Complex Metal Borohydrides: From Laboratory Oddities to Prime Candidates in Energy Storage Applications
Abstract
1. Introduction
2. Hydrogen Storage Options: Physical vs. Chemical Storage
2.1. Physical Storage of Hydrogen
2.2. Chemical Storage of Hydrogen
3. General Synthesis Strategies for Metal Borohydrides M(BH4)x
3.1. Solid-State—Mechanochemical Synthesis
3.2. Wet Chemistry—Solvent-Assisted Synthesis
3.3. Nanoconfined Hydrides
3.4. Derivatives—Formation of Adducts of M(BH4)x
4. Structural Considerations of M(BH4)x
4.1. Framework and Crystal Structure
4.2. Stability of MBH
4.3. Multi-Cationic Borohydrides
4.4. Anion Substitution of MBH: From Light to Heavy Halides and Pseudo-Halide Substitution
4.5. Stabilization of M(BH4)x by Coordination of Neutral Molecules (NH3, N2H4, H2O, (CH3)2S)
5. Physical and Chemical Properties of M(BH4)x
5.1. Electrochemistry of Metal Hydrides and M(BH4)x: Electrodes, Electrolytes (Li+, Mg2+, Ca2+), Complex Metal Hydrides
5.2. Optical and Magnetic Properties
5.3. M(BH4)x as Semi- and Superconductors
5.4. CO2 Capture
6. M(BH4)x as Hydrogen Storage Materials
6.1. Thermodynamic Properties of MBH
6.2. Destabilizing Methods for Complex Metal Borohydrides
| System | Catalyst | td (°C) | Obs. | Reference |
|---|---|---|---|---|
| LiBH4–LiNH2 | - via metastable Li2[BH4][NH2] and Li4[BH4][NH2]3 | 95melt-160onset-315peak, 230mean | 5.8 wt.%H2; H2 major; small traces of NH3 (TPD-MS data) | [310] |
| LiBH4-2LiNH2 | - (LiH possible intermediate) | 249 | 7.8 wt.%H2 LiBH4: 75 kJ/molH2; LiBH4-2LiNH2: 23 kJ/molH2 | [314] |
| x NaBH4–NaNH2 (x = 1,2,3,4) | - via α-/β- Na2[BH4][NH2] | 265onset-350peak,297 (2:1); 400 (1:1) | 8 wt.% | [311] |
| γ- Mg(BH4)2-0.5LiH | - | 380–420 | 15.5 wt.%exp., 14.78 wt.% (theoretical) * * higher wt.% exp. due to usage of solvated γ- Mg(BH4)2 | [315] |
| Ca(BH4)2–4 LiNH2 | - | 250onset-320peak (288mean) | 8 wt.% | [312,313] |
| Ca(BH4)2–4LiNH2-5 wt.% CoCl2 | Co2+ (CoCl2) | 150onset-207peak (178mean) | >7 wt.% | [312] |
| Ca(BH4)2-2Mg(NH2)2 | - | 220–480 (270, 290 and 310–multistep) | 8.3 (8.8 wt.% theoretical) not reversible (50-bar H2, 20–300 °C) | [309] |
| Ca(BH4)2-2Ca(NH2)2 | - | 220–480 (270, 290 and 310–multistep) | 6.8 (7.5 wt.% theoretical) not reversible (50-bar H2, 20–300 °C) | [309] |
| 5Ca(BH4)2-2LiBH4 | - | 83 | 6.7 wt.% * * theoretical | [306] |
| 5Mg(BH4)2-2LiBH4 | - | −29 | 8.4 wt.%* * theoretical | [306] |
| 2Mg(BH4)2-Ca(BH4)2 | - | 272, 326, 346, 398 (rehydrogenation at 288onset, 273peak) | 10.5 wt.% * * theoretical | [316,317,318] |
| 5Mg(BH4)2-Ca(BH4)2 | - | −18 °C (p = 1atm) * * predicted based on DFT calculations; 150 °C | 7.73 wt.%H2 * * predicted based on DFT calculations | [306]* [316,317] |
| LiBH4 + 0.2 MgCl2 + 0.1 TiCl3; LiBH4 + 0.076 MgCl2 + 0.047 TiCl3 | MgCl2, TiCl3 | 60onset-400peak | 5 wt.%H2 | [319,320] |
| LiBH4 + 0.09 TM oxides (TiO2, V2O3) | TiB2-possible active intermediate | 200 | 7–9 wt.%H2; reversible | [319] |
| LiBH4 + 0.2 M (M = Mg, Al) | Mg/Al | 60–300fast-600 | 9 wt.%H2; Al-based yields material probably volatile, only recharges to 3.5 wt.% capacity | [320] |
| LiBH4 + 0.0897 Al | Al | 450 | 12.4 wt.%, partially reversible | [321] |
7. Model Systems for Hydrogen Storage of MBH and Other RMH (Reactive Metal Hydrides): Nanoconfinement vs. Bulk Behavior for Improved Thermodynamics and Kinetics
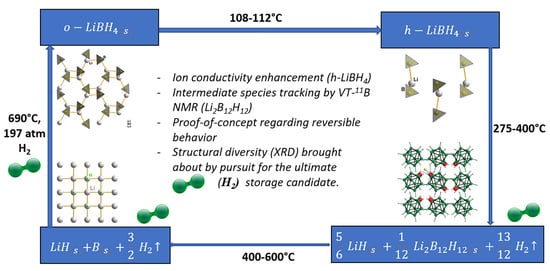
7.1. LiBH4
7.2. LiBH4 + MgH2
7.3. NaBH4
7.4. Mg(BH4)2
7.5. Ca(BH4)2
7.6. Ammonia Borane NH3BH3
8. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energ. Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Lai, Q.W.; Sun, Y.H.; Wang, T.; Modi, P.; Cazorla, C.; Demirci, U.B.; Ares Fernandez, J.R.; Leardini, F.; Aguey-Zinsou, K.F. How to design hydrogen storage materials? Fundamentals, synthesis, and storage tanks. Adv. Sustain. Syst. 2019, 3, 1900043. [Google Scholar] [CrossRef]
- Ong, B.C.; Kamarudin, S.K.; Basri, S. Direct liquid fuel cells: A review. Int. J. Hydrogen Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Mohtadi, R.; Orimo, S.I. The renaissance of hydrides as energy materials. Nat. Rev. Mater. 2017, 2, 16091. [Google Scholar] [CrossRef]
- Veras, T.D.; Mozer, T.S.; dos Santos, D.D.R.M.; Cesar, A.D. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrogen Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- He, T.; Pachfule, P.; Wu, H.; Xu, Q.; Chen, P. Hydrogen carriers. Nat. Rev. Mater. 2016, 1, 16067. [Google Scholar] [CrossRef]
- Lai, Q.W.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.F.; Mao, J.F.; Huang, Z.G.; Liu, H.K.; et al. Hydrogen storage materials for mobile and stationary applications: Current state of the art. ChemSusChem 2015, 8, 2789–2825. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Cheng, Y.H.; Zhang, J.Y. A review of high density solid hydrogen storage materials by pyrolysis for promising mobile applications. Ind. Eng. Chem. Res. 2021, 60, 2737–2771. [Google Scholar] [CrossRef]
- Schüth, F.; Bogdanović, B.; Felderhoff, M. Light metal hydrides and complex hydrides for hydrogen storage. Chem. Commun. 2004, 20, 2249–2258. [Google Scholar] [CrossRef]
- Schneemann, A.; White, J.L.; Kang, S.Y.; Jeong, S.; Wan, L.F.; Cho, E.S.; Heo, T.W.; Prendergast, D.; Urban, J.J.; Wood, B.C.; et al. Nanostructured metal hydrides for hydrogen storage. Chem. Rev. 2018, 118, 10775–10839. [Google Scholar] [CrossRef]
