Characterization of the Flux System: Lithium-Aluminum Silicate (Li)–Alkali Feldspars (Na,K); Magnesium (Mg) and Calcium (Ca)–Silicates
Abstract
:1. Introduction
2. Materials and Methods
2.1. High-Temperature Microscopy
- Shrinkage temperature Tg (sintering),
- Softening temperature Ta (corner rounding-end of sintering),
- Melting temperature Tb (hemisphere effect-melting),
- Spreading temperature Tc (sample base >200% or 1/3 height),
- Sintering interval (corner rounding temperature Ta—sintering temperature Tg),
- Melting interval (hemisphere temperature Tb—corner rounding temperature Ta),
2.2. Selected Thermal Parameters of Mineral Flux Systems
2.3. Selected Physical Parameters
3. Results and Discussion
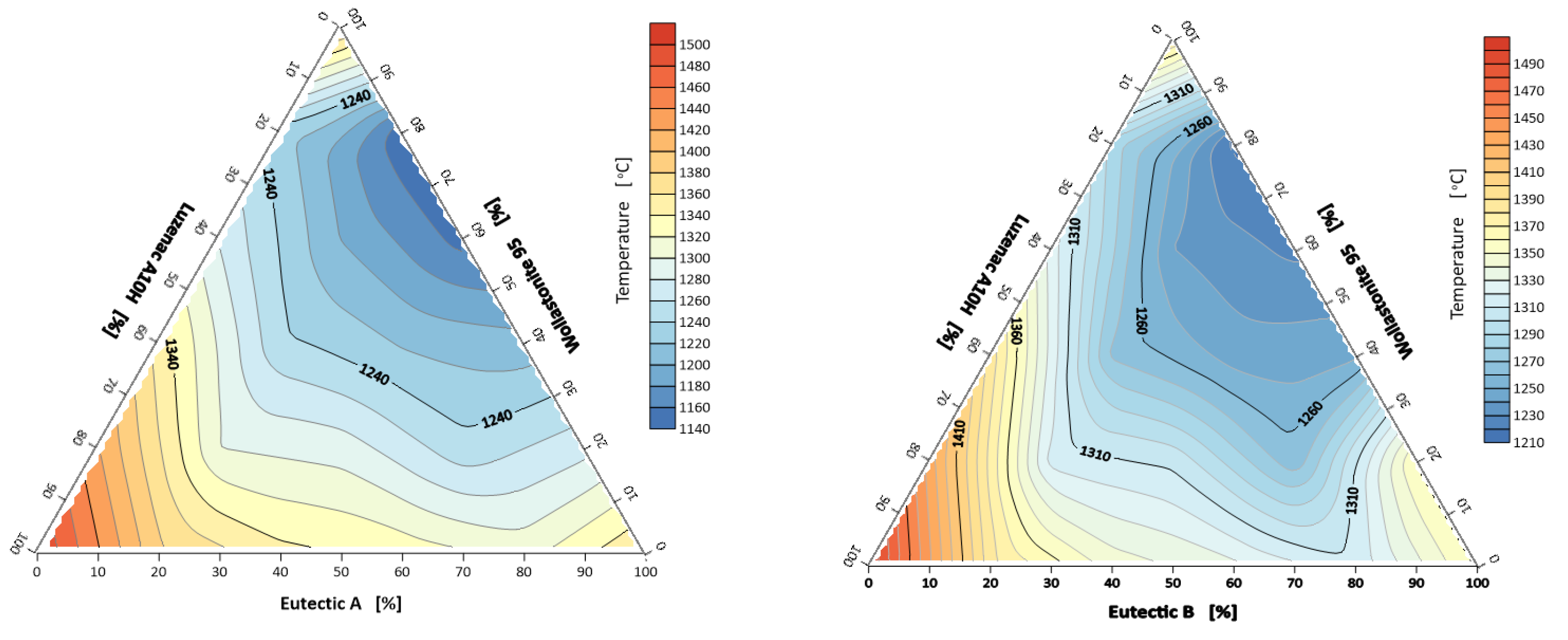
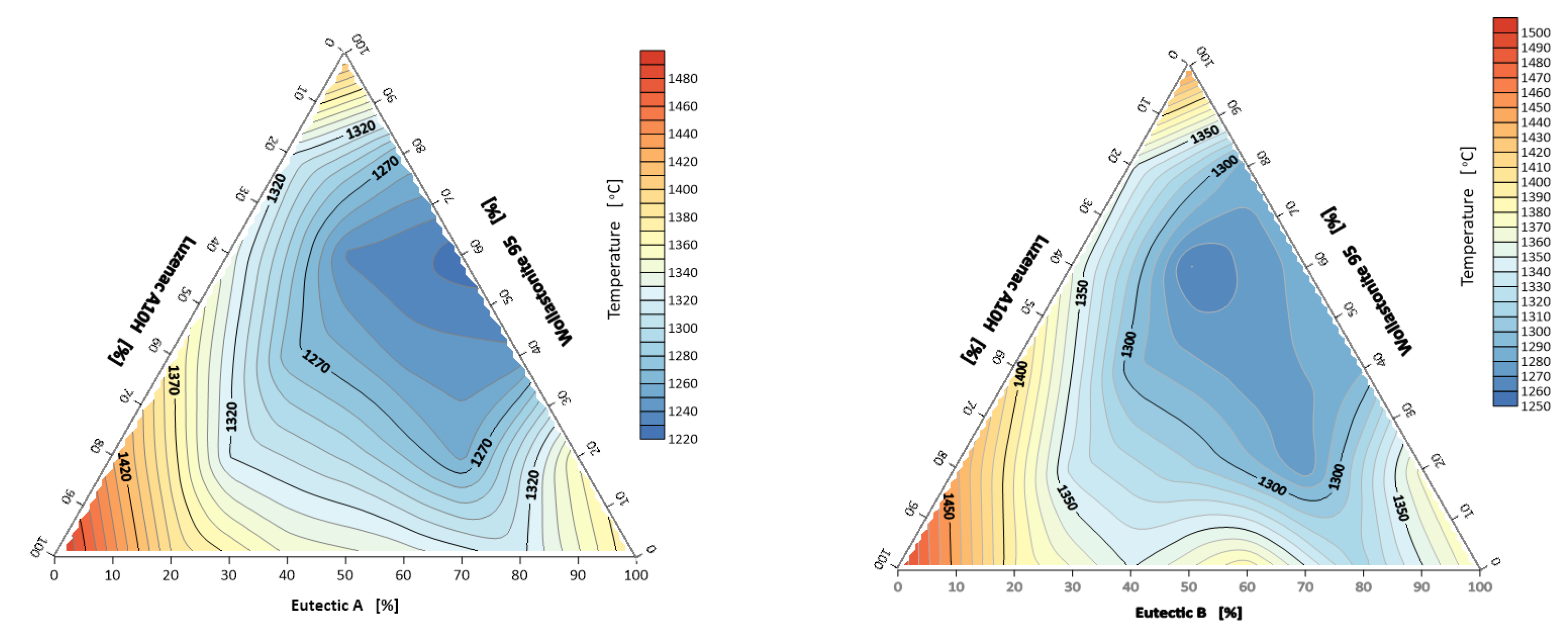
3.1. Visualization of Thermal Parameters Obtained from a High-Temperature Microscope of a Three-Component System
3.1.1. Characteristic Temperatures of the System: A-Eutectic–Talc–Wollastonite and B-Eutectic–Talc–Wollastonite
3.1.2. Melting and Flow Intervals of the System A-Eutectic–Talc–Wollastonite and B-Eutectic–Talc–Wollastonite
