Assessing Collagen D-Band Periodicity with Atomic Force Microscopy
Abstract
1. Introduction
1.1. General
1.2. Collagen
1.2.1. Collagen Superfamily and Collagen-Related Pathological Conditions
1.2.2. Collagen Type I
1.2.3. Collagen D-Band Periodicity
1.2.4. Collagen-Based Biomaterials
1.2.5. Imaging Collagen and Collagen-Based Biomaterials
1.3. Atomic Force Microscopy
1.3.1. General
1.3.2. AFM Basic Principles
1.3.3. AFM Modes
1.3.4. AFM Limitations
1.3.5. AFM and Collagen
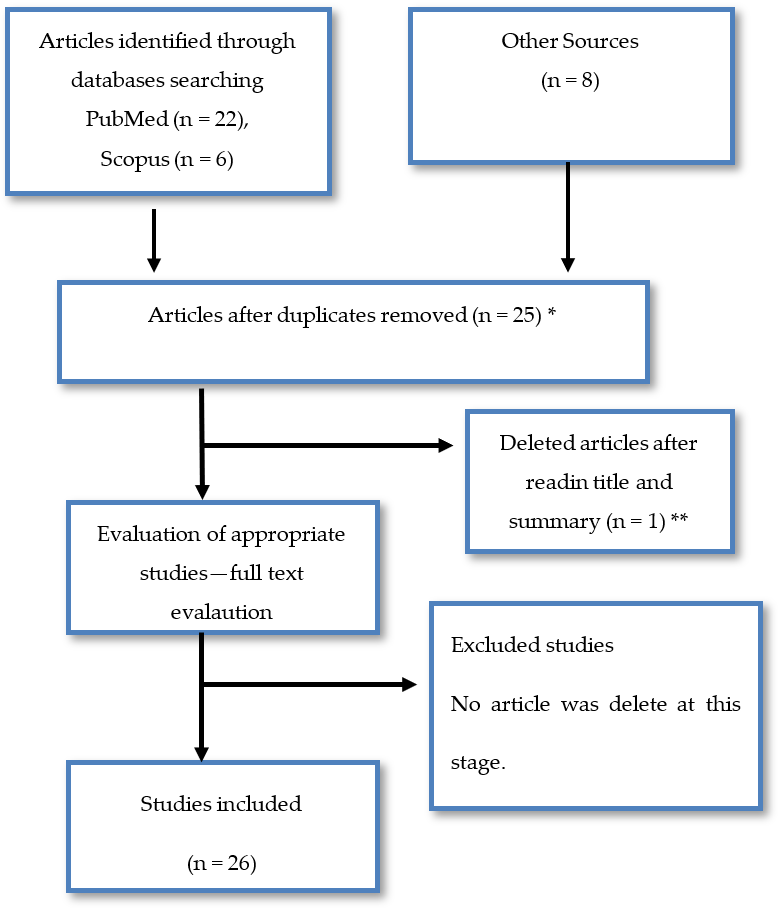
2. Materials and Methods
- (1)
- No systematic studies or review publications;
- (2)
- No conference proceedings;
- (3)
- Be in English language (publications in any other language were not included);
- (4)
- Studies that did not use AFM were not included;
- (5)
- Studies that did not study the D-band were not included.
3. Results
- Year of publication (and authors);
- Type of collagen that the researchers used in their work;
- Type of AFM mode that was applied;
- Environmental conditions under which the experiments were conducted;
- Major results, mainly concerning D-band periodicity.
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fratzl, P. Collagen Structure and Mechanics; Springer: New York, NY, USA, 2008. [Google Scholar]
- Tihan, G.T.; Rău, I.; Zgârian, R.G.; Ghica, M.V. Collagen-based biomaterials for ibuprofen delivery. Comptes Rendus Chim. 2016, 19, 389–393. [Google Scholar] [CrossRef]
- Walters, B.D.; Stegemann, J.P. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014, 10, 1488–1501. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Hulmes, D.J.S. Collagen diversity, synthesis and assembly. In Collagen: Structure and Mechanics; Springer: New York, NY, USA, 2008; pp. 15–47. [Google Scholar]
- Hasirci, V.; Vrana, E.; Zorlutuna, P.; Ndreu, A.; Yilgor, P.; Basmanav, F.B.; Aydin, E. Nanobiomaterials: A review of the existing science and technology, and new approaches. J. Biomater. Sci. Polym. Ed. 2006, 17, 1241–1268. [Google Scholar] [CrossRef]
- Stylianou, A.; Yova, D. Surface nanoscale imaging of collagen thin films by Atomic Force Microscopy. Mater. Sci. Eng. C 2013, 33, 2947–2957. [Google Scholar] [CrossRef]
- Stylianou, A.; Yova, D.; Politopoulos, K. Atomic force microscopy surface nanocharacterization of UV-irradiated collagen thin films. In Proceedings of the 12th IEEE International Conference on BioInformatics and BioEngineering, BIBE, Larnaca, Cyprus, 11–13 November 2012; pp. 602–607. [Google Scholar]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef]
- Morris, V.J.; Kirby, A.R.; Gunning, A.P. Atomic Force Microscopy for Biologists; Imperial College Press: London, UK, 2008. [Google Scholar]
- Gadegaard, N. Atomic force microscopy in biology: Technology and techniques. Biotech. Histochem. 2006, 81, 87–97. [Google Scholar] [CrossRef]
- Stylianou, A.; Yova, D.; Alexandratou, E. Nanotopography of collagen thin films in correlation with fibroblast response. J. Nanophotonics 2013, 7, 073590. [Google Scholar] [CrossRef]
- Engel, J.; Bächinger, H.P. Structure, stability and folding of the collagen triple helix. Top. Curr. Chem. 2005, 247, 7–33. [Google Scholar]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Bozec, L.; Knowles, J.C.; Syed, O.; Mudera, V.; Day, R.; Hyun, J.K. Collagen—Emerging collagen based therapies hit the patient. Adv. Drug Deliv. Rev. 2013, 65, 429–456. [Google Scholar] [CrossRef] [PubMed]
- Åsling, B.; Jirholt, J.; Hammond, P.; Knutsson, M.; Walentinsson, A.; Davidson, G.; Agreus, L.; Lehmann, A.; Lagerström-Fermer, M. Collagen type III alpha I is a gastro-oesophageal reflux disease susceptibility gene and a male risk factor for hiatus hernia. Gut 2009, 58, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a double-edged sword in tumor progression. Tumor Biol. 2014, 35, 2871–2882. [Google Scholar] [CrossRef] [PubMed]
- Gkretsi, V.; Stylianou, A.; Papageorgis, P.; Polydorou, C.; Stylianopoulos, T. Remodeling components of the tumor microenvironment to enhance cancer therapy. Front. Oncol. 2015, 5, 214. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A.; Gkretsi, V.; Louca, M.; Zacharia, L.; Stylianopoulos, T. Collagen Content and Extracellular Matrix Stiffness Remodels Pancreatic Fibroblasts Cytoskeleton. J. R. Soc. Interface 2019, 16, 20190226. [Google Scholar] [CrossRef]
- Stylianou, A.; Gkretsi, V.; Stylianopoulos, T. Transforming Growth Factor-β modulates Pancreatic Cancer Associated Fibroblasts cell shape, stiffness and invasion. Biochim. Biophys. Acta 2018, 1862, 1537–1546. [Google Scholar] [CrossRef]
- Stylianou, A.; Stylianopoulos, T. Atomic Force Microscopy Probing of Cancer Cells and Tumor Microenvironment Components. BioNanoScience 2016, 6, 33–46. [Google Scholar] [CrossRef]
- Birk, D.E.; Bruckner, P. Collagen suprastructures. Top. Curr. Chem. 2005, 247, 185–205. [Google Scholar]
- Brinckmann, J. Collagens at a glance. In Collagen; Brinckmann, J., Mueller, P.K., Notbohm, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 247, pp. 1–6. [Google Scholar]
- Ricard-Blum, S.; Ruggiero, F.; van der Rest, M. The collagen superfamily. Top. Curr. Chem. 2005, 247, 35–84. [Google Scholar]
- Bozec, L.; van der Heijden, G.; Horton, M. Collagen Fibrils: Nanoscale Ropes. Biophys. J. 2007, 92, 70–75. [Google Scholar] [CrossRef]
- Petruska, J.A.; Hodge, A.J. A Subunit Model for the Tropocollagen Macromolecule. Proc. Natl. Acad. Sci. USA 1964, 51, 871–876. [Google Scholar] [CrossRef]
- Wallace, J.M.; Orr, B.G.; Marini, J.C.; Holl, M.M.B. Nanoscale morphology of Type I collagen is altered in the Brtl mouse model of Osteogenesis Imperfecta. J. Struct. Biol. 2011, 173, 146–152. [Google Scholar] [CrossRef]
- Grant, C.A.; Phillips, M.A.; Thomson, N.H. Dynamic mechanical analysis of collagen fibrils at the nanoscale. J. Mech. Behav. Biomed. Mater. 2012, 5, 165–170. [Google Scholar] [CrossRef]
