduction
2. Data Processing
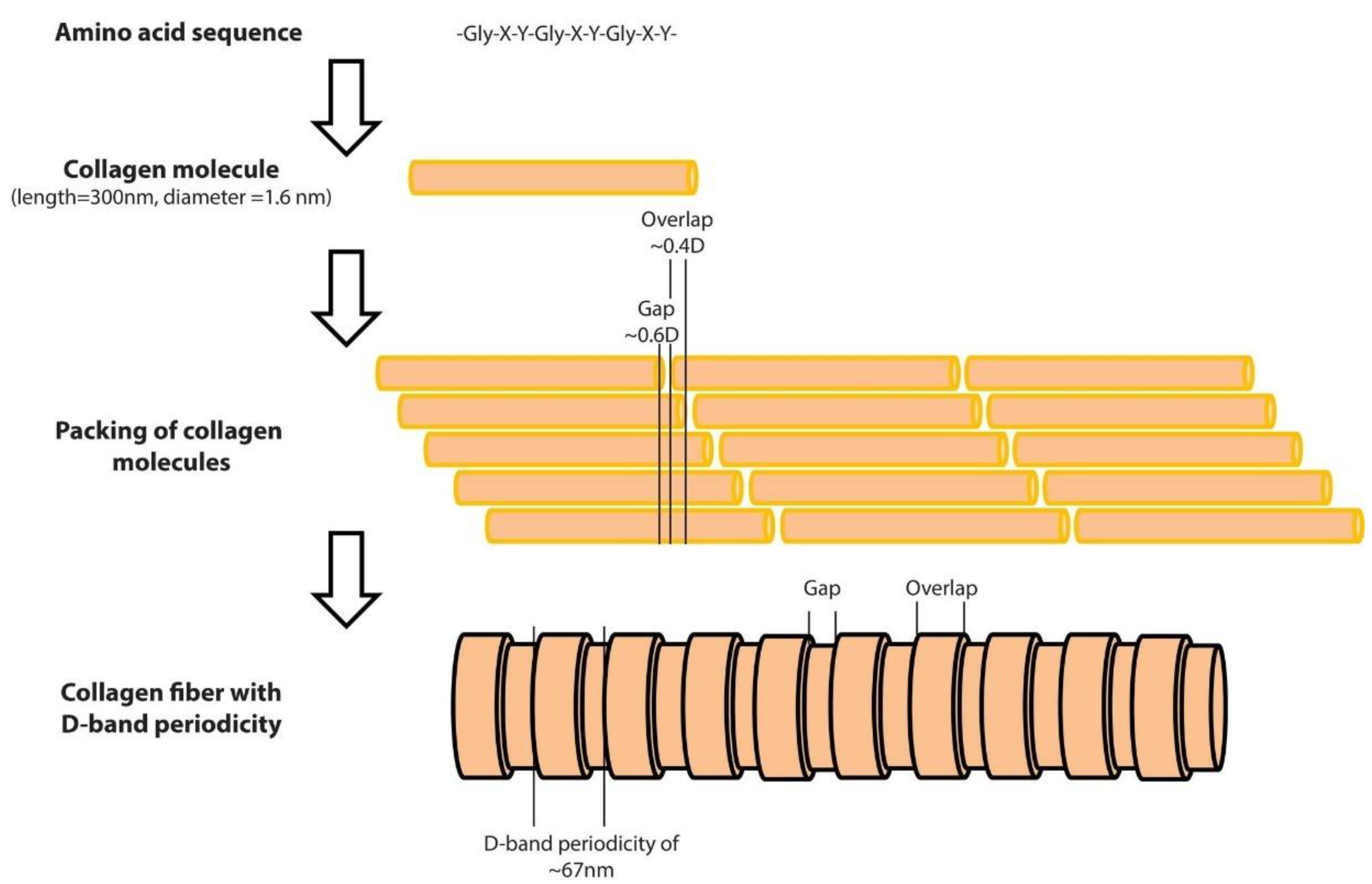
2.1. Collagen Sample Preparation
2.2. The Calibration of Probe Parameters
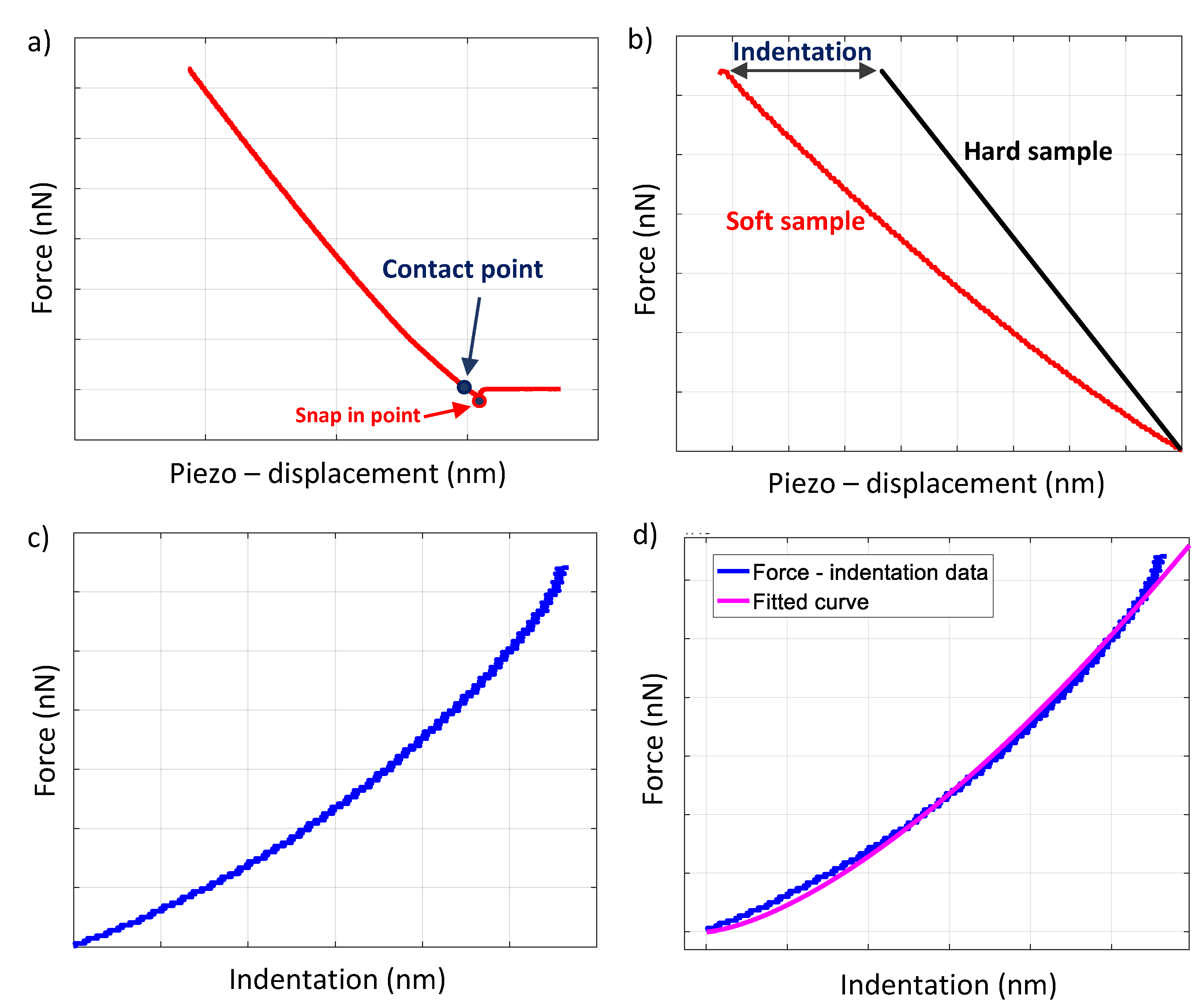
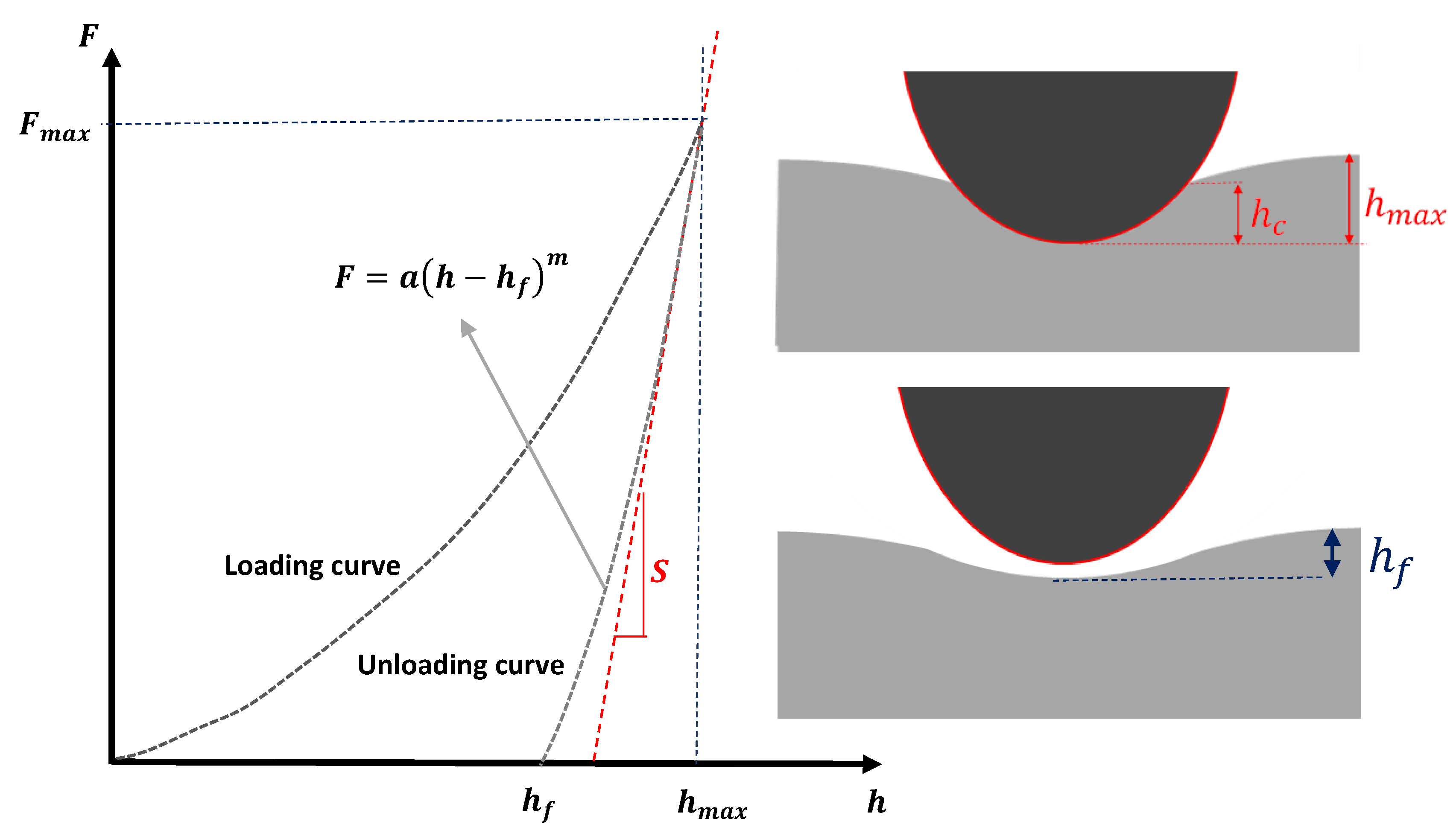