- Grahame, A.; Aguey-Zinsou, K.F. properties and applications of metal (M) dodecahydro-closo-dodecaborates (Mn=1,2B12H12) and their implications for reversible hydrogen storage in the borohydrides. Inorganics 2018, 6, 106. [Google Scholar] [CrossRef]
- Wang, K.; Pan, Z.X.; Yu, X.B. Metal B-N-H hydrogen-storage compound: Development and perspectives. J. Alloys Compd. 2019, 794, 303–324. [Google Scholar] [CrossRef]
- Kumar, R.; Karkamkar, A.; Bowden, M.; Autrey, T. Solid-state hydrogen rich boron-nitrogen compounds for energy storage. Chem. Soc. Rev. 2019, 48, 5350–5380. [Google Scholar] [CrossRef]
- Ley, M.B.; Jepsen, L.H.; Lee, Y.S.; Cho, Y.W.; von Colbe, J.M.B.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage—New perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Ouyang, L.Z.; Chen, K.; Jiang, J.; Yang, X.S.; Zhu, M. Hydrogen storage in light-metal based systems: A review. J. Alloys Compd. 2020, 829, 154597. [Google Scholar] [CrossRef]
- Singh, R.; Altaee, A.; Gautam, S. Nanomaterials in the advancement of hydrogen energy storage. Heliyon 2020, 6, e04487. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C. Sodium borohydride as a fuel for the future. Renew. Sustain. Energ. Rev. 2011, 15, 3980–4001. [Google Scholar] [CrossRef]
- Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles (accessed on 31 January 2022).
- Available online: https://www.energy.gov/sites/default/files/2017/05/f34/fcto_targets_onboard_hydro_storage_explanation.pdf (accessed on 31 January 2022).
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Cerny, R.; Ravnsbæk, D.B.; Filinchuk, Y.; Dornheim, M.; Besenbacher, F.; Jensen, T.R. Metal borohydrides and derivatives—Synthesis, structure and properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N. A review on hydrogen generation from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 726–765. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.Y.; Li, D.S.; Zhang, S.Q.; Zhang, Q.C. Recent advances in the “on-off” approaches for on-demand liquid-phase hydrogen evolution. J. Mater. Chem. A 2021, 9, 18164–18174. [Google Scholar] [CrossRef]
- Nunes, H.X.; Silva, D.L.; Rangel, C.M.; Pinto, A.M.F.R. Rehydrogenation of sodium borates to close the NaBH4-H2 cycle: A review. Energies 2021, 14, 3567. [Google Scholar] [CrossRef]
- Gras, M.; Lota, G. Control of hydrogen release during borohydride electrooxidation with porous carbon materials. RSC Adv. 2021, 11, 15639–15655. [Google Scholar] [CrossRef]
- Yao, Q.L.; Ding, Y.Y.; Lu, Z.H. Noble-metal-free nanocatalysts for hydrogen generation from boron- and nitrogen-based hydrides. Inorg. Chem. Front. 2020, 7, 3837–3874. [Google Scholar] [CrossRef]
- Deng, J.F.; Chen, S.P.; Wu, X.J.; Zheng, J.; Li, X.G. Recent progress on materials for hydrogen generation via hydrolysis. J. Inorg. Mater. 2021, 36, 1–8. [Google Scholar] [CrossRef]
- Dionne, M.; Hao, S.; Gambarotta, S. Preparation and characterization of a new series of Cr(ll) hydroborates. Can. J. Chem. 1995, 73, 1126–1134. [Google Scholar] [CrossRef]
- Nöth, H.; Seitz, M. Preparation and characterization of chromium(ii) tetrahydroborate-tetrahydrofuran (1/2). J. Chem. Soc. Chem. Commun. 1976, 24, 1004a. [Google Scholar] [CrossRef]
- Soulie, J.P.; Renaudin, G.; Cerny, R.; Yvon, K. Lithium borohydride LiBH4 I. Crystal structure. J. Alloys Compd. 2002, 346, 200–205. [Google Scholar] [CrossRef]
- Puszkiel, J.; Gasnier, A.; Amica, G.; Gennari, F. Tuning LiBH4 for hydrogen storage: Destabilization, additive, and nanoconfinement approaches. Molecules 2020, 25, 163. [Google Scholar] [CrossRef]
- Skripov, A.V.; Soloninin, A.V.; Babanova, O.A.; Skoryunov, R.V. Anion and cation dynamics in polyhydroborate salts: NMR studies. Molecules 2020, 25, 2940. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Pistidda, C.; Nguyen, V.H.; Singh, P.; Raizada, P.; Klassen, T.; Dornheim, M. Nanoconfinement effects on hydrogen storage properties of MgH2 and LiBH4. Int. J. Hydrogen Energy 2021, 46, 23723–23736. [Google Scholar] [CrossRef]
- Skoryunov, R.V.; Babanova, O.A.; Soloninin, A.V.; Grinderslev, J.B.; Skripov, A.V.; Jensen, T.R. Dynamical properties of lithium borohydride-ammine composite LiBH4.NH3: A nuclear magnetic resonance study. J. Alloys Compd. 2022, 894, 162446. [Google Scholar] [CrossRef]
- Ye, J.K.; Xia, G.L.; Yu, X.B. In-situ constructed destabilization reaction of LiBH4 wrapped with graphene toward stable hydrogen storage reversibility. Mater. Today Energy 2021, 22, 100885. [Google Scholar] [CrossRef]
- Yang, G.Y.; Xie, C.; Li, Y.T.; Li, H.W.; Liu, D.M.; Chen, J.G.; Zhang, Q.G. Enhancement of the ionic conductivity of lithium borohydride by silica supports. Dalton Trans. 2021, 50, 15352–15358. [Google Scholar] [CrossRef] [PubMed]
- De Kort, L.M.; Gulino, V.; de Jongh, P.E.; Ngene, P. Ionic conductivity in complex metal hydride-based nanocomposite materials: The impact of nanostructuring and nanocomposite formation. J. Alloys Compd. 2022, 901, 163474. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, Z.Q.; Shi, X.M.; Zhang, C.; Liu, K.; Zhang, J.; Zhou, L.; Ma, C.J.; Du, Y.P. Recent advances on rare earths in solid lithium ion conductors. J. Rare Earths 2021, 39, 1–10. [Google Scholar] [CrossRef]