3.2. Thermal Parameters of the Eutectic System
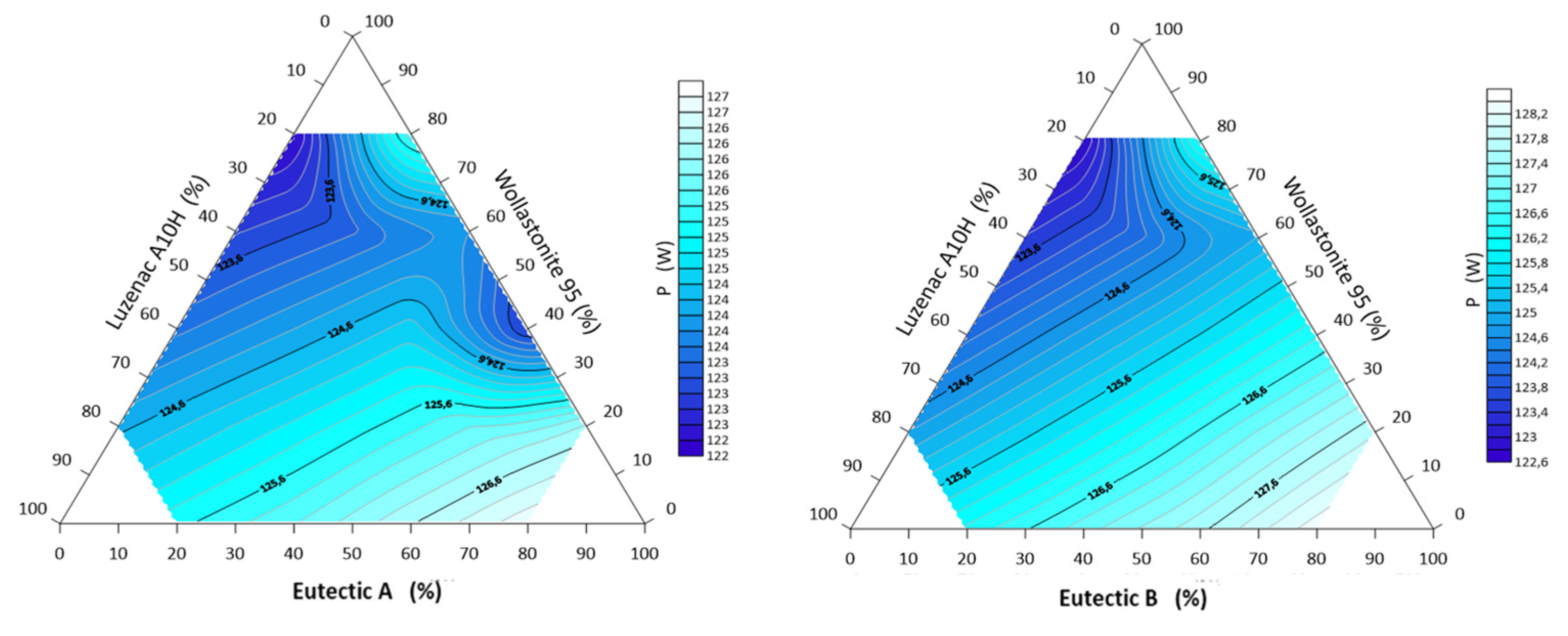
3.3. Characteristics of Other Parameters of Alloys of the System: A-Eutectic–Talc–Wollastonite and B-Eutectic–Talc–Wollastonite
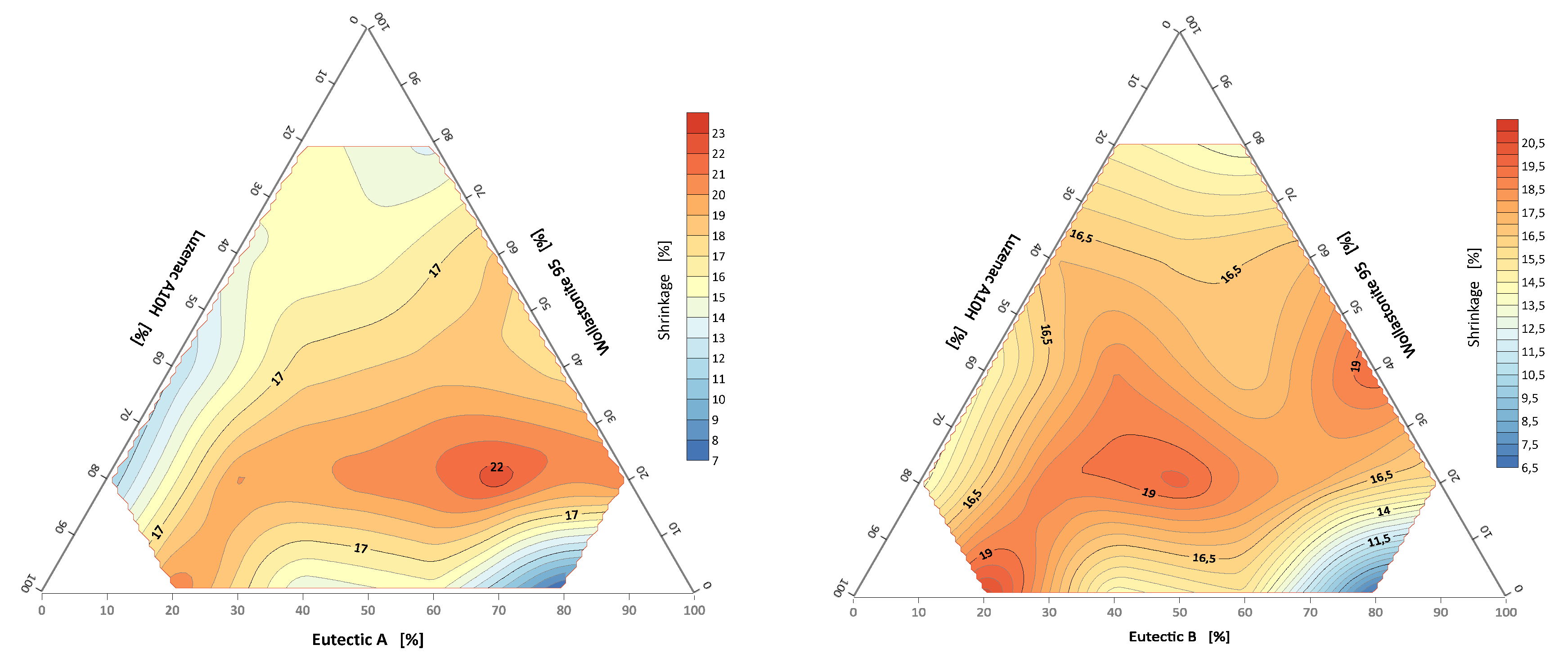
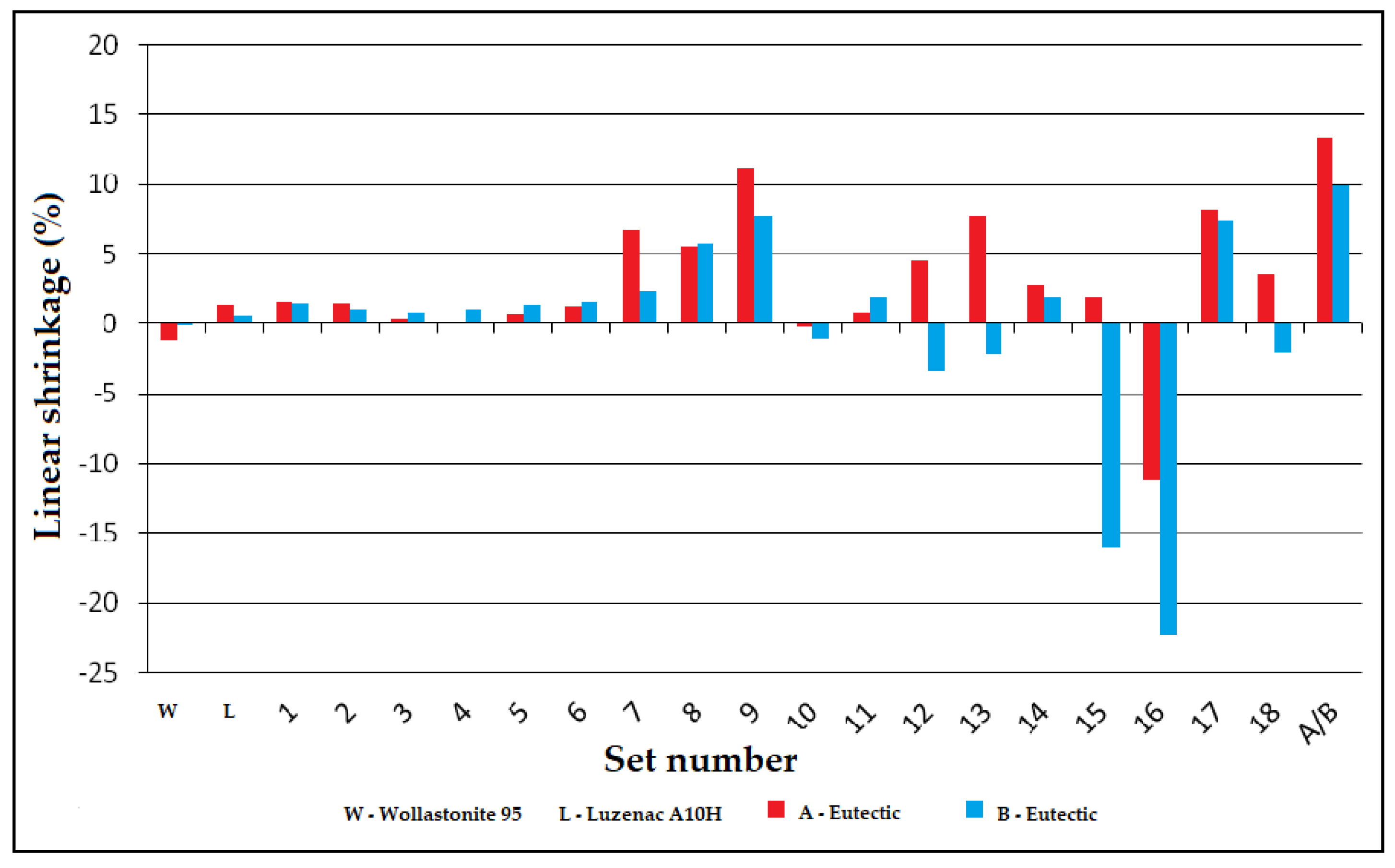
3.3.1. Linear Shrinkage of Alloys of the System: A/B-Eutectic–Talk–Wollastonite
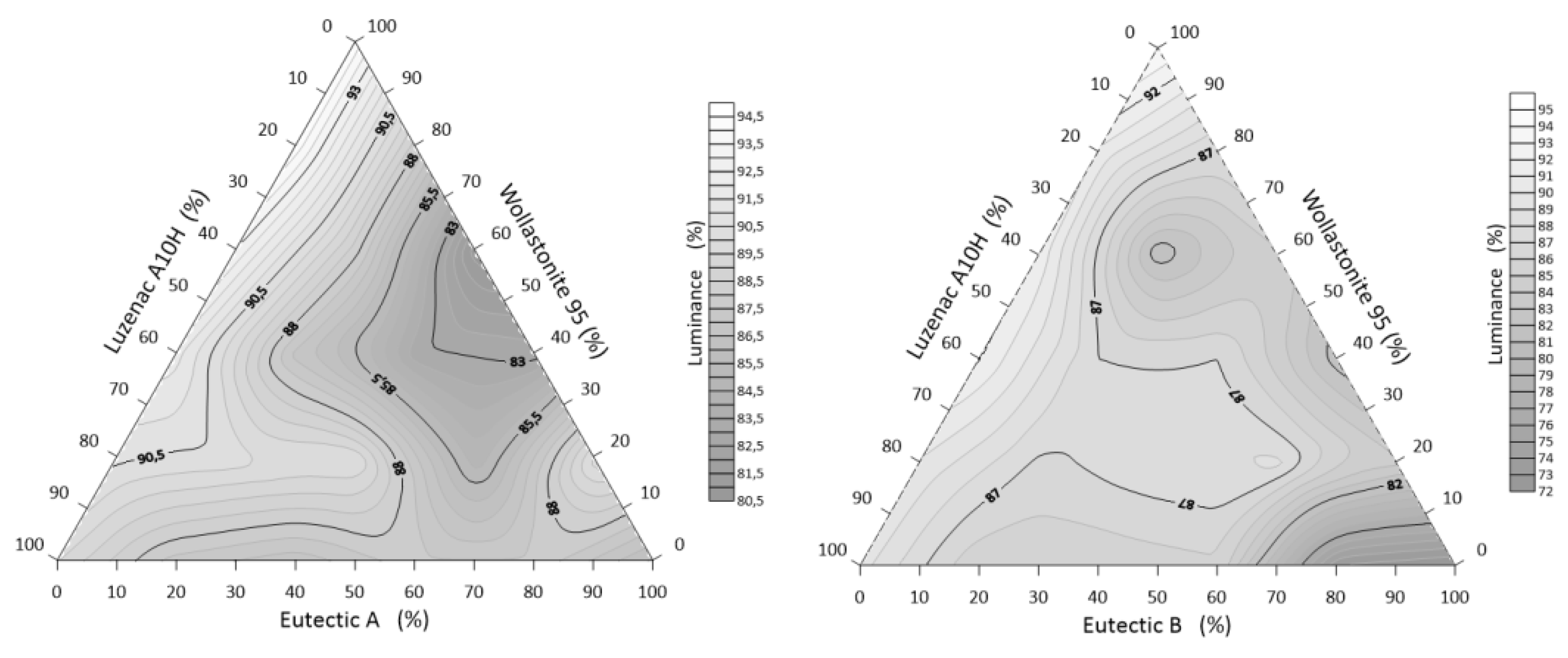
3.3.2. Whiteness of Alloys: A/B-Eutectic–Talc–Wollastonite
4. Conclusions
- The lowest melting point was observed for the composition of A-eutectic 20% and wollastonite 80% and it was 1140 °C. Similarly, for the system with B-eutectic, the lowest melting point of 1210 °C was recorded for the point with the same composition.
- An increase in the talc content of the flux system increases the characteristic temperatures. Wollastonite improves the sintering of the system.
- From the technological point of view, the flow interval is important, and the most favorable was again the point A-eutectic 20% and wollastonite 80% (set number 9) with the interval of 123 °C.