2.3. Constructing a Force–Indentation Curve and Curve Fitting
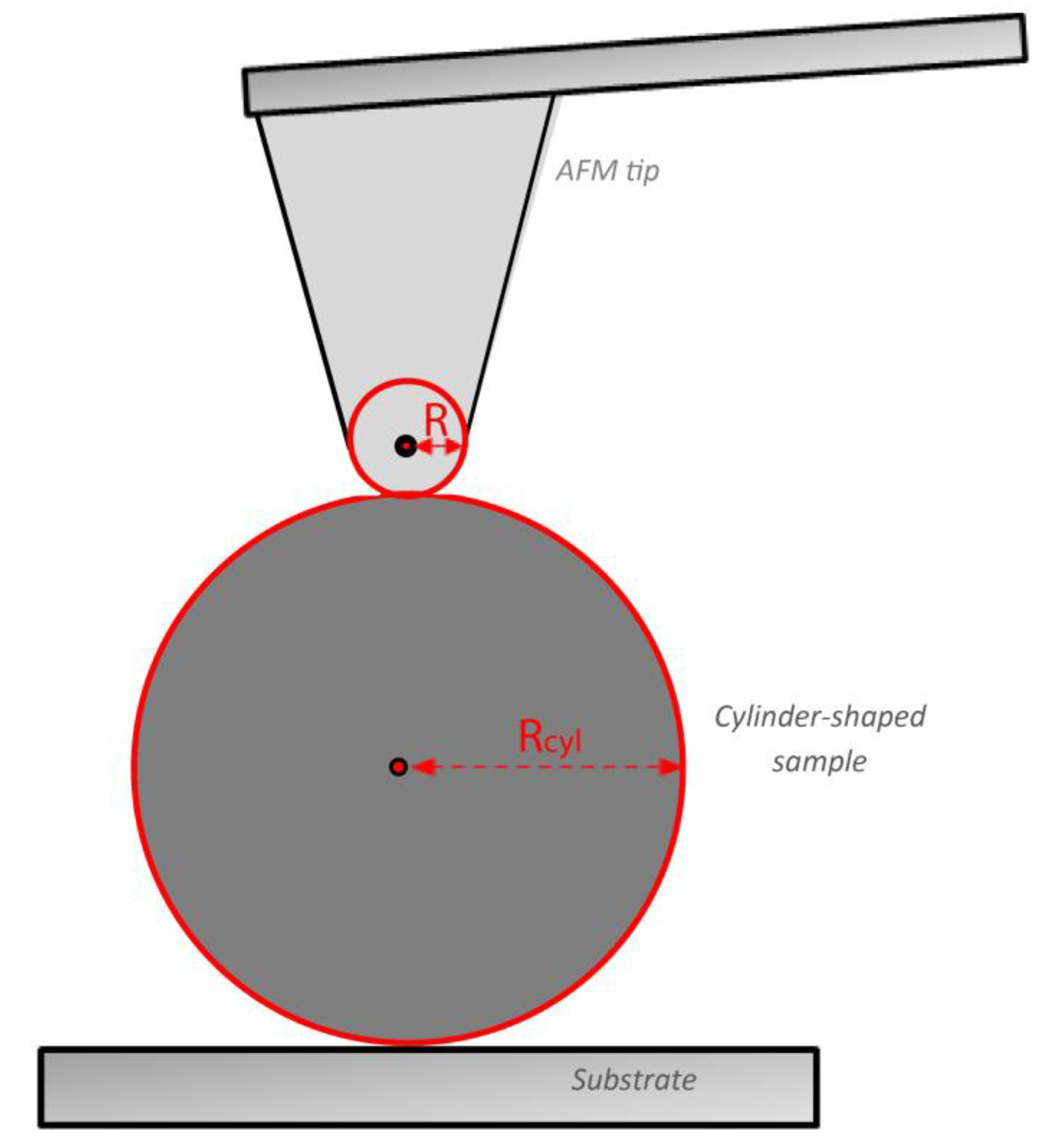
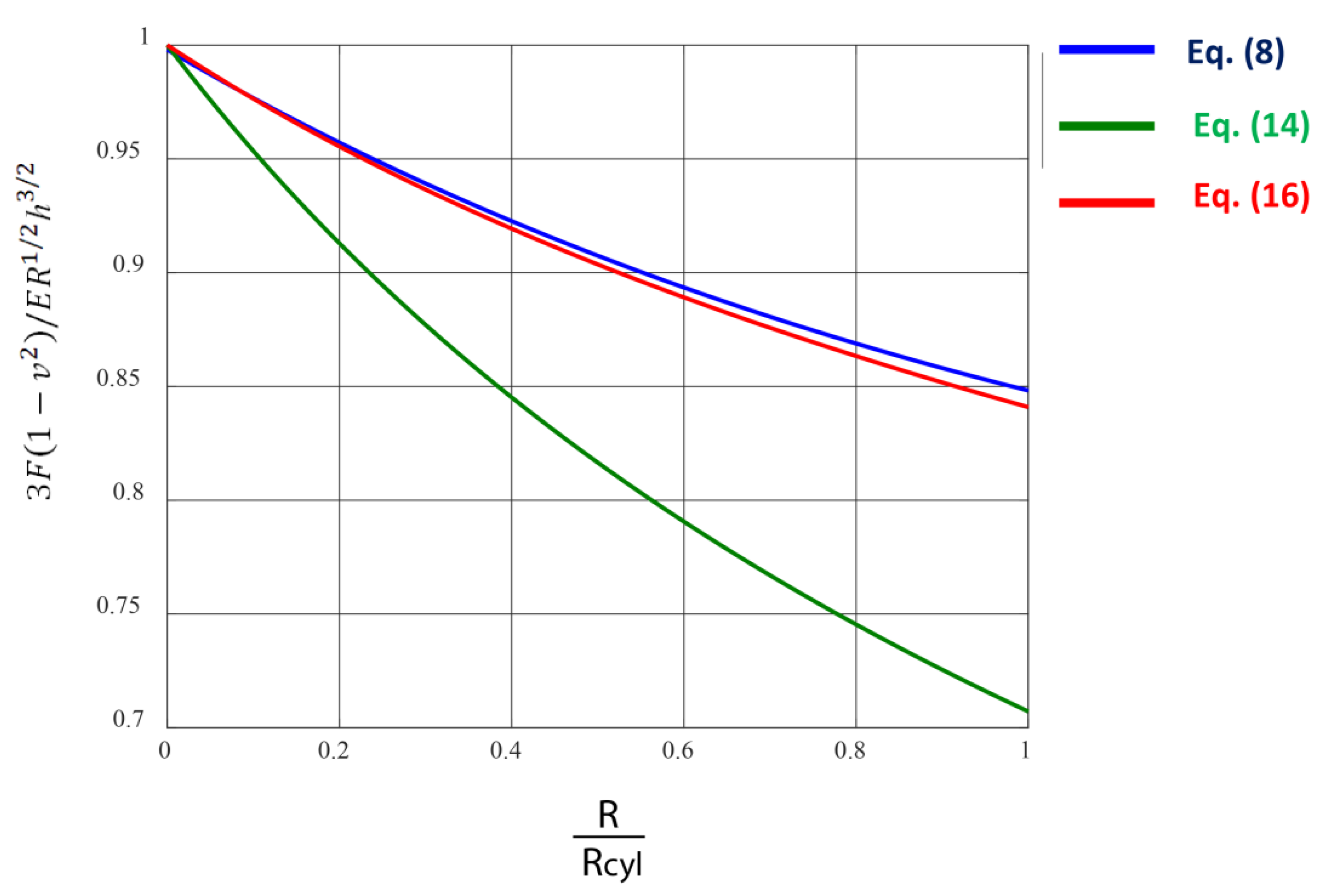
2.4. The Purely Elastic Sphere–Cylinder Interaction
2.5. The Elastic–Plastic Contact
2.6. Mechanical Properties Maps
