Comparative Study on Electrical Conductivity of CeO2-Doped AlN Ceramics Sintered by Hot-Pressing and Spark Plasma Sintering
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Raw Powders
2.2. Sintering and Characterizations
3. Results
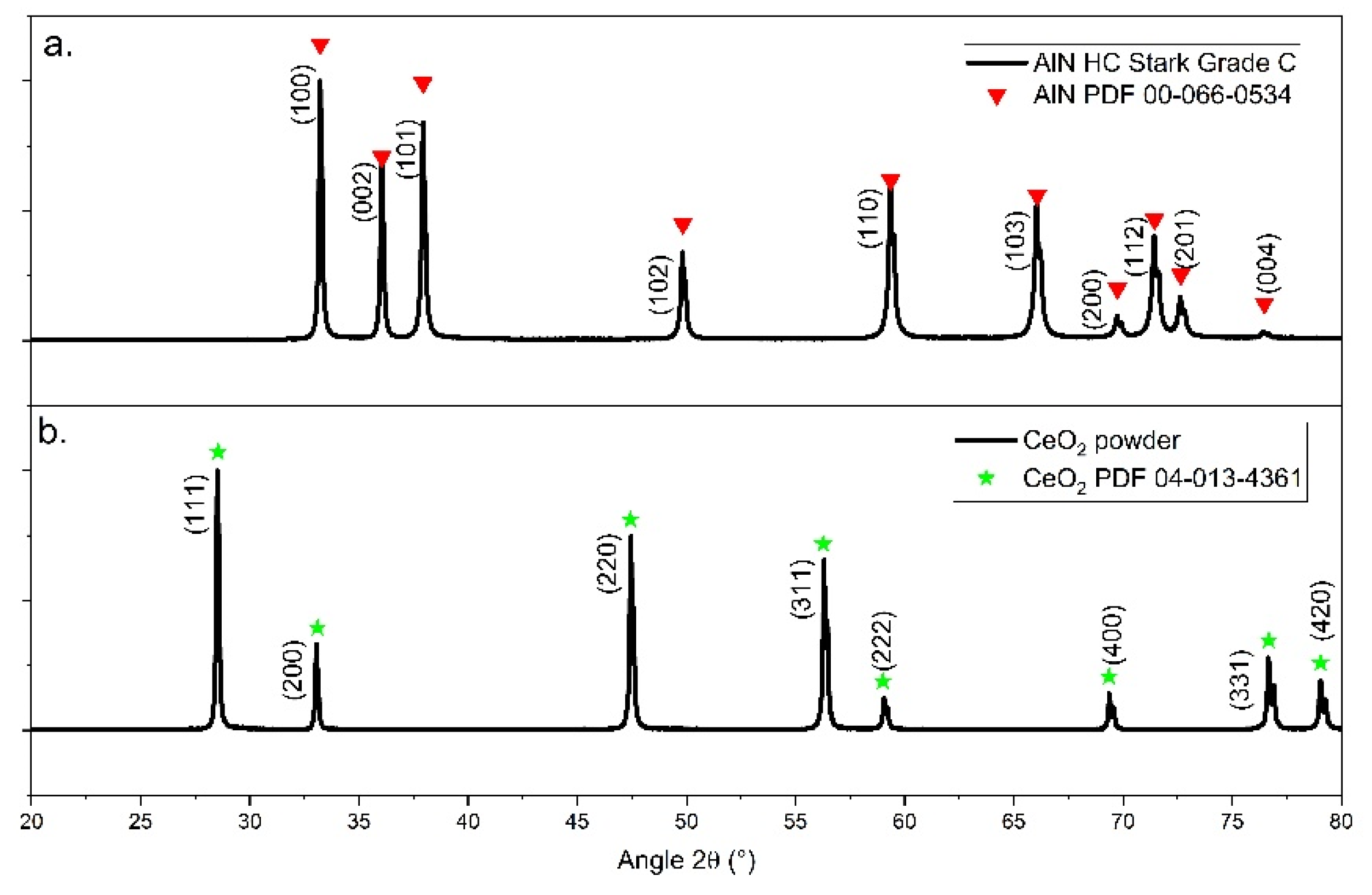
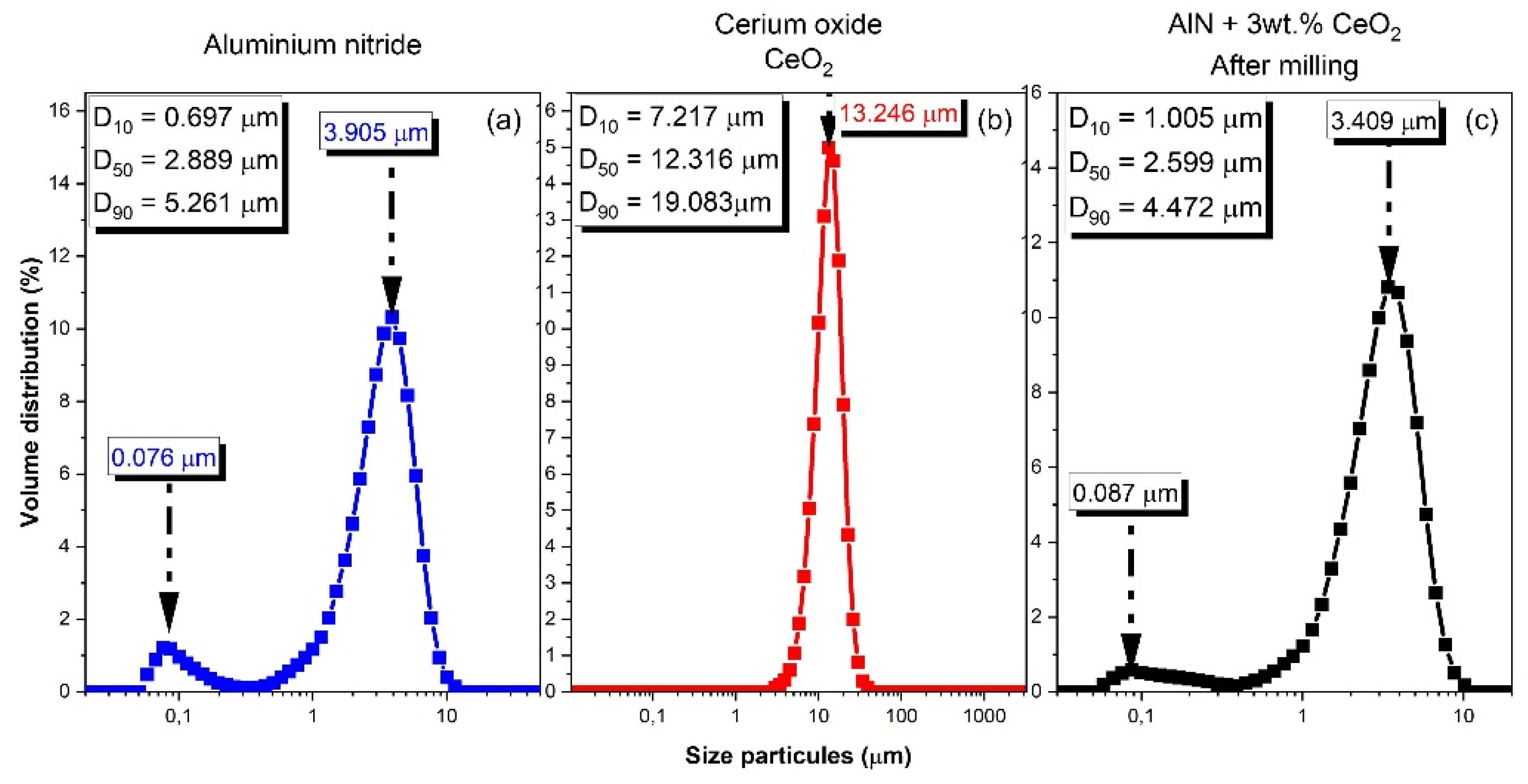
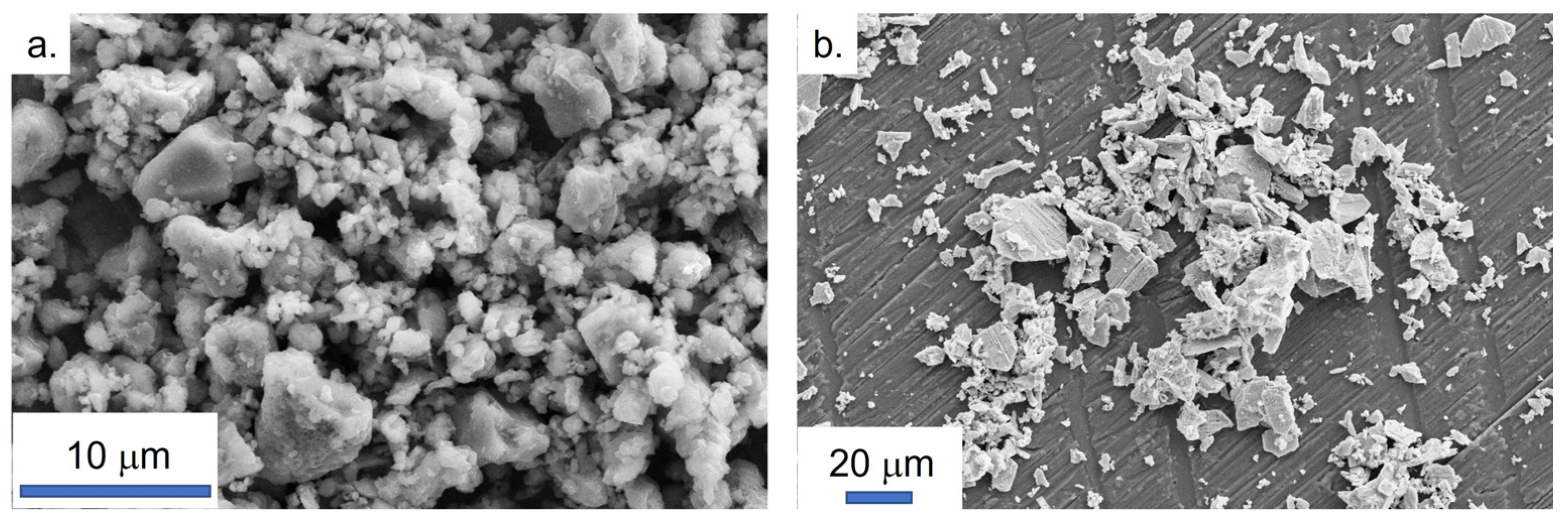
3.1. Raw Powders Characterizations
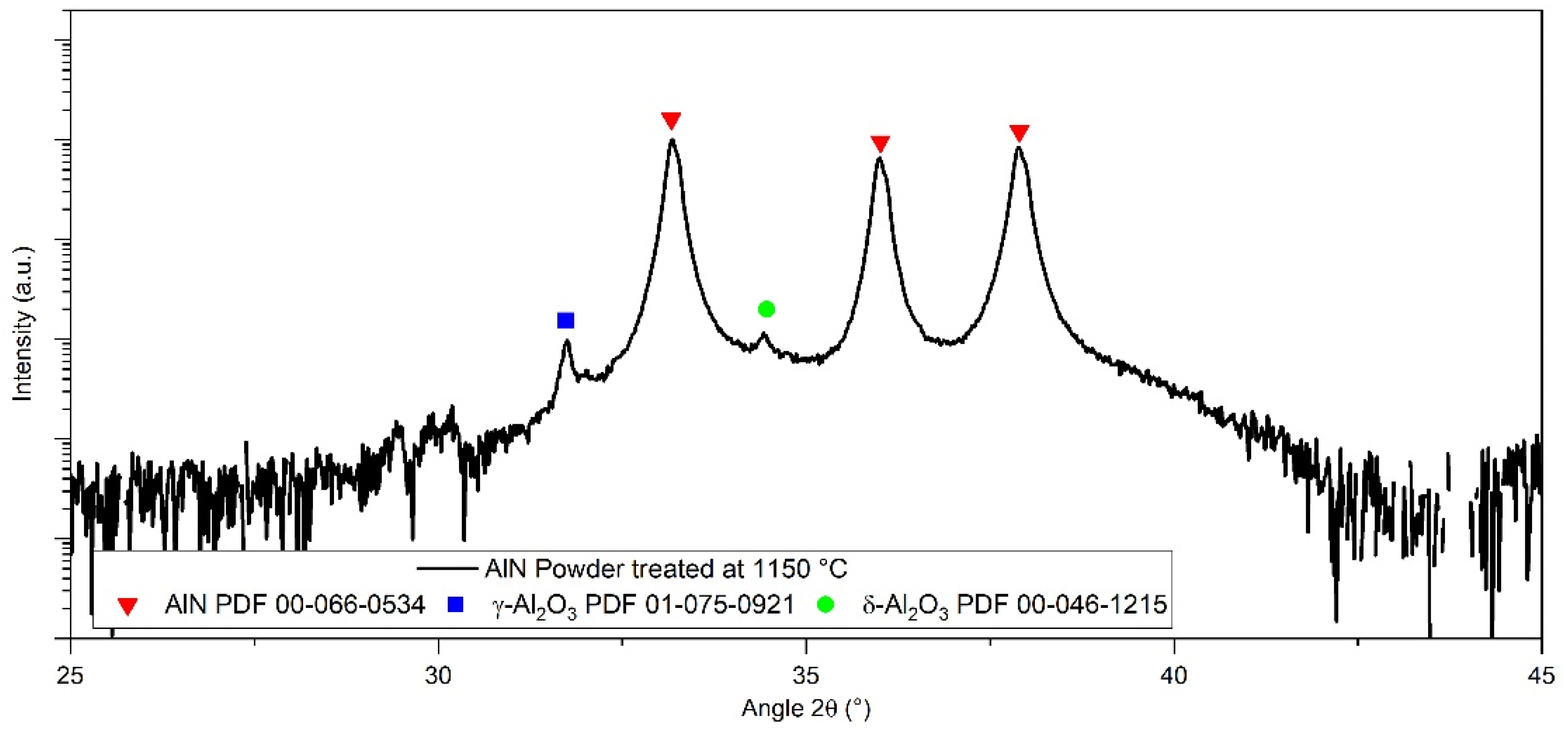
3.2. Specific Focus on Aluminium Nitride Powder
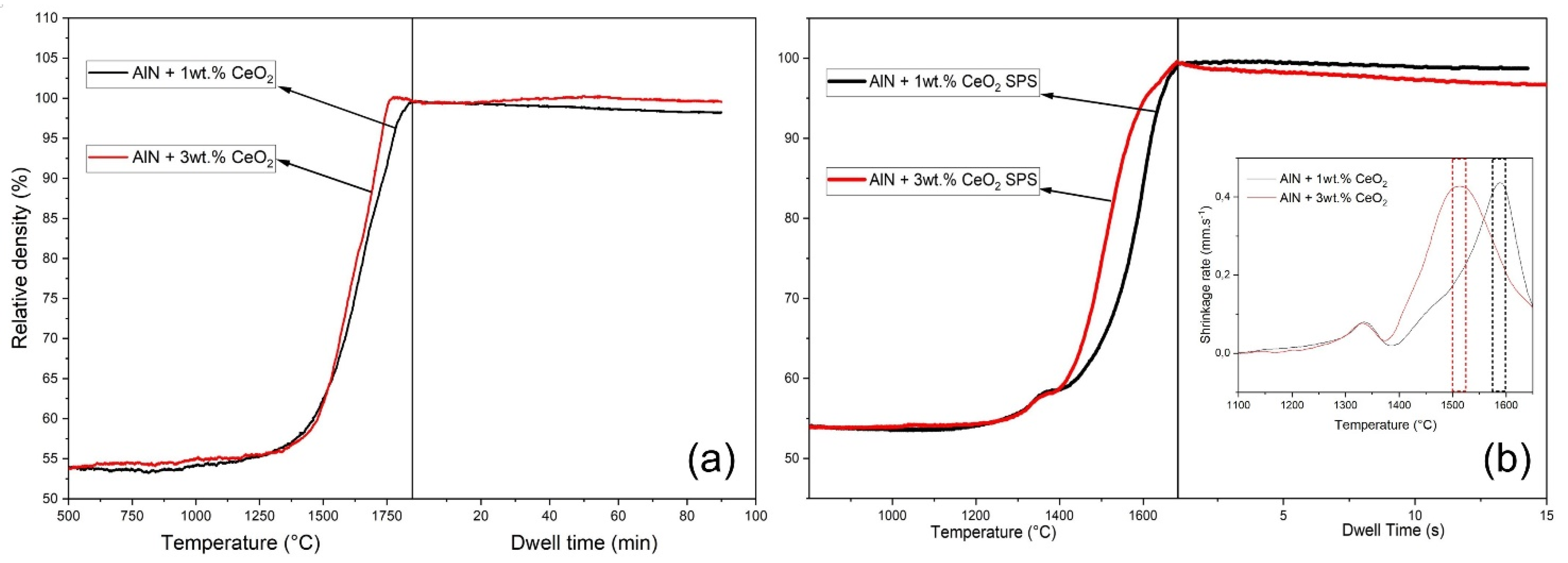
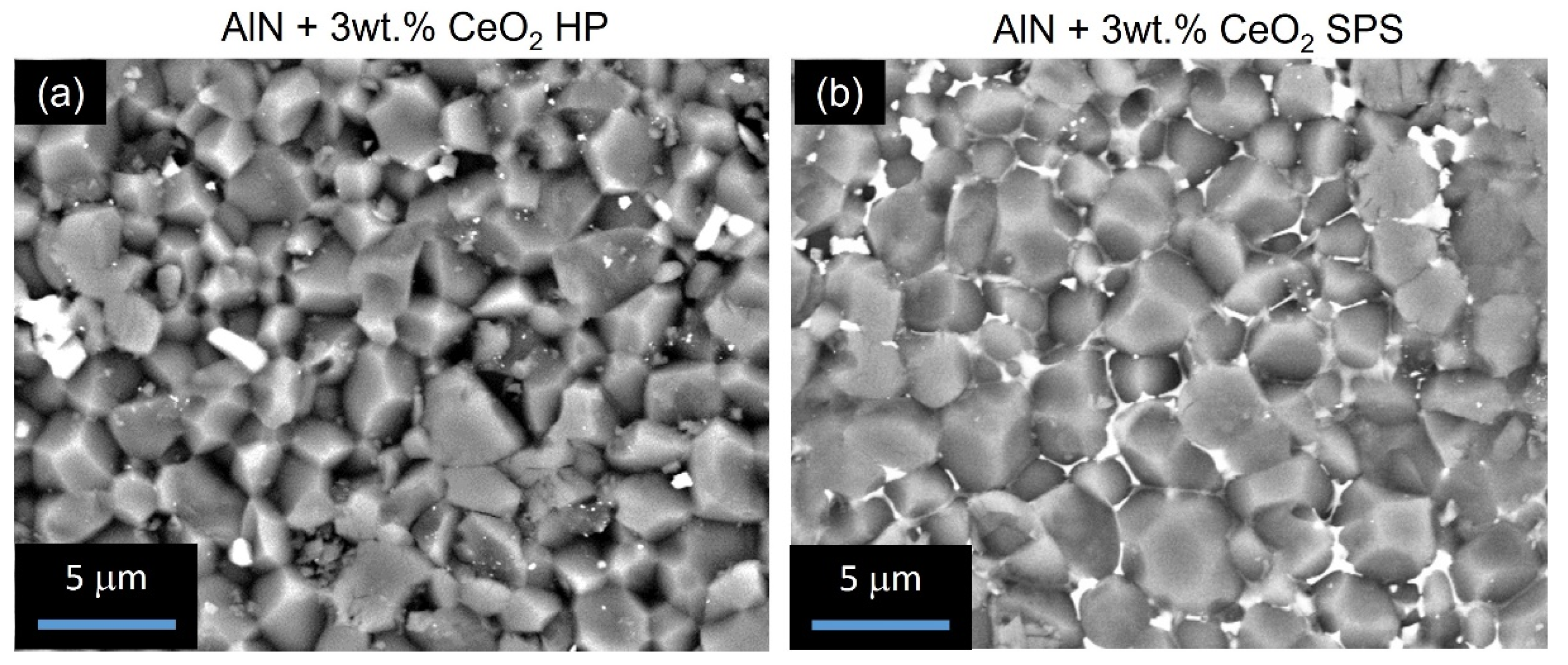
3.3. Densification and Microstructure of CeO2 Doped Aluminium Nitride Ceramics
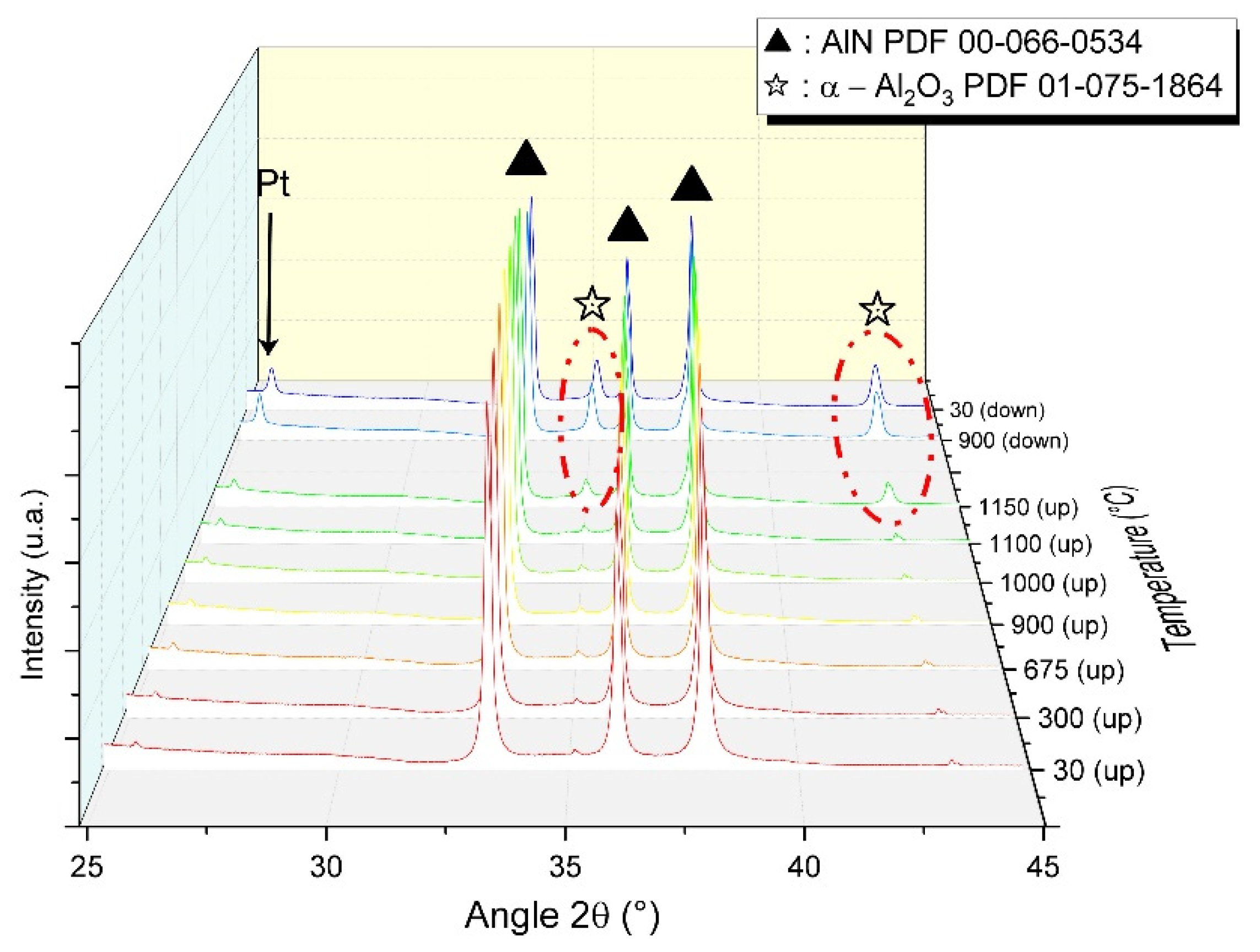
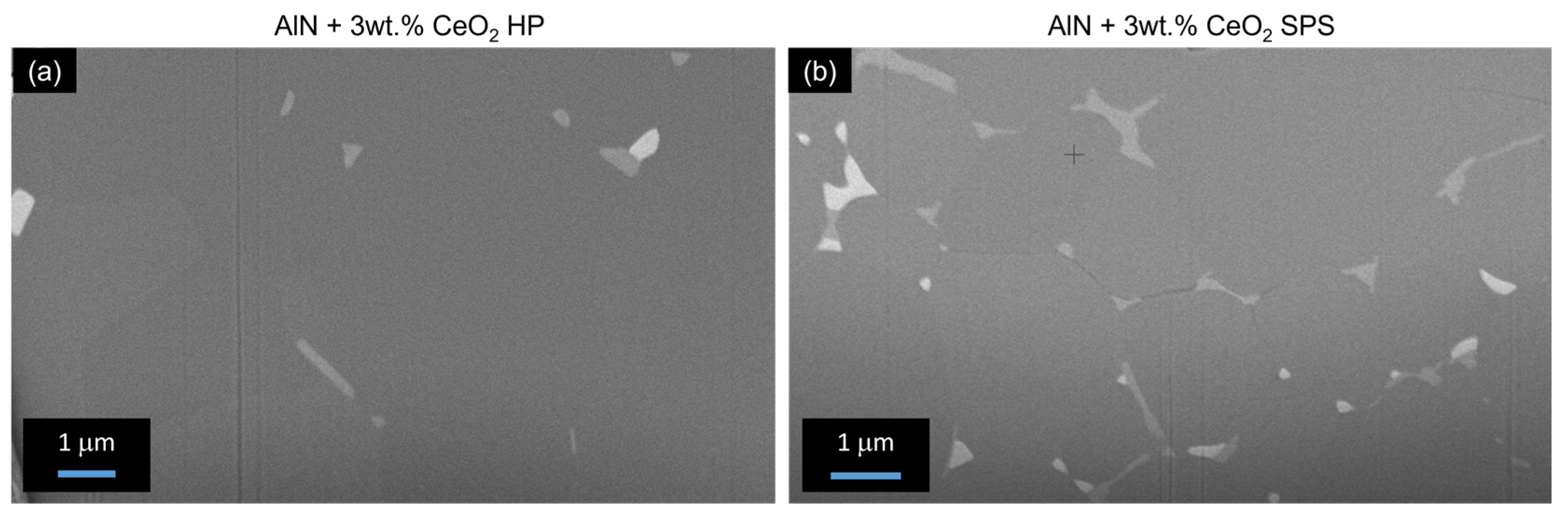
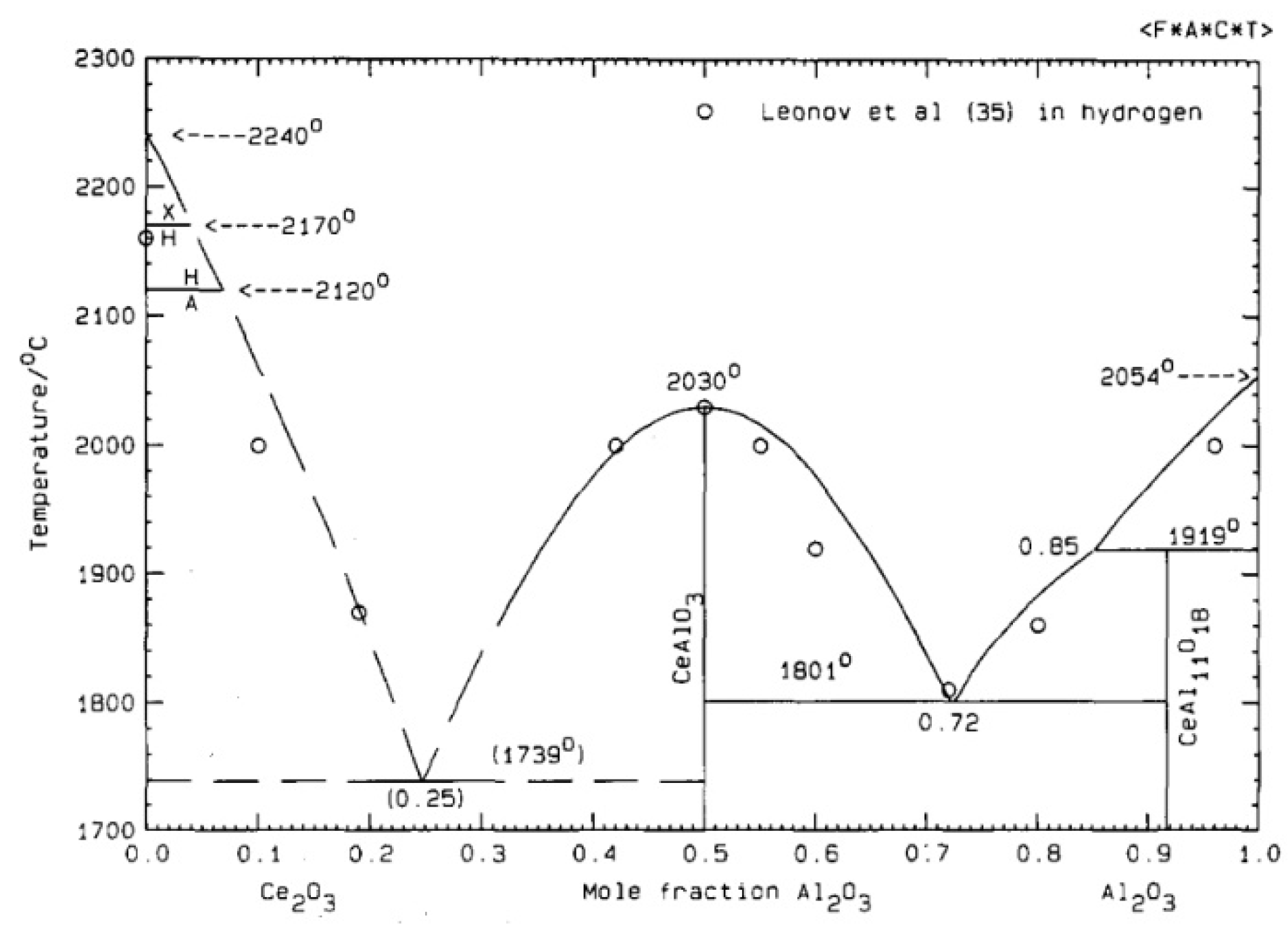
3.4. Secondary Phase Formation
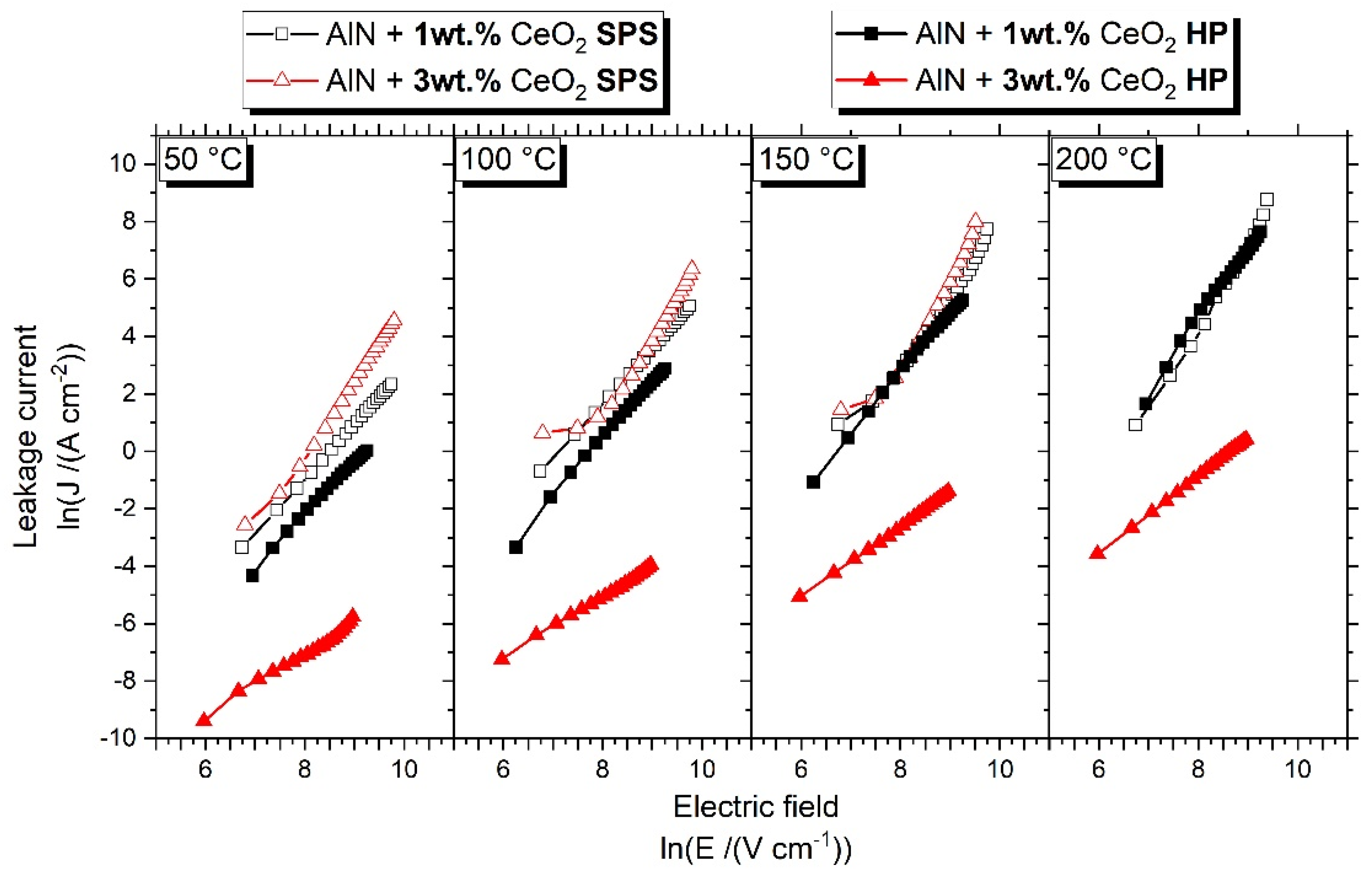
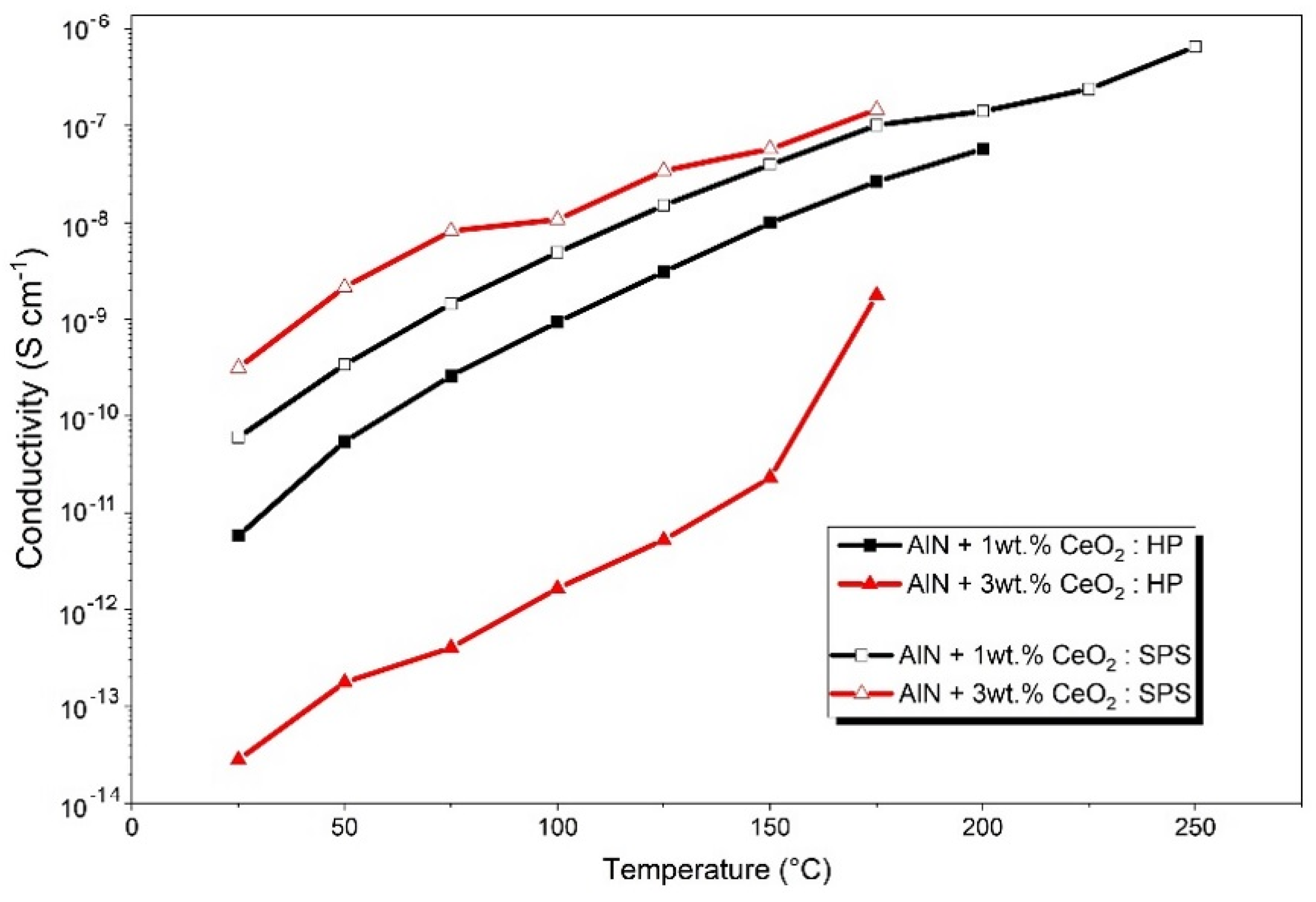
3.5. Electrical Properties of Sintered Ceramics
4. Discussions
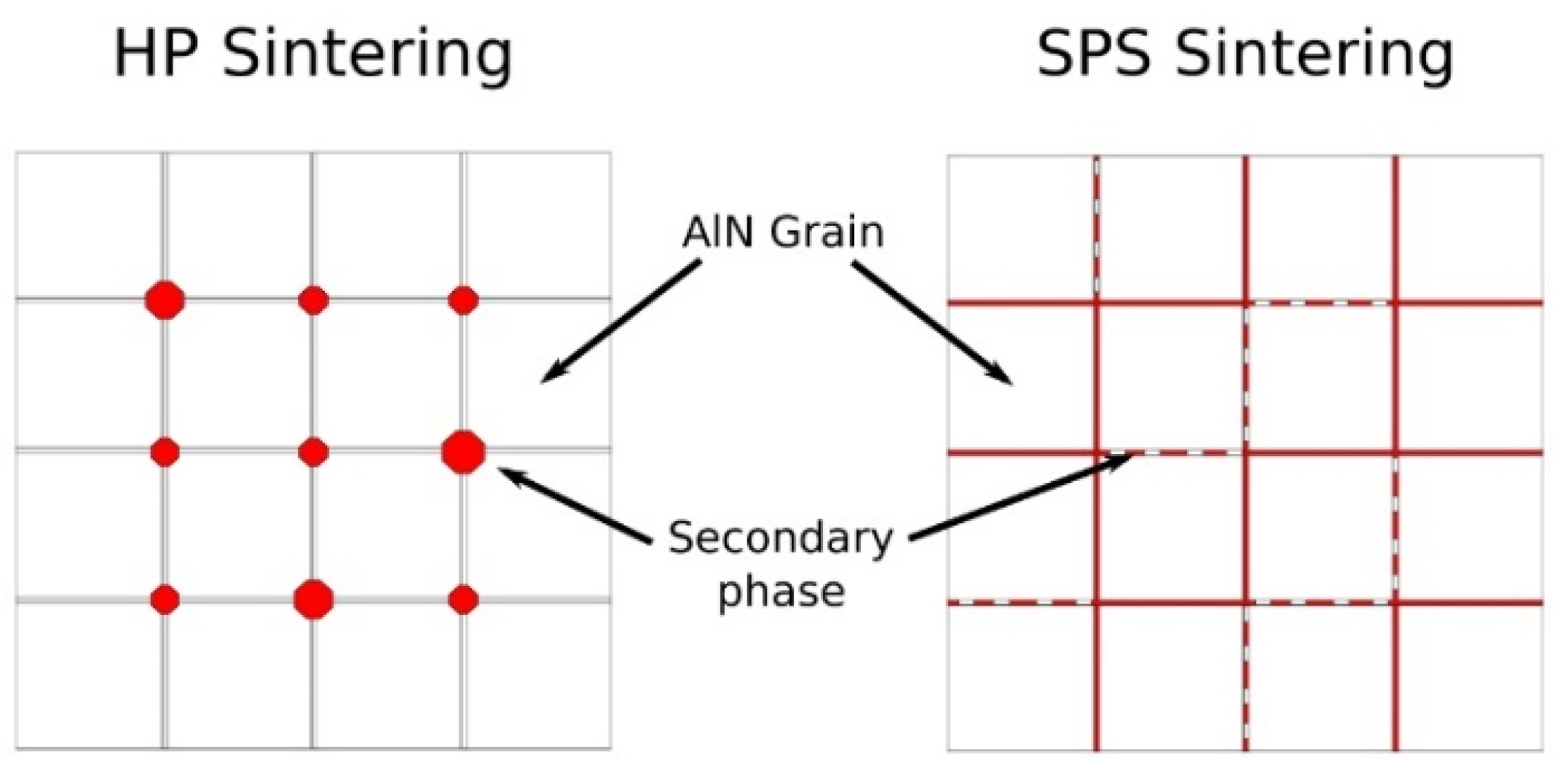
4.1. Sintering of CeO2 Doped Aluminium Nitride Ceramics
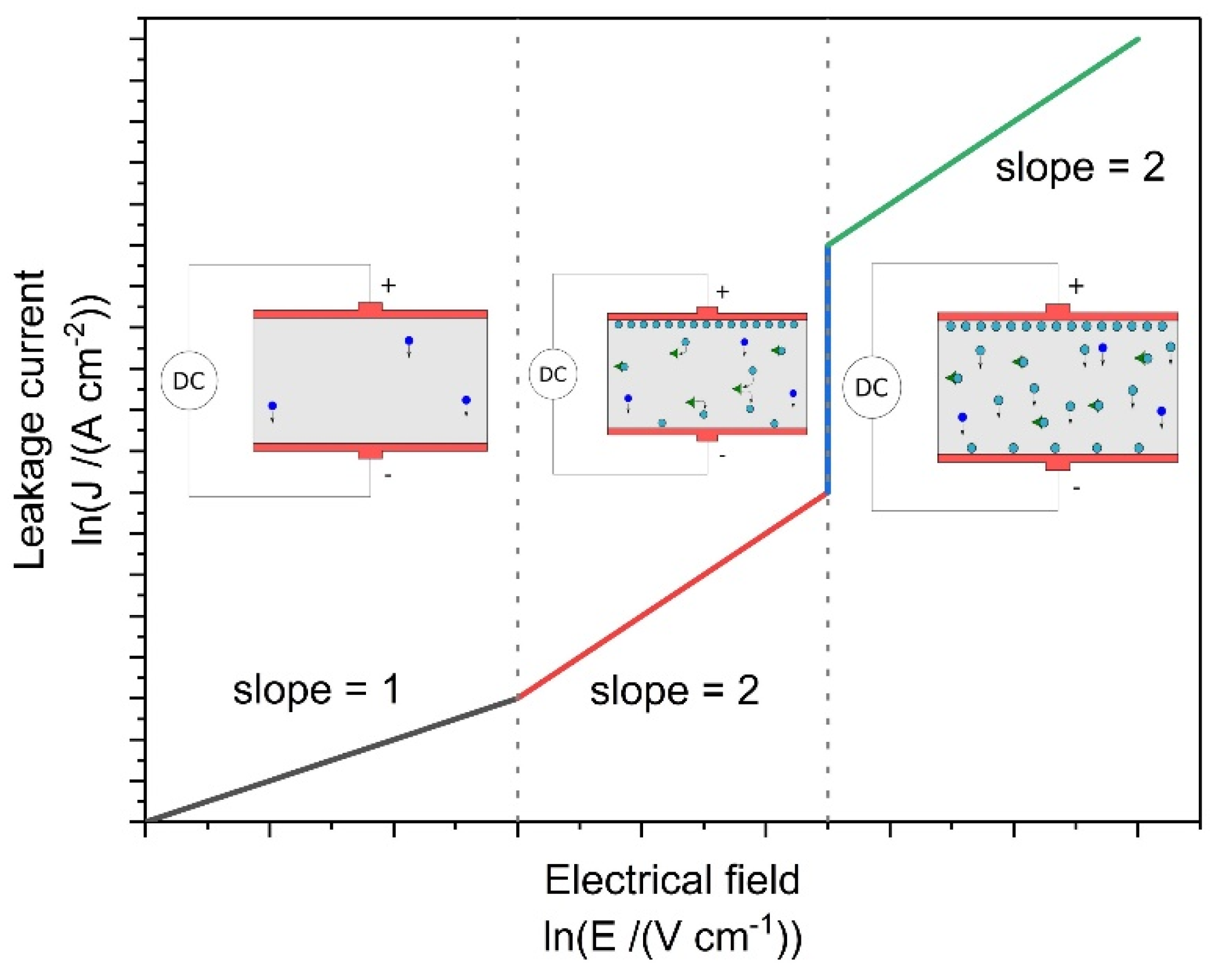
4.2. Influence of Secondary Phases on Electrical Property of Ceramics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slack, G.A. Nonmetallic crystals with high thermal conductivity. J. Phys. Chem. Solids 1973, 34, 321–335. [Google Scholar] [CrossRef]