- Fratzl, P. Collagen: Structure and mechanics, an introduction. In Collagen: Structure and Mechanics; Springer: Boston, MA, USA, 2008; pp. 1–13. [Google Scholar]
- Ivanova, V.P.; Krivchenko, A.I. A current viewpoint on structure and evolution of collagens. I. Fibrillar collagens. J. Evol. Biochem. Physiol. 2012, 48, 127–139. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen tissue engineering: Development of novel biomaterials and applications. Pediatr. Res. 2008, 63, 492–496. [Google Scholar] [CrossRef]
- Bender, E.; Silver, F.H.; Hayashi, K. Model conformations of the carboxyl telopeptides in vivo based on type I collagen fibral banding patterns. Coll. Relat. Res. 1983, 3, 407–418. [Google Scholar] [CrossRef]
- Bruns, R.R.; Gross, J. High-resolution analysis of the modified quarter-stagger model of the collagen fibril. Biopolymers 1974, 13, 931–941. [Google Scholar] [CrossRef]
- Ortolani, F.; Giordano, M.; Marchini, M. A model for type II collagen fibrils: Distinctive D-band patterns in native and reconstituted fibrils compared with sequence data for helix and telopeptide domains. Biopolymers 2000, 54, 448–463. [Google Scholar] [CrossRef]
- Stark, M.; Miller, E.J.; Kühn, K. Comparative electron-microscope studies on the collagens extracted from cartilage, bone, and skin. Eur. J. Biochem. 1972, 27, 192–196. [Google Scholar] [CrossRef]
- Wiedemann, H.; Chung, E.; Fujii, T.; Miller, E.J.; Kühn, K. Comparative electron-microscope studies on type-III and type-I collagens. Eur. J. Biochem. 1975, 51, 363–368. [Google Scholar] [CrossRef]
- Kühn, K. Segment-long-spacing crystallites, a powerful tool in collagen research. Coll. Relat. Res. 1982, 2, 61–80. [Google Scholar] [CrossRef]
- Mallinger, R.; Schmut, O. Reaggregation behavior of different types of collagen in vitro: Variations in the occurrence and structure of dimeric segment long-spacing collagen. J. Ultrastruct. Mol. Struct. Res. 1988, 98, 11–18. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hashimoto, Y.; Hayakawa, T.; Hoshino, T. Further evidence for the correlation between the primary structure and the stain exclusion banding pattern of the segment-long-spacing crystallites of collagen. J. Ultrastruct. Mol. Struct. Res. 1988, 100, 255–262. [Google Scholar] [CrossRef]
- Ortolani, F.; Marchini, M. Cartilage type II collagen fibrils show distinctive negative-staining band patterns differences between type II and type I unfixed or glutaraldehyde-fixed collagen fibrils. J. Electron Microsc. 1995, 44, 365–375. [Google Scholar]
- Adachi, E.; Hayashi, T. Comparison of axial banding patterns in fibrils of type V collagen and type I collagen. Coll. Relat. Res. 1987, 7, 27–38. [Google Scholar] [CrossRef]
- Brodsky, B.; Eikenberry, E.F.; Cassidy, K. An unusual collagen periodicity in skin. Biochim. Biophys. Acta 1980, 621, 162–166. [Google Scholar] [CrossRef]
- Marchini, M.; Morocutti, M.; Ruggeri, A.; Koch, M.H.; Bigi, A.; Roveri, N. Differences in the fibril structure of corneal and tendon collagen. An electron microscopy and X-ray diffraction investigation. Connect. Tissue Res. 1986, 15, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.A.; Armitage, P.M. An analysis of fibrous long spacing forms of collagen. Connect. Tissue Res. 1972, 1, 31–37. [Google Scholar] [CrossRef]
- Loo, R.W.; Goh, J.B.; Cheng, C.C.H.; Su, N.; Cynthia Goh, M. In vitro synthesis of native, fibrous long spacing and segmental long spacing collagen. J. Vis. Exp. 2012, 67, e4417–e4425. [Google Scholar] [CrossRef]
- Nakanishi, I.; Masuda, S.; Kitamura, T.; Moriizumi, T.; Kajikawa, K. Distribution of fibrous long-spacing fibers in normal and pathological lymph nodes. Pathol. Int. 1981, 31, 733–745. [Google Scholar] [CrossRef]
- Wen, C.K.; Goh, M.C. Fibrous long spacing type collagen fibrils have a hierarchical internal structure. Proteins Struct. Funct. Genet. 2006, 64, 227–233. [Google Scholar] [CrossRef]
- Highberger, J.H.; Gross, J.; Schmitt, F.O. Electron microscope observations of certain fibrous structures obtained from connective tissue extracts. J. Am. Chem. Soc. 1950, 72, 3321–3322. [Google Scholar] [CrossRef]
- Jakus, M.A. Studies on the cornea. II. The fine structure of Descement’s membrane. J. Biophys. Biochem. Cytol. 1956, 2, 243–252. [Google Scholar] [CrossRef]
- Cauna, N.; Ross, L.L. The fine structure of Meissner’s touch corpuscles of human fingers. J. Biophys. Biochem. Cytol. 1960, 8, 467–482. [Google Scholar] [CrossRef]
- Luse, S.A. Electron microscopic studies of brain tumors. Neurology 1960, 10, 881–905. [Google Scholar] [CrossRef]
- Poole, K.; Khairy, K.; Friedrichs, J.; Franz, C.; Cisneros, D.A.; Howard, J.; Mueller, D.; Baumeister, W. Molecular-scale topographic cues induce the orientation and directional movement of fibroblasts on two-dimensional collagen surfaces. J. Mol. Biol. 2005, 349, 380–386. [Google Scholar] [CrossRef]
- Stamov, D.R.; Müller, A.; Wegrowski, Y.; Brezillon, S.; Franz, C.M. Quantitative analysis of type I collagen fibril regulation by lumican and decorin using AFM. J. Struct. Biol. 2013, 183, 394–403. [Google Scholar] [CrossRef]
- Fang, M.; Holl, M.M.B. Variation in type I collagen fibril nanomorphology: The significance and origin. Bonekey Rep. 2013, 2, 394. [Google Scholar] [CrossRef]
- Gross, J.; Schmitt, F.O. The structure of human skin collagen as studied with the electron microscope. J. Exp. Med. 1948, 88, 555–568. [Google Scholar] [CrossRef]
- Beniash, E.; Traub, W.; Veis, A.; Weiner, S. A transmission electron microscope study using vitrified ice sections of predentin: Structural changes in the dentin collagenous matrix prior to mineralization. J. Struct. Biol. 2000, 132, 212–225. [Google Scholar] [CrossRef]
- Habelitz, S.; Balooch, M.; Marshall, S.J.; Balooch, G.; Marshall, G.W., Jr. In situ atomic force microscopy of partially demineralized human dentin collagen fibrils. J. Struct. Biol. 2002, 138, 227–236. [Google Scholar] [CrossRef]