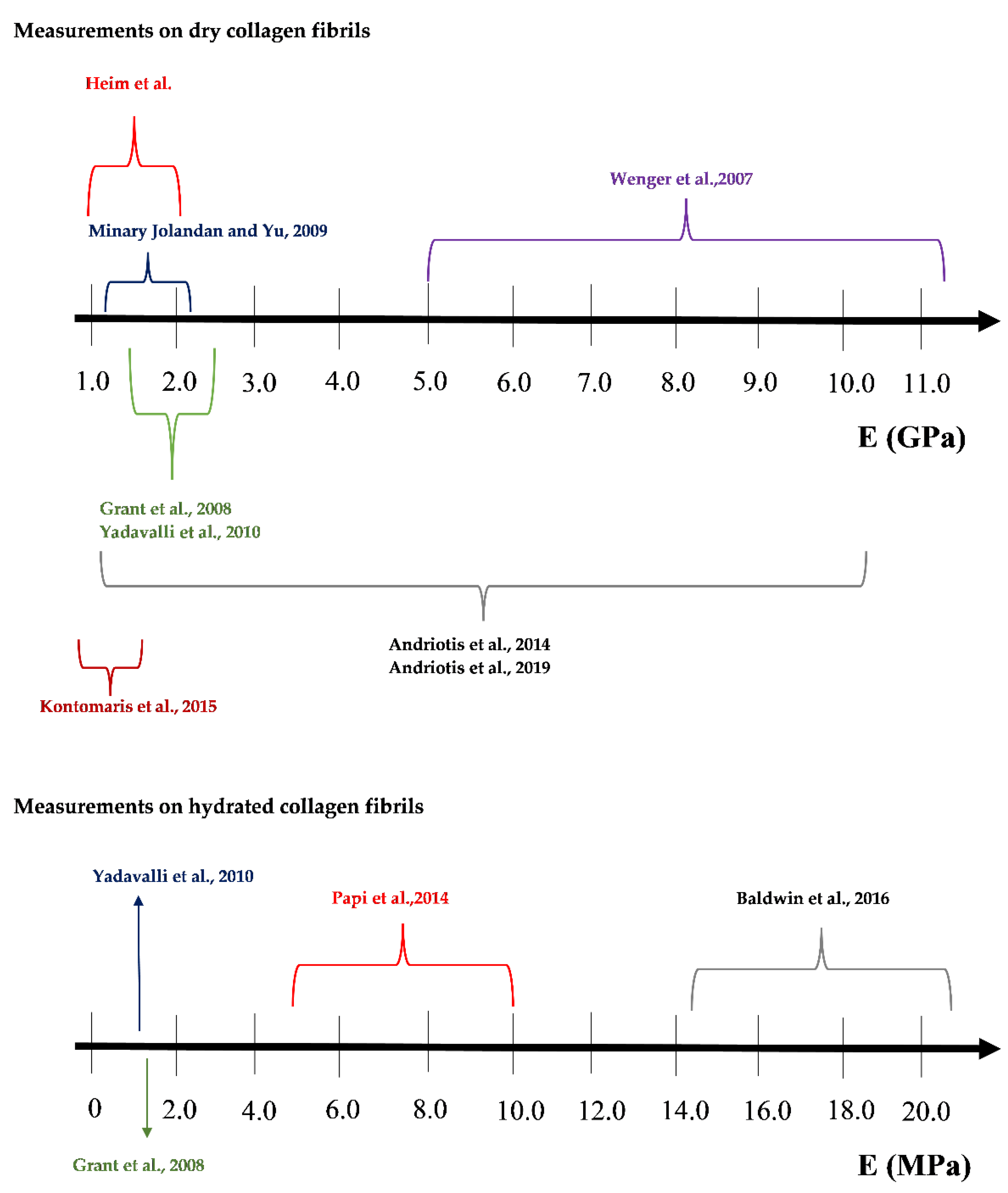
3. A Discussion Regarding the Extended Range of Young’s Modulus Values Found in the Literature
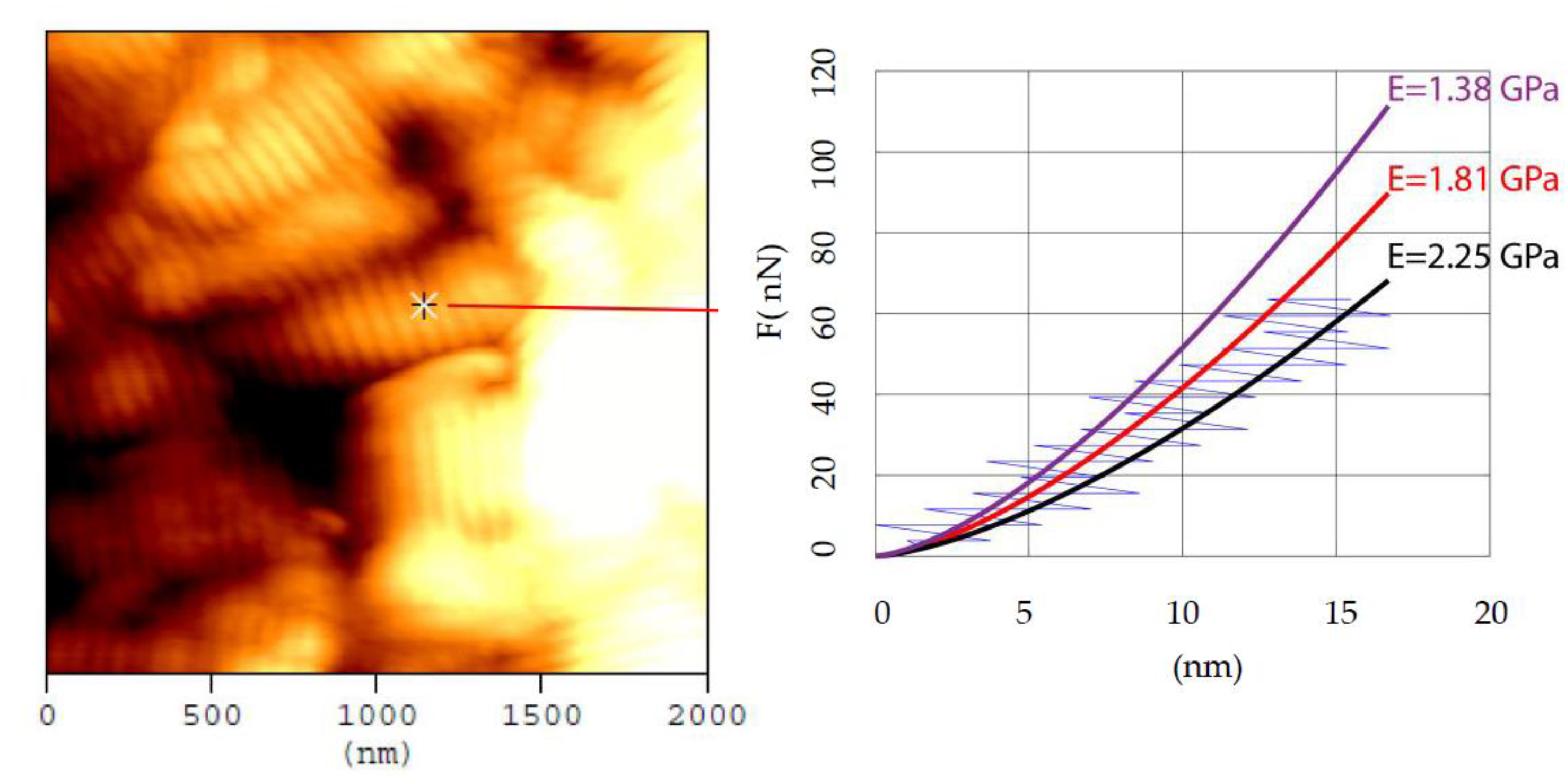
3.1. The Mechanical Heterogeneity of Collagen Fibrils
3.2. Errors in Calibration Procedures
3.3. Errors in Fitting Process
3.4. Errors due to Various Misuses of Contact Mechanics Models
3.5. The Importance of Research on Collagen
4. Conclusions
- Errors in calibration procedures.
