Young’s Modulus and Vickers Hardness of the Hydroxyapatite Bioceramics with a Small Amount of the Multi-Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sintering of HAp–CNT Composite
2.2. Characterization of the Samples
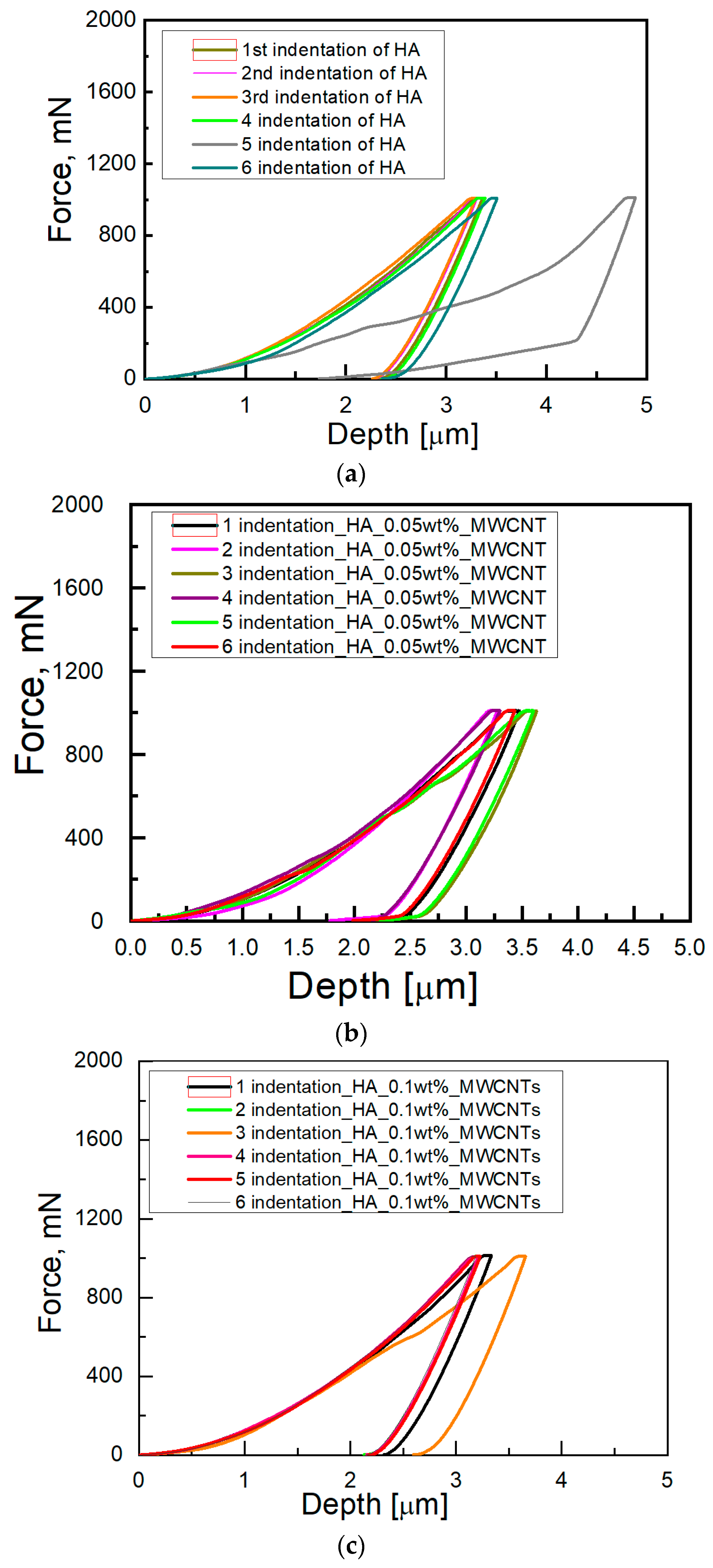
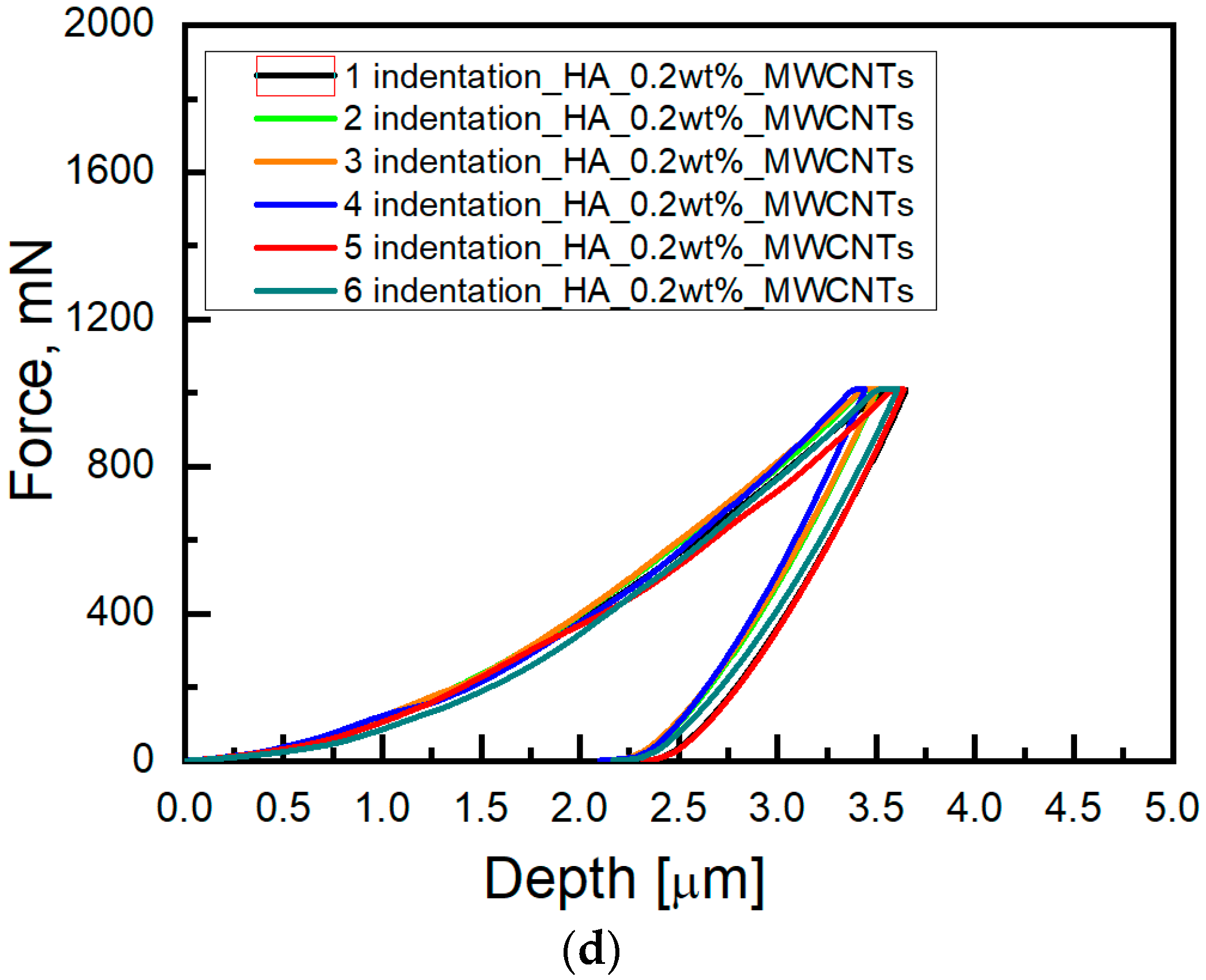
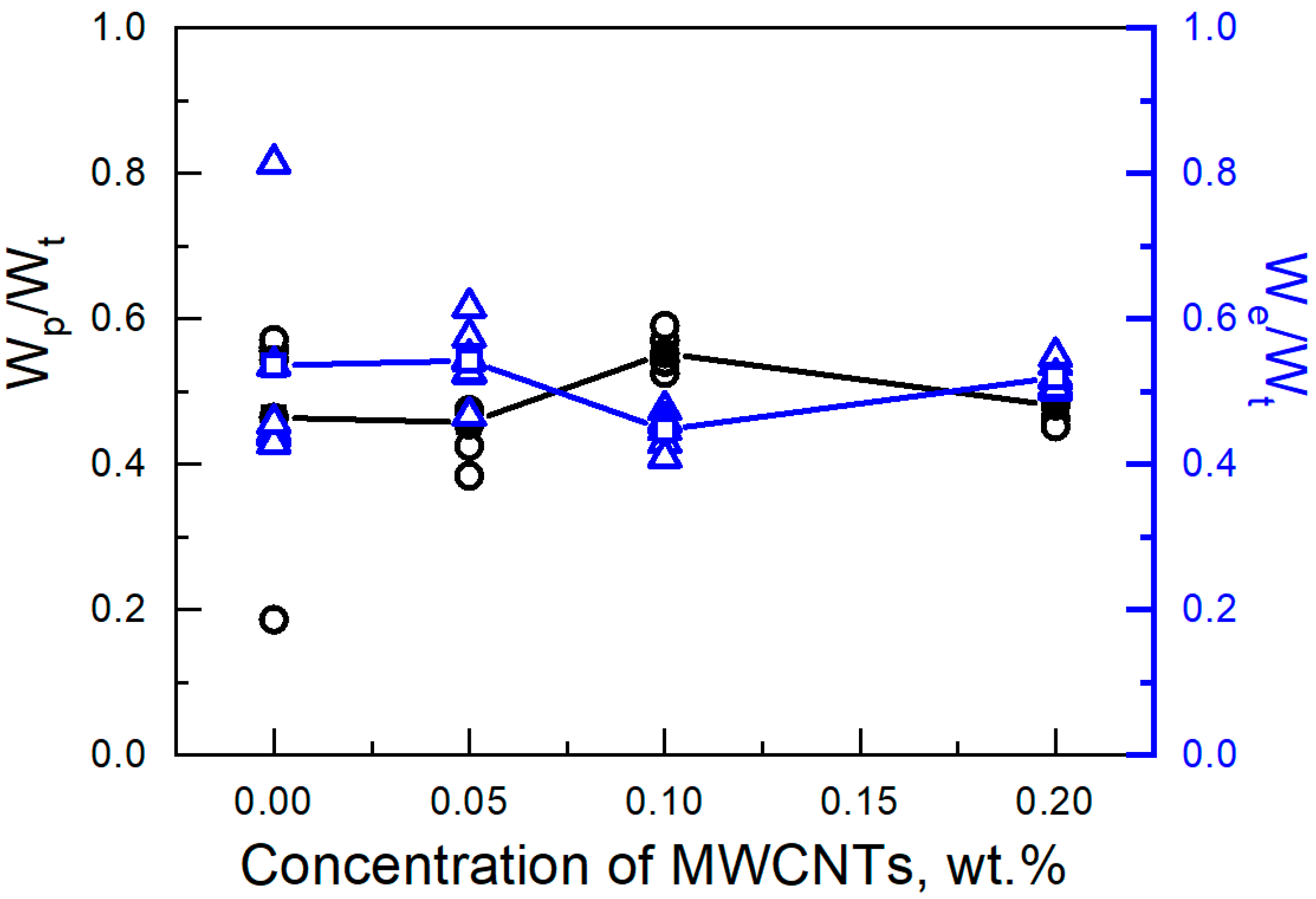
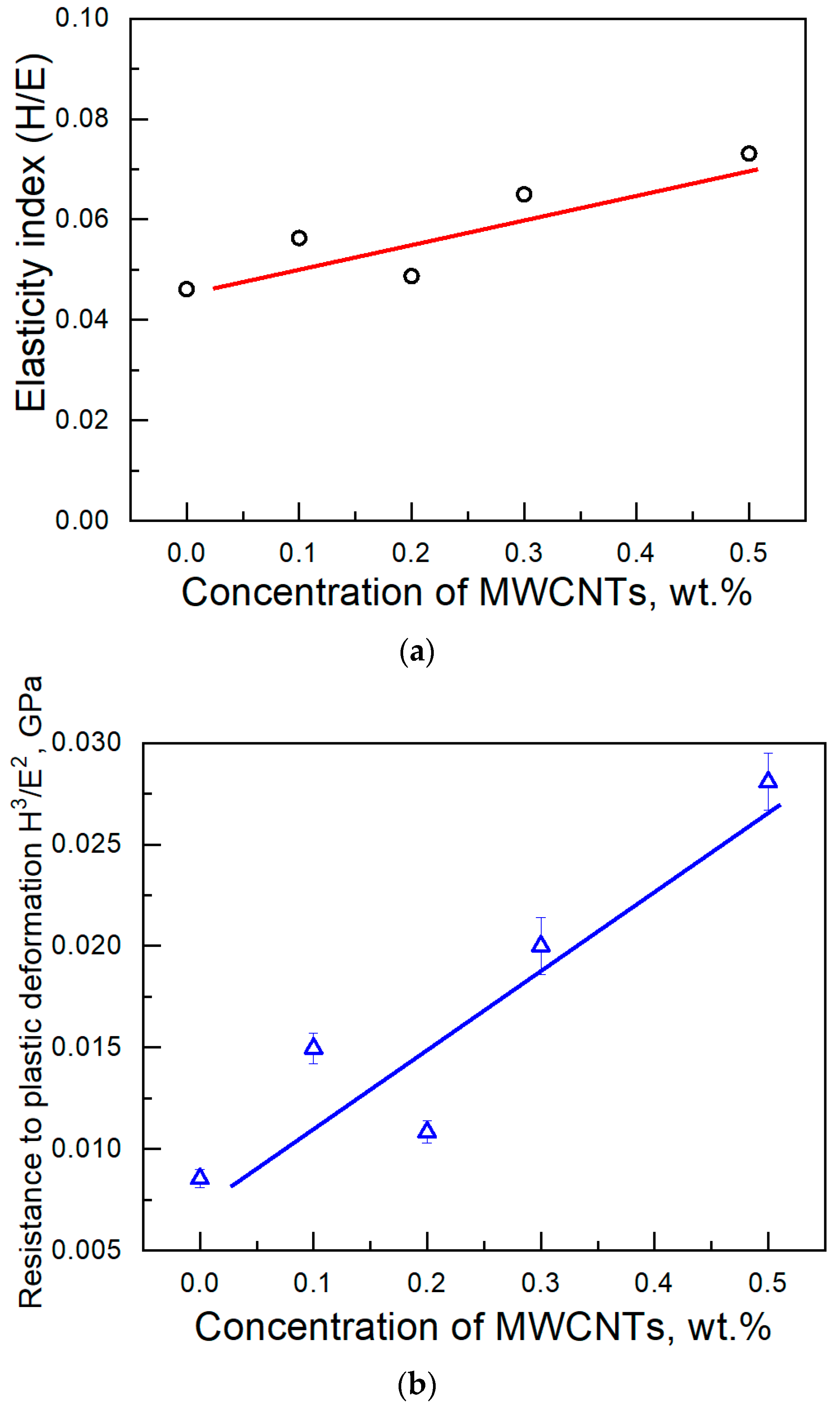
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdollahi, S.; Paryab, A.; Khalilifard, R.; Khalilifard, R.; Anousheh, M.; Khachatourian, A.M. The fabrication and characterization of bioactive Akermanite/Octacalcium phosphate glass-ceramic scaffolds produced via PDC method. Ceram. Intern. 2021, 47, 6653–6662. [Google Scholar] [CrossRef]
- Ribas, R.G.; Schatkoski, V.M.; do Amaral Montanheiro, T.L.; de Menezes, B.R.C.; Stegemann, C.; Leite, D.M.G.; Thim, G.P. Current advances in bone tissue engineering concerning ceramic and bioglass scaffolds: A review. Ceram. Intern. 2019, 45, 21051–21061. [Google Scholar] [CrossRef]
- Zhao, X.; Zheng, J.; Zhang, W.; Chen, X.; Gui, Z. Preparation of silicon coated-carbon fiber reinforced HA bio-ceramics for application of load-bearing bone. Ceram. Intern. 2020, 46, 7903–7911. [Google Scholar] [CrossRef]
- Khalid, P.; Suman, V.B. Carbon Nanotube-Hydroxyapatite Composite for Bone Tissue Engineering and Their Interaction with Mouse Fibroblast L929 In Vitro. J. Bionanosci. 2017, 11, 233–240. [Google Scholar] [CrossRef]
- Ananth, H.; Kundapur, V.; Mohammed, H.S.; Anand, M.; Amarnath, G.S.; Mankar, S. A review on biomaterials in dental implantology. Int. J. Biomed. Sci. 2015, 11, 113–120. [Google Scholar] [PubMed]
- Boteanu, R.M.; Suica, V.I.; Ivan, L.; Safciuc, F.; Uyy, E.; Dragan, E.; Croitoru, S.M.; Grumezescu, V.; Chiritoiu, M.; Sima, L.E.; et al. Proteomics of regenerated tissue in response to a titanium implant with a bioactive surface in a rat tibial defect model. Nature 2020, 10, 18493. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Ma, P.; Sutrisno, L.; Luo, Z.; Hu, Y.; Yu, Y.; Tao, B.; Li, C.; Cai, K. Fabrication of magnesium/zinc-metal organic framework on titanium implants to inhibit bacterial infection and promote bone regeneration. Biomaterials 2019, 212, 1–16. [Google Scholar] [CrossRef]