- Slack, G.A.; Tanzilli, R.A.; Pohl, R.O.; Vandersande, J.W. The intrinsic thermal conductivity of AIN. J. Phys. Chem. Solids 1987, 48, 641–647. [Google Scholar] [CrossRef]
- Shackelford, J.F. CRC Materials Science and Engineering Handbook, 4th ed.; CRC Press, Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2016; ISBN 978-1-4822-1653-0. [Google Scholar]
- Werdecker, W.; Aldinger, F. Aluminum nitride: An alternative ceramic substrate for high power applications in microcircuits. IEEE Trans. Comp. Hybrids Manufact. Technol. 1984, 7, 399–404. [Google Scholar] [CrossRef]
- Miyashiro, F.; Iwase, N.; Tsuge, A.; Ueno, F.; Nakahashi, M.; Takahashi, T. High thermal conductivity aluminum nitride ceramic substrates and packages. IEEE Trans. Compon. Hybrids Manuf. Technol. 1990, 13, 313–319. [Google Scholar] [CrossRef]
- Lee, H.-K.; Lee, H.M.; Kim, D.K. AC Impedance spectroscopy of CaF2-doped AlN ceramics. J. Am. Ceram. Soc. 2014, 97, 805–810. [Google Scholar] [CrossRef]
- Kang, S.B.; Chung, D.C.; Kim, S.-J.; Chung, J.-K.; Park, S.-Y.; Kim, K.-C.; Kwak, M.H. Terahertz characterization of Y2O3-added AlN ceramics. Appl. Surf. Sci. 2016, 388, 741–745. [Google Scholar] [CrossRef]
