Clinical and Radiographic Outcomes of Zirconia Dental Implants—A Clinical Case Series Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Description
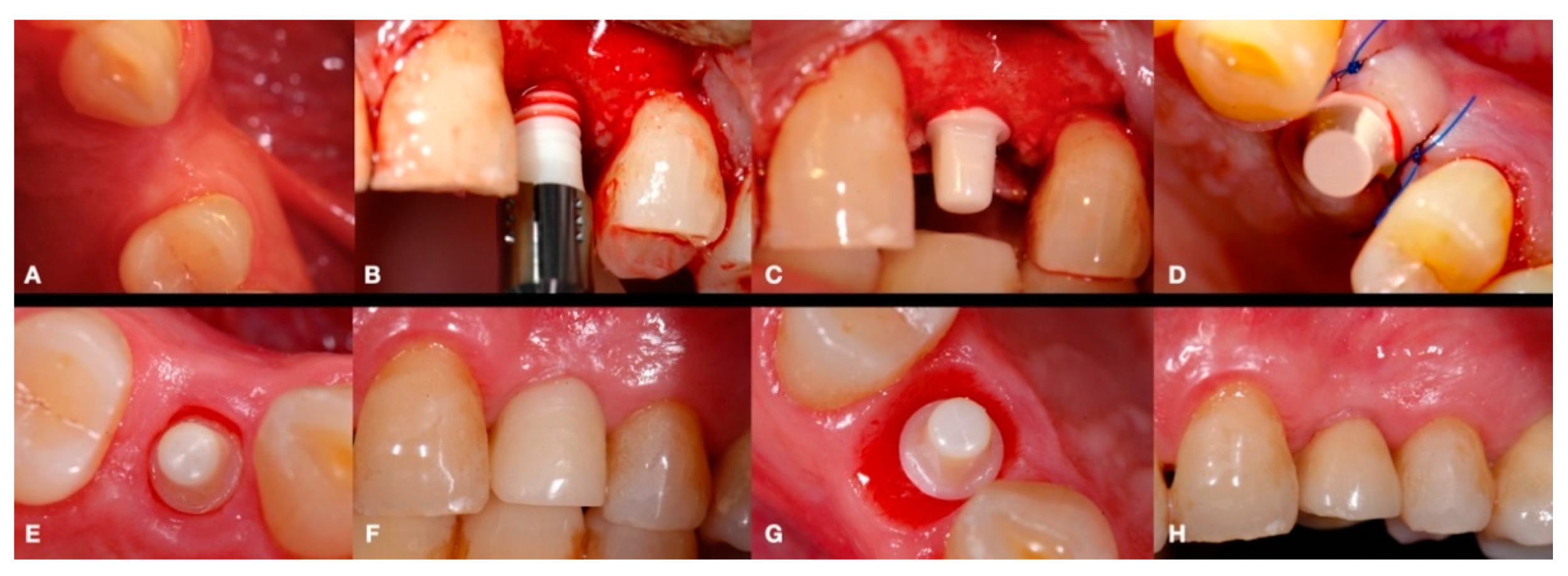
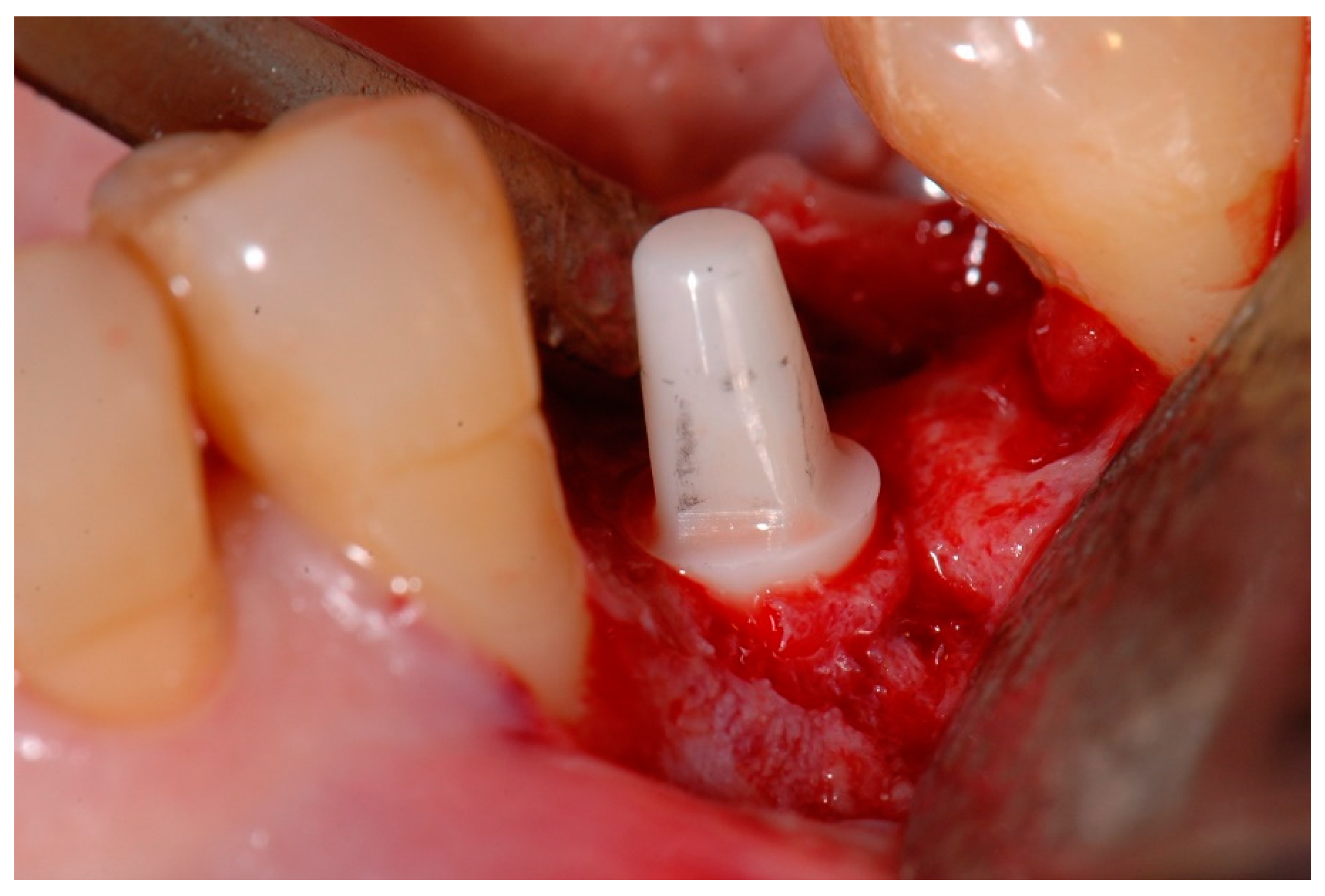
2.2. Surgical Procedures
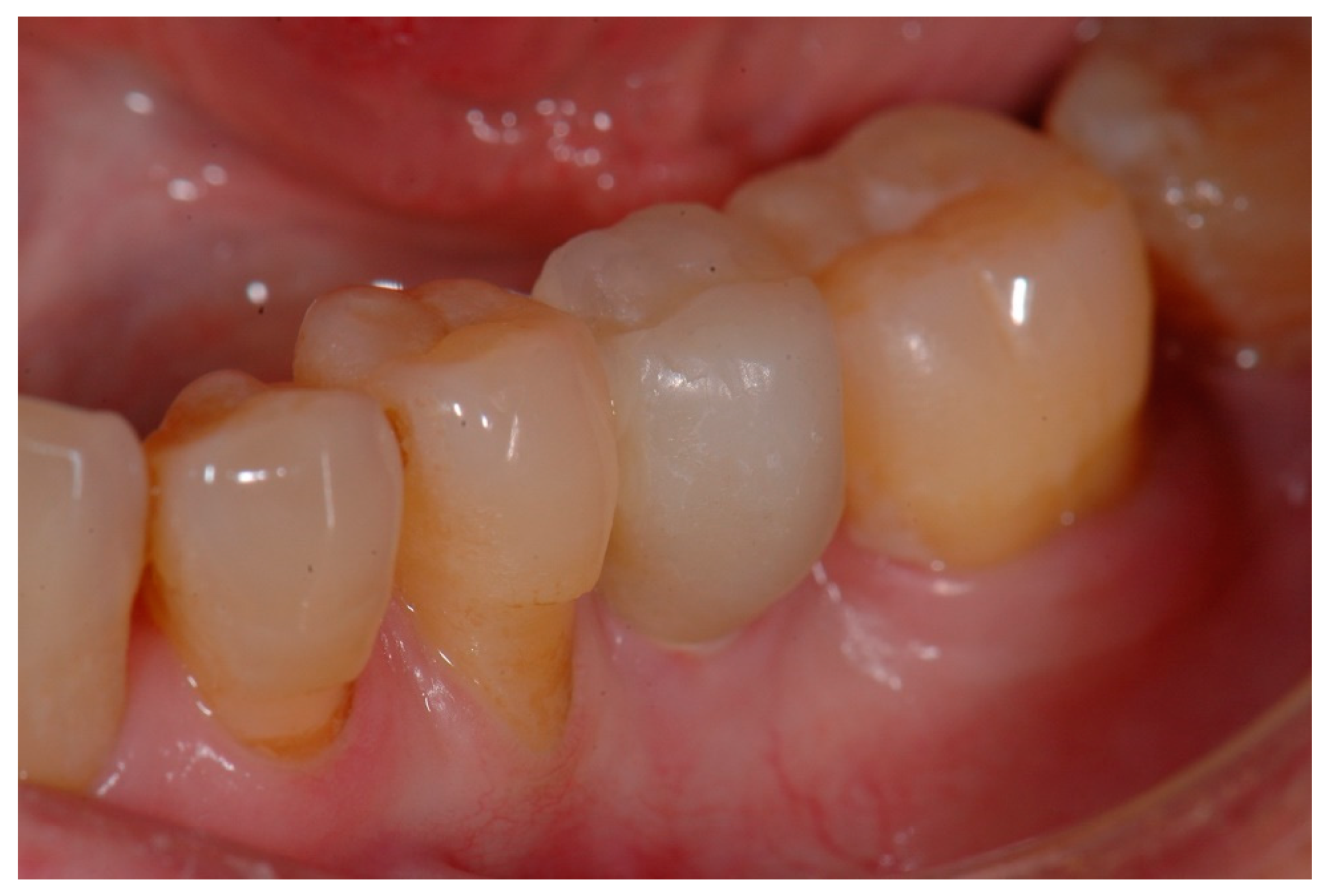
2.3. Prosthetic Restoration
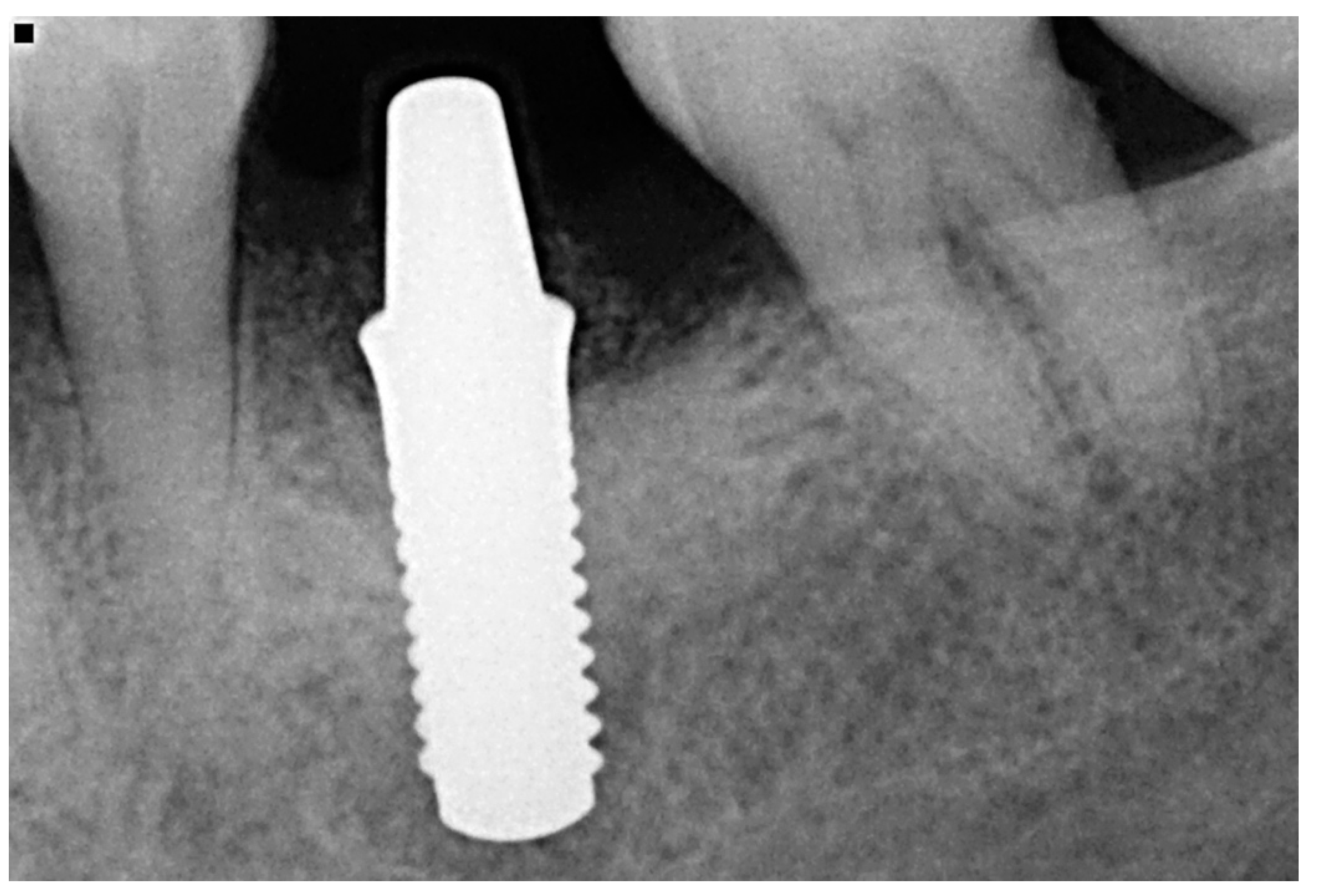
2.4. Clinical and Radiographic Parameters
3. Results
3.1. Sample Analysis
3.2. Clinical and Radiological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buser, D.; Janner, S.F.; Wittneben, J.G.; Bragger, U.; Ramseier, C.A.; Salvi, G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant. Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin. Oral Impants Res. 2008, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Safioti, L.M.; Kotsakis, G.A.; Pozhitkov, A.E.; Chung, W.O.; Daubert, D.M. Increased levels of dissolved titanium are associated with peri-implantitis—A cross-sectional study. J. Periodontol. 2017, 88, 436–442. [Google Scholar] [CrossRef]
- Halperin-Sternfeld, M.; Sabo, E.; Akrish, S. The pathogenesis of implant-related reactive lesions: A clinical, histologic and polarized light microscopy study. J. Periodontol. 2016, 87, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, D.G.; Paparella, M.L.; Brandizzi, D.; Cabrini, R.L. Reactive lesions of peri-implant mucosa associated with titanium dental implants: A report of 2 cases. Int. J. Oral Maxillofac. Surg. 2010, 39, 503–507. [Google Scholar] [CrossRef]
- Liu, F.X.; Wu, C.L.; Zhu, Z.A.; Li, M.Q.; Mao, Y.Q.; Liu, M.; Wang, X.Q.; Yu, D.G.; Tang, T.T. Calcineurin/nfat pathway mediates wear particle-induced tnf-alpha release and osteoclastogenesis from mice bone marrow macrophages in vitro. Acta Pharmacol. Sin. 2013, 34, 1457–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, K.; Kato, T.; Ito, T.; Oda, T.; Sekine, H.; Yoshinari, M.; Yajima, Y. Influence of titanium ions on cytokine levels of murine splenocytes stimulated with periodontopathic bacterial lipopolysaccharide. Int. J. Oral Maxillofac. Implants 2014, 29, 472–477. [Google Scholar]