- Koller, M.; Steyer, E.; Theisen, K.; Stagnell, S.; Jakse, N.; Payer, M. Two-piece zirconia versus titanium implants after 80 months: Clinical outcomes from a prospective randomized pilot trial. Clin. Oral Impl. Res. 2020, 31, 388–396. [Google Scholar] [CrossRef] [Green Version]
- Nhlapo, N.; Dzogbewy, T.C.; De Smidt, O. A systematic review on improving the biocompatibility of titanium implants using nanoparticles. Manuf. Rev. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Sansone, V.; Pagani, D.; Melato, M. The effects on bone cells of metal ions released from orthopedic implants. A review. Clin. Cas. Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar]
- Patil, N.A.; Kandasubramanian, B. Biological and mechanical enhancement of zirconium dioxide for medical applications. Ceram. Intern. 2020, 46, 4041–4057. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, W.; Chu, F.; Zhou, F.; Liang, S.; Ma, C.; Hu, Y. Hydroxyapatite/polyureananocomposite: Preparation and multiple performance enhancements. Compos. Part A Appl. Sci. Manuf. 2020, 128, 105681. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.; Ghazali, S.; Heim, H.P.; Feldmann, M. Impact modified PLAhydroxyapatite composites—Thermo-mechanical properties. Compos. Part A Appl. Sci. Manuf. 2018, 107, 326–333. [Google Scholar] [CrossRef]
- Ruiz-Aguilar, C.; Olivares-Pinto, U.; Alfonso, I. Novel β-TCP scaffold production using NaCl as a porogen for bone tissue applications. Ceram. Intern. 2021, 47, 2244–2254. [Google Scholar] [CrossRef]
- Cho, S.; Kim, J.; Lee, S.B.; Choi, M.; Kim, D.H.; Jo, I.; Kwon, H.; Kim, Y. Fabrication of functionally graded hydroxyapatite and structurally graded porous hydroxyapatite by using multi-walled carbon nanotubes. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106138. [Google Scholar] [CrossRef]
- Taromsari, S.M.; Salari, M.; Bagheri, R.; Sani, M.A.F. Optimizing tribological, tensile & in-vitro biofunctional properties of UHMWPE based nanocomposites with simultaneous incorporation of graphene nanoplatelets (GNP) & hydroxyapatite (HAp) via a facile approach for biomedical applications. Compos. Part B Eng. 2019, 175, 107181. [Google Scholar]