- Virkar, A.V.; Jackson, T.B.; Cutler, R.A. Thermodynamic and kinetic effects of oxygen removal on the thermal conductivity of aluminum nitride. J. Am. Ceram. Soc. 1989, 72, 2031–2042. [Google Scholar] [CrossRef]
- Qiao, L.; Zhou, H.; Fu, R. Thermal conductivity of AlN ceramics sintered with CaF2 and YF3. Ceram. Int. 2003, 29, 893–896. [Google Scholar] [CrossRef]
- AlShaikhi, A.; Srivastava, G.P. Role of additives in enhancing the thermal conductivity of AlN ceramics. J. Phys. D Appl. Phys. 2008, 41, 185407. [Google Scholar] [CrossRef]
- Molisani, A.L.; Yoshimura, H.N.; Goldenstein, H. Sintering mechanisms in aluminum nitride with Y or Ca-containing additive. J. Mater. Sci. Mater. Electron. 2009, 20, 1–8. [Google Scholar] [CrossRef]
- Choi, H.-S.; Im, H.-N.; Kim, Y.-M.; Chavan, A.; Song, S.-J. Structural, thermal and mechanical properties of aluminum nitride ceramics with CeO2 as a sintering aid. Ceram. Int. 2016, 42, 11519–11524. [Google Scholar] [CrossRef]
- Xu, X.; Zhuang, H.; Li, W.; Xu, S.; Zhang, B.; Fu, X. Improving thermal conductivity of Sm 2O3-doped AlN ceramics by changing sintering conditions. Mater. Sci. Eng. A 2003, 342, 104–108. [Google Scholar] [CrossRef]