- Pettersson, M.; Kelk, P.; Belibasakis, G.N.; Bylund, D.; Molin Thoren, M.; Johansson, A. Titanium ions form particles that activate and execute interleukin-1beta release from lipopolysaccharide-primed macrophages. J. Periodontal Res. 2017, 52, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Van Brakel, R.; Noordmans, H.J.; Frenken, J.; de Roode, R.; de Wit, G.C.; Cune, M.S. The effect of zirconia and titanium implant abutments on light reflection of the supporting soft tissues. Clin. Oral Implants Res. 2011, 22, 1172–1178. [Google Scholar] [CrossRef] [Green Version]
- Andreiotelli, M.; Wenz, H.J.; Kohal, R.J. Are ceramic implants a viable alternative to titanium implants? A systematic literature review. Clin. Oral Implants Res. 2009, 20 (Suppl. 4), 32–47. [Google Scholar] [CrossRef]
- Payer, M.; Arnetzl, V.; Kirmeier, R.; Koller, M.; Arnetzl, G.; Jakse, N. Immediate provisional restoration of single-piece zirconia implants: A prospective case series—Results after 24 months of clinical function. Clin. Oral Implants Res. 2013, 24, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Manzano, G.; Herrero, L.R.; Montero, J. Comparison of clinical performance of zirconia implants and titanium implants in animal models: A systematic review. Int. J. Oral Maxillofac. Implants 2014, 29, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depprich, R.; Naujoks, C.; Ommerborn, M.; Schwarz, F.; Kubler, N.R.; Handschel, J. Current findings regarding zirconia implants. Clin. Implant. Dent. Relat. Res. 2014, 16, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.J.; Langhoff, J.D.; Voelter, K.; von Rechenberg, B.; Scharnweber, D.; Bierbaum, S.; Schnabelrauch, M.; Kautz, A.R.; Frauchiger, V.M.; Mueller, T.L.; et al. Biomechanical comparison of different surface modifications for dental implants. Int. J. Oral Maxillofac. Implants 2008, 23, 1037–1046. [Google Scholar]