- The lowest transformation energy of the system ΔQ 808kJ was recorded for the point 40% A-eutectic and 60% wollastonite, at the same time the sets A/B-eutectic 20% and wollastonite 80% in which the eutectic point was recorded to have the highest transformation energy 1018 kJ.
- Maximum shrinkage occurs at the same points for the A/B-eutectic –wollastonite–talc system (set 9).
- The most promising from the point of view of industrial implementation seems to be set 9, i.e., 80% wollastonite with eutectic A. This set has the lowest melting point and the highest density.
- Currently, technological (strength, water absorbability, density) and microstructural studies are carried out on the obtained lithium aluminum silicate (Li)–alkali feldspars (Na,K)–magnesium (Mg) and calcium (Ca) silicates.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Handke, M. Krystalochemia Krzemianów; Wydawnictwa AGH: Kraków, Poland, 2008. [Google Scholar]
- Wyszomirski, P.; Galos, K. Surowce Mineralne i Chemiczne Przemysłu Ceramicznego; AGH Uczelniane Wydawnistwo Naukowo-Dydaktyczne: Kraków, Poland, 2007. [Google Scholar]
- Bozdoǧan, I.; Göknel, I. Turkish feldspar. Ceramika/Ceramics 2004, 84, 115–118. [Google Scholar]
- Wyszomirski, P.; Gacki, F.; Szydłak, T. Turkish feldspar raw materials in polish production of ceramic tiles. Gospod. Surowcami Miner. Miner. Resour. Manag. 2012, 28, 5–18. [Google Scholar] [CrossRef]
- Xia, J.; Geng, R.; Chen, Z. The Effect of K-feldspar and Silica as Fluxing Agent on the Production Process of Phosphorus Furnace. Silicon 2018, 11, 233–239. [Google Scholar] [CrossRef]
- Sokolář, R.; Keršnerová, L.; Šveda, M. The effect of different fluxing agents on the sintering of dry pressed porcelain bodies. J. Asian Ceram. Soc. 2017, 5, 290–294. [Google Scholar] [CrossRef]
- Peng, L.; Qin, S. Sintering behavior and technological properties of low-temperature porcelain tiles prepared using a lithium ore and silica waste. Minerals 2019, 9, 731. [Google Scholar] [CrossRef] [Green Version]
- Stempkowska, A. Silicate Mineral Eutectics with Special Reference to Lithium. Materials 2021, 14, 4334. [Google Scholar] [CrossRef]
- Boyd, J.E. Pyrometric Properties of Spodumene-Felspar Mixtures. J. Am. Ceram. Soc. 1938, 21, 385–388. [Google Scholar] [CrossRef]
- Kunasz, I.A. Lithium Resources. In Industrial Minerals and Rocks; SME (Society Mining Metallurgy and Exploration): Englewood, CO, USA, 2006; pp. 599–614. [Google Scholar]
- Timoshevskii, A.N.; Ktalkherman, M.G.; Emel’Kin, V.A.; Pozdnyakov, B.A.; Zamyatin, A.P. High-temperature decomposition of lithium carbonate at atmospheric pressure. High Temp. 2008, 46, 414–421. [Google Scholar] [CrossRef]
- Liu, W.; Chu, G.W.; Li, S.C.; Bai, S.; Luo, Y.; Sun, B.C.; Chen, J.F. Preparation of lithium carbonate by thermal decomposition in a rotating packed bed reactor. Chem. Eng. J. 2019, 377, 119929. [Google Scholar] [CrossRef]
- Shi, L.; Qu, T.; Liu, D.; Deng, Y.; Yang, B.; Dai, Y. Process of Thermal Decomposition of Lithium Carbonate. In Materials Processing Fundamentals; Lee, J., Wagstaff, S., Lambotte, G., Allanore, A., Tesfaye, F., Eds.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Cioch, A.; Izak, P.; Stempkowska, A.; Thiery, D. Właściwości stopu układu: Spodumen-skaleń potasowy-skaleń sodowy. Szkło Ceram. 2009, 1, 29–32. [Google Scholar]