- He, X.; Ye, F.; Zhang, H.; Liu, L. Effect of Sm2O3 content on microstructure and thermal conductivity of spark plasma sintered AlN ceramics. J. Alloy. Compd. 2009, 482, 345–348. [Google Scholar] [CrossRef]
- Terao, R.; Tatami, J.; Meguro, T.; Komeya, K. Fracture behavior of AlN ceramics with rare earth oxides. J. Eur. Ceram. Soc. 2002, 22, 1051–1059. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Wu, C.; Cheng, X. Microstructure and Mechanical Properties of Aluminum Nitride Co-Doped with Cerium oxide via hot-pressing sintering. J. Alloys Compd. 2015, 640, 275–279. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X.-H.; Fan, G.-F.; Lei, W.; Fu, M.; Wang, X.-C.; Liang, F.; Lu, W.-Z. Effect of hot-pressing sintering on thermal and electrical properties of aln ceramics with impedance spectroscopy and dielectric relaxations analysis. J. Eur. Ceram. Soc. 2019, 39, 5174–5180. [Google Scholar] [CrossRef]
- Coeffe-Desvaux, M.; Tabares-Medina, T.; Pradeilles, N.; Marchet, P.; Joinet, M.; Maitre, A. New insight on the effect of yttria based secondary phases on sintering and electrical behaviour of aluminium nitride ceramics. J. Mater. Eng. Perform. 2022. [Google Scholar] [CrossRef]