- Langhoff, J.D.; Voelter, K.; Scharnweber, D.; Schnabelrauch, M.; Schlottig, F.; Hefti, T.; Kalchofner, K.; Nuss, K.; von Rechenberg, B. Comparison of chemically and pharmaceutically modified titanium and zirconia implant surfaces in dentistry: A study in sheep. Int. J. Oral Maxillofac. Surg. 2008, 37, 1125–1132. [Google Scholar] [CrossRef]
- Koch, F.P.; Weng, D.; Kramer, S.; Biesterfeld, S.; Jahn-Eimermacher, A.; Wagner, W. Osseointegration of one-piece zirconia implants compared with a titanium implant of identical design: A histomorphometric study in the dog. Clin. Oral Implants Res. 2010, 21, 350–356. [Google Scholar] [CrossRef]
- Gahlert, M.; Gudehus, T.; Eichhorn, S.; Steinhauser, E.; Kniha, H.; Erhardt, W. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clin. Oral Implants Res. 2007, 18, 662–668. [Google Scholar] [CrossRef]
- Sennerby, L.; Dasmah, A.; Larsson, B.; Iverhed, M. Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin. Implant. Dent. Relat. Res. 2005, 7, S13–S20. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, B. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Gahlert, M.; Röhling, S.; Wieland, M.; Eichhorn, S.; Küchenhoff, H.; Kniha, H. A comparison study of the osseointegration of zirconia and titanium dental implants. A biomechanical evaluation in the maxilla of pigs. Clin. Implant. Dent. Relat. Res. 2010, 12, 297–305. [Google Scholar] [CrossRef]
- Krautwald, L.; Smeets, R.; Stolzer, C.; Rutkowski, R.; Guo, L.; Reitmeier, A.; Gosau, M.; Henningsen, A. Osseointegration of zirconia implants after UV-light or cold atmospheric plasma surface treatment in vivo. Materials 2022, 15, 496. [Google Scholar] [CrossRef] [PubMed]