- Gao, C.; Feng, P.; Peng, S.; Shuai, C. Carbon nanotube, graphene and boron nitride nanotube reinforced bioactive ceramics for bone repair. Acta Biomater. 2017, 61, 1–20. [Google Scholar] [CrossRef]
- Usui, Y.; Aoki, K.; Narita, N.; Murakami, N.; Nakamura, I.; Nakamura, K.; Ishigaki, N.; Yamazaki, H.; Horiuchi, H.; Kato, H. Carbon nanotubes with high bone-tissue compatibility and bone-formation acceleration effects. Biocomp. Mater. 2008, 4, 240–246. [Google Scholar] [CrossRef]
- Khalid, P.; Hussain, M.A.; Suman, V.B.; Arun, A.B. Toxicology of carbon nanotubes—A review. Int. J. App. Eng. Res. 2016, 11, 148–157. [Google Scholar]
- Khalid, P.; Hussain, M.A.; Rekha, P.D.; Arun, A.B. Carbon nanotube-reinforced hydroxyapatite composite and their interaction with human osteoblast in vitro. Hum. Exp. Toxicol. 2014, 34, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Kotchey, G.P.; Zhao, Y.; Kagan, V.E.; Star, A. Peroxidasemediated Biodegradation of carbon nanotubes in vitro and in vivo. Adv. Drug. Deliv. Rev. 2013, 65, 1921–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Yang, M.; Nakajima, H.; Yudasaka, M.; Iijima, S.; Okazaki, T. Diameter-dependent degradation of 11 types of carbon nanotubes: Safety implications. ACS Appl. Nano Mater. 2019, 2, 4293–4301. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, M. Biodegradation of carbon nanotubes by macrophages. Front. Mater. 2019, 6, 225. [Google Scholar] [CrossRef]
- Elumeeva, K.V.; Kuznetsov, V.L.; Ischenko, A.V. Reinforcement of CVD grown multi-walled carbon nanotubes by high temperature annealing. AIP Adv. 2013, 3, 112101. [Google Scholar] [CrossRef]
- Barabashko, M.S.; Tkachenko, M.V.; Neiman, A.A.; Ponomarev, A.N.; Rezvanova, A.E. Variation of Vickers microhardness and compression strength of the bioceramics based on hydroxyapatite by adding the multi-walled carbon nanotubes. Appl. Nanosci. 2020, 10, 2601–2608. [Google Scholar] [CrossRef]