- Izak, P.; Cioch, A.; Thiery, D. Charakterystyka układu spodumen-skaleń potasowy-skaleń sodowy. Ceramika 2008, 103, 1201–1206. [Google Scholar]
- Cultrone, G.; Rodrguez-Navarro, C.; Sebastian, E.; Cazalla, O.; De la Torre, M.J. Carbonate and silicate phase reactions during ceramic firing. Eur. J. Mineral. 2001, 13, 621–634. [Google Scholar] [CrossRef] [Green Version]
- Tufar, W. Talc. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2000. [Google Scholar]
- Hammas, A.; Lecomte-Nana, G.; Daou, I.; Tessier-Doyen, N.; Peyratout, C.; Zibouche, F. Kaolinite-Magnesite or Kaolinite–Talc-Based Ceramics. Part II: Microstructure and the Final Properties Related Sintered Tapes. Minerals 2020, 10, 1080. [Google Scholar] [CrossRef]
- Sallam, E.H.; Naga, S.M.; Ibrahim, D.M. Mode of talc addition and its effect on the properties of ceramic bodies. Ceram. Int. 1984, 10, 87–92. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Z.; Wei, Z.; Chen, Y.; Li, J.; Luo, L. Effect of talc and titania on the microstructure and mechanical properties of alumina ceramics. Int. J. Appl. Ceram. Technol. 2018, 15, 633–642. [Google Scholar] [CrossRef]
- Valášková, M.; Blahůšková, V.; Martaus, A.; Študentová, S.; Vallová, S.; Tokarský, J. Effect of Talc in Mixtures with Fly Ash on Sintering Crystalline Phases and Porosity of Mullite-Cordierite Ceramics. Minerals 2021, 11, 154. [Google Scholar] [CrossRef]
- Bragança, S.R.; Lengler, H.C.M.; Bergmann, C.P. Wollastonite as a Flux for Ceramics Bodies. Mater. Sci. Forum 2012, 727–728, 1016–1021. [Google Scholar] [CrossRef]
- Yoon, S.D.; Lee, J.U.; Lee, J.H.; Yun, Y.H.; Yoon, W.J. Characterization of Wollastonite Glass-ceramics Made from Waste Glass and Coal Fly Ash. J. Mater. Sci. Technol. 2013, 29, 149–153. [Google Scholar] [CrossRef]
- Owoeye, S.S.; Abegunde, S.M.; Folorunso, D.O.; Adigun, B.O.; Kingsley, U. Microstructure, phase and physical evaluation of non-bioactive wollastonite glass–Ceramic prepared from waste glass by sintering method. Open Ceram. 2021, 5, 100062. [Google Scholar] [CrossRef]
- Fiocco, L.; Elsayed, H.; Daguano, J.K.M.F.; Soares, V.O.; Bernardo, E. Silicone resins mixed with active oxide fillers and Ca–Mg Silicate glass as alternative/integrative precursors for wollastonite–diopside glass-ceramic foams. J. Non-Cryst. Solids 2015, 416, 44–49. [Google Scholar] [CrossRef]
- Naoki, O.; Arisa, T.; Hirotaka, M.; Akiko, O.; Yasutoshi, N.; Toshihiro, K. Tuning of ion-release capability from bio-ceramic-polymer composites for enhancing cellular activity. R. Soc. Open Sci. 2019, 6, 190612. [Google Scholar] [CrossRef] [Green Version]
- No, Y.J.; Li, J.J.; Zreiqat, H. Doped Calcium Silicate Ceramics: A New Class of Candidates for Synthetic Bone Substitutes. Materials 2017, 10, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Zhao, T.; Qi, Y.; Luo, J.; Fang, J.; Yang, X.; Liu, X.; Xu, T.; Yang, Q.; Gou, Z.; et al. In Vitro Chondrocyte Responses in Mg-doped Wollastonite/Hydrogel Composite Scaffolds for Osteochondral Interface Regeneration. Sci. Rep. 2018, 8, 17911. [Google Scholar] [CrossRef] [PubMed]
- Ahmadipour, M.; Mohammadi, H.; Pang, A.L.; Arjmand, M.; Otitoju, T.A.; Okoye, P.U.; Rajitha, B. A review: Silicate ceramic-polymer composite scaffold for bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–16. [Google Scholar] [CrossRef]