- Vohra, F.; Al-Kheraif, A.A.; Ab Ghani, S.M.; Abu Hassan, M.I.; Alnassar, T.; Javed, F. Crestal bone loss and periimplant inflammatory parameters around zirconia implants: A systematic review. J. Prosthet. Dent. 2015, 114, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontol. 2000 2017, 73, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Elnayef, B.; Lazaro, A.; Suarez-Lopez Del Amo, F.; Galindo-Moreno, P.; Wang, H.L.; Gargallo-Albiol, J.; Hernandez-Alfaro, F. Zirconia implants as an alternative to titanium: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implants 2017, 32, e125–e134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gahlert, M.; Burtscher, D.; Pfundstein, G.; Grunert, I.; Kniha, H.; Roehling, S. Dental zirconia implants up to three years in function: A retrospective clinical study and evaluation of prosthetic restorations and failures. Int. J. Oral Maxillofac. Implants 2013, 28, 896–904. [Google Scholar] [CrossRef] [Green Version]
- Borgonovo, A.E.; Censi, R.; Vavassori, V.; Dolci, M.; Calvo-Guirado, J.L.; Delgado Ruiz, R.A.; Maiorana, C. Evaluation of the success criteria for zirconia dental Implants: A four-year clinical and radiological study. Int. J. Dent. 2013, 2013, 463073. [Google Scholar] [CrossRef]
- Vilor-Fernández, V.; García-de-la-Fuente, A.M.; Marichalar-Mendia, X.; Estefanía-Fresco, R.; Aguirre-Zorzano, L.A. Single tooth restoration in the maxillary esthetic zone using a one-piece ceramic implant with 1 year of follow-up: Case series. Int. J. Implant Dent. 2021, 7, 26. [Google Scholar] [CrossRef]
- Balmer, M.; Spies, B.C.; Vach, K.; Ralf-Joachim, K.; Hämmerle, C.H.F.; Jung, R.E. Three-year analysis of zirconia implants used for single-tooth replacement and three unit fixed dental prostheses: A prospective multicenter study. Clin. Oral Implants Res. 2018, 29, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Hammerle, C.H.; Chen, S.T.; Wilson, T.G., Jr. Consensus statements and recommended clinical procedures regarding the placement of implants in extraction sockets. Int. J. Oral Maxillofac. Implants 2004, 19, 26–28. [Google Scholar]