- Bogdanovic, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloys Compd. 1997, 253, 1–9. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Sanderson, R.T.; Burg, A.B. A volatile compound of aluminum, boron and hydrogen. J. Am. Chem. Soc. 1939, 61, 536. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Filinchuk, Y.; Cerenius, Y.; Jakobsen, H.J.; Besenbacher, F.; Skibsted, J.; Jensen, T.R. A series of mixed-metal borohydrides. Angew. Chem. Int. Ed. 2009, 48, 6659–6663. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, H.; Cerny, R. Synthetic approaches to inorganic borohydrides. Dalton Trans. 2010, 39, 6006–6012. [Google Scholar] [CrossRef]
- Wang, T.; Aguey-Zinsou, K.F. Synthesis of borohydride nanoparticles at room temperature by precipitation. Int. J. Hydrogen Energy 2021, 46, 24286–24292. [Google Scholar] [CrossRef]
- Suarez-Alcantara, K.; Garcia, J.R.T. Metal borohydrides beyond groups I and II: A review. Materials 2021, 14, 2561. [Google Scholar] [CrossRef] [PubMed]
- Cerny, R.; Brighi, M.; Murgia, F. The crystal chemistry of inorganic hydroborates. Chemistry 2020, 2, 53. [Google Scholar] [CrossRef]
- Gu, T.T.; Gu, J.; Zhang, Y.; Ren, H. Metal borohydride-based system for solid-state hydrogen storage. Prog. Chem. 2020, 32, 665–686. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Dornheim, M.; Eigen, N.; Barkhordarian, G.; Klassen, T.; Bormann, R. Tailoring hydrogen storage materials towards application. Adv. Eng. Mater 2006, 8, 377–385. [Google Scholar] [CrossRef]
- Eigen, N.; Keller, C.; Dornheim, M.; Klassen, T.; Bormann, R. Industrial production of light metal hydrides for hydrogen storage. Scr. Mater. 2007, 56, 847–851. [Google Scholar] [CrossRef]
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen storage technologies for stationary and mobile applications: Review, analysis and perspectives. Renew. Sustain. Energ. Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- Takacs, L. Self-sustaining reactions induced by ball milling. Prog. Mater. Sci. 2002, 47, 355–414. [Google Scholar] [CrossRef]
- Balema, V.P.; Wiench, J.W.; Pruski, M.; Pecharsky, V.K. Solvent-free mechanochemical synthesis of phosphonium salts. Chem. Commun. 2002, 7, 724–725. [Google Scholar] [CrossRef]
- Pistidda, C.; Santhosh, A.; Jerabek, P.; Shang, Y.Y.; Girella, A.; Milanese, C.; Dore, M.; Garroni, S.; Bordignon, S.; Chierotti, M.R.; et al. Hydrogenation via a low energy mechanochemical approach: The MgB2 case. J. Phys. Energy 2021, 3, 044001. [Google Scholar] [CrossRef]
- Balema, V.P. Mechanical processing in hydrogen storage research and development. Mater. Matter. 2007, 2, 16–18. Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/190/337/material-matters-v2n2.pdf (accessed on 31 January 2022). [CrossRef]
- Bateni, A.; Scherpe, S.; Acar, S.; Somer, M. Novel approach for synthesis of Magnesium Borohydride, Mg(BH4)2. Energy Procedia 2012, 29, 26–33. [Google Scholar] [CrossRef]
- Xu, D.Y.; Zhang, Y.; Guo, Q.J. Research progress on catalysts for hydrogen generation through sodium borohydride alcoholysis. Int. J. Hydrogen Energy 2022, 47, 5929–5946. [Google Scholar] [CrossRef]
- Ouyang, L.Z.; Jiang, J.; Chen, K.; Zhu, M.; Liu, Z.W. Hydrogen production via hydrolysis and alcoholysis of light metal-based materials: A review. Nano-Micro Lett. 2021, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Al-Msrhad, T.M.H.; Devrim, Y.; Uzundurukan, A.; Budak, Y. Investigation of hydrogen production from sodium borohydride by carbon nano tube-graphene supported PdRu bimetallic catalyst for PEM fuel cell application. Int. J. Energy Res. 2021. [Google Scholar] [CrossRef]
- Faghihi, M.; Akbarbandari, F.; Zabihi, M.; Pazouki, M. Synthesis and characterization of the magnetic supported metal-organic framework catalysts (CuCoBTC@MAC and CuBTC@MAC) for the hydrogen production from sodium borohydride. Mater. Chem. Phys. 2021, 267, 124599. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Hyde, E.K. The preparation of other borohydrides by metathetical reactions utilizing the alkali metal borohydrides. J. Am. Chem. Soc. 1953, 75, 209–213. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Hoekstra, H.R.; Rapp, L.R. Reactions of diborane with alkali metal hydrides and their addition compounds. New syntheses of borohydrides. Sodium and potassium borohydrides. J. Am. Chem. Soc. 1953, 75, 199–204. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E. The preparation of sodium borohydride by the high temperature reaction of sodium hydride with borate esters. J. Am. Chem. Soc. 1953, 75, 205–209. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C. Uranium(IV) Borohydride. J. Am. Chem. Soc. 1953, 75, 219–221. [Google Scholar] [CrossRef]
- Payandeh Gharibdoust, S.; Heere, M.; Nervi, C.; Sørby, M.H.; Hauback, B.C.; Jensen, T.R. Synthesis, structure, and polymorphic transitions of praseodymium(III) and neodymium(III) borohydride, Pr(BH4)3 and Nd(BH4)3. Dalt. Trans. 2018, 47, 8307–8319. [Google Scholar] [CrossRef] [PubMed]
- Frommen, C.; Aliouane, N.; Deledda, S.; Fonneløp, J.E.; Grove, H.; Lieutenant, K.; Llamas-Jansa, I.; Sartori, S.; Sørby, M.H.; Hauback, B.C. Crystal structure, polymorphism, and thermal properties of yttrium borohydride Y(BH4)3. J. Alloys Compd. 2010, 496, 710–716. [Google Scholar] [CrossRef]
- Yang, C.H.; Tsai, W.T.; Chang, J.K. Hydrogen desorption behavior of vanadium borohydride synthesized by modified mechanochemical process. Int. J. Hydrogen Energy 2011, 36, 4993–4999. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Filinchuk, Y.; Cerny, R.; Ley, M.B.; Haase, D.; Jakobsen, H.J.; Skibsted, J.; Jensen, T.R. Thermal polymorphism and decomposition of Y(BH4)3. Inorg. Chem. 2010, 49, 3801–3809. [Google Scholar] [CrossRef]