- Xu, J.; Hu, X.; Jiang, S.; Wang, Y.; Parungao, R.; Zheng, S.; Nie, Y.; Liu, T.; Song, K. The Application of Multi-Walled Carbon Nanotubes in Bone Tissue Repair Hybrid Scaffolds and the Effect on Cell Growth In Vitro. Polymers 2019, 11, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.; Devi, S.; Pandey, V.S.; Bharj, R.S.; Tyagi, S. Synthesis and Characterization of Carbon Nanotubes Doped Hydroxyapatite Nanoceramic for Orthopedic Applications. Trans. Indian Inst. Met. 2017, 71, 177–183. [Google Scholar] [CrossRef]
- Abrishamchian, A.; Hooshmand, T.; Mohammadi, M.; Najafi, F. Preparation and characterization of multi-walled carbon nanotube/hydroxyapatite nanocomposite film dip coated on Ti–6Al–4V by sol–gel method for biomedical applications: An in vitro study. Mat. Sci. Eng. C 2013, 33, 2002–2010. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.Q.; Zhan, T.H.; Gan, C.H.; Zheng, C.Y.; Yu, G. Carbon nanotube reinforced hydroxyapatite composite coatings produced through laser surface alloying. Carbon 2006, 44, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Mohajernia, S.; Pour-Ali, S.; Hejazi, S.; Saremi, M.; Kiani-Rashid, A.R. Hydroxyapatite coating containing multi-walled carbon nanotubes on AZ31 magnesium: Mechanical-electrochemical degradation in a physiological environment. Ceram. Intern. 2018, 44, 8297–8305. [Google Scholar] [CrossRef]
- Balani, K.; Anderson, R.; Laha, T.; Andara, M.; Tercero, J.; Crumpler, E.; Agarwal, A. Plasma-sprayed carbon nanotube reinforced hydroxyapatite coatings and their interaction with human osteoblasts in vitro. Biomaterials 2007, 28, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi-Rad, M. Effect of dispersants on the electrophoretic deposition of hydroxyapatite-carbon nanotubes nanocomposite coatings. J. Am. Ceram. Soc. 2016, 99, 2947–2955. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Su, Y.; Guo, Q.; Zhang, L. Preparation and properties of in-situ growth of carbon nanotubes reinforced hydroxyapatite coating for carbon/carbon composites. Mat. Sci. Eng. C 2017, 70, 805–811. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Zhang, L.; Liu, Q.; Wang, Y.; Zhang, W.; Zheng, J. Preparation of Nano-Hydroxyapatite Coated Carbon Nanotube Reinforced Hydroxyapatite Composites. Coatings 2018, 8, 357. [Google Scholar] [CrossRef] [Green Version]