- Bornstein, M.M.; Lauber, R.; Sendi, P.; von Arx, T. Comparison of periapical radiography and limited cone-beam computed tomography in mandibular molars for analysis of anatomical landmarks before apical surgery. J. Endod. 2011, 37, 151–157. [Google Scholar] [CrossRef]
- Kohal, R.J.; Knauf, M.; Larsson, B.; Sahlin, H.; Butz, F. One-piece zirconia oral implants: One-year results from a prospective cohort study. 1. Single tooth replacement. J. Clin. Periodontol. 2012, 39, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Roehling, S.; Woelfler, H.; Hicklin, S.; Kniha, H.; Gahlert, M. A retrospective clinical study with regard to survival and success rates of zirconia implants up to and after 7 years of loading. Clin. Implant. Dent. Relat. Res. 2016, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Belser, U.; Buser, D.; Higginbottom, F. Consensus statements and recommended clinical procedures regarding esthetics in implant dentistry. Int. J. Oral Maxillofac. Implants 2004, 19, 73–74. [Google Scholar] [PubMed]
- Glauser, R.; Sailer, I.; Wohlwend, A.; Studer, S.; Schibli, M.; Scharer, P. Experimental zirconia abutments for implant-supported single-tooth restorations in esthetically demanding regions: 4-year results of a prospective clinical study. Int. J. Prosthodont. 2004, 17, 285–290. [Google Scholar] [PubMed]
- Rimondini, L.; Cerroni, L.; Carrassi, A.; Torricelli, P. Bacterial colonization of zirconia ceramic surfaces: An in vitro and in vivo study. Int. J. Oral Maxillofac. Implants 2002, 17, 793–798. [Google Scholar] [PubMed]
- Chappuis, V.; Buser, R.; Bragger, U.; Bornstein, M.M.; Salvi, G.E.; Buser, D. Long-term outcomes of dental implants with a titanium plasma-sprayed surface: A 20-year prospective case series study in partially edentulous patients. Clin. Implant Dent. Relat. Res. 2013, 15, 780–790. [Google Scholar] [CrossRef]
- Rodriguez, A.E.; Monzavi, M.; Yokoyama, C.L.; Nowzari, H. Zirconia dental implants: A clinical and radiographic evaluation. J. Esthet. Restor. Dent. 2018, 30, 538–544. [Google Scholar] [CrossRef]