- Sato, T.; Miwa, K.; Nakamori, Y.; Ohoyama, K.; Li, H.; Noritake, T.; Aoki, M.; Towata, S.I.; Orimo, S.I. Experimental and computational studies on solvent-free rare-earth metal borohydrides R(BH4)3 (R=Y, Dy, and Gd). Phys. Rev. B 2008, 77, 104114. [Google Scholar] [CrossRef]
- Wegner, W.; Jaron, T.; Grochala, W. Polymorphism and hydrogen discharge from holmium borohydride, Ho(BH4)3, and KHo(BH4)4. Int. J. Hydrogen Energy 2014, 39, 20024–20030. [Google Scholar] [CrossRef]
- Wegner, W.; Jaron, T.; Grochala, W. Preparation of a series of lanthanide borohydrides and their thermal decomposition to refractory lanthanide borides. J. Alloys Compd. 2018, 744, 57–63. [Google Scholar] [CrossRef]
- Andrade-Gamboa, J.; Puszkiel, J.A.; Fernández-Albanesi, L.; Gennari, F.C. A novel polymorph of gadolinium tetrahydroborate produced by mechanical milling. Int. J. Hydrogen Energy 2010, 35, 10324–10328. [Google Scholar] [CrossRef]
- Ley, M.B.; Paskevicius, M.; Schouwink, P.; Richter, B.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Novel solvates M(BH4)3S(CH3)2 and properties of halide-free M(BH4)3 (M = Y or Gd). Dalton Trans. 2014, 43, 13333–13342. [Google Scholar] [CrossRef]
- Grinderslev, J.B.; Møller, K.T.; Bremholm, M.; Jensen, T.R. Trends in synthesis, crystal structure, and thermal and magnetic properties of rare-earth metal borohydrides. Inorg. Chem. 2019, 58, 5503–5517. [Google Scholar] [CrossRef] [PubMed]
- Jaron, T.; Grochala, W. Y(BH4)3—An old–new ternary hydrogen store akalearning from a multitude of failures. Dalton Trans. 2010, 39, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Shim, J.-H.; Cho, Y.W. Polymorphism and thermodynamics of Y(BH4)3 from first principles. J. Phys. Chem. C 2010, 114, 12833–12837. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.W.; Sato, T.; Umeda, N.; Miwa, K.; Towata, S.; Orimo, S. Dehydriding and rehydriding properties of yttrium borohydride Y(BH4)3 prepared by liquid-phase synthesis. Int. J. Hydrogen Energy 2009, 34, 5732–5736. [Google Scholar] [CrossRef]
- Haaland, A.; Shorokhov, D.J.; Tutukin, A.V.; Volden, H.V.; Swang, O.; McGrady, G.S.; Kaltsoyannis, N.; Downs, A.J.; Tang, C.Y.; Turner, J.F.C. Molecular structures of two metal tetrakis(tetrahydroborates), Zr(BH4)4 and U(BH4)4: Equilibrium conformations and barriers to internal rotation of the triply bridging BH4 groups. Inorg. Chem. 2002, 41, 6646–6655. [Google Scholar] [CrossRef] [PubMed]
- Banks, R.H.; Edelstein, N.M. Synthesis and Characterization of Protactinium(IV), Neptunium(IV), and Plutonium (IV) Borohydrides. In Lanthanide and Actinide Chemistry and Spectroscopy; American Chemical Society Publications: Washington, DC, USA, 1980; pp. 331–348. [Google Scholar] [CrossRef]
- Rajnak, K.; Gamp, E.; Shinomoto, R.; Edelstein, N. Optical and magnetic properties of uranium borohydride and tetrakismethylborohydride. J. Chem. Phys. 1984, 80, 5942–5950. [Google Scholar] [CrossRef][Green Version]
- Banks, R.H.; Edelstein, N.M.; Rietz, R.R.; Templeton, D.H.; Zalkin, A. Preparation and properties of the actinide borohydrides: Protactinium(IV), neptunium(IV), and plutonium(IV) borohydrides. J. Am. Chem. Soc. 1978, 100, 1957–1958. [Google Scholar] [CrossRef]
- Desch, C.H. Henry Louis Le Chatelier, 1850–1936; Royal Society: London, UK, 1938; Volume 2, pp. 250–259. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J. Physical Chemistry, 8th ed.; Chemical Equilibrium; Oxford University Press: Oxford, UK, 2006; ISBN 0716787598. [Google Scholar]
- Aldridge, S.; Blake, A.J.; Downs, A.J.; Gould, R.O.; Parsons, S.; Pulham, C.R. Some tetrahydroborate derivatives of aluminium: Crystal structures of dimethylaluminium tetrahydroborate and the α and β phases of aluminium tris(tetrahydroborate) at low temperature. J. Chem. Soc. Dalton Trans. 1997, 6, 1007–1012. [Google Scholar] [CrossRef]
- Downs, A.J.; Thomas, P.D.P. Gallium borohydrides: The synthesis and properties of HGa(BH4)2. J. Chem. Soc. Chem. Commun. 1976, 20, 825–827. [Google Scholar] [CrossRef]
- Pulham, C.R.; Brain, P.T.; Downs, A.J.; Rankin, D.W.H.; Robertson, H.E. Gallaborane, H2Ga(µ-H)2BH2: Synthesis, properties, and structure of the gaseous molecule as determined by electron diffraction. J. Chem. Soc. Chem. Commun. 1990, 2, 177–178. [Google Scholar] [CrossRef]
- Wiberg, E.; Nöth, H. Notizen: Über Wasserstoff-Verbindungen des Indiums. Z. Naturforsch. B 1957, 12, 59–60. [Google Scholar] [CrossRef]
- Wiberg, E.; Dittmann, O.; Nöth, H.; Schmidt, M. Notizen: Über Wasserstoff-Verbindungen des Thalliums. V. Zur Kenntnis eines Thallium(I)-boranats TlBH4 und Thallium(I)-alanats TlAlH4. Z. Naturforsch. B 1957, 12, 62–63. [Google Scholar] [CrossRef]
- Wiberg, E.; Nöth, H. Notizen: Über Wasserstoff-Verbindungen des Thalliums. VI. Zur Kenntnis eines Thallium(III)-boranats TlCl(BH4)2. Z. Naturforsch. B 1957, 12, 63–65. [Google Scholar]
- Mosegaard, L.; Møller, B.; Jørgensen, J.; Filinchuk, Y.; Cerenius, Y.; Hanson, J.C.; Dimasi, E.; Besenbacher, F.; Jensen, T.R. Reactivity of LiBH4: In Situ Synchrotron Radiation Powder X-ray Diffraction Study. J. Phys. Chem. C 2008, 112, 1299–1303. [Google Scholar] [CrossRef]
- Goerrig, D. Verfahren zur Herstellung von Boranaten. German Patent No. DE 000001077644 A, 17 March 1960. [Google Scholar]