- Nezhad, E.Z.; Qu, X.; Musharavati, F.; Jaber, F.; Appleford, M.R.; Bae, S.; Uzun, K.; Struthers, M.; Chowdhury, M.E.H.; Khandakar, A. Effects of titanium and carbon nanotubes on nano/micromechanical properties of HA/TNT/CNT nanocomposites. Appl. Surf. Sci. 2021, 538, 148123. [Google Scholar] [CrossRef]
- Lahiri, D.; Singh, V.; Keshri, A.K.; Seal, S.; Agarwal, A. Carbon nanotube toughened hydroxyapatite by spark plasma sintering: Microstructural evolution and multiscale tribological properties. Carbon 2010, 48, 3103–3120. [Google Scholar] [CrossRef]
- Kalmodia, S.; Goenka, S.; Laha, T.; Lahiri, D.; Basu, B.; Balani, K. Microstructure, mechanical properties, and in vitro biocompatibility of spark plasma sintered hydroxyapatite–aluminum oxide–carbon nanotube composite. Mat. Sci. Eng. C 2010, 30, 1162–1169. [Google Scholar] [CrossRef]
- Kealley, C.S.; Latella, B.A.; Van Riessen, A.; Elcombe, M.M.; Ben-Nissan, B. Micro- and Nano-Indentation of a Hy-droxyapatite-Carbon Nanotube Composite. J. Nanosci. Nanotechnol. 2008, 8, 3936–3941. [Google Scholar] [CrossRef]
- Zyman, Z.Z.; Tkachenko, M.V.; Polevodin, D.V. Preparation and characterization of biphasic calcium phosphate ceramics of desired composition. J. Mat. Sci. Mat. Med. 2008, 19, 2819–2825. [Google Scholar] [CrossRef]
- Usoltseva, A.; Kuznetsov, V.; Rudina, N.; Moroz, E.; Haluska, M.; Roth, S. Influence of catalysts’ activation on their activity and selectivity in carbon nanotubes synthesis. Phys. Stat. Solidi (b) 2007, 244, 3920–3924. [Google Scholar] [CrossRef]
- Kuznetsov, V.L.; Krasnikov, D.V.; Schmakov, A.N.; Elumeeva, K.V. In situ and ex situ time resolved study of multi-component Fe-Co oxide catalyst activation during MWNT synthesis. Phys. Stat. Solidi (b) 2012, 249, 2390–2394. [Google Scholar] [CrossRef]
- Ramesh, S.; Tan, C.Y.; Hamdi, M.; Sopyan, I.; Teng, W.D. The influence of Ca/P ratio on the properties of hydroxy-apatite bioceramics. Intern. Conf. Smart Mat. Nanotech. Eng. 2007, 6423, 855–860. [Google Scholar]
- PourAkbar Saffar, K.; Sudak, L.J.; Federico, S. A biomechanical evaluation of CNT-grown bone. J. Biomed. Mater. Res. Part A 2015, 104, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Golokhvast, K.S.; Kuznetsov, V.L.; Kusaikin, M.I.; Yelumeeva, K.V.; Mishakov, I.V.; Starokon, Y.V.; Chaika, V.V.; Nikiforov, P.A.; Panichev, A.M.; Gulkov, A.N. The influence of some synthetic and natural nanoparticles on sea hedgehog larvae development. Nanotechnol. Health 2013, 5, 36–39. (In Russian) [Google Scholar]