- Borgonovo, A.E.; Fabbri, A.; Vavassori, V.; Censi, R.; Maiorana, C. Multiple teeth replacement with endosseous one-piece yttrium-stabilized zirconia dental implants. Med. Oral Patol. Oral Cir. Bucal. 2012, 17, e981–e987. [Google Scholar] [CrossRef]
- Kohorst, P.; Borchers, L.; Strempel, J.; Stiesch, M.; Hassel, T.; Bach, F.W.; Hubsch, C. Low-temperature degradation of different zirconia ceramics for dental applications. Acta Biomater. 2012, 8, 1213–1220. [Google Scholar] [CrossRef]
- Lughi, V.; Sergo, V. Low temperature degradation -aging- of zirconia: A critical review of the relevant aspects in dentistry. Dent. Mater. 2010, 26, 807–820. [Google Scholar] [CrossRef]
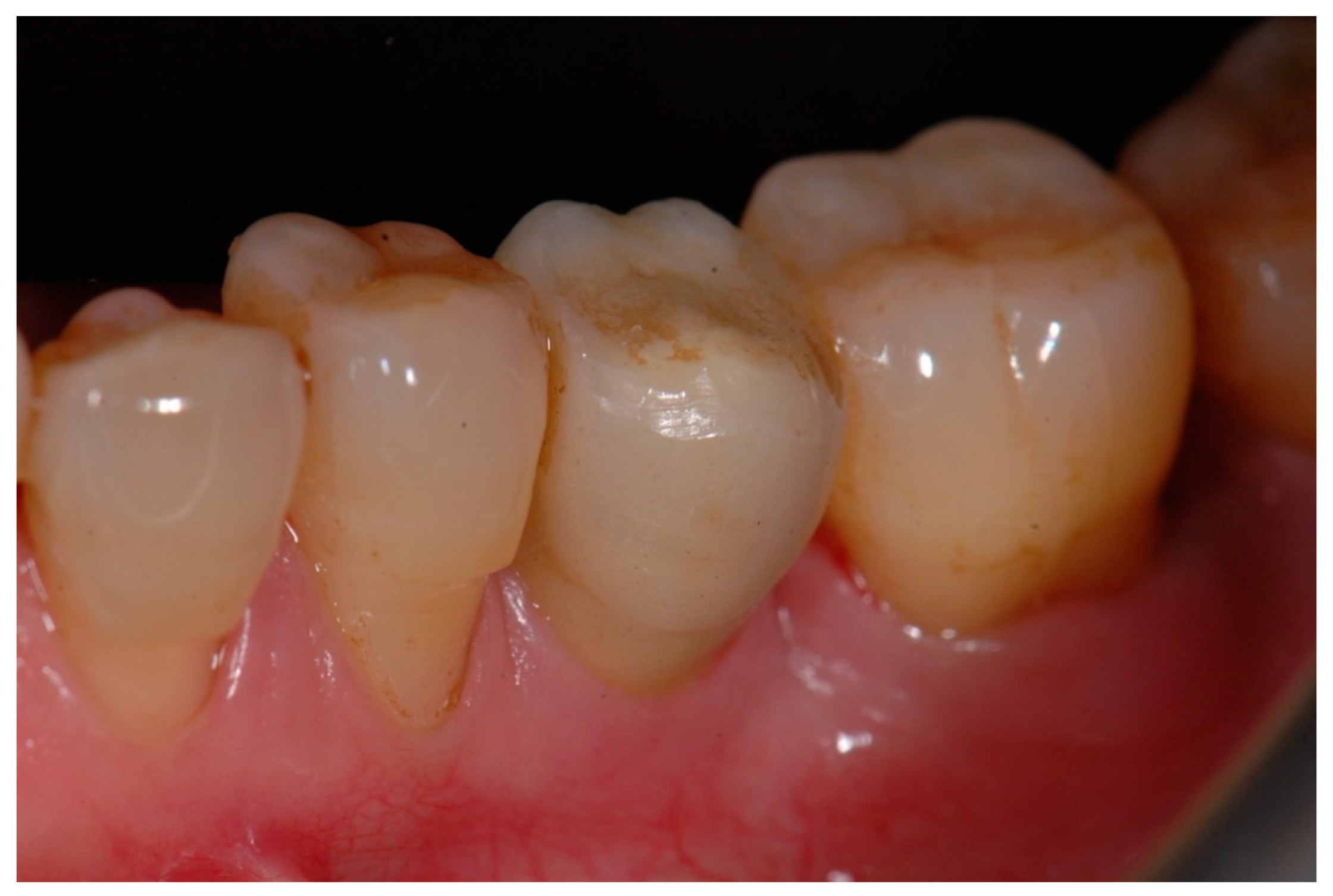
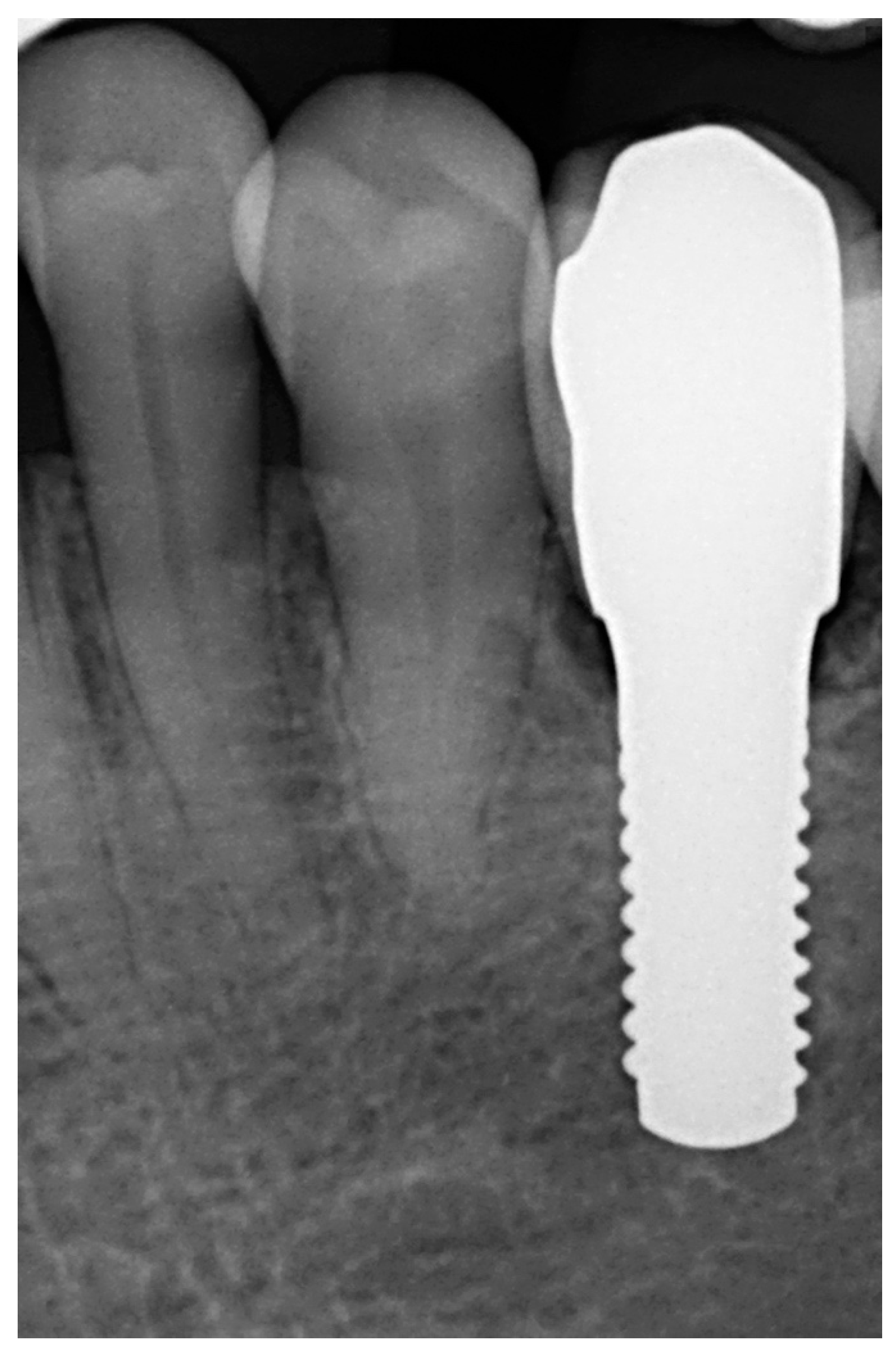
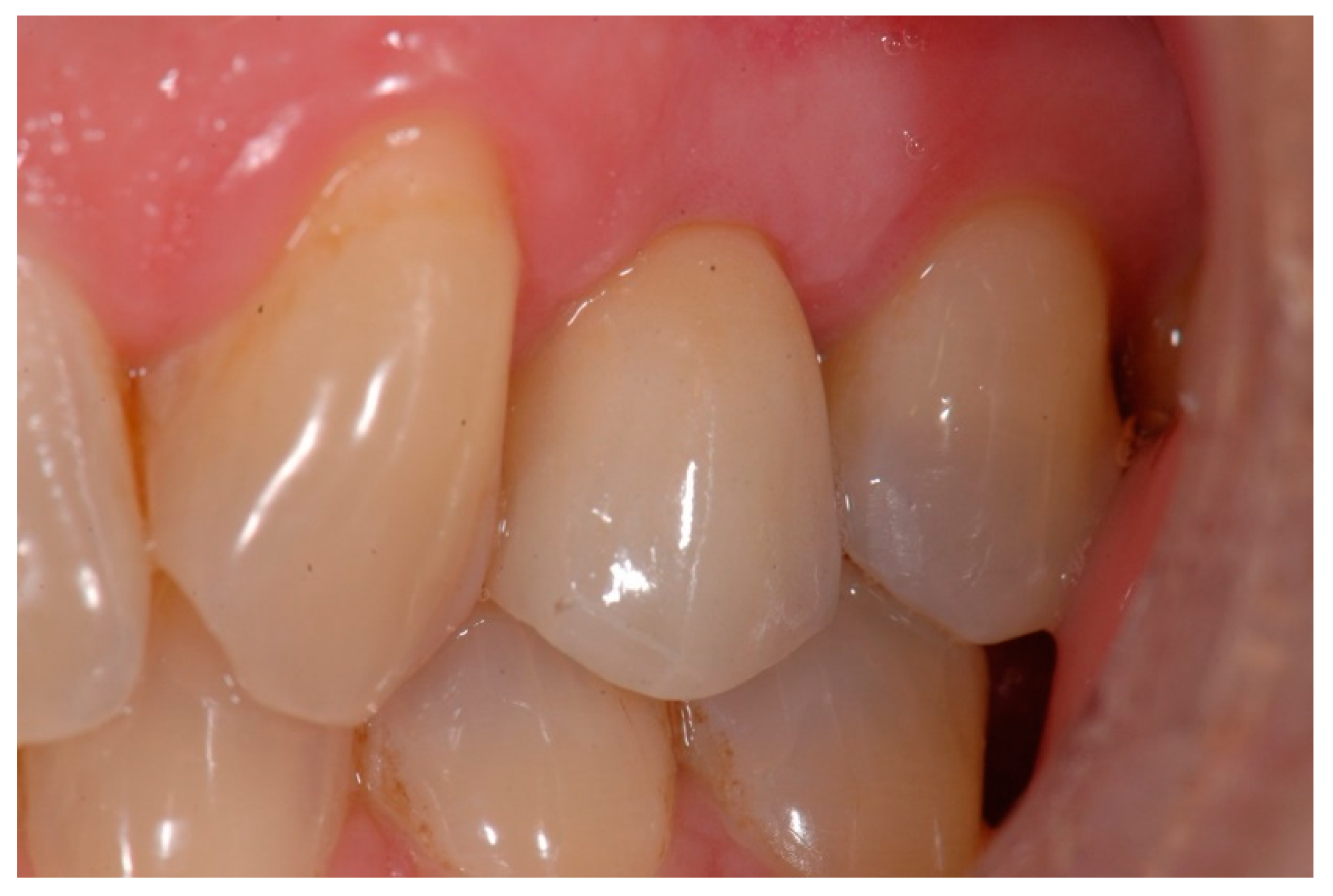
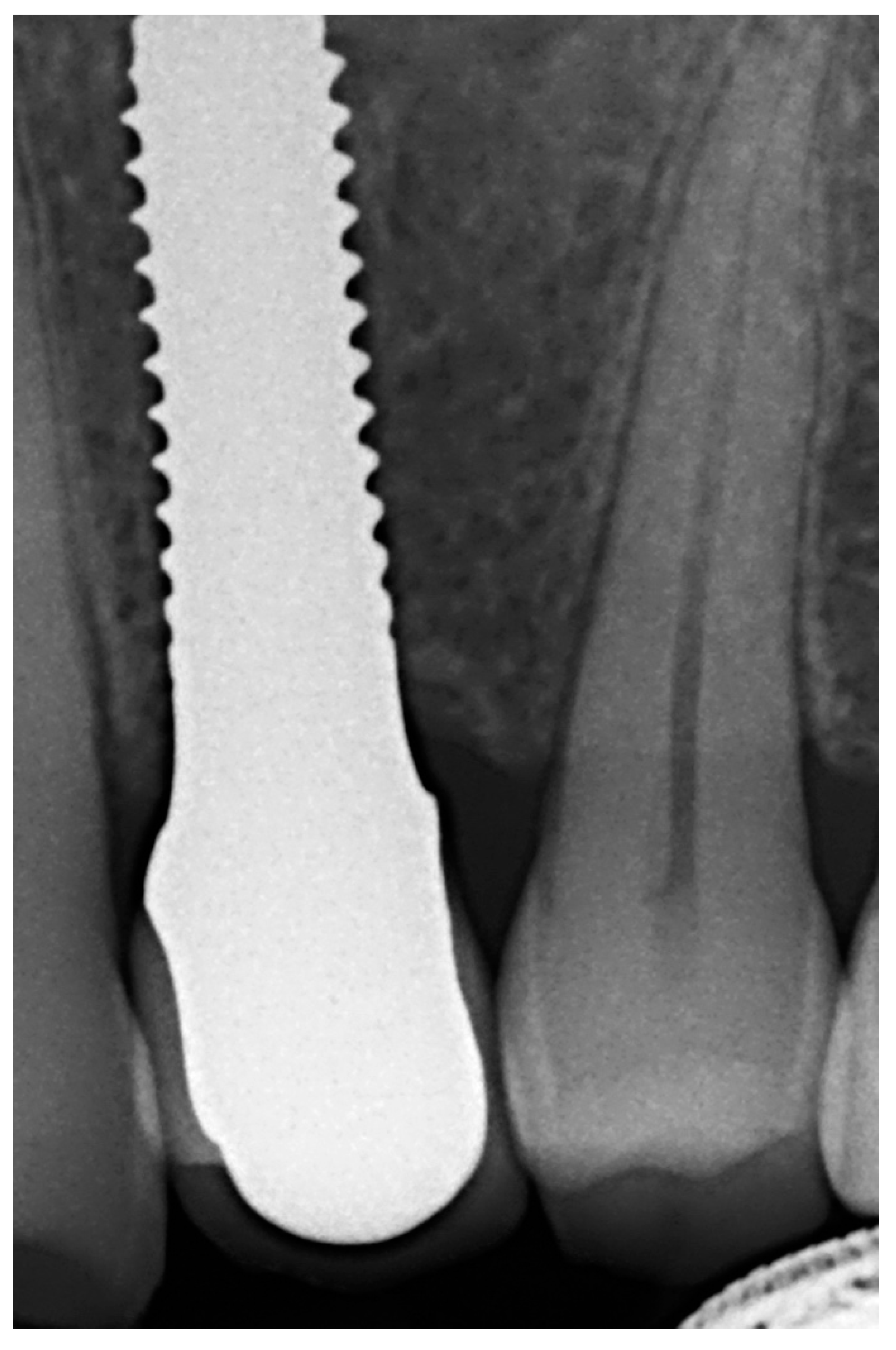
| N | Index Score | Parameters |
|---|---|---|
| Gingival index (GI) | 1 | Normal gingiva: natural coral pink with no inflammation |
| 2 | Mild inflammation: slight changes in color, slight edema. No bleeding on probing | |
| 3 | Moderate inflammation: redness, edema and glazing | |
| Modified plaque index (mPI) | 0 | No detection of plaque |
| 1 | Plaque only recognized by running a probe across the smooth marginal surface of the implant | |
| 2 | Plaque can be seen by the naked eye | |
| 3 | Abundance of soft matter | |
| Modified sulcus bleeding index (mBI) | 0 | No bleeding |
| 1 | Isolated bleeding spots visible | |
| 2 | Blood forms a confluent red line on margin | |
| 3 | Heavy or profuse bleeding |
| N | Gender | Age | Smoking (>10 cig/Day) | Implant Position | Implant ∅ (mm) | Implant Length (mm) | Bone Augmentation | Definitive Restoration Placement (Months) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 70 | Y | 15 | 4.1 | 10 | N | 2.5 |
| 2 | M | 70 | Y | 26 | 4.1 | 10 | Y | 2.5 |
| 3 | F | 47 | N | 36 | 4.1 | 12 | Y | 3 |
| 4 | F | 47 | N | 46 | 4.1 | 12 | Y | 3 |