- The fitting processes.
- The contact mechanics models.
- The structural and mechanical heterogeneity of collagen fibrils.
- the indentation depth (i.e., across the radial direction of fibrils);
- the direction along the fibril (it is expected to lead in a periodic function due to the alternating gap and overlapping regions);
- the moisture content within the fibril.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fratzl, P. Collagen Structure and Mechanics; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Hulmes, D.J.S. Collagen diversity, synthesis and assembly. In Collagen: Structure and Mechanics; Springer: Boston, MA, USA, 2008; pp. 15–47. [Google Scholar] [CrossRef]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef] [PubMed]
- Bozec, L.; van der Heijden, G.; Horton, M. Collagen Fibrils: Nanoscale Ropes. Biophys. J. 2007, 92, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Hasirci, V.; Vrana, E.; Zorlutuna, P.; Ndreu, A.; Yilgor, P.; Basmanav, F.B.; Aydin, E. Nanobiomaterials: A review of the existing science and technology, and new approaches. J. Biomater. Sci. Polym. Ed. 2006, 17, 1241–1268. [Google Scholar] [CrossRef]
- Harley, R.; James, D.; Miller, A.; White, J.W. Phonons and the elastic moduli of collagen and muscle. Nature 1977, 267, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Cusack, S.; Miller, A. Determination of the elastic constants of collagen by Brillouin light scattering. J. Mol. Biol. 1979, 135, 39–51. [Google Scholar] [CrossRef]
- Van Der Rijt, J.A.J.; Van Der Werf, K.O.; Bennink, M.L.; Dijkstra, P.J.; Feijen, J. Micromechanical testing of individual collagen fibrils. Macromol. Biosci. 2006, 6, 697–702. [Google Scholar] [CrossRef]
- Sasaki, N.; Odajima, S. Stress-strain curve and Young’s modulus of a collagen molecule as determined by the X-ray diffraction technique. J. Biomech. 1996, 29, 655–658. [Google Scholar] [CrossRef]
- Eppell, S.J.; Smith, B.N.; Kahn, H.; Ballarini, R. Nano measurements with micro-devices: Mechanical properties of hydrated collagen fibrils. J. R. Soc. Interface 2006, 3, 117–121. [Google Scholar] [CrossRef]
- Shen, Z.L.; Dodge, M.R.; Kahn, H.; Ballarini, R.; Eppell, S.J. Stress-strain experiments on individual collagen fibrils. Biophys. J. 2008, 95, 3956–3963. [Google Scholar] [CrossRef]
- Shen, Z.L.; Kahn, H.; Ballarini, R.; Eppell, S.J. Viscoelastic properties of isolated collagen fibrils. Biophys. J. 2011, 100, 3008–3015. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, A.C.; Caffarena, E.R. Elastic properties, Young’s modulus determination and structural stability of the tropocollagen molecule: A computational study by steered molecular dynamics. J. Biomech. 2005, 38, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Svensson, R.B.; Hassenkam, T.; Hansen, P.; Peter Magnusson, S. Viscoelastic behavior of discrete human collagen fibrils. J. Mech. Behav. Biomed. Mater. 2010, 3, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Hang, F.; Barber, A.H. Nano-mechanical properties of individual mineralized collagen fibrils from bone tissue. J. R. Soc. Interface 2011, 8, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A.; Kontomaris, S.V.; Alexandratou, E.; Grant, C. Atomic Force Microscopy on biological materials related to pathological conditions. Scanning 2019, 2019, 8452851. [Google Scholar] [CrossRef]
- Plodinec, M.; Loparic, M.; Monnier, C.A.; Obermann, E.C.; Zanetti-Dallenbach, R.; Oertle, P.; Hyotyla, J.T.; Aebi, U.; Bentires-Alj, M.; Lim, R.Y.H.; et al. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 2012, 7, 757–765. [Google Scholar] [CrossRef]
- Stolz, M.; Gottardi, R.; Raiteri, R.; Miot, S.; Martin, I.; Imer, R.; Staufer, U.; Raducanu, A.; Düggelin, M.; Baschong, W.; et al. Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force microscopy. Nat. Nanotechnol. 2009, 4, 186–192. [Google Scholar] [CrossRef]
- Grant, C.A.; Brockwell, D.J.; Radford, S.E.; Thomson, N.H. Effects of hydration on the mechanical response of individual collagen fibrils. Appl. Phys. Lett. 2008, 92, 233902. [Google Scholar] [CrossRef]
- Wenger, M.P.E.; Bozec, L.; Horton, M.A.; Mesquidaz, P. Mechanical properties of collagen fibrils. Biophys. J. 2007, 93, 1255–1263. [Google Scholar] [CrossRef]
- Heim, A.J.; Matthews, W.G.; Koob, T.J. Determination of the elastic modulus of native collagen fibrils via radial indentation. Appl. Phys. Lett. 2006, 89, 181902. [Google Scholar] [CrossRef]
- Minary-Jolandan, M.; Yu, M.F. Nanomechanical heterogeneity in the gap and overlap regions of type I collagen fibrils with implications for bone heterogeneity. Biomacromolecules 2009, 10, 2565–2570. [Google Scholar] [CrossRef]
- Yadavalli, V.K.; Svintradze, D.V.; Pidaparti, R.M. Nanoscale measurements of the assembly of collagen to fibrils. Int. J. Biol. Macromol. 2010, 46, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Andriotis, O.G.; Manuyakorn, W.; Zekonyte, J.; Katsamenis, O.L.; Fabri, S.; Howarth, P.H.; Davies, D.E.; Thurner, P.J. Nanomechanical assessment of human and murine collagen fibrils via atomic force microscopy cantilever-based nanoindentation. J. Mech. Behav. Biomed. Mater. 2014, 39, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Kontomaris, S.V.; Stylianou, A.; Yova, D.; Balogiannis, G. The effects of UV irradiation on collagen D-band revealed by atomic force microscopy. Scanning 2015, 37, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Andriotis, O.G.; Elsayad, K.; Smart, D.E.; Nalbach, M.; Davies, D.E.; Thurner, P.J. Hydration and nanomechanical changes in collagen fibrils bearing advanced glycation end-products. Biomed. Opt. Express 2019, 10, 1841–1855. [Google Scholar] [CrossRef]