- Friedrichs, O.; Buchter, F.; Zwicky, C.N.; Borgschulte, A.; Remhof, A.; Mauron, P.; Bielmann, M.; Zuttel, A. Direct synthesis of Li[BH4] and Li[BD4] from the elements. Acta Mater. 2008, 56, 949–954. [Google Scholar] [CrossRef]
- Barkhodarian, G.; Klassen, T.; Dornheim, M.; Bormann, R. Unexpected kinetic effect of MgB2 in reactive hydride composites containing complex borohydrides. J. Alloys Compd. 2007, 440, L18–L21. [Google Scholar] [CrossRef]
- Friedrichs, O.; Remhof, A.; Borgschulte, A.; Buchter, F.; Orimo, S.I.; Züttel, A. Breaking the passivation—the road to a solvent free borohydride synthesis. Phys. Chem. Chem. Phys. 2010, 12, 10919–10922. [Google Scholar] [CrossRef] [PubMed]
- Barkhordarian, G.; Jensen, T.R.; Doppiu, S.; Bosenberg, U.; Borgschulte, A.; Gremaud, R.; Cerenius, Y.; Dornheim, M.; Klassen, T.; Bormann, R. Formation of Ca(BH4)2 from Hydrogenation of CaH2+MgB2 Composite. J. Phys. Chem. C 2008, 112, 2743–2749. [Google Scholar] [CrossRef]
- Ronnebro, E.; Majzoub, E.H. Calcium borohydride for hydrogen storage: Catalysis and reversibility. J. Phys. Chem. B 2007, 111, 12045–12047. [Google Scholar] [CrossRef]
- Yan, Y.; Rentsch, D.; Remhof, A. Controllable decomposition of Ca(BH4)2 for reversible hydrogen storage. Phys. Chem. Chem. Phys. 2017, 19, 7788–7792. [Google Scholar] [CrossRef]
- Pinkerton, F.E.; Meyer, M.S. Reversible hydrogen storage in the lithium borohydride—Calcium hydride coupled system. J. Alloys Compd. 2008, 464, L1–L4. [Google Scholar] [CrossRef]
- Sahle, C.J.; Sternemann, C.; Giacobbe, C.; Yan, Y.; Weis, C.; Harder, M.; Forov, Y.; Spiekermann, G.; Tolan, M.; Krisch, M.; et al. Formation of CaB6 in the thermal decomposition of the hydrogen storage material Ca(BH4)2. Phys. Chem. Chem. Phys. 2016, 18, 19866–19872. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Martinez, C.A.; Moury, R.; Ould-Amara, S.; Demirci, U.B. Destabilization of boron-based compounds for hydrogen storage in the solid-state: Recent advances. Energies 2021, 14, 7003. [Google Scholar] [CrossRef]
- Rongeat, C.; D’Anna, V.; Hagemann, H.; Borgschulte, A.; Zuttel, A.; Schultz, L.; Gutfleisch, O. Effect of additives on the synthesis and reversibility of Ca(BH4)2. J. Alloys Compd. 2010, 493, 281–287. [Google Scholar] [CrossRef]
- Nakamori, Y.; Li, H.W.; Kikuchi, K.; Aoki, M.; Miwa, K.; Towata, S.; Orimo, S. Thermodynamical stabilities of metal-borohydrides. J. Alloys Compd. 2007, 446–447, 296–300. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C. Metallo Borohydrides. III. Lithium borohydride. J. Am. Chem. Soc. 1940, 62, 3429–3435. [Google Scholar] [CrossRef]
- Kollonitsch, J.; Fuchs, O. Preparation of aluminium borohydride and its applications in organic reductions. Nature 1955, 176, 1081. [Google Scholar] [CrossRef]
- Brown, H.C.; Choi, Y.M.; Narasimhan, S. Addition compounds of alkali metal hydrides. 22. Convenient procedures for the preparation of lithium borohydride from sodium borohydride and borane-dimethyl sulfide in simple ether solvents. Inorg. Chem. 1982, 21, 3657–3661. [Google Scholar] [CrossRef]
- Rittmeyer, P.; Wietelmann, U. Ullmann’s Encyclopaedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Cerny, R.; Filinchuk, Y.; Hagemann, H.; Yvon, K. Magnesium borohydride: Synthesis and crystal structure. Angew. Chem. Int. Ed. 2007, 46, 5765–5767. [Google Scholar] [CrossRef]
- Soloveichik, G.L.; Andrus, M.; Gao, Y.; Zhao, J.-C.; Kniajanski, S. Magnesium borohydride as a hydrogen storage material: Synthesis of unsolvated Mg(BH4)2. Int. J. Hydrogen Energy 2009, 34, 2144–2152. [Google Scholar] [CrossRef]
- Huang, H.X.; Liu, B.G.; Lv, Y.J.; Lv, W.; Yuan, J.G.; Wu, Y. Double regulation of Mg95Ni5 hydride in suppressing ammonia and promoting hydrogen evolution for Mg(BH4)2.2NH3. J. Alloys Compd. 2022, 901, 163468. [Google Scholar] [CrossRef]
- Li, H.W.; Kikuchi, K.; Nakamori, Y.; Miwa, K.; Towata, S.; Orimo, S. Effects of ball milling and additives on dehydriding behaviors of well-crystallized Mg(BH4)2. Scr. Mater. 2007, 57, 679–682. [Google Scholar] [CrossRef]
- Zanella, P.; Crociani, L.; Masciocchi, N.; Giunchi, G. Facile high-yield synthesis of pure, crystalline Mg(BH4)2. Inorg. Chem. 2007, 46, 9039–9041. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, E.; Hartwimmer, R.Z. Zur Kenntnis von Erdalkaliboranaten Me[BH4]2 III. Synthese aus Erdalkalihydriden und Diboran. Z. Naturforsch. B Chem. Sci. 1955, 10, 295–296. [Google Scholar] [CrossRef]
- Wiberg, E.; Noth, H.; Hartwimmer, R.Z. Zur Kenntnis von Erdalkaliboranaten Me[BH4]2 I. Darstellung aus Erdalkali-tetramethoxoboraten und Diboran. Z. Naturforsch. B Chem. Sci. 1955, 10, 292–294. [Google Scholar] [CrossRef]
- Wiberg, E. Zur kenntnis eines kupfer-bor-wasserstoffs CuBH4. Z. Naturforsch. B 1952, 7b, 582. [Google Scholar]
- Wiberg, E.; Henle, W. Notizen: Zur Kenntnis eines Silber-bor-wasserstoffs AgBH4. Z. Naturforsch. B 1952, 7, 575–576. [Google Scholar] [CrossRef]
- Wiberg, E. Neuere Ergebnisse der praparativen Hydrid-Forschung. Angew. Chem. 1953, 65, 16–33. [Google Scholar] [CrossRef]
- Musaev, D.G.; Morokuma, K. Does the Tetrahydroborate Species AuBH4 Exist? Ab Initio MO Study of the Structure and Stability of CuBH4, AgBH4, and AuBH4. Organometallics 1995, 14, 3327–3334. [Google Scholar] [CrossRef]
- Jain, I.P.; Jain, P.; Jain, A. Novel hydrogen storage materials: A review of lightweight complex hydrides. J. Alloys Compd. 2010, 503, 303–339. [Google Scholar] [CrossRef]