- Roske, L. Packaging de Composants Grand Gap Haute Température et Haute Tension. Ph.D. Thesis, Université de Toulouse, Toulouse, France, 2015. [Google Scholar]
- Breit, F.; Lebey, T.; Agnel, S.; Toureille, A. Investigations on DC Conductivity of AlN. In Proceedings of the 2001 Annual Report Conference on Electrical Insulation and Dielectric Phenomena (Cat. No.01CH37225), Kitchener, Canada, 14–17 October 2001; pp. 71–74. [Google Scholar]
- Xiong, Y.; Wang, H.; Fu, Z. Transient liquid-phase sintering of AlN ceramics with CaF2 additive. J. Eur. Ceram. Soc. 2013, 33, 2199–2205. [Google Scholar] [CrossRef]
- Tabares-Medina, T. Pressure Assisted Sintering Mechanisms and Electrical Properties for AlN Based Composites: Role of the Secondary Phases. Ph.D. Thesis, Université de Limoges, Limoges, France, 2019. [Google Scholar]
- Roumiguier, L. Frittage Par Spark Plasma Sintering de Céramiques de Carbure de Bore: Modélisation Numérique du Procédé et Optimisation des Nano-, Microstructures Pour l’amélioration des Performances des Absorbants en Réacteurs à Neutrons Rapides. Ph.D. Thesis, Université de Limoges, Limoges, France, 2019. [Google Scholar]
- Laadoua, H. Impact de L’utilisation de Polycarbosilane dans l’Elaboration de Composites ZrC/SiC Par Frittage SPS. Ph.D. Thesis, Université de Limoges, Limoges, France, 2019. [Google Scholar]