- Papi, M.; Paoletti, P.; Geraghty, B.; Akhtar, R. Nanoscale characterization of the biomechanical properties of collagen fibrils in the sclera. Appl. Phys. Lett. 2014, 104, 103703. [Google Scholar] [CrossRef]
- Baldwin, S.J.; Kreplak, L.; Lee, J.M. Characterization via atomic force microscopy of discrete plasticity in collagen fibrils from mechanically overloaded tendons: Nano-scale structural changes mimic rope failure. J. Mech. Behav. Biomed. Mater. 2016, 60, 356–366. [Google Scholar] [CrossRef]
- Kazaili, A.; Al-Hindy, H.A.A.; Madine, J.; Akhtar, R. Nano-scale stiffness and collagen fibril deterioration: Probing the cornea following enzymatic degradation using peakforce-qnm afm. Sensors 2021, 21, 1629. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Malamou, A. Hertz model or Oliver & Pharr analysis? Tutorial regarding AFM nanoindentation experiments on biological samples. Mater. Res. Express 2020, 7, 033001. [Google Scholar] [CrossRef]
- Sobola, D.; Ramazanov, S.; Koneĉnỳ, M.; Orudzhev, F.; Kaspar, P.; Papež, N.; Knápek, A.; Potoĉek, M. Complementary SEM-AFM of swelling Bi-Fe-O film on HOPG substrate. Materials 2020, 13, 2402. [Google Scholar] [CrossRef]
- Quigley, A.S.; Veres, S.P.; Kreplak, L. Bowstring stretching and quantitative imaging of single collagen fibrils via atomic force microscopy. PLoS ONE 2016, 11, e0161951. [Google Scholar] [CrossRef]
- Stylianou, A.; Gkretsi, V.; Patrickios, C.S.; Stylianopoulos, T. Exploring the Nano-Surface of Collagenous and Other Fibrotic Tissues with AFM. In Fibrosis: Methods and Protocols; Rittié, L., Ed.; Springer: New York, NY, USA, 2017; pp. 453–489. [Google Scholar] [CrossRef]
- Jiang, F.; Khairy, K.; Poole, K.; Howard, J.; Müller, D.J. Creating nanoscopic collagen matrices using atomic force microscopy. Microsc. Res. Tech. 2004, 64, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A. Atomic force microscopy for collagen-based nanobiomaterials. J. Nanomater. 2017, 2017, 9234627. [Google Scholar] [CrossRef]
- Stylianou, A.; Kontomaris, S.B.; Kyriazi, M.; Yova, D. Surface characterization of collagen films by atomic force microscopy. In 12th Mediterranean Conference on Medical and Biological Engineering and Computing, MEDICON 2010; Springer: Berlin/Heidelberg, Germany, 2010; Volume 29, pp. 612–615. [Google Scholar] [CrossRef]
- Stylianou, A.; Kontomaris, S.V.; Yova, D. Assessing Collagen Nanoscale Thin Films Heterogeneity by AFM Multimode Imaging and Nanoindetation for NanoBioMedical Applications. Micro Nanosyst. 2014, 6, 95–102. [Google Scholar] [CrossRef]
- Stylianou, A.; Yova, D. Surface nanoscale imaging of collagen thin films by Atomic Force Microscopy. Mater. Sci. Eng. C 2013, 33, 2947–2957. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, S.; Vahdat, A.S.; Trotsenko, O.; Minko, S.; Babu, S.V. AFM-based study of the interaction forces between ceria, silicon dioxide and polyurethane pad during non-prestonian polishing of silicon dioxide films. ECS J. Solid State Sci. Technol. 2015, 4, P5016–P5020. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Chung, K.H. Effect of tip shape on nanomechanical properties measurements using AFM. Ultramicroscopy 2019, 202, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.; Taylor, L.; Duncan, W.; Melmed, A.J. Fast, high-resolution atomic force microscopy using a quartz tuning fork as actuator and sensor. J. Appl. Phys. 1997, 82, 980–984. [Google Scholar] [CrossRef][Green Version]
- Giessibl, F.J. Atomic resolution on Si(111)-(7×7) by noncontact atomic force microscopy with a force sensor based on a quartz tuning fork. Appl. Phys. Lett. 2000, 76, 1470–1472. [Google Scholar] [CrossRef]
- Giessibl, F.J.; Hembacher, S.; Bielefeldt, H.; Mannhart, J. Subatomic features on the silicon (111)-(7 × 7) surface observed by atomic force microscopy. Science 2000, 289, 422–425. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Stylianou, A. Atomic force microscopy for university students: Applications in biomaterials. Eur. J. Phys. 2017, 38, 033003. [Google Scholar] [CrossRef]
- Hermanowicz, P.; Sarna, M.; Burda, K.; Gabryś, H. AtomicJ: An open source software for analysis of force curves. Rev. Sci. Instrum. 2014, 85, 063703. [Google Scholar] [CrossRef] [PubMed]
- Lekka, M. Discrimination Between Normal and Cancerous Cells Using AFM. BioNanoScience 2016, 6, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Koruk, H. Modelling small and large displacements of a sphere on an elastic half-space exposed to a dynamic force. Eur. J. Phys. 2021, 42, 055006. [Google Scholar] [CrossRef]
- Radmacher, M. Studying the Mechanics of Cellular Processes by Atomic Force Microscopy. Methods Cell Biol. 2007, 83, 347–372. [Google Scholar] [PubMed]
- Krieg, M.; Fläschner, G.; Alsteens, D.; Gaub, B.M.; Roos, W.H.; Wuite, G.J.L.; Gaub, H.E.; Gerber, C.; Dufrêne, Y.F.; Müller, D.J. Atomic force microscopy-based mechanobiology. Nat. Rev. Phys. 2019, 1, 41–57. [Google Scholar] [CrossRef]