- Riktor, M.D.; Sørby, M.H.; Chlopek, K.; Fichtner, M.; Buchter, F.; Zuttel, A.; Hauback, B.C. In situ synchrotron diffraction studies of phase transitions and thermal decomposition of Mg(BH4)2 and Ca(BH4)2. J. Mater. Chem. 2007, 17, 4939–4942. [Google Scholar] [CrossRef]
- Jeon, E.; Cho, Y.W. Mechanochemical synthesis and thermal decomposition of zinc borohydride. J. Alloys Compd. 2006, 422, 273–275. [Google Scholar] [CrossRef]
- Srinivasan, S.; Escobar, D.; Jurczyk, M.; Goswami, Y.; Stefanakos, E. Nanocatalyst doping of Zn(BH4)2 for on-board hydrogen storage. J. Alloys Compd. 2008, 462, 294–302. [Google Scholar] [CrossRef]
- Wiberg, E.; Henle, W. Notizen: Zur Kenntnis eines ätherlöslichen Zink-bor-wasser-stoffs Zn(BH4)2. Z. Naturforsch. B 1952, 7, 579–580. [Google Scholar] [CrossRef]
- Nöth, H.; Wiberg, E.; Winter, L.P. Boranate und Boranato-metallate. I. Zur Kenntnis von Solvaten des Zinkboranats. Z. Anorg. Allg. Chem. 1969, 370, 209–223. [Google Scholar] [CrossRef]
- Banus, M.D.; Bragdon, R.W.; Hinckley, A.A. Potassium, rubidium and cesium borohydrides. J. Am. Chem. Soc. 1954, 76, 3848–3849. [Google Scholar] [CrossRef]
- Hagemann, H.; Gomes, S.; Renaudin, G.; Yvon, K. Raman studies of reorientation motions of [BH4]− anions in alkali borohydrides. J. Alloys Compd. 2004, 363, 129–132. [Google Scholar] [CrossRef]
- Friedrichs, O.; Borgschulte, A.; Kato, S.; Buchter, F.; Gremaud, R.; Remhof, A.; Zuttel, A. Low-temperature synthesis of LiBH4 by gas-solid reaction. Chem. Eur. J. 2009, 15, 5531–5534. [Google Scholar] [CrossRef]
- James, B.D.; Wallbridge, M.G.H. Metal tetrahydroborates. Prog. Inorg. Chem. 1970, 11, 99–231. [Google Scholar]
- Paskevicius, M.; Sheppard, D.A.; Buckley, C.E. Thermodynamic changes in mechanochemically synthesized magnesium hydride nanoparticles. J. Am. Chem. Soc. 2010, 132, 5077–5083. [Google Scholar] [CrossRef]
- Berube, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res 2007, 31, 637–663. [Google Scholar] [CrossRef]
- Gertsman, V.; Birringer, R. On the room-temperature grain growth in nanocrystalline copper. Scr. Metall. Mater. 1994, 30, 577–581. [Google Scholar] [CrossRef]
- Vigeholm, B.; Kjoller, J.; Larsen, B.; Schroder Pedersen, A. Hydrogen sorption performance of pure magnesium during continued cycling. Int. J. Hydrogen Energy 1983, 8, 809–817. [Google Scholar] [CrossRef]
- Berlouis, L.; Cabrera, E.; Hall-Barientos, E.; Hall, P.; Dodd, S.; Morris, S.; Imam, M. Thermal analysis investigation of hydriding properties of nanocrystalline Mg–Ni- and Mg–Fe-based alloys prepared by high-energy ball milling. J. Mater. Res. 2001, 16, 45–57. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Besenbacher, F.; Jensen, T.R. Nanoconfined hydrides for energy storage. Nanoscale 2011, 3, 2086–2098. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Manickam, K.; Hirscher, M.; Besenbacher, F.; Jensen, T.R. Confinement of MgH2 Nanoclusters within Nanoporous Aerogel Scaffold Materials. ACS Nano 2009, 3, 3521–3528. [Google Scholar] [CrossRef]
- Zhang, S.; Gross, A.F.; Atta, S.L.; Lopez, M.; Liu, P.; Ahn, C.C.; Vajo, J.J.; Jensen, C.M. The synthesis and hydrogen storage properties of a MgH2 incorporated carbon aerogel scaffold. Nanotechnology 2009, 20, 204027. [Google Scholar] [CrossRef] [PubMed]
- de Gennes, P.G. Wetting: Statics and dynamics. Rev. Mod. Phys. 1985, 57, 827–863. [Google Scholar] [CrossRef]
- Dujardin, E.; Ebbesen, T.W.; Hiura, H.; Tanigaki, K. Capillarity and wetting of carbon nanotubes. Science 1994, 265, 1850–1852. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Adelhelm, P.; Verkuijlen, M.H.W.; Rongeat, C.; Herrich, M.; van Bentum, P.J.M.; Gutfleisch, O.; Kentgens, A.P.M.; de Jong, K.P.; de Jongh, P.E. Confinement of NaAlH4 in nanoporous carbon: Impact on h2 release, reversibility, and thermodynamics. J. Phys. Chem. C. 2010, 114, 4675–4682. [Google Scholar] [CrossRef]
- Dilts, J.A.; Ashby, E.C. A study of the thermal decomposition of complex metal hydrides. Inorg. Chem. 1972, 11, 1230–1236. [Google Scholar] [CrossRef]
- Bogdanović, B.; Brand, R.A.; Marjanovic, A.; Schwickardi, M.; Tölle, J. Metal-doped sodium aluminium hydrides as potential new hydrogen storage materials. J. Alloys Compd. 2000, 302, 36–58. [Google Scholar] [CrossRef]
- Verkuijlen, M.H.W.; Gao, J.; Adelhelm, P.; van Bentum, P.J.M.; de Jongh, P.E.; Kentgens, A.P.M. Solid-state NMR studies of the local structure of NaAlH4/C nanocomposites at different stages of hydrogen desorption and rehydrogenation. J. Phys. Chem. C 2010, 114, 4683–4692. [Google Scholar] [CrossRef]
- Lohstroh, W.; Roth, A.; Hahn, H.; Fichtner, M. Thermodynamic effects in nanoscale NaAlH4. ChemPhysChem 2010, 11, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.D.; Gross, A.F.; Atta, S.L.; Vajo, J.J.; Pinkerton, F.E. The kinetic enhancement of hydrogen cycling in NaAlH4 by melt infusion into nanoporous carbon aerogel. Nanotechnology 2009, 20, 204018. [Google Scholar] [CrossRef]
- Comanescu, C.; Capurso, G.; Maddalena, A. Nanoconfinement in activated mesoporous carbon of calcium borohydride for improved reversible hydrogen storage. Nanotechnology 2012, 23, 385401. [Google Scholar] [CrossRef]
- Gutowski, M.; Autrey, T. Computational studies of boron/nitrogen and aluminum/nitrogen compounds for chemical hydrogen storage. Prepr. Pap. Am. Chem. Soc. Div. Fuel Chem. 2004, 49, 275–276. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.459.4955&rep=rep1&type=pdf (accessed on 31 January 2022).