- Meek, K.M. The cornea and sclera. In Collagen: Structure and Mechanics; Springer: Boston, MA, USA, 2008; pp. 359–396. [Google Scholar]
- Plant, A.L.; Bhadriraju, K.; Spurlin, T.A.; Elliott, J.T. Cell response to matrix mechanics: Focus on collagen. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 893–902. [Google Scholar] [CrossRef]
- Loesberg, W.A.; te Riet, J.; van Delft, F.C.M.J.M.; Schön, P.; Figdor, C.G.; Speller, S.; van Loon, J.J.W.A.; Walboomers, X.F.; Jansen, J.A. The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion. Biomaterials 2007, 28, 3944–3951. [Google Scholar] [CrossRef]
- Lisboa, P.; Villiers, M.B.; Brakha, C.; Marche, P.N.; Valsesia, A.; Colpo, P.; Rossi, F. Fabrication of bio-functionalised polypyrrole nanoarrays for bio-molecular recognition. Micro Nanosyst. 2011, 3, 83–89. [Google Scholar] [CrossRef][Green Version]
- Brouwer, K.M.; van Rensch, P.; Harbers, V.E.; Geutjes, P.J.; Koens, M.J.; Wijnen, R.M.; Daamen, W.F.; van Kuppevelt, T.H. Evaluation of methods for the construction of collagenous scaffolds with a radial pore structure for tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 501–504. [Google Scholar] [CrossRef]
- Silver, F.H.; Freeman, J.W.; Seehra, G.P. Collagen self-assembly and the development of tendon mechanical properties. J. Biomech. 2003, 36, 1529–1553. [Google Scholar] [CrossRef]
- Phong, H.Q.; Wang, S.L.; Wang, M.J. Cell behaviors on micro-patterned porous thin films. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2010, 169, 94–100. [Google Scholar] [CrossRef]
- Tay, C.Y.; Irvine, S.A.; Boey, F.Y.C.; Tan, L.P.; Venkatraman, S. Micro-/nano-engineered cellular responses for soft tissue engineering and biomedical applications. Small 2011, 7, 1361–1378. [Google Scholar] [CrossRef]
- Stylianou, A. Atomic force microscopy for collagen-based nanobiomaterials. J. Nanomater. 2017, 2017, 9234627. [Google Scholar] [CrossRef]
- Stylianou, A.; Kontomaris, S.V.; Yova, D. Assessing Collagen Nanoscale Thin Films Heterogeneity by AFM Multimode Imaging and Nanoindetation for NanoBioMedical Applications. Micro Nanosyst. 2014, 6, 95–102. [Google Scholar] [CrossRef]
- Stylianou, A.; Kontomaris, S.B.; Kyriazi, M.; Yova, D. Surface characterization of collagen films by atomic force microscopy. In Proceedings of the 12th Mediterranean Conference on Medical and Biological Engineering and Computing, MEDICON, Chalkidiki, Greece, 23–30 May 2010; Volume 29, pp. 612–615. [Google Scholar]
- Garcia, A.M.; Magalhes, F.L.; Soares, J.S.; Junior, E.P.; de Lima, M.F.R.; Mamede, M.; de Paula, A.M. Second harmonic generation imaging of the collagen architecture in prostate cancer tissue. Biomed. Phys. Eng. Express 2018, 4, 025026. [Google Scholar] [CrossRef]
- Kim, B.M.; Eichler, J.; Reiser, K.M.; Rubenchik, A.M.; Da Silva, L.B. Collagen structure and nonlinear susceptibility: Effects of heat, glycation, and enzymatic cleavage on second harmonic signal intensity. Lasers Surg. Med. 2000, 27, 329–335. [Google Scholar] [CrossRef]
- Han, M.; Giese, G.; Bille, J.F. Second harmonic generation imaging of collagen fibrils in cornea and sciera. Opt. Express 2005, 13, 5791–5797. [Google Scholar] [CrossRef]
- Tuer, A.E.; Krouglov, S.; Prent, N.; Cisek, R.; Sandkuijl, D.; Yasufuku, K.; Wilson, B.C.; Barzda, V. Nonlinear optical properties of type i collagen fibers studied by polarization dependent second harmonic generation microscopy. J. Phys. Chem. B 2011, 115, 12759–12769. [Google Scholar] [CrossRef]
- Psilodimitrakopoulos, S.; Filippidis, G.; Kouloumentas, C.; Alexandratou, E.; Yova, D. Combined two photon excited fluorescence and second harmonic generation imaging microscopy of collagen structures. Proc. SPIE 2006, 6089, 60891P. [Google Scholar]
- Drifka, C.R.; Loeffler, A.G.; Mathewson, K.; Mehta, G.; Keikhosravi, A.; Liu, Y.; Lemancik, S.; Ricke, W.A.; Weber, S.M.; Kao, W.J.; et al. Comparison of Picrosirius Red Staining With Second Harmonic Generation Imaging for the Quantification of Clinically Relevant Collagen Fiber Features in Histopathology Samples. J. Histochem. Cytochem. 2016, 64, 519–529. [Google Scholar] [CrossRef]
- Rittié, L. Method for Picrosirius Red-Polarization Detection of Collagen Fibers in Tissue Sections. Methods Mol. Biol. 2017, 1627, 395–407. [Google Scholar]
- Stylianou, A.; Voutouri, C.; Mpekris, F.; Stylianopoulos, T. Pancreatic cancer collagen-based optical signatures. In Proceedings of the Polarized Light and Optical Angular Momentum for Biomedical Diagnostics, online, 6–12 March 2021; International Society for Optics and Photonics (SPIE): Bellingham, WA, USA, 2021; Volume 11646. [Google Scholar]
- Starborg, T.; Kalson, N.S.; Lu, Y.; Mironov, A.; Cootes, T.F.; Holmes, D.F.; Kadler, K.E. Using transmission electron microscopy and 3View to determine collagen fibril size and three-dimensional organization. Nat. Protoc. 2013, 8, 1433–1448. [Google Scholar] [CrossRef]
- Starborg, T.; Lu, Y.; Kadler, K.E.; Holmes, D.F. Chapter 17 Electron Microscopy of Collagen Fibril Structure In Vitro and In Vivo Including Three-Dimensional Reconstruction. In Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 2008; Volume 88, pp. 319–345. [Google Scholar]
- Maurer, T.; Stoffel, M.H.; Belyaev, Y.; Stiefel, N.G.; Vidondo, B.; Küker, S.; Mogel, H.; Schäfer, B.; Balmer, J. Structural characterization of four different naturally occurring porcine collagen membranes suitable for medical applications. PLoS ONE 2018, 13, e0205027. [Google Scholar] [CrossRef]
- Hodge, A.J.; Schmitt, F.O. The charge profile of the tropocollagen macromolecule and the packing arrangement in native-type collagen fibrils. Proc. Natl. Acad. Sci. USA 1960, 46, 186–197. [Google Scholar] [CrossRef]
- Ushiki, T. Collagen Fibers, Reticular Fibers and Elastic Fibers. A Comprehensive Understanding from a Morphological Viewpoint. Arch. Histol. Cytol. 2002, 65, 109–126. [Google Scholar] [CrossRef]