- Rezvanova, A.E.; Barabashko, M.S.; Tkachenko, M.V.; Ponomarev, A.N.; Neiman, A.A.; Belosludtseva, A.A. Experimental measurements and calculation of fracture toughness coefficient of a hydroxyapatite composite with small concentrations of additives of multi-walled carbon nanotubes. AIP Conf. Proc. 2020, 2310, 020277. [Google Scholar]
- Ponomarev, A.N.; Barabashko, M.S.; Rezvanova, A.E.; Evtushenko, E.P. Influence of porosity on fracture toughness of hydroxyapatite/multi-walled carbon nanotubes biocomposite materials. Rus. Phys. J. 2021, 63, 1885–1890. [Google Scholar] [CrossRef]
- White, A.A.; Best, S.M.; Kinloch, I.A. Hydroxyapatite-carbon nanotube composites for biomedical applications: A review. Int. J. Appl. Ceram. Technol. 2007, 4, 1–13. [Google Scholar] [CrossRef]
- Barabashko, M.S.; Tkachenko, M.V.; Rezvanova, A.E.; Ponomarev, A.N. Analysis of temperature gradients in the hy-droxyapatite ceramics with the additives of multi-walled carbon nanotubes. Rus. J. Phys. Chem. A 2021, 95, 1017–1022. [Google Scholar] [CrossRef]
- Rao, W.R.; Boehm, R.F. A Study of Sintered Apatites. J. Dent. Res. 1974, 53, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Muralithran, G.; Ramesh, S. The effects of sintering temperature on the properties of hydroxyapatite. Ceram. Intern. 2000, 26, 221–230. [Google Scholar] [CrossRef]
- Barralet, J.E.; Best, S.M.; Bonfield, W. Effect of sintering parameters on the density and microstructure of carbonate hydroxyapatite. J. Mat. Sci. Mat. Med. 2000, 11, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.M.; Shih, W.J.; Hon, M.H.; Wang, M.C. The Properties of Sintered Calcium Phosphate with [Ca]/[P] = 1.50. Int. J. Mol. Sci. 2012, 13, 13569–13586. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Kundu, B.; Sen, S.; Chanda, A. Improved properties of hydroxyapatite–carbon nanotube biocomposite: Mechanical, in vitro bioactivity and biological studies. Ceram. Intern. 2014, 40, 5635–5643. [Google Scholar] [CrossRef]