- Gutowska, A.; Li, L.; Shin, Y.; Wang, C.M.; Li, X.S.; Linehan, J.C.; Smith, R.S.; Kay, B.D.; Schmid, B.; Shaw, W.; et al. Nanoscaffold mediates hydrogen release and the reactivity of ammonia borane. Angew. Chem. Int. Ed. 2005, 44, 3578–3582. [Google Scholar] [CrossRef] [PubMed]
- Filinchuk, Y.; Ronnebro, E.; Chandra, D. Crystal structures and phase transformations in Ca(BH4)2. Acta Mater. 2009, 57, 732–738. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Nickels, E.A.; Cerny, R.; Olesen, C.H.; David, W.I.F.; Edwards, P.P.; Filinchuk, Y.; Jensen, T.R. Novel Alkali Earth Borohydride Sr(BH4)2 and Borohydride-Chloride Sr(BH4)Cl. Inorg. Chem. 2013, 52, 10877–10885. [Google Scholar] [CrossRef]
- Sharma, M.; Didelot, E.; Spyratou, A.; Daku, L.M.L.; Cerny, R.; Hagemann, H. Halide Free M(BH4)2 (M = Sr, Ba, and Eu) Synthesis, Structure, and Decomposition. Inorg. Chem. 2016, 55, 7090–7097. [Google Scholar] [CrossRef] [PubMed]
- Ravnsbæk, D.B.; Sørensen, L.H.; Filinchuk, Y.; Besenbacher, F.; Jensen, T.R. Screening of Metal Borohydrides by Mechanochemistry and Diffraction. Angew. Chem. Int. Ed. 2012, 51, 3582–3586. [Google Scholar] [CrossRef]
- Wiberg, E.; Henle, W. Zur Kenntnis eines Cadmium-bor-wasserstoffs Cd(BH4)2. Z. Naturforschg. B 1952, 7, 582. [Google Scholar] [CrossRef]
- Nöth, H.; Winter, L.P. Metallboranate und Boranato-metallate. V. Solvate des Cadmiumboranats und Boranatocadmate. Z. Anorg. Allg. Chem. 1972, 389, 225–234. [Google Scholar] [CrossRef]
- Stockmayer, W.H.; Rice, D.W.; Stephenson, C.C. Thermodynamic properties of sodium borohydride and aqueous borohydride ion. J. Am. Chem. Soc. 1955, 77, 1980–1983. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Hagemann, H. Structure and properties of NaBH4·2H2O and NaBH4. Eur. J. Inorg. Chem. 2008, 20, 3127–3133. [Google Scholar] [CrossRef]
- Hagemann, H.; D’Anna, V.; Carbonniere, P.; Bardaji, E.G.; Fichtner, M. Synthesis and characterization of NaBD3H, a potential structural probe for hydrogen storage materials. J. Phys. Chem. A 2009, 113, 13932–13936. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.E.; Kenson, R.E. Boron Hydrides. XII. The synthesis and infrared spectra of NaBH3D and NaBD3H. Proc. Indiana Acad. Sci. 1966, 76, 236–240. [Google Scholar]
- Chłopek, K.; Frommen, C.; Leon, A.; Zabara, O.; Fichtner, M. Synthesis and properties of magnesium tetrahydroborate, Mg(BH4)2. J. Mater. Chem. 2007, 17, 3496–3503. [Google Scholar] [CrossRef]
- Buchter, F.; Łodziana, Z.; Remhof, A.; Friedrichs, O.; Borgschulte, A.; Mauron, P.; Zuttel, A.; Sheptyakov, D.; Barkhordarian, G.; Bormann, R.; et al. Structure of Ca(BD4)2 β-phase from combined neutron and synchrotron x-ray powder diffraction data and density functional calculations. J. Phys. Chem. B 2008, 112, 8042–8048. [Google Scholar] [CrossRef] [PubMed]
- Filinchuk, Y.; Richter, B.; Jensen, T.R.; Dmitriev, V.; Chernyshov, D.; Hagemann, H. Porous and dense magnesium borohydride frameworks: Synthesis, stability, and reversible absorption of guest species. Angew. Chem. Int. Ed. 2011, 50, 11162–11166. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, R.; Rice, G. The chemistry of alane. XII. The lithium tetrahydroalanate-triethylamine complex. Inorg. Chem. 1966, 5, 1284–1286. [Google Scholar] [CrossRef]
- Plešek, J.; Heřmánek, S. Chemistry of boranes. IV. On preparation, properties, and behavior towards Lewis bases of magnesium borohydride. Collect. Czech. Chem. Commun. 1966, 31, 3845–3858. [Google Scholar] [CrossRef]
- Brown, H.C.; Yoon, N.M. Selective reductions. X. reaction of aluminum hydride with selected organic compounds containing representative functional groups. Comparison of the reducing characteristics of lithium aluminum hydride and its derivatives. J. Am. Chem. Soc. 1966, 88, 1464–1472. [Google Scholar] [CrossRef]
- Ruff, J.K.; Hawthorne, M.F. The amine complexes of aluminum hydride. II. J. Am. Chem. Soc. 1961, 83, 535–538. [Google Scholar] [CrossRef]
- Kuzdrowska, M.; Annunziata, L.; Marks, S.; Schmid, M.; Jaffredo, C.G.; Roesky, P.W.; Guillaume, S.M.; Maron, L. Organometallic calcium and strontium borohydrides as initiators for the polymerization of epsilon-caprolactone and L-lactide: Combined experimental and computational investigations. Dalton Trans. 2013, 42, 9352–9360. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Remhof, A.; Hwang, S.-J.; Li, H.-W.; Mauron, P.; Orimo, S.-I.; Zuttel, A. Pressure and temperature dependence of the decomposition pathway of LiBH4. Phys. Chem. Chem. Phys. 2012, 14, 6514–6519. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, S.C.; Kalnajs, J. The lattice constants of the alkali borohydrides and the low-temperature phase of sodium borohydride. J. Chem. Phys. 1954, 22, 434–436. [Google Scholar] [CrossRef]
- Kumar, R.S.; Kim, E.; Cornelius, A.L. Structural Phase Transitions in the Potential Hydrogen Storage Compound KBH4 under Compression. J. Phys. Chem. C 2008, 112, 8452–8457. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Talyzin, A.V.; Hagemann, H.; Dmitriev, V.; Chernyshov, D.; Sundqvist, B. Cation Size and Anion Anisotropy in Structural Chemistry of Metal Borohydrides. The Peculiar Pressure Evolution of RbBH4. Inorg. Chem. 2010, 49, 5285–5292. [Google Scholar] [CrossRef]
- Babanova, O.A.; Soloninin, A.V.; Stepanov, A.P.; Skripov, A.V.; Filinchuk, Y. Structural and Dynamical Properties of NaBH4 and KBH4: NMR and Synchrotron X-ray Diffraction Studies. J. Phys. Chem. C 2010, 114, 3712–3718. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Talyzin, A.V.; Chernyshov, D.; Dmitriev, V. High-pressure phase of NaBH4: Crystal structure from synchrotron powder diffraction data. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 76, 092104. [Google Scholar] [CrossRef]