- Ruprecht, J.; Nield, J. Determining the structure of biological macromolecules by transmission electron microscopy, single particle analysis and 3D reconstruction. Prog. Biophys. Mol. Biol. 2001, 75, 121–164. [Google Scholar] [CrossRef]
- Baumeister, W.; Grimm, R.; Walz, J. Electron tomography of molecules and cells. Trends Cell Biol. 1999, 9, 81–85. [Google Scholar] [CrossRef]
- Allison, D.P.; Mortensen, N.P.; Sullivan, C.J.; Doktycz, M.J. Atomic force microscopy of biological samples. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 618–634. [Google Scholar] [CrossRef]
- Stylianou, A.; Yova, D.; Politopoulos, K. Atomic force microscopy quantitative and qualitative nanoscale characterization of collagen thin films. In Proceedings of the 5th International Conference on Emerging Technologies in Non-Destructive Testing, NDT, Ioannina, Greece, 10–21 September 2011; pp. 415–420. [Google Scholar]
- Stylianou, A.; Politopoulos, K.; Yova, D. Atomic force microscopy imaging of the nanoscale assembly of type i collagen on controlled polystyrene particles surfaces. In Proceedings of the 5th European Conference of the International Federation for Medical and Biological Engineering, Budapest, Hungary, 14–18 September 2011; Volume 37, pp. 1058–1061. [Google Scholar]
- Kontomaris, S.V.; Stylianou, A.; Yova, D.; Politopoulos, K. Mechanical properties of collagen fibrils on thin films by Atomic Force Microscopy nanoindentation. In Proceedings of the 12th IEEE International Conference on BioInformatics and BioEngineering, BIBE, Lancarna, Cyprus, 11–13 November 2012; pp. 608–613. [Google Scholar]
- Stylianou, A.; Kontomaris, S.V.; Yova, D.; Balogiannis, G. AFM Multimode Imaging and Nanoindetation Method for Assessing Collagen Nanoscale Thin Films Heterogeneity. IFMBE Proc. 2014, 41, 407–410. [Google Scholar]
- Kontomaris, S.V.; Stylianou, A.; Yova, D.; Balogiannis, G. The effects of UV irradiation on collagen D-band revealed by atomic force microscopy. Scanning 2015, 37, 101–111. [Google Scholar] [CrossRef]
- Keysight-Technologies Keysight 5500 Scanning Probe Microscope-User’s Guide. Available online: http://nano.em.keysight.com/PDFs/5500%20User%20Guide%20Dec%202015%20REV%20H.pdf (accessed on 16 October 2021).
- Han, W.; Serry, F.M. Force Spectroscopy with the Atomic Force Microscope-Application Note; Agilent Technologies: Santa Clara, CA, USA, 2008. [Google Scholar]
- Stolz, M.; Raiteri, R.; Daniels, A.U.; VanLandingham, M.R.; Baschong, W.; Aebi, U. Dynamic Elastic Modulus of Porcine Articular Cartilage Determined at Two Different Levels of Tissue Organization by Indentation-Type Atomic Force Microscopy. Biophys. J. 2004, 86, 3269–3283. [Google Scholar] [CrossRef]
- Stolz, M.; Gottardi, R.; Raiteri, R.; Miot, S.; Martin, I.; Imer, R.; Staufer, U.; Raducanu, A.; Düggelin, M.; Baschong, W.; et al. Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force microscopy. Nat. Nanotechnol. 2009, 4, 186–192. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Darling, E.M. Force scanning: A rapid, high-resolution approach for spatial mechanical property mapping. Nanotechnology 2011, 22, 175707. [Google Scholar] [CrossRef]
- Braunsmann, C.; Seifert, J.; Rheinlaender, J.; Schäffer, T.E. High-speed force mapping on living cells with a small cantilever atomic force microscope. Rev. Sci. Instrum. 2014, 85, 073703. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Yova, D.; Stylianou, A.; Politopoulos, K. The significance of the percentage differences of young’s modulus in the AFM nanoindentation procedure. Micro Nanosyst. 2015, 7, 86–97. [Google Scholar] [CrossRef]
- Hertz, H. Ueber die Berührung fester elastischer Körper. J. Für Die Reine Und Angew. Math. 1882, 1882, 156–171. [Google Scholar]
- Mackay, J.L.; Kumar, S. Measuring the elastic properties of living cells with atomic force microscopy indentation. Methods Mol. Biol. 2013, 931, 313–329. [Google Scholar]
- Kontomaris, S.V.; Stylianou, A. Atomic force microscopy for university students: Applications in biomaterials. Eur. J. Phys. 2017, 38, 033003. [Google Scholar] [CrossRef]
- Graham, H.K.; Hodson, N.W.; Hoyland, J.A.; Millward-Sadler, S.J.; Garrod, D.; Scothern, A.; Griffiths, C.E.M.; Watson, R.E.B.; Cox, T.R.; Erler, J.T.; et al. Tissue section AFM: In situ ultrastructural imaging of native biomolecules. Matrix Biol. 2010, 29, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ushiki, T.; Hitomi, J.; Ogura, S.; Umemoto, T.; Shigeno, M. Atomic force microscopy in histology and cytology. Arch. Histol. Cytol. 1996, 59, 421–431. [Google Scholar] [PubMed]
- Strange, A.P.; Aguayo, S.; Ahmed, T.; Mordan, N.; Stratton, R.; Porter, S.R.; Parekh, S.; Bozec, L. Quantitative nanohistological investigation of scleroderma: An atomic force microscopy-based approach to disease characterization. Int. J. Nanomed. 2017, 12, 411–420. [Google Scholar] [CrossRef][Green Version]
- Gisbert, V.G.; Benaglia, S.; Uhlig, M.R.; Proksch, R.; Garcia, R. High-Speed Nanomechanical Mapping of the Early Stages of Collagen Growth by Bimodal Force Microscopy. ACS Nano 2021, 15, 1850–1857. [Google Scholar] [CrossRef]
- Cisneros, D.A.; Friedrichs, J.; Taubenberger, A.; Franz, C.M.; Muller, D.J. Creating ultrathin nanoscopic collagen matrices for biological and biotechnological applications. Small 2007, 3, 956–963. [Google Scholar] [CrossRef]
- Chernoff, E.A.G.; Chernoff, D.A. Atomic force microscope images of collagen fibers. J. Vac. Sci. Technol. A 1992, 10, 596–599. [Google Scholar] [CrossRef]
- Baselt, D.R.; Revel, J.P.; Baldeschwieler, J.D. Subfibrillar structure of type I collagen observed by atomic force microscopy. Biophys. J. 1993, 65, 2644–2655. [Google Scholar] [CrossRef]
- Aragno, I.; Odetti, P.; Altamura, F.; Cavalleri, O.; Rolandi, R. Structure of rat tail tendon collagen examined by atomic force microscope. Experientia 1995, 51, 1063–1067. [Google Scholar] [CrossRef]