- Digne, M.; Sautet, P.; Raybaud, P.; Toulhoat, H.; Artacho, E. Structure and stability of aluminum hydroxides: A theoretical study. J. Phys. Chem. B 2002, 106, 5155–5162. [Google Scholar] [CrossRef]
- Wu, P.; Pelton, A.D. Coupled thermodynamic-phase diagram assessment of the rare earth oxide-aluminium oxide binary systems. J. Alloys Compd. 1992, 179, 259–287. [Google Scholar] [CrossRef]
- Tas, A.C.; Akinc, M. Phase relations in the system Ce2O3-Al2O3 in inert and reducing atmospheres. J. Am. Ceram. Soc. 1994, 77, 2961–2967. [Google Scholar] [CrossRef]
- Naga, S.M.; El-Maghraby, H.F.; Awaad, M.; Kern, F.; Gadow, R.; Hassan, A.M. Preparation and characterization of tough cerium hexaaluminate bodies. Mater. Lett. 2019, 254, 402–406. [Google Scholar] [CrossRef]
- Roske, L.; Lebey, T.; Valdez-Nava, Z. High Temperature Behavior of Ceramic Substrates for Power Electronics Applications. In Proceedings of the 2013 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Chenzhen, China, 20–23 October 2013; pp. 595–598. [Google Scholar]
- Rose, A. Space-charge-limited currents in solids. Phys. Rev. 1955, 97, 1538–1544. [Google Scholar] [CrossRef]

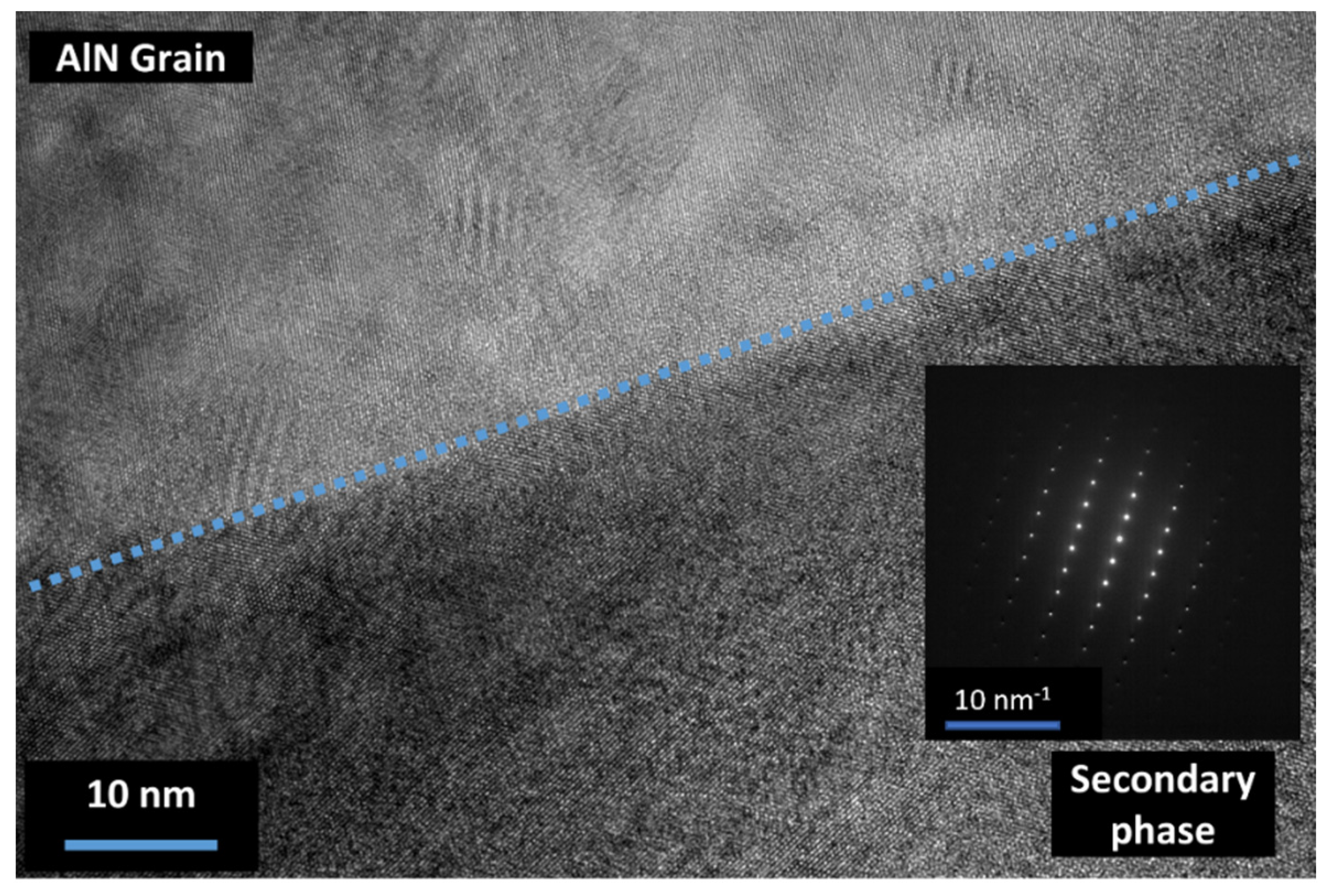
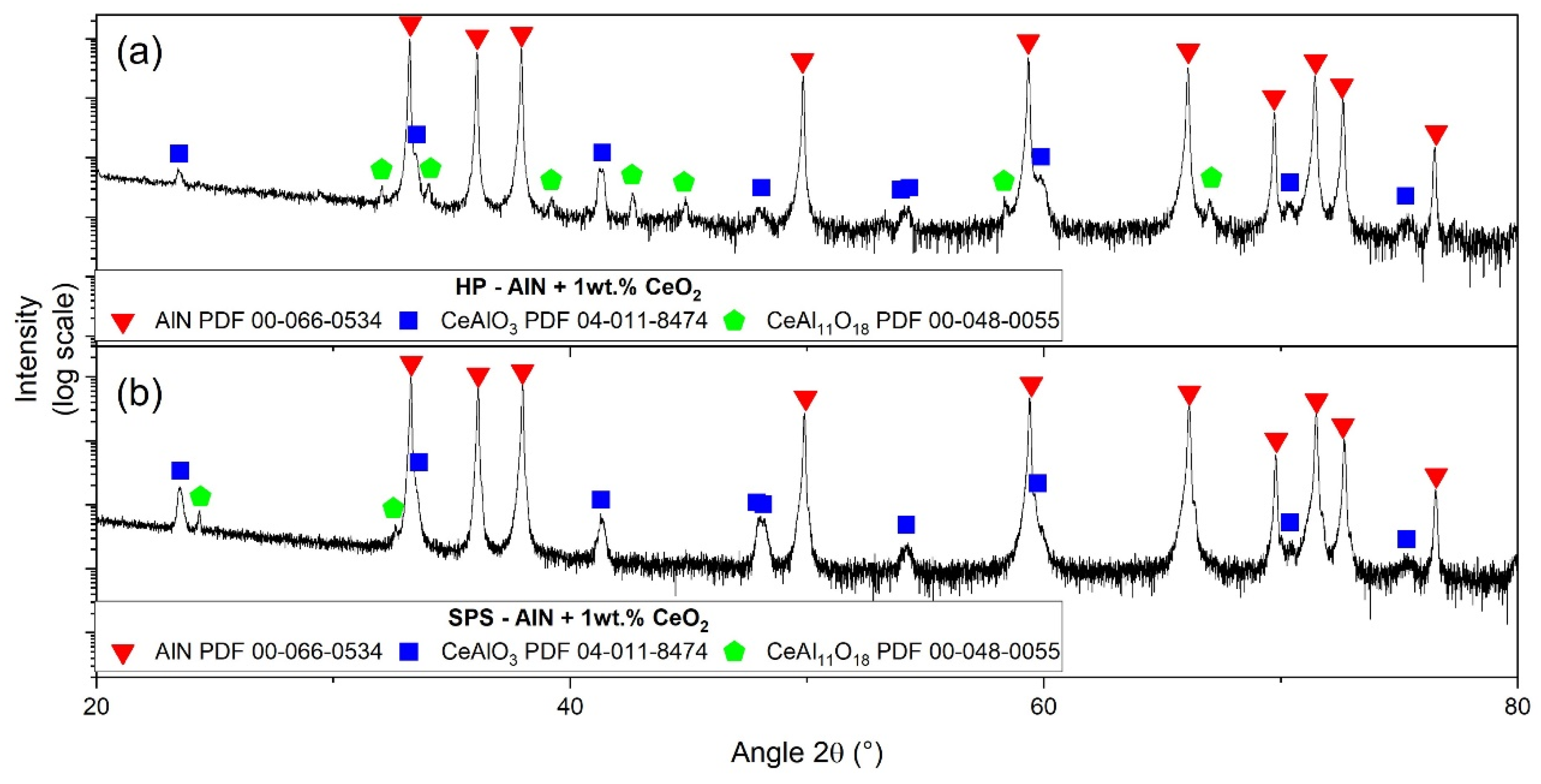