| 5 | M | 50 | N | 16 | 4.1 | 8 | N | 3 |
| 6 | M | 50 | N | 46 | 4.1 | 10 | N | 3 |
| 7 | F | 45 | N | 36 | 4.1 | 10 | N | 2.5 |
| 8 | M | 63 | N | 17 | 4.1 | 10 | N | 1.5 |
| 9 | M | 63 | N | 16 | 3.3 | 10 | Y | 1.5 |
| 10 | M | 63 | N | 27 | 4.1 | 10 | N | 1.5 |
| 11 | M | 63 | N | 26 | 4.1 | 10 | Y | 1.5 |
| 12 | M | 63 | N | 37 | 3.3 | 10 | Y | 1.5 |
| 13 | M | 63 | N | 36 | 3.3 | 10 | Y | 1.5 |
| 14 | M | 63 | N | 47 | 3.3 | 10 | Y | 1.5 |
| 15 | M | 63 | N | 46 | 3.3 | 10 | Y | 1.5 |
| 16 | F | 61 | N | 24 | 4.1 | 12 | N | 3 |
| 17 | M | 75 | N | 24 | 4.1 | 12 | N | 4 |
| 18 | F | 48 | N | 15 | 4.1 | 12 | Y | 4 |
| 19 | F | 69 | N | 35 | 4.1 | 10 | Y | 4.0 |
| 20 | F | 79 | N | 24 | 4.1 | 10 | Y | 7.0 |
| Mean | 60.75 | 2.67 |
| N | Follow-Up (Months) | GI (B, L, M, D) | Mean GI | mPI (B, L, M, D) | Mean mPI | mSB (B, L) | Mean mSB | PD (B, L, M, D) | Mean PD | Implant Shoulder-First BIC (M; D) | Mean Implant Shoulder-First BIC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24 | 0, 0, 1, 0 | 0.25 | 0, 0, 0, 0 | 0 | 0, 0 | 0 | 0, 0, 0, 2 | 0.5 | 0.54; 0.17 | 0.355 |
| 2 | 24 | 0, 1, 0, 1 | 0.5 | 0, 0, 0, 0 | 0 | 0, 1 | 0.5 | 0, 2, 3, 4 | 2.25 | 1.8; 2.1 | 1.95 |
| 3 | 24 | 0, 2, 0, 1 | 0.75 | 0, 1, 2, 2 | 1.25 | 2, 1 | 1.5 | 3, 4, 3, 4 | 3.5 | 2.2; 2.2 | 2.2 |
| 4 | 24 | 0, 0, 2, 1 | 0.75 | 0, 0, 1, 1 | 0.5 | 0, 1 | 0.5 | 2, 3, 5, 4 | 3.5 | 2.3; 2.4 | 2.35 |
| 5 | 24 | 0, 0, 1, 0 | 0.25 | 0, 0, 1, 1 | 0.5 | 0, 1 | 0.5 | 1, 2, 1, 1 | 1.25 | 1.53; 1.84 | 1.685 |
| 6 | 24 | 0, 0, 0, 1 | 0.25 | 0, 0, 1, 1 | 0.5 | 0, 1 | 0.5 | 1, 2, 1, 2 | 1.5 | 2.32; 2.15 | 2.235 |
| 7 | 24 | 1, 0, 1, 0 | 0.5 | 1, 1, 0, 0 | 0.5 | 0, 0 | 0 | 2, 1, 4, 3 | 2.5 | 1.66; 2.01 | 1.835 |
| 8 | 24 | 0, 0, 1, 0 | 0.25 | 1, 0, 1, 0 | 0.5 | 2, 3 | 2.5 | 4, 2, 3, 2 | 2.75 | 0.81; 0.9 | 0.855 |
| 9 | 24 | 0, 1, 0, 0 | 0.25 | 0, 1, 0, 1 | 0.5 | 2, 2 | 2 | 3, 3, 2, 2 | 2.5 | 0.45; 0.29 | 0.37 |
| 10 | 24 | 0, 0, 0, 1 | 0.25 | 0, 0, 0, 0 | 0 | 2, 1 | 1.5 | 4, 3, 5, 3 | 3.75 | 2.2; 1.49 | 1.845 |