- Odetti, P.; Aragno, I.; Altamura, F.; Rolandi, R. Study of aging rat tail collagen using atomic force microscope. Aging Clin. Exp. Res. 1995, 7, 352–357. [Google Scholar] [CrossRef]
- Odetti, P.; Aragno, I.; Rolandi, R.; Garibaldi, S.; Valentini, S.; Cosso, L.; Traverso, N.; Cottalasso, D.; Pronzato, M.A.; Marinari, U.M. Scanning force microscopy reveals structural alterations in diabetic rat collagen fibrils: Role of protein glycation. Diabetes Metab. Res. Rev. 2000, 16, 74–81. [Google Scholar] [CrossRef]
- Siperko, L.M.; Landis, W.J. Aspects of mineral structure in normally calcifying avian tendon. J. Struct. Biol. 2001, 135, 313–320. [Google Scholar] [CrossRef]
- Wang, H.; Layton, B.E.; Sastry, A.M. Nerve collagens from diabetic and nondiabetic Sprague-Dawley and biobreeding rats: An atomic force microscopy study. Diabetes Metab. Res. Rev. 2003, 19, 288–298. [Google Scholar] [CrossRef]
- Jastrzebska, M.; Zalewska-Rejdak, J.; Mróz, I.; Barwinski, B.; Wrzalik, R.; Kocot, A.; Nozynski, J. Atomic force microscopy and FT-IR spectroscopy investigations of human heart valves. Gen. Physiol. Biophys. 2006, 25, 231–244. [Google Scholar]
- Cisneros, D.A.; Hung, C.; Franz, C.M.; Muller, D.J. Observing growth steps of collagen self-assembly by time-lapse high-resolution atomic force microscopy. J. Struct. Biol. 2006, 154, 232–245. [Google Scholar] [CrossRef]
- Heinemann, S.; Ehrlich, H.; Douglas, T.; Heinemann, C.; Worch, H.; Schatton, W.; Hanke, T. Ultrastructural studies on the collagen of the marine sponge Chondrosia reniformis Nardo. Biomacromolecules 2007, 8, 3452–3457. [Google Scholar] [CrossRef]
- Strasser, S.; Zink, A.; Janko, M.; Heckl, W.M.; Thalhammer, S. Structural investigations on native collagen type I fibrils using AFM. Biochem. Biophys. Res. Commun. 2007, 354, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Minary-Jolandan, M.; Yu, M.F. Nanomechanical heterogeneity in the gap and overlap regions of type I collagen fibrils with implications for bone heterogeneity. Biomacromolecules 2009, 10, 2565–2570. [Google Scholar] [CrossRef]
- Hurng, J.M.; Kurylo, M.P.; Marshall, G.W.; Webb, S.M.; Ryder, M.I.; Ho, S.P. Discontinuities in the human bone-PDL-cementum complex. Biomaterials 2011, 32, 7106–7117. [Google Scholar] [CrossRef]
- Fang, M.; Liroff, K.G.; Turner, A.S.; Les, C.M.; Orr, B.G.; Holl, M.M.B. Estrogen depletion results in nanoscale morphology changes in dermal collagen. J. Investig. Dermatol. 2012, 132, 1791–1797. [Google Scholar] [CrossRef]
- Fang, M.; Goldstein, E.L.; Turner, A.S.; Les, C.M.; Orr, B.G.; Fisher, G.J.; Welch, K.B.; Rothman, E.D.; Banaszak Holl, M.M. Type I collagen D-spacing in fibril bundles of dermis, tendon, and bone: Bridging between nano- and micro-level tissue hierarchy. ACS Nano 2012, 6, 9503–9514. [Google Scholar] [CrossRef]
- Erickson, B.; Fang, M.; Wallace, J.M.; Orr, B.G.; Les, C.M.; Banaszak Holl, M.M. Nanoscale structure of type I collagen fibrils: Quantitative measurement of D-spacing. Biotechnol. J. 2013, 8, 117–126. [Google Scholar] [CrossRef]
- Gudzenko, T.; Franz, C.M. Inverting adherent cells for visualizing ECM interactions at the basal cell side. Ultramicroscopy 2013, 128, 1–9. [Google Scholar] [CrossRef]
- Hammond, M.A.; Gallant, M.A.; Burr, D.B.; Wallace, J.M. Nanoscale changes in collagen are reflected in physical and mechanical properties of bone at the microscale in diabetic rats. Bone 2013, 60, 26–32. [Google Scholar] [CrossRef]
- Stylianou, A.; Yova, D.; Alexandratou, E. Investigation of the influence of UV irradiation on collagen thin films by AFM imaging. Mater. Sci. Eng. C 2014, 45, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Spitzner, E.C.; Röper, S.; Zerson, M.; Bernstein, A.; Magerle, R. Nanoscale Swelling Heterogeneities in Type I Collagen Fibrils. ACS Nano 2015, 9, 5683–5694. [Google Scholar] [CrossRef]
- Wallace, J.M. Effects of fixation and demineralization on bone collagen D-spacing as analyzed by atomic force microscopy. Connect. Tissue Res. 2015, 56, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Watanabe-Nakayama, T.; Itami, M.; Kodera, N.; Ando, T.; Konno, H. High-speed atomic force microscopy reveals strongly polarized movement of clostridial collagenase along collagen fibrils. Sci. Rep. 2016, 6, 28975. [Google Scholar] [CrossRef]
- Uhlig, M.R.; Magerle, R. Unraveling capillary interaction and viscoelastic response in atomic force microscopy of hydrated collagen fibrils. Nanoscale 2017, 9, 1244–1256. [Google Scholar] [CrossRef]
- Stylianou, A.; Gkretsi, V.; Stylianopoulos, T. Atomic force microscopy nano-characterization of 3D collagen gels with tunable stiffness. MethodsX 2018, 5, 503–513. [Google Scholar] [CrossRef]
- Peacock, C.J.; Kreplak, L. Nanomechanical mapping of single collagen fibrils under tension. Nanoscale 2019, 11, 14417–14425. [Google Scholar] [CrossRef]
- Baldwin, S.J.; Kreplak, L.; Lee, J.M. MMP-9 selectively cleaves non-D-banded material on collagen fibrils with discrete plasticity damage in mechanically-overloaded tendon. J. Mech. Behav. Biomed. Mater. 2019, 95, 67–75. [Google Scholar] [CrossRef]
- Cauble, M.A.; Mancini, N.S.; Kalinowski, J.; Lykotrafitis, G.; Moss, I.L. Atomic force microscopy imaging for nanoscale and microscale assessments of extracellular matrix in intervertebral disc and degeneration. JOR Spine 2020, 3, e1125. [Google Scholar] [CrossRef]
- Zhong, Q.; Inniss, D.; Kjoller, K.; Elings, V.B. Fractured polymer/silica fiber surface studied by tapping mode atomic force microscopy. Surf. Sci. 1993, 290, L688–L692. [Google Scholar] [CrossRef]

| Term | Key Words 1 |
|---|---|
| Collagen D-band | 1. “Collagen D-band” 2. “Collagen D band” 3. “Collagen D-periodicity” 4. “Collagen D periodicity” 5. “Collagen D-spacing” 6. “Collagen D spacing” |
| 7. #1 OR #2 OR #3 OR #4 OR #5 OR #6 | |
| Atomic Force Microscopy | 8. “Atomic Force Microscopy” 9. “AFM” |
| 10. #8 OR #9 | |
| 11. #7 AND #10 | |