- Sainz, M.A.; Pena, P.; Serena, S.; Caballero, A. Influence of design on bioactivity of novel CaSiO3–CaMg(SiO3)2 bioceramics: In vitro simulated body fluid test and thermodynamic simulation. Acta Biomater. 2010, 6, 2797–2807. [Google Scholar] [CrossRef] [PubMed]
- Grima, L.; Díaz-Pérez, M.; Gil, J.; Sola, D.; Peña, J.I. Generation of a Porous Scaffold with a Starting Composition in the CaO–SiO2–MgO–P2O5 System in a Simulated Physiological Environment. Appl. Sci. 2019, 10, 312. [Google Scholar] [CrossRef] [Green Version]
- Boccaccini, A.R.; Hamann, B. Review In Situ high-temperature optical microscopy. J. Mater. Sci. 1999, 34, 5419–5436. [Google Scholar] [CrossRef]
- Amarante, M.M.; Botelho de Sousa, A.; Machado Lite, M. Technical note processing a spodumene ore to obtain lithium concentrates for addition to glass and ceramic bodies. Miner. Eng. 1999, 12, 433–436. [Google Scholar] [CrossRef]
- Grimvall, G. Thermophysical Properties of Materials; Elsevier: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Kalinowski, E. Termodynamika; Wydawnictwo Politechniki Wrocławskiej: Wrocław, Poland, 1994. [Google Scholar]
- Więckowski, J. Właściwości Cieplne i Magnetyczne Wybranych Związków Kobaltu o Strukturze Warstwowej. Ph.D. Thesis, Polska Akademia Nauk, Warszawa, Poland, 2013. [Google Scholar]
- Szargut, J. Termodynamika Techniczna; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2000. [Google Scholar]
- Mielicki, J. Zarys Wiadomości o Barwie; Fundacja Rozwoju Polskiej Kolorystyki: Łódź, Poland, 1997. [Google Scholar]
- Stockman, H.M.G.; Gevers, T. Color measurement by imaging spectrometry. Comput. Vis. Image Underst. 2000, 79, 236–249. [Google Scholar] [CrossRef]
- Stempkowska, A.; Izak, P.; Mastalska-Popławska, J.; Staszewska, M. The analysis of thermal properties of selected rock materials by thermovision methods. J. Pol. Miner. Eng. Soc. 2018, 20, 337–344. [Google Scholar]
- Stempkowska, A.; Mastalska-Popławska, J.; Izak, P.; Wójcik, Ł.; Gawenda, T.; Karbowy, M. Research on the Thermal Properties of Fireplace Concrete Materials Containing Various Mineral Aggregates Enriched by Organic and Inorganic Fibers. Materials 2021, 14, 904. [Google Scholar] [CrossRef]
- Zergadło, B.; Halicka, A. Analiza właściwości cieplnych betonu z kruszywem z odpadów ceramiki sanitarnej. Bud. Archit. 2011, 9, 39–49. [Google Scholar]
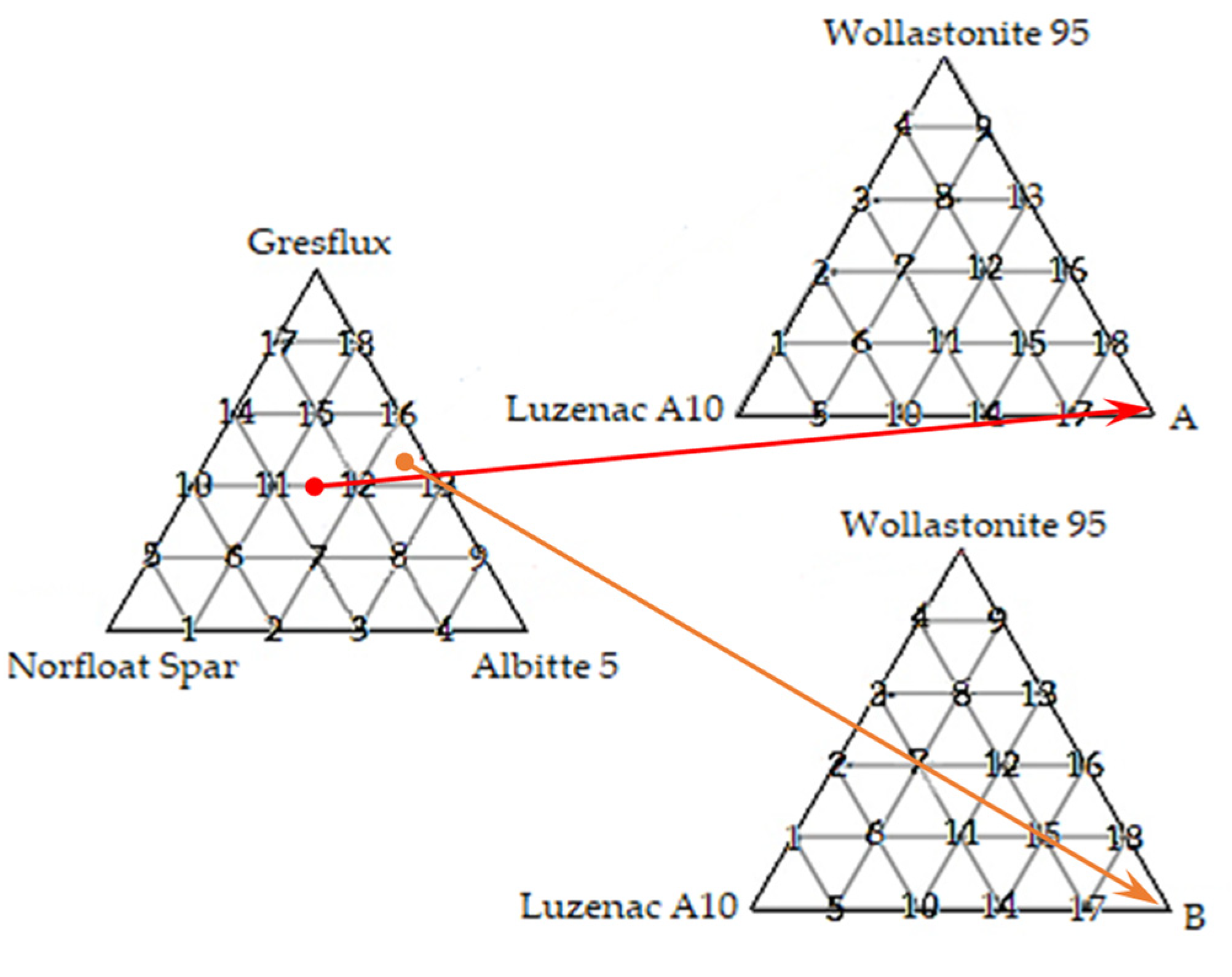



| Raw Material | Wollastonite 95 | Luzenac A10H | Eutectic A | Eutectic B |
|---|---|---|---|---|
| Chemical Composition (%wt.) | ||||
| SiO2 | 50.98 | 61.0 | 67.1 | 67.49 |
| Al2O3 | 0.41 | 0.3 | 19.09 | 20.19 |
| CaO | 45.54 | 0.4 | 0.49 | 0.50 |
| MgO | 1.20 | 32.0 | 0.5 | 0.69 |
| TiO2 | - | - | 0.11 | 0.16 |
| Fe2O3 | 0.29 | 0.2 | 0.44 | 0.53 |
| MnO | - | - | 0.08 | 0.1 |
| P2O5 | 0.11 | - | 0.18 | 0.21 |
| Na2O | 0.19 | 0.1 | 4.12 | 4.92 |
| K2O | 0.12 | - | 4.06 | 1.19 |
| Li2O | - | - | 2.44 | 3.05 |
| LOI | 1.15 | 6.0 | 1.49 | 1.02 |
| Metlig Point | 1450 °C | 1500 °C | 1263 °C | 1376 °C |
| Dominant Mineral | wollastonite (95%) | talc (95%) | - | - |
| Oxide | cv (J/kg·K) |
|---|---|
| SiO2 | 742 |
| Al2O3 | 775 |
| Fe2O3 | 655 |
| MgO | 924 |
| CaO | 750 |
| Na2O | 1115 |
| K2O | 764 |
| Li2O | 1811 |
| Specific Heat cv | Wollastonite 95 | Luzenac A10H | Eutectic A | Eutectic B |
|---|---|---|---|---|
| (J/kg K) | 742.69 | 765.06 | 778.26 | 790.00 |