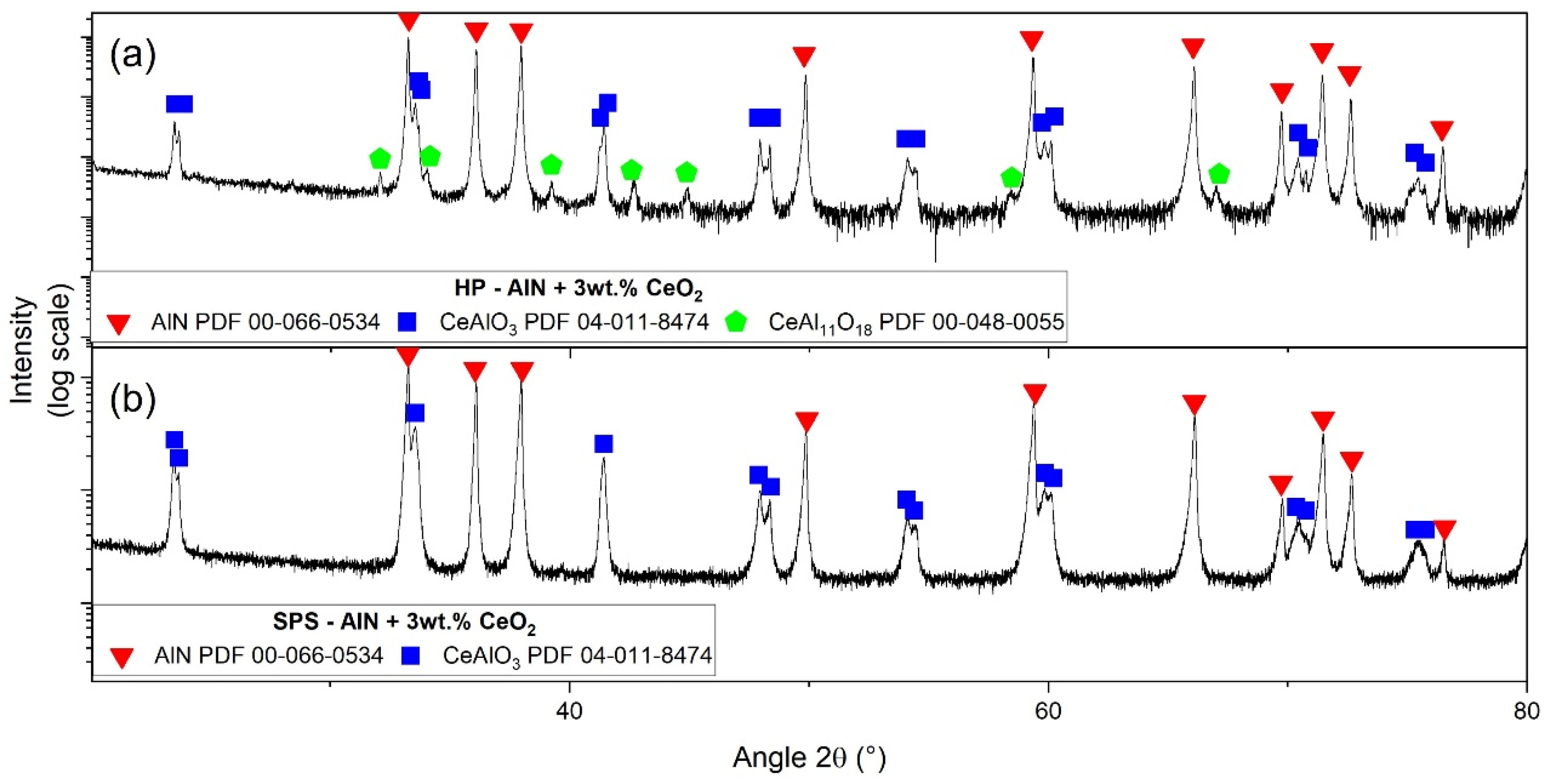
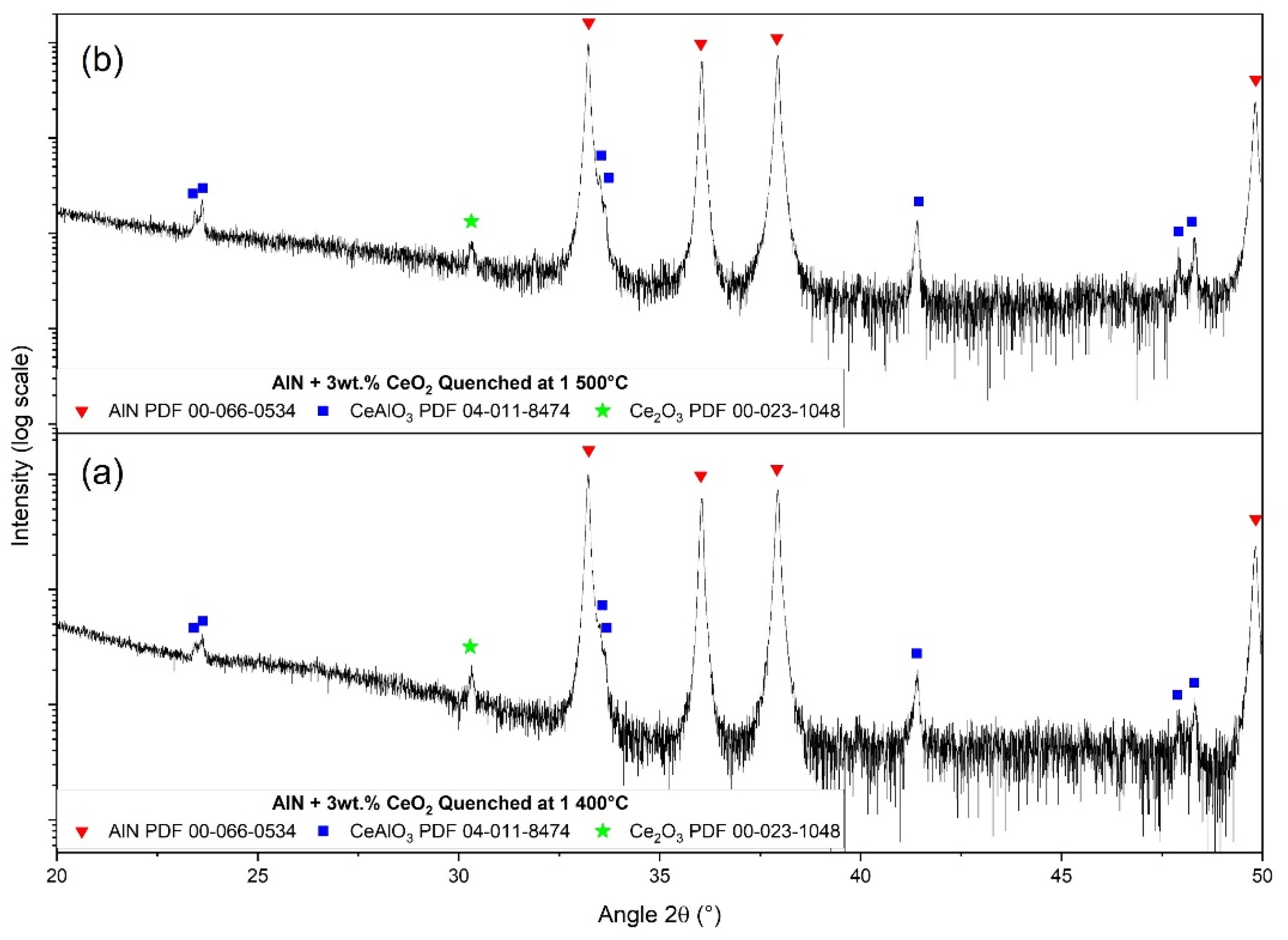
| Elementary Analysis IGA (¤) and GDMS | AlN Raw Powder | CeO2 Raw Powder |
| O: 1.10 wt.% (¤) | P: 540 ppm | |
| H: 0.05 wt.% (¤) | Al: 490 ppm | |
| Si: 21 ppm | Na: 120 ppm | |
| Cl: 20 ppm | Fe: 67 ppm | |
| Fe: 18 ppm | Si: 62 ppm |
| Sintering Process | Sample | Beginning of Shrinkage | Maximal Shrinkage |
|---|---|---|---|
| HP | AlN + 1 wt.% CeO2 | 1320 °C ± 30 °C | 1660 °C ± 20 °C |
| AlN + 3 wt.% CeO2 | 1350 °C ± 20 °C | 1680 °C ± 20 °C | |
| SPS | AlN + 1 wt.% CeO2 | 1180 °C ± 20 °C | 1590 °C ± 10 °C |
| AlN + 3 wt.% CeO2 | 1200 °C ± 20 °C | 1520 °C ± 10 °C |
| Sintering Process | Sample | Density (g cm−3) | Relative Density | Open Porosity |
|---|---|---|---|---|
| HP | AlN + 1 wt.% CeO2 | 3.27 | 99.8% | < 0.5% |
| AlN + 3 wt.% CeO2 | 3.31 | 99.7% | ||
| SPS | AlN + 1 wt.% CeO2 | 3.27 | 99.6% | |
| AlN + 3 wt.% CeO2 | 3.31 | 99.5% |
| Sintering Process | Composition | 50 °C | 100 °C | 150 °C | 200 °C |
|---|---|---|---|---|---|
| HP | AlN + 1 wt.% CeO2 | 1.8 | 1.9 | 2.1 | 2.5 |
| AlN + 3 wt.% CeO2 | 1.0 (L) 1.6 (H) | 1.0 | 1.2 | 1.3 | |
| SPS | AlN + 1 wt.% CeO2 | 1.9 | 1.9 | 2.4 (L) 3.2 (H) | 2.5 (L) 3.1 (H) |