| A/A | Authors (Year) Reference | Collagen | AFM | Environment | Results Concerning D-Band | |
|---|---|---|---|---|---|---|
| D-Band Values (nm) | General Results | |||||
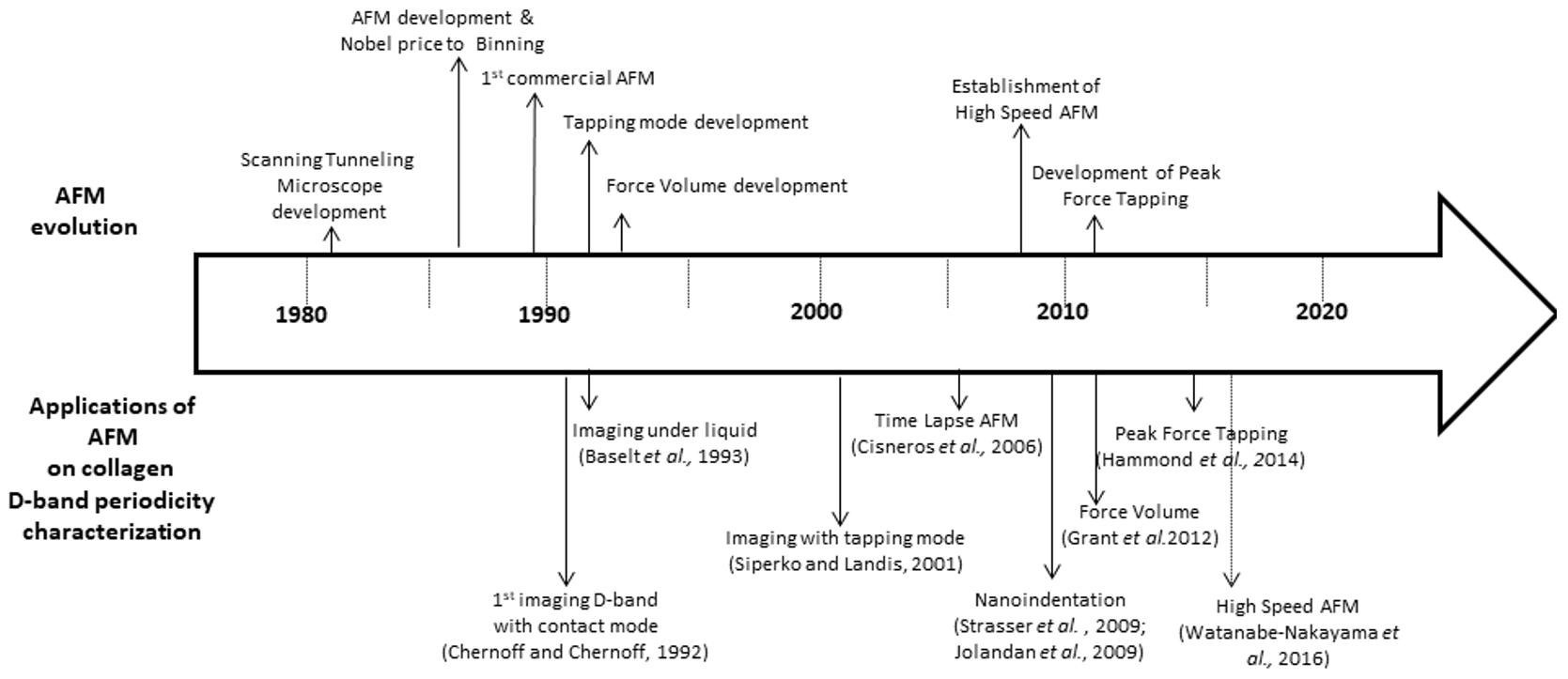
| 1 | Chernoff and Chernoff (1992) [108] | Fibril-forming and monomeric type I collagen from type I bovine skin collagen | Not specified [contact mode] | Air | 70 | Collagen fibrils that were imaged with AFM presented the characteristic D-band periodicity (~70 nm). [Probably the first demonstration of collagen D-band periodicity with AFM] |
| 2 | Baselt et al. (1993) [109] | Native rat tail and reconstituted bovine dermal type I collagen | Not specified [contact mode] | Air/Water | 60–70 | The authors performed AFM imaging of the D periodicity (60–70 nm) in collagen type I from native rat tail and reconstituted bovine dermal fibrils. They also found a “minor” period and “microfibrils” that were arranged either parallel to or inclined −5° to the fibril axis. |
| 3 | Aragno et al. (1995) [110] | Collagen (type I) from rat tail tendon fibers | Contact mode in height mode | Air | 67.0 ± 0.1 | Study collagen fibrils at different fibrillogenesis times. Although, the fibrils presented an increasing size with time, the band interval did not change. In addition, the depth of the D-band periodicity remained stable after an initial increase. |
| 4 | Odetti et al. (1995) [111] | Collagen (type I) from young and old rat tail tendons. | Contact mode in height mode | Air | Young rats 67.0 ± 0.1 Old rats 66.5 ± 0.1 | Collagen from young and old rats presented the same D-band interval, but collagen in the aged rats was characterized by lower widths and heights. Furthermore, collagen from old rats presented a higher value in the depth of gap between two overlaps. |
| 5 | Odetti et al. (2000) [112] | Tendon fibrils (collagen type I) from tails of rats which developed diabetes and from diabetes-resistant rats of the same strain. | Not specified [probably contact mode] | Air at room temperature and a humidity range of 40 ± 50%. | Non-diabetic 67.0 ± 0.1 Diabetic 67.0 ± 0.1 Glucose-incubated fibrils 67.3 ± 0.2 | They studied modification in collagen structure caused by high glycose concentration. Non-diabetic fibrils present differences in radius and gap depth compared to the diabetic and glucose incubated fibrils. Although alterations were observed in gap depth, the D-band periodicity was the same in the studied groups, demonstrating that non-enzymatic glycation did not alter the axial organization. |
| 6 | Siperko and Landis (2001) [113] | Collagen and minerals in tendons from turkey (not mentioned what collagen, but in tendons is type I) | Tapping Mode | Air (ambient laboratory conditions) | 62 ± 8 | AFM images showed numerous mineralized collagen fibrils that were align parallel to each other and they were presenting the characteristic D-band periodicity. In addition, in some occasions, mineral plates were observed between and in intimate contact with collagen fibrils. |
| 7 | Wang et al. (2003) [114] | Nerve and tail tendon collagen from control and diabetic rats. (Sprague-Dawley rats—SD and biobreeding rats—BB.) Collagen type is not specified (probably type I) | Not specified [probably contact mode] | Air | 63–69 Sciatic nerve SD rats Control 66.8 ± 3.8 (epi) 63.6 ± 3.6 (endo) Diabetic 64.7 ± 4.3 (epi) 63.8 ± 4.9 (endo) BB rats Control 67.8 ± 3.2 (epi) 65.5 ± 4.0 (endo) Diabetic 68.1 ± 2.8 (epi) 68.5 ± 3.8 (endo) Tail tendon BB rat Control 69.4 ± 2.4 Diabetic 67.9 ± 2.2 | Collagen fibrils from diabetic rats presented higher diameters compared to collagen from control rats. No significant changes were observed in D-band periodicity that varied from 63 to 69 nm. |
| 8 | Jastrzebska et al. (2006) [115] | Collagen structure in human aortic, mitral, tricuspid and pulmonary heart valves (the collagen component of the specimens is predominantly type I (74%) and type III (24%)) | Contact mode | Air | 70–80 | The valves with different origins demonstrated a significant heterogeneity as far the collagen fibrils’ surface topography. Almost all the fibrils acquired the characteristic D-band periodicity, with the band interval presenting a wide range of values. |
| 9 | Cisneros et al. (2006) [116] | Solubilized bovine dermal collagen (type I) | Time-lapse Tapping Mode | Buffer | ~67 | In this study, the researchers achieved the in vitro assembly of collagen fibrils with the natural occurring D-band periodicity of ~67 nm. It was shown that the assembly is a two-step process, where the molecules firstly assemble with each other and, subsequently, the molecules are rearranged into microfibrils. |
| 10 | Heinemann et al. (2007) [117] | Ιsolated fibrils of Chondrosia reniformis sponge collagen. | Tapping mode | Air | Chondrosia collagen 67–69 (but consisting by different gap and overlap zones) | In this investigation, by using AFM, the morphology of collagen from sponge was compared with the morphology of other fibril-forming collagens. It was found that sponge collagen presented a quite different D-band periodicity consisting of overlap zones followed by 2 identical gap zones. |
| 11 | Strasser et al. 2009 [118] | Collagen solution from calf skin (collagen type I, in vitro fibrous long spacing collagen fibers). | Non-Contact mode Nanoindentation | Air (with control humidity) | 78 | In this work, the authors report the AFM microdissection technique, where they cut collagen fibrils in order to study both the core and the outer shell of the fibril. The results concerning collagen fibrils’ structure demonstrated that the D-band periodicity can be found also in the core and not only on the shell of the fibrils. In addition, nanomechanical measurements indicated that the D-band periodicity both in the core and the shell have the same Young’s modulus values. |