- Stylianos-Vasileios, K. The Hertz Model in AFM Nanoindentation Experiments: Applications in Biological Samples and Biomaterials. Micro Nanosyst. 2018, 10, 11–22. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Puricelli, L.; Galluzzi, M.; Schulte, C.; Podestà, A.; Milani, P. Nanomechanical and topographical imaging of living cells by atomic force microscopy with colloidal probes. Rev. Sci. Instrum. 2015, 86, 033705. [Google Scholar] [CrossRef]
- Sneddon, I.N. The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int. J. Eng. Sci. 1965, 3, 47–57. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Malamou, A. A novel approximate method to calculate the force applied on an elastic half space by a rigid sphere. Eur. J. Phys. 2021, 42, 025010. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Malamou, A. The harmonic motion of a rigid cylinder on an elastic half-space. Eur. J. Phys. 2020, 41, 015003. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Malamou, A. Revisiting the theory behind AFM indentation procedures. Exploring the physical significance of fundamental equations. Eur. J. Phys. 2022, 43, 015010. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Stylianou, A.; Malamou, A.; Stylianopoulos, T. A discussion regarding the approximation of cylindrical and spherical shaped samples as half spaces in AFM nanoindentation experiments. Mater. Res. Express 2018, 5, 085402. [Google Scholar] [CrossRef]
- Hrouz, J.; Vojta, V.; Ilavský, M. Penetration behavior of the system sphere-cylinder. Polym. Eng. Amp. Sci. 1980, 20, 402–405. [Google Scholar] [CrossRef]
- Pharr, G.M.; Brotzen, F.R. On the generality of the relationship among contact stiffness, contact area, and elastic modulus during indentation. J. Mater. Res. 1992, 7, 613–617. [Google Scholar] [CrossRef]
- Fu, G. On the fundamental relations used in the analysis of nanoindentation data. J. Mater. Sci. 2004, 39, 745–746. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Stylianou, A.; Nikita, K.S.; Malamou, A.; Stylianopoulos, T. A simplified approach for the determination of fitting constants in Oliver–Pharr method regarding biological samples. Phys. Biol. 2019, 16, 056003. [Google Scholar] [CrossRef]
- Bueckle, H.; Westbrook, J.W.; Conrad, H. The Science of Hardness Testing and Its Research Applications; Metals, A.S., Ed.; American Society for Metals: Metals Park, OH, USA, 1973. [Google Scholar]
- Kontomaris, S.V.; Malamou, A. An extension of the general nanoindentation equation regarding cylindrical—shaped samples and a simplified model for the contact ellipse determination. Mater. Res. Express 2018, 5, 125403. [Google Scholar] [CrossRef]
- Darling, E.M. Force scanning: A rapid, high-resolution approach for spatial mechanical property mapping. Nanotechnology 2011, 22, 175707. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Stylianou, A.; Yova, D. Investigation of the mechanical properties of collagen fibrils under the influence of low power red laser irradiation. Biomed. Phys. Eng. Express 2016, 2, 064002. [Google Scholar] [CrossRef]
- Jacobs, T.D.B.; Mathew Mate, C.; Turner, K.T.; Carpick, R.W. Understanding the tip-sample contact: An overview of contact mechanics from the macro- to the nanoscale. In Scanning Probe Microscopy for Industrial Applications: Nanomechanical Characterization; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 15–48. [Google Scholar] [CrossRef]
- Spitzner, E.C.; Röper, S.; Zerson, M.; Bernstein, A.; Magerle, R. Nanoscale Swelling Heterogeneities in Type I Collagen Fibrils. ACS Nano 2015, 9, 5683–5694. [Google Scholar] [CrossRef]
- Layton, B.E.; Sastry, A.M.; Wang, H.; Sullivan, K.A.; Feldman, E.L.; Komorowski, T.E.; Philbert, M.A. Differences between collagen morphologies, properties and distribution in diabetic and normal biobreeding and Sprague-Dawley rat sciatic nerves. J. Biomech. 2004, 37, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Calò, A.; Romin, Y.; Srouji, R.; Zambirinis, C.P.; Fan, N.; Santella, A.; Feng, E.; Fujisawa, S.; Turkekul, M.; Huang, S.; et al. Spatial mapping of the collagen distribution in human and mouse tissues by force volume atomic force microscopy. Sci. Rep. 2020, 10, 15664. [Google Scholar] [CrossRef] [PubMed]
- Brett, E.A.; Sauter, M.A.; Machens, H.-G.; Duscher, D. Tumor-associated collagen signatures: Pushing tumor boundaries. Cancer Metab. 2020, 8, 14. [Google Scholar] [CrossRef]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Friedl, A.; Keely, P.J. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 2011, 178, 1221–1232. [Google Scholar] [CrossRef]
- Erkan, M.; Hausmann, S.; Michalski, C.W.; Fingerle, A.A.; Dobritz, M.; Kleeff, J.; Friess, H. The role of stroma in pancreatic cancer: Diagnostic and therapeutic implications. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 454–467. [Google Scholar] [CrossRef]
- Stylianou, A.; Gkretsi, V.; Louca, M.; Zacharia, L.; Stylianopoulos, T. Collagen Content and Extracellular Matrix Stiffness Remodels Pancreatic Fibroblasts Cytoskeleton. J. R. Soc. Interface 2019, 16, 20190226. [Google Scholar] [CrossRef]
- Stylianou, A.; Gkretsi, V.; Stylianopoulos, T. Transforming Growth Factor-β modulates Pancreatic Cancer Associated Fibroblasts cell shape, stiffness and invasion. Biochim. Biophys. Acta 2018, 1862, 1537–1546. [Google Scholar] [CrossRef]
- Choi, S.; Cheong, Y.; Shin, J.H.; Lee, H.J.; Lee, G.J.; Choi, S.K.; Jin, K.H.; Park, H.K. Short-term nanostructural effects of high radiofrequency treatment on the skin tissues of rabbits. Lasers Med. Sci. 2012, 27, 923–933. [Google Scholar] [CrossRef]