| 11 | 24 | 0, 1, 0, 0 | 0.25 | 0, 1, 0, 0 | 0.25 | 2, 2 | 2 | 4, 4, 3, 2 | 3.25 | 0.15; 2.88 | 1.515 |
| 12 | 24 | 0, 0, 1, 1 | 0.5 | 0, 1, 1, 1 | 0.75 | 1, 3 | 2 | 4, 3, 4, 4 | 3.75 | 0.43; 0.35 | 0.39 |
| 13 | 24 | 1, 0, 1, 1 | 0.75 | 0, 0, 0, 1 | 0.25 | 2, 3 | 2.5 | 4, 4, 4, 4 | 4 | 2.75; 0.92 | 1.835 |
| 14 | 24 | 0, 0, 0, 2 | 0.5 | 0, 0, 0, 1 | 0.25 | 2, 3 | 2.5 | 5, 3, 3, 3 | 3.5 | 0.48; 0.74 | 0.61 |
| 15 | 24 | 0, 0, 1, 1 | 0.5 | 1, 0, 0, 1 | 0.5 | 2, 3 | 2.5 | 5, 4, 4, 3 | 4 | 2.2; 2.94 | 2.57 |
| 16 | 36 | 0, 1, 1, 1 | 0.75 | 0, 0, 0, 0 | 0 | 0, 0 | 0 | 2, 2, 3, 3 | 2.5 | 1.9; 2.1 | 2 |
| 17 | 72 | 0, 0, 0, 0 | 0 | 0, 0, 0, 0 | 0 | 0, 0 | 0 | 1, 2, 1, 1 | 1.25 | 1.8; 2.1 | 1.95 |
| 18 | 72 | 1, 1, 0, 0 | 0.5 | 0, 0, 0, 0 | 0 | 0, 0 | 0 | 1, 2, 1, 1 | 1.25 | 0.9; 1.8 | 1.35 |
| 19 | 84 | 0, 0, 0, 0 | 0 | 1, 0, 0, 1 | 0.5 | 1, 0 | 0.5 | 2, 1, 1, 1 | 1.25 | 1.5–1.8 | 1.65 |
| 20 | 57 | 0, 0, 0, 0 | 0 | 0, 0, 0, 0 | 0 | 0, 0 | 0 | 2, 3, 2, 3 | 2.5 | 0.3–0.4 | 0.35 |
| Mean | 34.05 | 0.38 | 0.33 | 1.075 | 2.56 | 1.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gargallo-Albiol, J.; Böhm, K.; Wang, H.-L. Clinical and Radiographic Outcomes of Zirconia Dental Implants—A Clinical Case Series Study. Materials 2022, 15, 2437. https://doi.org/10.3390/ma15072437
Gargallo-Albiol J, Böhm K, Wang H-L. Clinical and Radiographic Outcomes of Zirconia Dental Implants—A Clinical Case Series Study. Materials. 2022; 15(7):2437. https://doi.org/10.3390/ma15072437
Chicago/Turabian StyleGargallo-Albiol, Jordi, Karl Böhm, and Hom-Lay Wang. 2022. "Clinical and Radiographic Outcomes of Zirconia Dental Implants—A Clinical Case Series Study" Materials 15, no. 7: 2437. https://doi.org/10.3390/ma15072437
APA StyleGargallo-Albiol, J., Böhm, K., & Wang, H.-L. (2022). Clinical and Radiographic Outcomes of Zirconia Dental Implants—A Clinical Case Series Study. Materials, 15(7), 2437. https://doi.org/10.3390/ma15072437