| 12 | Jolandan et al. 2009 [119] | Type I collagen fibrils from bovine Achilles tendon | Tapping mode Contact mode Dynamic Nanoindentation Static Nanoindentation | Air | ~67 nm | The authors demonstrated the mechanical heterogeneity along the axial direction of a single isolated collagen fibril from tendon and showed that, within the D period, the gap and overlapping regions have significantly different elastic and energy dissipation properties, correlating the significantly different molecular structures in these two regions |
| 13 | Hurng et al. (2011) [120] | Collagen from human fibrous joints (specific type of collagen is not provided). | Contact mode | Air and hydrated conditions | Not provided | AFM demonstrated structural reorganization of the periodontal ligament (PDL), collagen spacing, organic-dominant areas at the PDL- cementum and PDL-bone entheses and within cementum and bone. |
| 14 | Wallace et al. 2011 [27] | Collagen type I from murine femurs in strains that present D-band periodicity alterations related to osteogenesis imperfecta | Tapping mode | Air | Wild Type 67.6 Heterozygous (Brtl/+) mice 67.4 | AFM was used to image and quantitatively characterize the D-band periodicity of type I collagen fibrils related to osteogenesis imperfecta. |
| 15 | Fang et al. (2012) [121] | Ovine dermal sections (type I collagen) | Contact mode | Air | Ovariectomized61.9 Control 62.3 | Using AFM, it was shown that after estrogen depletion, nano-scale morphological alterations of dermis collagen fibrils occurred. After 2 years of ovariectomy in ovine dermal samples, a new subpopulation of fibrils with D-band periodicity was found. In addition, it was found that the overall width of the distribution was increased. |
| 16 | Grant et al. 2012 [28] | Collagen type I fibrils from rat tail tendons | Force Volume Imaging | Air | 67.4 ± 1.8 | The authors conducted low-frequency dynamic mechanical analysis on individual collagen type I fibrils in order for elastic and viscous responses to be correlated to the D-band periodicity. |
| 17 | Fang et al. (2012) [122] | Specimen from ovine dermis and bone. Human skin biopsies and tendons were also acquired. (Collagen type I) | Contact mode | Air | 58–69 | In this research work, it was found that the D-band periodicity of collagen fibrils, within a single bundle, from specimens of different origins, was nearly identical and frequently differs by less than 1 nm. In addition, similarity in D-band periodicity for up to 40 μm in bundle length and width was observed, and it was demonstrated that D-band periodicity presents differences at the bundle level, independent of species or tissue types (dermis, tendon, and bone). |
| 18 | Erickson et al. (2013) [123] | Type I collagen morphology in disease models from dermal sheep skin of estrogen depletion and osteogenesis imperfecta | Tapping mode | Air and water | Air 63.1 ± 1.9 Water 62.2 ± 2.0 | In this work, a quantitative approach that combined AFM and 2D Fast Fourier Transform was used for measuring D-band periodicity. The authors demonstrated that in the case of estrogen depletion (that is correlated with osteogenesis imperfecta and early stage of osteoporosis), it is the D-band periodicity that presented statistically significant differences and not the D-spacing mean. |
| 19 | Gudzenko and Franz (2013) [124] | Bovine collagen type I monomers Mixed with ITC- conjugated bovine collagen type I | Contact mode | Liquid (PBS) | 67 | The authors of this study investigated the adherent cells/ECM interactions at the basal cell side. A number of different substrates were used, including collagen substrates presenting natural characteristics, such as the D-band periodicity. |
| 20 | Hammond et al. (2014) [125] | Right tibiae and tails from control and diabetic rats (collagen type I). | (Peak force) tapping mode | Air | Bone Control 66.1 ± 0.8 Diabetic 66.5 ± 1.5 Tendon Control 68.4 ± 0.2 Diabetic 68.6 ± 1.2 | Diabetic bones had had a noticeable different distribution of collagen D-band periodicity than controls. The distribution from the diabetic bones was characterized by variability and higher values. The shift to higher values in D-band periodicity distribution was more evident in the case of tendons. Diabetes in rat promotes alterations to the nanoscale morphology of collagen, which results in nano-mechanical and compositional effects in bones. |
| 21 | Stylianou et al. (2014) [126] | Collagen solution and thin films formed with spin coating procedure from type I collagen from bovine Achilles tendon. | Contact and Tapping Mode | Air | 67 | UV irradiation was applied for both collagen solution and thin films. For short irradiation times, AFM imaging showed modification in surface roughness. Additionally, different effects were found when UV irradiation was performed on collagen solution compared to irradiation on thin films. Fibroblasts responded on surface alterations after UV irradiation of both collagen solution and films. Long irradiation intervals deformed fibrils revealing a number of inner shells. In addition, it was found that collagen D-band periodicity is presented not only in the outer shells but also to the inner ones. |
| 22 | Spitzner et al. (2015) [127] | Type I collagen isolated from bovine hide | MUSIC-mode AFM/ Nano-indentation | Air with control humidity | 67 | In this study, they investigated the impact of water on collagen type I. It was found that during swelling, gap and overlap present a difference in the water uptake. This result is direct evidence for different amounts of bound and free water within the gap and overlap regions. On the one hand, in the dry state, the D-band periodicity that is recorded by AFM imaging is due to height corrugations along a fibril’s axis. On the other hand, in the hydrated state, the surface of the fibril is smoother and D-band periodicity presents different nanomechanical properties in the gap/overlap regions. |