| (g/cm3) | 2.98 | 2.71 | 2.79 | 2.87 |
| Set Number | Eutectic A–Luzenac A10H–Wollastonite 95 | Eutetic B–Luzenac A10H–Wollastonite 95 | ||||
|---|---|---|---|---|---|---|
| B MJ/(m3K) | t sec | ∆Q kJ | b M J/(m3K) | t sec | ∆Q kJ | |
| 1 | 2.098 | 6774 | 843 | 2.098 | 7656 | 955 |
| 2 | 2.127 | 7164 | 888 | 2.127 | 7788 | 966 |
| 3 | 2.155 | 7794 | 961 | 2.155 | 7872 | 971 |
| 4 | 2.182 | 8046 | 987 | 2.182 | 8088 | 992 |
| 5 | 2.090 | 6372 | 799 | 2.110 | 7398 | 934 |
| 6 | 2.119 | 6948 | 868 | 2.137 | 7368 | 924 |
| 7 | 2.148 | 7308 | 909 | 2.166 | 7584 | 946 |
| 8 | 2.176 | 7656 | 947 | 2.194 | 7656 | 950 |
| 9 | 2.256 | 8082 | 1018 | 2.275 | 8052 | 1018 |
| 10 | 2.112 | 6414 | 808 | 2.148 | 6900 | 876 |
| 11 | 2.141 | 7122 | 893 | 2.177 | 7092 | 895 |
| 12 | 2.170 | 7572 | 945 | 2.206 | 7308 | 917 |
| 13 | 2.200 | 7788 | 967 | 2.234 | 7794 | 974 |
| 14 | 2.133 | 6930 | 877 | 2.186 | 6420 | 819 |
| 15 | 2.163 | 7368 | 928 | 2.216 | 6948 | 882 |
| 16 | 2.156 | 7566 | 932 | 2.245 | 7164 | 905 |
| 17 | 2.155 | 7398 | 940 | 2.226 | 6372 | 818 |
| 18 | 2.185 | 7482 | 946 | 2.256 | 6780 | 866 |
| Set Number | Eutectic A–Luzenac A10H–Wollastonite 95 | Eutectic B–Luzenac A10H–Wollastonite 95 | ||||
|---|---|---|---|---|---|---|
| L (%) | a (%) | b (%) | L (%) | a (%) | b (%) | |
| 1 | 94.49 | 0.69 | 7.34 | 91.12 | 0.79 | 13.49 |
| 2 | 93.09 | 1.69 | 8.95 | 92.01 | 0.89 | 11.50 |
| 3 | 91.78 | 2.60 | 10.40 | 92.23 | 1.53 | 13.22 |
| 4 | 90.63 | 3.24 | 11.83 | 89.94 | 2.39 | 14.91 |
| 5 | 88.30 | −0.82 | 10.57 | 87.08 | −0.96 | 9.15 |
| 6 | 88.27 | −0.24 | 11.51 | 81.25 | −0.14 | 16.20 |
| 7 | 86.85 | −0.49 | 12.58 | 87.01 | 0.62 | 14.52 |
| 8 | 90.26 | 0.79 | 11.03 | 86.80 | 0.75 | 16.20 |
| 9 | 87.15 | 0.80 | 9.44 | 85.07 | 1.79 | 12.07 |
| 10 | 80.58 | −0.60 | 1.71 | 84.94 | −1.46 | 4.00 |
| 11 | 83.19 | −0.75 | 9.59 | 87.00 | −0.78 | 13.46 |
| 12 | 90.00 | −0.03 | 10.63 | 87.79 | −0.08 | 11.10 |
| 13 | 86.50 | 1.25 | 10.10 | 85.15 | 1.40 | 11.62 |
| 14 | 82.80 | −1.53 | 2.26 | 81.30 | −2.70 | 1.81 |
| 15 | 84.68 | −0.51 | 8.37 | 88.21 | −0.78 | 7.10 |
| 16 | 87.70 | 0.65 | 9.07 | 85.71 | 0.15 | 8.45 |
| 17 | 90.38 | −0.33 | 6.81 | 84.18 | −0.96 | 4.79 |
| 18 | 87.70 | −2.55 | 1.12 | 72.45 | −1.93 | 1.67 |
| Wollastonit | 94.49 | 0.31 | 6.09 | 94.49 | 0.31 | 6.09 |
| Talk | 89.53 | 3.85 | 12.33 | 89.53 | 3.85 | 12.33 |
| A/B | 85.61 | −0.03 | 2.02 | 71.41 | −0.16 | 1.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stempkowska, A. Characterization of the Flux System: Lithium-Aluminum Silicate (Li)–Alkali Feldspars (Na,K); Magnesium (Mg) and Calcium (Ca)–Silicates. Materials 2021, 14, 7386. https://doi.org/10.3390/ma14237386
Stempkowska A. Characterization of the Flux System: Lithium-Aluminum Silicate (Li)–Alkali Feldspars (Na,K); Magnesium (Mg) and Calcium (Ca)–Silicates. Materials. 2021; 14(23):7386. https://doi.org/10.3390/ma14237386
Chicago/Turabian StyleStempkowska, Agata. 2021. "Characterization of the Flux System: Lithium-Aluminum Silicate (Li)–Alkali Feldspars (Na,K); Magnesium (Mg) and Calcium (Ca)–Silicates" Materials 14, no. 23: 7386. https://doi.org/10.3390/ma14237386
APA StyleStempkowska, A. (2021). Characterization of the Flux System: Lithium-Aluminum Silicate (Li)–Alkali Feldspars (Na,K); Magnesium (Mg) and Calcium (Ca)–Silicates. Materials, 14(23), 7386. https://doi.org/10.3390/ma14237386