- Sionkowska, A.; Wess, T. Mechanical properties of UV irradiated rat tail tendon (RTT) collagen. Int. J. Biol. Macromol. 2004, 34, 9–12. [Google Scholar] [CrossRef]
- Stylianou, A.; Politopoulos, K.; Kyriazi, M.; Yova, D. Combined information from AFM imaging and SHG signal analysis of collagen thin films. Biomed. Signal Processing Control. 2011, 6, 307–313. [Google Scholar] [CrossRef]
- Stylianou, A.; Yova, D. Atomic Force Microscopy Investigation of the Interaction of Low-Level Laser Irradiation of Collagen Thin Films in Correlation with Fibroblast Response. Lasers Med. Sci. 2015, 30, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A.; Yova, D.; Alexandratou, E. Investigation of the influence of UV irradiation on collagen thin films by AFM imaging. Mater. Sci. Eng. C 2014, 45, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A.; Yova, D.; Alexandratou, E.; Petri, A. Atomic force imaging microscopy investigation of the interaction of ultraviolet radiation with collagen thin films. In Proceedings of the Nanoscale Imaging, Sensing, and Actuation for Biomedical Applications X, San Francisco, CA, USA, 6–7 February 2013. [Google Scholar]
- Stylianou, A.; Yova, D.; Politopoulos, K. Atomic force microscopy surface nanocharacterization of UV-irradiated collagen thin films. In Proceedings of the 12th IEEE International Conference on BioInformatics and BioEngineering, BIBE, Larnaca, Cyprus, 11–13 November 2012; pp. 602–607. [Google Scholar] [CrossRef]
- Baron, E.D.; Suggs, A.K. Introduction to photobiology. Dermatol. Clin. 2014, 32, 255–266. [Google Scholar] [CrossRef]
- Markovitsi, D.; Sage, E.; Lewis, F.D.; Davies, J. Interaction of UV radiation with DNA. Photochem. Photobiol. Sci. 2013, 12, 1256–1258. [Google Scholar] [CrossRef]
- Fessel, G.; Wernli, J.; Li, Y.; Gerber, C.; Snedeker, J.G. Exogenous collagen cross-linking recovers tendon functional integrity in an experimental model of partial tear. J. Orthop. Res. 2012, 30, 973–981. [Google Scholar] [CrossRef]
- Gaspar, A.; Moldovan, L.; Constantin, D.; Stanciuc, A.M.; Sarbu Boeti, P.M.; Efrimescu, I.C. Collagen-based scaffolds for skin tissue engineering. J. Med. Life 2011, 4, 172–177. [Google Scholar]
- Suh, H.; Park, J.C. Evaluation of calcification in porcine valves treated by ultraviolet ray and glutaraldehyde. Mater. Sci. Eng. C 2000, 13, 65–73. [Google Scholar] [CrossRef]
- Fathima, N.N.; Suresh, R.; Rao, J.R.; Nair, B.U.; Ramasami, T. Effect of UV irradiation on stabilized collagen: Role of chromium(III). Colloids Surf. B Biointerfaces 2008, 62, 11–16. [Google Scholar] [CrossRef]
- Jariashvili, K.; Madhan, B.; Brodsky, B.; Kuchava, A.; Namicheishvili, L.; Metreveli, N. Uv damage of collagen: Insights from model collagen peptides. Biopolymers 2012, 97, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Metreveli, N.O.; Jariashvili, K.K.; Namicheishvili, L.O.; Svintradze, D.V.; Chikvaidze, E.N.; Sionkowska, A.; Skopinska, J. UV-vis and FT-IR spectra of ultraviolet irradiated collagen in the presence of antioxidant ascorbic acid. Ecotoxicol. Environ. Saf. 2010, 73, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Skopinska-Wisniewska, J.; Sionkowska, A.; Kaminska, A.; Kaznica, A.; Jachimiak, R.; Drewa, T. Surface characterization of collagen/elastin based biomaterials for tissue regeneration. Appl. Surf. Sci. 2009, 255, 8286–8292. [Google Scholar] [CrossRef]
- Choong, J.K.; Hampson, A.J.; Brody, K.M.; Lo, J.; Bester, C.W.; Gummer, A.W.; Reynolds, N.P.; O’Leary, S.J. Nanomechanical mapping reveals localized stiffening of the basilar membrane after cochlear implantation: Basilar membrane stiffness after CI. Hear. Res. 2020, 385, 107846. [Google Scholar] [CrossRef] [PubMed]
- Kontomaris, S.V.; Georgakopoulos, A.; Malamou, A.; Stylianou, A. The average Young’s modulus as a physical quantity for describing the depth-dependent mechanical properties of cells. Mech. Mater. 2021, 158, 103846. [Google Scholar] [CrossRef]
| Z–Factor (Equation (10)) | Z–Factor (Equation (10)) | ||
|---|---|---|---|
| 0.100 | 1.024 | 0.850 | 1.158 |
| 0.150 | 1.034 | 0.900 | 1.165 |
| 0.200 | 1.045 | 0.950 | 1.172 |
| 0.250 | 1.055 | 1.000 | 1.179 |
| 0.300 | 1.065 | 1.050 | 1.185 |
| 0.350 | 1.074 | 1.100 | 1.191 |
| 0.400 | 1.084 | 1.150 | 1.197 |
| 0.450 | 1.093 | 1.200 | 1.203 |
| 0.500 | 1.103 | 1.250 | 1.208 |
| 0.550 | 1.111 | 1.300 | 1.214 |
| 0.600 | 1.112 | 1.350 | 1.219 |
| 0.650 | 1.128 | 1.400 | 1.223 |
| 0.700 | 1.135 | 1.450 | 1.228 |
| 0.750 | 1.143 | 1.500 | 1.232 |
| 0.800 | 1.151 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontomaris, S.V.; Stylianou, A.; Malamou, A. Atomic Force Microscopy Nanoindentation Method on Collagen Fibrils. Materials 2022, 15, 2477. https://doi.org/10.3390/ma15072477
Kontomaris SV, Stylianou A, Malamou A. Atomic Force Microscopy Nanoindentation Method on Collagen Fibrils. Materials. 2022; 15(7):2477. https://doi.org/10.3390/ma15072477
Chicago/Turabian StyleKontomaris, Stylianos Vasileios, Andreas Stylianou, and Anna Malamou. 2022. "Atomic Force Microscopy Nanoindentation Method on Collagen Fibrils" Materials 15, no. 7: 2477. https://doi.org/10.3390/ma15072477
APA StyleKontomaris, S. V., Stylianou, A., & Malamou, A. (2022). Atomic Force Microscopy Nanoindentation Method on Collagen Fibrils. Materials, 15(7), 2477. https://doi.org/10.3390/ma15072477