| 23 | Wallace (2015) [128] | Specimens from the anterior diaphysis of a pig femur. Samples were demineralized to expose collagen. Also, mouse tail tendons were characterized. (collagen type I) | (Peak force) tapping mode | Air (dry and wet samples) | Control 64.9 ± 0.4 Fixed 65.8 ± 0.2 | The aim of this work was to explore whether the fixation of bone maintain collagen ultrastructure. Specimens were studied with AFM and the results showed that after fixation D-band periodicity variability was decreased. In addition, it was found that D-band periodicity had higher average periodicity compared to control samples. Furthermore, data from tendons showed that after fixation of drying do not significantly affect collagen structure as it presents characteristics similar with those of its native state. |
| 24 | Kontomaris et al. (2015) [91] | UV irradiated type I collagen from bovine Achilles tendon | Force–distance (FD) and contact mode AFM imaging | Air | 67 | The results showed that the UV irradiation influence the height level differences between the gap and overlap regions. In addition, it was found that the Young’s modulus values were reduced after irradiation, confirming that UV affects collagen fibrils mechanical properties. |
| 25 | Watanabe-Nakayama et al. (2016) [129] | Rat tail type I collagen | High speed Tapping mode | Water | 67 Overlap 36.18 Gap 30.82 | They studied the movement of clostridial collagenase along collagen fibrils |
| 26 | Uhlig and Magerle (2017) [130] | Reconstituted type I collagen fibrils (acid-extracted, purified type I collagen from bovine calf hide) | force–distance (FD) and amplitude-phasedistance (APD) measurements | Air with controlled relative humidity | [not measured]. | The research results of this study showed that differences in the mechanical properties of the gap and ovelrap regions are only observed in the top 2 nm but not in the fibril’s bulk. |
| 27 | Stylianou et al. (2018) [131] | 3D collagen type I gels (rat tail) | force–distance (FD), tapping and contact mode AFM imaging | Ari histological sections from collagen gels | [not mentioned] | Developed protocol for imaging and measuring mechanical properties of collagen fibers with D-band with AFM. |
| 28 | Peacock and Kreplak (2019) [132] | Tendon from the forelimb of an 18–24 months old steer. [Collagen type is not mentioned but tendons are rich in collagen type I]. | Force–distance curves (obtained in Peak Force QNM) | Liquid (PBS) | 67 ± 2 | In this work, single collagen fibrils under tension were studied. The results demonstrated that upon 5–30% stretching a radial stiffening is observed consistent with the fibrils being under tension. This is correlated with an increase in D-band length. Furthermore, the indentation modulus contrast which is relevant with the D-band periodicity was increased linearly with D-band strain. |
| 29 | Baldwin et al. (2019) [133] | Tendons from bovine tails (steers aged 18–24 months). [Collagen type is not specified but tendons are rich in collagen type I]. | Tapping mode | Air (hydrated) | [Not measured]. | In this work, AFM was used in order to study trypsin and MMP-9 enzymatic removal of material from fibrils which were: (i) untreated, (ii) partially heat denatured, (iii) or displaying discrete plasticity damaged after repeated mechanical overload. The results demonstrated that both enzymes removed material from the two groups and not the untreated groups. Interestingly, the researchers showed that MMP-9 presented selective removal of non-D-banded material, especially in the case of the damaged fibrils. |
| 30 | Cauble et al. (2020) [134] | Mouse tails from a murine model of degeneration of the intervertebral disc. The intervertebral disc (IVD) consists of: -the annulus fibrosus (AF), comprised of type I collagen -nucleus pulposus (NP), comprised of proteoglycan and type II collagen. | Contact mode | Air | Nondegenerate annulus fibrosus 61.1 ± 2.9 Degenerate annulus fibrosus 62.6 ± 2.3 Degenerate nucleus pulposus 62.6 ± 2.3 | The researchers showed that in the case of degenerative discs, the fibril D-band periodicity distribution shifted to higher values in the annulus fibrosus, as well as in nucleus pulposus. In addition, a novel microstructural feature, collagen toroids, defined by a topographical pit enclosed by fibril forming matrix was observed in the nucleus pulposus. After degeneration alterations were observed, including increase in the number of these structures, while they were reshaping to oval microstructures instead of circular ones. |
| 31 | Gisbert et al. 2021 [106] | Monomeric bovine collagen type I | High-speed bimodal AFM | Buffer | 67 | The authors reported the development of a high-speed bimodal AFM. This system can offer maps with high spatial resolution of the: elastic modulus, loss tangent, topography. The developed microscope was used for investigating the initial stages of collagen self-assembly. The researchers, based on alteration in the physical properties, found 4 distinct stages: (i) nucleation and growth of collagen precursors, (ii) formation of tropocollagen molecules, (iii) assembly of tropocollagens into microfibrils, and (iv) alignment of microfibrils to generate microribbons). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stylianou, A. Assessing Collagen D-Band Periodicity with Atomic Force Microscopy. Materials 2022, 15, 1608. https://doi.org/10.3390/ma15041608
Stylianou A. Assessing Collagen D-Band Periodicity with Atomic Force Microscopy. Materials. 2022; 15(4):1608. https://doi.org/10.3390/ma15041608
Chicago/Turabian StyleStylianou, Andreas. 2022. "Assessing Collagen D-Band Periodicity with Atomic Force Microscopy" Materials 15, no. 4: 1608. https://doi.org/10.3390/ma15041608
APA StyleStylianou, A. (2022). Assessing Collagen D-Band Periodicity with Atomic Force Microscopy. Materials, 15(4), 1608. https://doi.org/10.3390/ma15041608