- White, A.A.; Kinloch, I.A.; Windle, A.H.; Best, S.M. Optimization of the sintering atmosphere for high-density hy-droxyapatite-carbon nanotube composites. J. R. Soc. Interface 2010, 7, S529–S539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, A.; Kingon, A.; Kukovecz, Á.; Konya, Z.; Vilarinho, P.M. Studies on the thermal decomposition of multiwall carbon nanotubes under different atmospheres. Mat. Lett. 2013, 90, 165–168. [Google Scholar] [CrossRef]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mat. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Kim, B.C.; Lee, J.H.; Kim, J.J.; Ikegami, T. Rapid rate sintering of nanocrystalline indium tin oxide ceramics: Particle size effect. Mat. Lett. 2002, 52, 114–119. [Google Scholar] [CrossRef]
- Barabashko, M.S.; Drozd, M.; Szewczyk, D.; Jeżowski, A.; Bagatskii, M.I.; Sumarokov, V.V.; Dolbin, A.V.; Nesov, S.N.; Korusenko, P.M.; Ponomarev, A.N. Calorimetric, NEXAFS and XPS studies of MWCNTs with low defectiveness. Fuller. Nanotub. Carbon Nanostruct. 2021, 29, 331–336. [Google Scholar] [CrossRef]
- Ponomarev, A.; Egorushkin, V.; Bobenko, N.; Barabashko, M.; Rezvanova, A.; Belosludtseva, A. On the Possible Nature of Armchair-Zigzag Structure Formation and Heat Capacity Decrease in MWCNTs. Materials 2022, 15, 518. [Google Scholar] [CrossRef]
- Mazov, I.; Kuznetsov, V.L.; Simonova, I.A.; Simonova, I.A.; Stadnichenko, A.I.; Ishchenko, A.V.; Romanenko, A.I.; Tkachev, E.N.; Anikeeva, O.B. Oxidation behavior of multiwall carbon nanotubes with different diameters and morphology. Appl. Surf. Sci. 2012, 258, 6272–6280. [Google Scholar] [CrossRef]
- Oyen, M.L. Handbook of Nanoindentation: With Biological Applications; Pan Stanford Publishing: Cambridge, UK, 2010. [Google Scholar]
- Imbeni, V.; Kruzic, J.J.; Marshall, G.W.; Marshall, S.J.; Ritchie, R.O. The dentin–enamel junction and the fracture of human teeth. Nat. Mat. 2005, 4, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley Publishing Company: Boston, MA, USA, 1978. [Google Scholar]