| AlN + 3 wt.% CeO2 | 2.7 | 3.0 | 3.3 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coëffe-Desvaux, M.; Pradeilles, N.; Marchet, P.; Vandenhende, M.; Joinet, M.; Maître, A. Comparative Study on Electrical Conductivity of CeO2-Doped AlN Ceramics Sintered by Hot-Pressing and Spark Plasma Sintering. Materials 2022, 15, 2399. https://doi.org/10.3390/ma15072399
Coëffe-Desvaux M, Pradeilles N, Marchet P, Vandenhende M, Joinet M, Maître A. Comparative Study on Electrical Conductivity of CeO2-Doped AlN Ceramics Sintered by Hot-Pressing and Spark Plasma Sintering. Materials. 2022; 15(7):2399. https://doi.org/10.3390/ma15072399
Chicago/Turabian StyleCoëffe-Desvaux, Mickael, Nicolas Pradeilles, Pascal Marchet, Marion Vandenhende, Mickael Joinet, and Alexandre Maître. 2022. "Comparative Study on Electrical Conductivity of CeO2-Doped AlN Ceramics Sintered by Hot-Pressing and Spark Plasma Sintering" Materials 15, no. 7: 2399. https://doi.org/10.3390/ma15072399
APA StyleCoëffe-Desvaux, M., Pradeilles, N., Marchet, P., Vandenhende, M., Joinet, M., & Maître, A. (2022). Comparative Study on Electrical Conductivity of CeO2-Doped AlN Ceramics Sintered by Hot-Pressing and Spark Plasma Sintering. Materials, 15(7), 2399. https://doi.org/10.3390/ma15072399