- Wang, Z.; Wang, K.; Xu, W.; Gong, X.; Zhang, F. Mapping the mechanical gradient of human dentin-enamel-junction at different intratooth locations. Dent. Mater. 2018, 34, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Case, E.D.; Ren, F.; Shu, Y.; Baumann, M.J. Part II: Fracture strength and elastic modulus as a function of porosity for hydroxyapatite and other brittle materials. J. Mech. Behav. Biomed. Mater. 2012, 8, 99–110. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Cheng, C.M. Scaling, dimensional analysis, and indentation measurements. Mater. Sci. Eng. R Rep. 2004, 44, 91–149. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Li, Z.; Cheng, C.M. Scaling relationships for indentation measurements. Phil. Mag. A 2002, 82, 1821–1829. [Google Scholar] [CrossRef]
- Dearnaley, G.; Arps, J.H. Biomedical applications of diamond-like carbon (DLC) coatings: A review. Surf. Coat. Technol. 2005, 200, 2518–2524. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Johnson, K.L. Contact Mechanics; Cambridge University Press: New York, NY, USA, 1987. [Google Scholar]


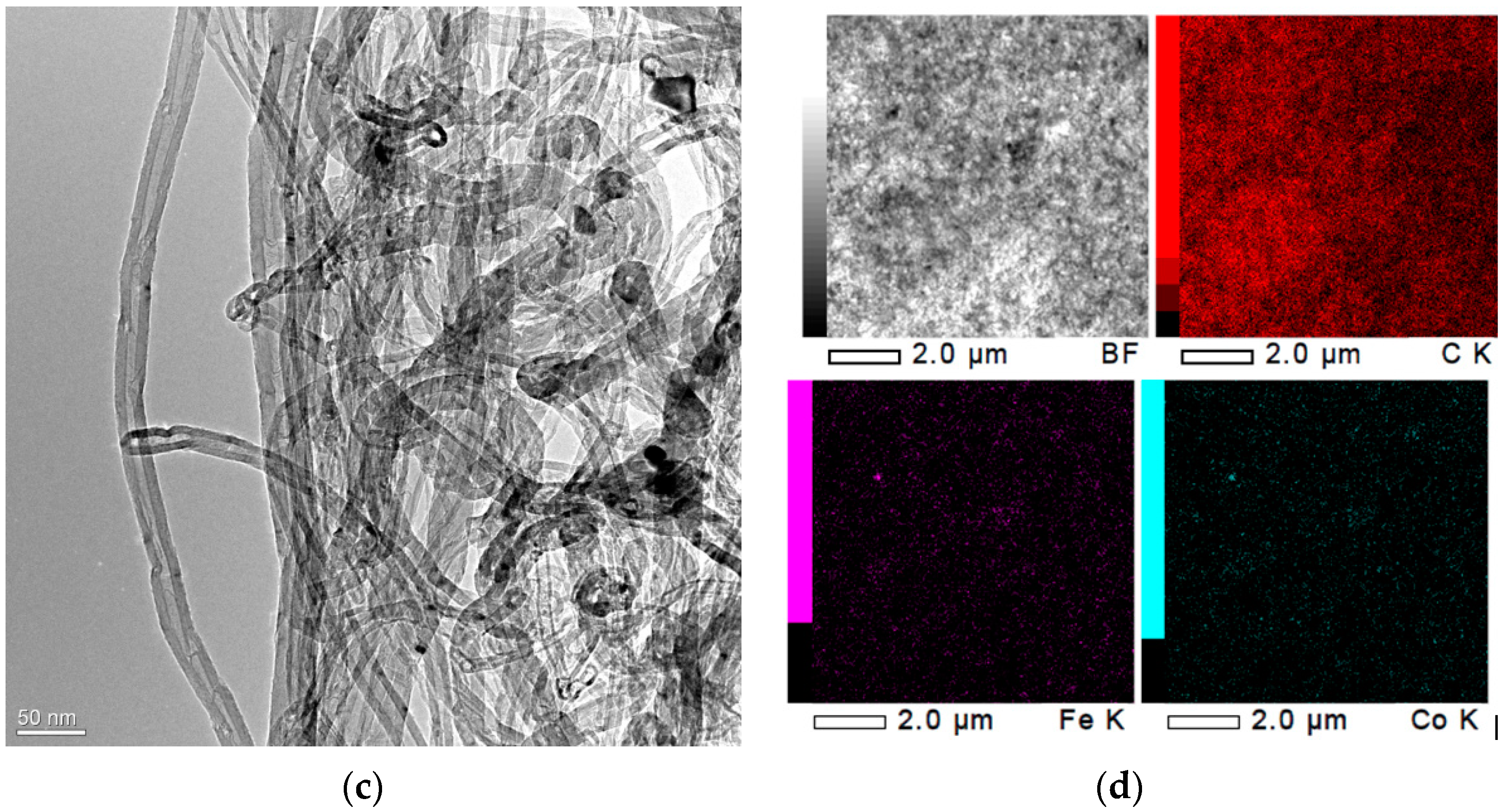
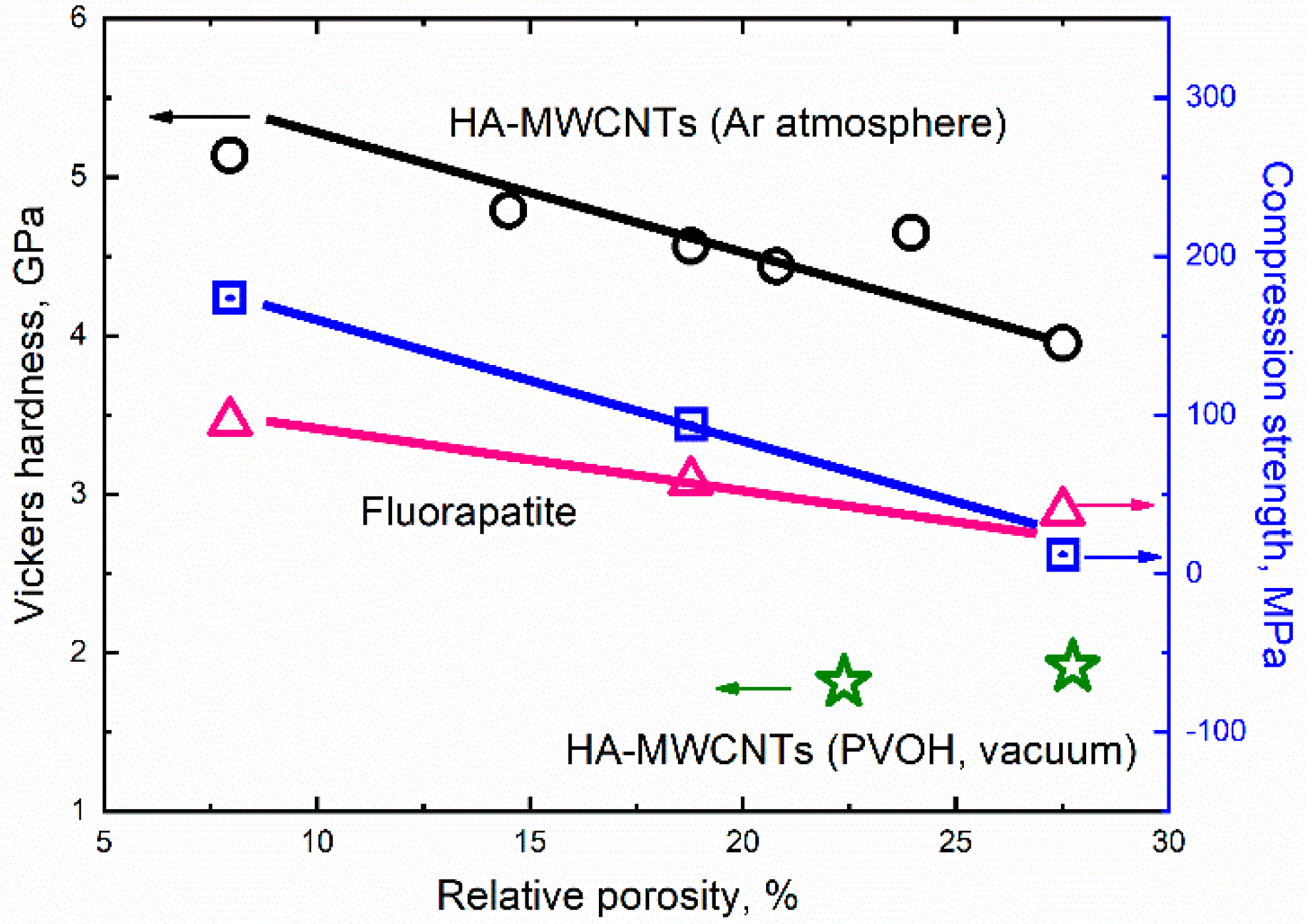
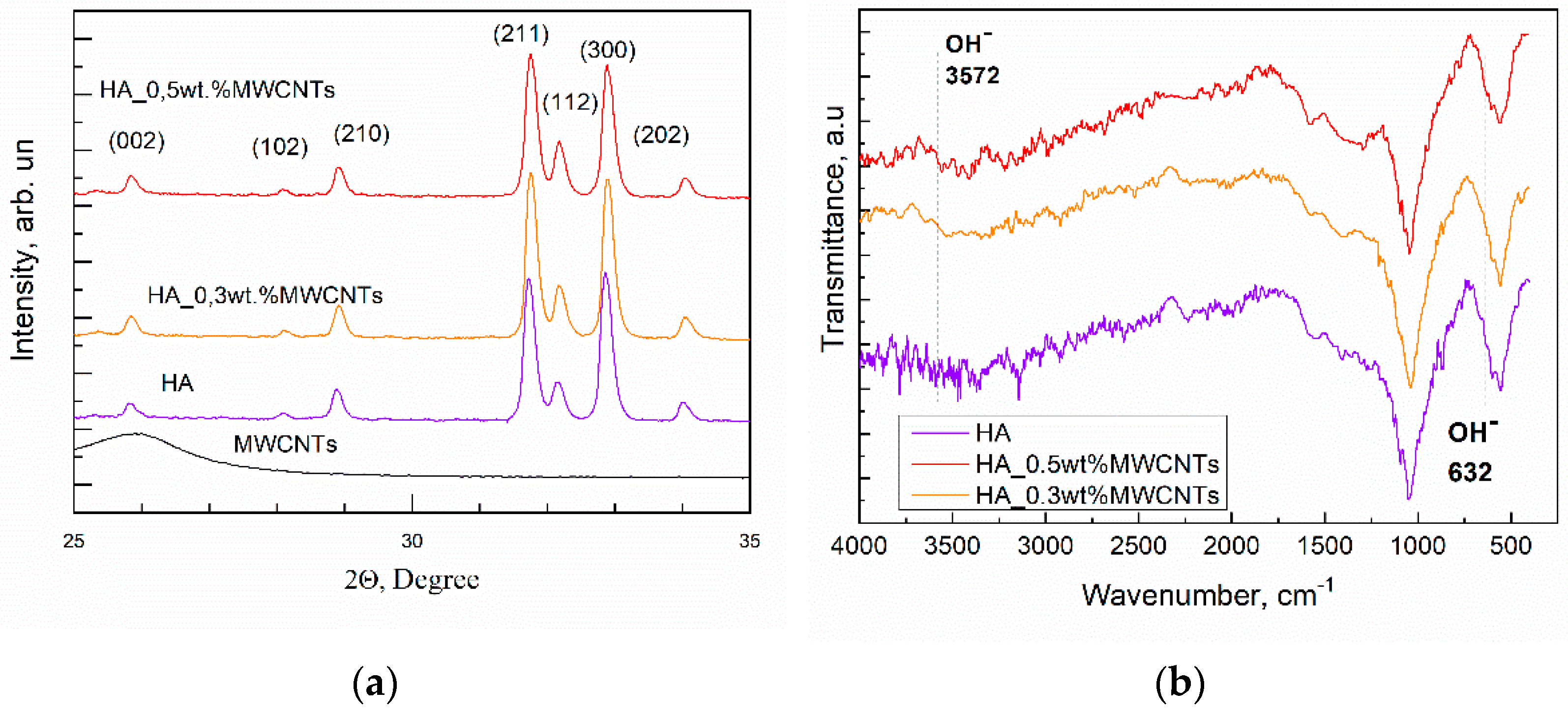
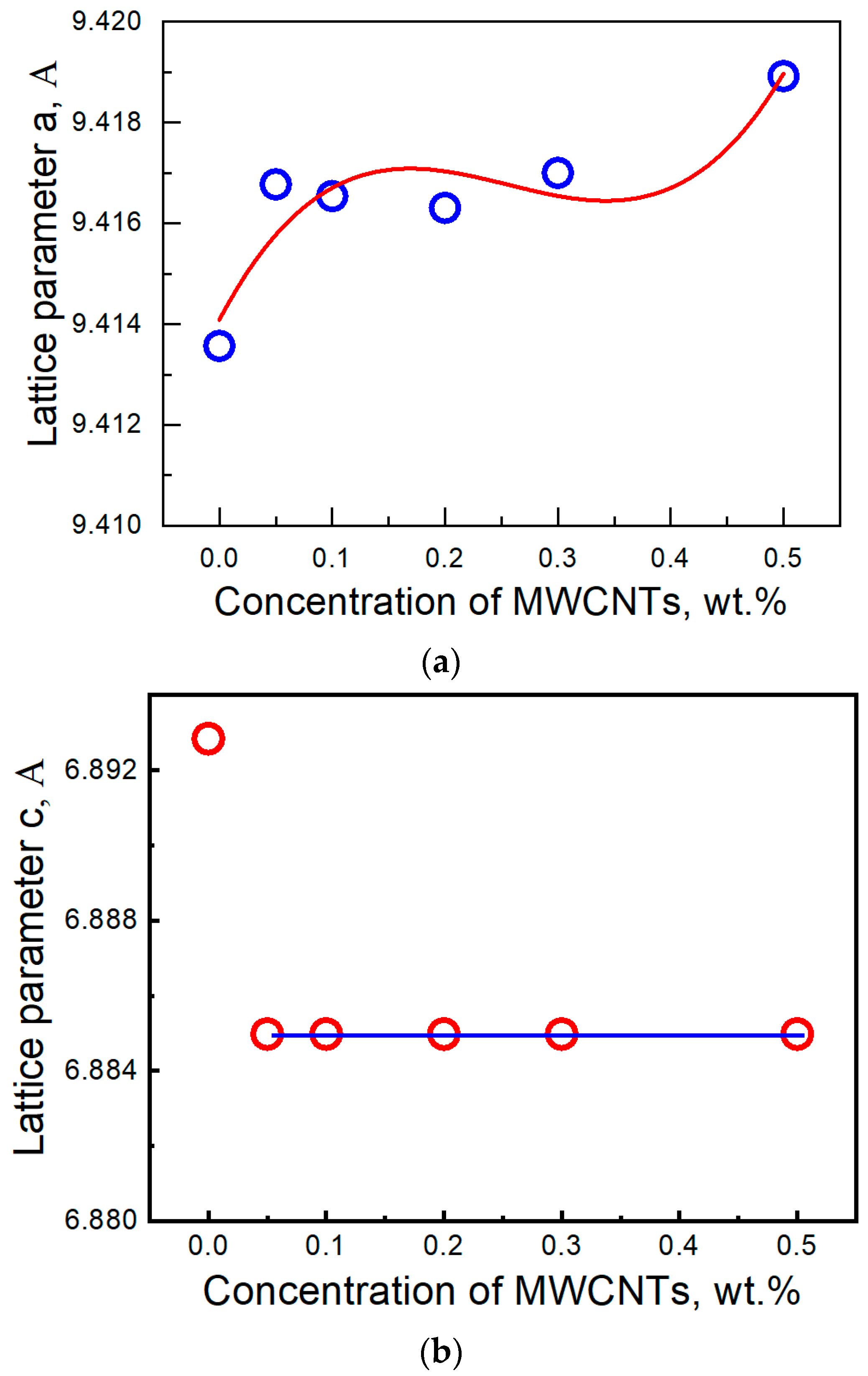

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barabashko, M.; Ponomarev, A.; Rezvanova, A.; Kuznetsov, V.; Moseenkov, S. Young’s Modulus and Vickers Hardness of the Hydroxyapatite Bioceramics with a Small Amount of the Multi-Walled Carbon Nanotubes. Materials 2022, 15, 5304. https://doi.org/10.3390/ma15155304
Barabashko M, Ponomarev A, Rezvanova A, Kuznetsov V, Moseenkov S. Young’s Modulus and Vickers Hardness of the Hydroxyapatite Bioceramics with a Small Amount of the Multi-Walled Carbon Nanotubes. Materials. 2022; 15(15):5304. https://doi.org/10.3390/ma15155304
Chicago/Turabian StyleBarabashko, Maksym, Alexander Ponomarev, Anastasiya Rezvanova, Vladimir Kuznetsov, and Sergey Moseenkov. 2022. "Young’s Modulus and Vickers Hardness of the Hydroxyapatite Bioceramics with a Small Amount of the Multi-Walled Carbon Nanotubes" Materials 15, no. 15: 5304. https://doi.org/10.3390/ma15155304
APA StyleBarabashko, M., Ponomarev, A., Rezvanova, A., Kuznetsov, V., & Moseenkov, S. (2022). Young’s Modulus and Vickers Hardness of the Hydroxyapatite Bioceramics with a Small Amount of the Multi-Walled Carbon Nanotubes. Materials, 15(15), 5304. https://doi.org/10.3390/ma15155304