- Marynick, D.S.; Lipscomb, W.N. Crystal structure of beryllium borohydride. Inorg. Chem. 1972, 11, 820–823. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Cerny, R.; Hagemann, H. Insight into Mg(BH4)2 with Synchrotron X-ray diffraction: Structure revision, crystal chemistry, and anomalous thermal expansion. Chem. Mater. 2009, 21, 925–933. [Google Scholar] [CrossRef]
- Her, J.H.; Stephens, P.W.; Gao, Y.; Soloveichik, G.L.; Rijssenbeek, J.; Andrus, M.; Zhao, J.C. Structure of unsolvated magnesium borohydride Mg(BH4)2. Acta Crystallogr. Sect. B Struct. Sci. 2007, 63, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Pitt, M.P.; Webb, C.J.; Paskevicius, M.; Sheptyakov, D.; Buckley, C.E.; Gray, E.M. In situ neutron diffraction study of the deuteration of isotopic Mg11B2. J. Phys. Chem. C 2011, 115, 22669–22679. [Google Scholar] [CrossRef]
- Cerny, R.; Penin, N.; Hagemann, H.; Filinchuk, Y. The first crystallographic and spectroscopic characterization of a 3d-metal borohydride: Mn(BH4)2. J. Phys. Chem. C 2009, 113, 9003–9007. [Google Scholar] [CrossRef]
- Tumanov, N.A.; Roedern, E.; Łodziana, Z.; Nielsen, D.B.; Jensen, T.R.; Talyzin, A.V.; Černy, R.; Chernyshov, D.; Dmitriev, V.; Palasyuk, T.; et al. High-Pressure study of Mn(BH4)2 reveals a stable polymorph with high hydrogen density. Chem. Mater. 2016, 28, 274–283. [Google Scholar] [CrossRef]
- Łodziana, Z. Multivalent metal tetrahydroborides of Al, Sc, Y., Ti, and Zr. Phys. Rev. B 2010, 81, 144108. [Google Scholar] [CrossRef]
- Dain, C.J.; Downs, A.J.; Goode, M.J.; Evans, D.G.; Nicholls, K.T.; Rankin, D.W.H.; Robertson, H.E. Molecular structure of gaseous titanium tris(tetrahydroborate), Ti(BH4)3: Experimental determination by electron diffraction and molecular orbital analysis of some Ti(BH4)3 derivatives. J. Chem. Soc. Dalt. Trans. 1991, 967–977. [Google Scholar] [CrossRef]
- Franz, K.; Fusstetter, H.; Nöth, H. Metallboranate und Boranatometallate. IX [1]; Äther-Addukte von Tris(boranato)-titan(III) und dimere Alkoxy-bis(boranato)-titan(III)-Verbindungen. Z. Anorg. Allg. Chem. 1976, 427, 97–113. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Ma, L.P.; Kang, X.D.; Wang, P.J.; Wang, P.; Cheng, H.M. In situ formation and rapid decomposition of Ti(BH4)3 by mechanical milling LiBH4 with TiF3. Appl. Phys. Lett. 2009, 94, 1–4. [Google Scholar] [CrossRef]
- Nöth, H. Anorganische Reaktionen der Alkaliboranate. Angew. Chem. 1961, 73, 371–383. [Google Scholar] [CrossRef]
- Hagemann, H.; Longhini, M.; Kaminski, J.W.; Wesolowski, T.A.; Cerny, R.; Penin, N.; Sørby, M.H.; Hauback, B.C.; Severa, G.; Jensen, C.M. LiSc(BH4)4: A novel salt of Li+ and dIscrete Sc(BH4)4− complex anions. J. Phys. Chem. A 2008, 112, 7551–7555. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Hwang, S.-J.; Bowman, R.C.J.; Reiter, J.W.; Zan, J.A.; Kulleck, J.G.; Kabbour, H.; Majzoub, E.H.; Ozolins, V. LiSc(BH4)4 as a hydrogen storage material: Multinuclear high-resolution solid-state nmr and first-principles density functional theory studies. J. Phys. Chem. C 2009, 113, 9956–9968. [Google Scholar] [CrossRef]
- Morris, J.H.; Smith, W.E. Synthesis and characterisation of a tetrahydrofuran derivative of scandium tetrahydroborate. J. Chem. Soc. D Chem. Commun. 1970, 245a. [Google Scholar] [CrossRef]
- Cerny, R.; Kim, K.C.; Penin, N.; D’Anna, V.; Hagemann, H.; Sholl, D.S. AZn2(BH4)5 (A = Li, Na) and NaZn(BH4)3: Structural studies. J. Phys. Chem. C 2010, 114, 19127–19133. [Google Scholar] [CrossRef]
- Møller, K.T.; Ley, M.B.; Schouwink, P.; Černý, R.; Jensen, T.R. Synthesis and thermal stability of perovskite alkali metal strontium borohydrides. Dalton Trans. 2016, 45, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Grinderslev, J.B.; Møller, K.T.; Yan, Y.; Chen, X.M.; Li, Y.; Li, H.W.; Zhou, W.; Skibsted, J.; Chen, X.; Jensen, T.R. Potassium octahydridotriborate: Diverse polymorphism in a potential hydrogen storage material and potassium ion conductor. Dalton Trans. 2019, 48, 8872–8881. [Google Scholar] [CrossRef]
- Fischer, P.; Zuttel, A. Order-disorder phase transition in NaBD4. Mater. Sci. Forum 2004, 443, 287–290. [Google Scholar] [CrossRef]
- Miwa, K.; Aoki, M.; Noritake, T.; Ohba, N.; Nakamori, Y.; Towata, S.; Zuttel, A.; Orimo, S. Thermodynamical stability of calcium borohydride Ca(BH4)2. Phys. Rev. B Condens. Matter Mater. Phys. 2006, 74, 155122. [Google Scholar] [CrossRef]
- Richter, B.; Ravnsbaek, D.B.; Tumanov, N.; Filinchuk, Y.; Jensen, T.R. Manganese borohydride; synthesis and characterization. Dalton Trans. 2015, 44, 3988–3996. [Google Scholar] [CrossRef]
- Severa, G.; Hagemann, H.; Longhini, M.; Kaminski, J.W.; Wesolowski, T.A.; Jensen, C.M. Thermal desorption, vibrational spectroscopic, and DFT computational studies of the complex manganese borohydrides Mn(BH4)2 and [Mn(BH4)4]2−. J. Phys. Chem. C 2010, 114, 15516–15521. [Google Scholar] [CrossRef]
- Makhaev, V.D.; Borisov, A.P.; Gnilomedova, T.P.; Lobkovskii, É.B.; Chekhlov, A.N. Production of manganese borohydride complexes of manganese solvated with THF, and the structure of Mn(BH4)2 (THF)3. Bull. Acad. Sci. USSR Div. Chem. Sci. 1987, 36, 1582–1586. [Google Scholar] [CrossRef]
- Schaeffer, G.W.; Roscoe, J.S.; Stewart, A.C. The Reduction of Iron(III) Chloride with Lithium Aluminohydride and Lithium Borohydride: Iron(II) Borohydride. J. Am. Chem. Soc. 1956, 78, 729–733. [Google Scholar] [CrossRef]
- Varin, R.A.; Bidabadi, A.S. Rapid, ambient temperature hydrogen generation from the solid state Li-B-Fe-H system by mechanochemical activation synthesis. J. Power Sources 2015, 284, 554–565. [Google Scholar] [CrossRef]
- Stewart, A.C.; Schaeffer, G.W. The reaction of cobalt(II) bromide with lithium borohydride and lithium aluminohydride. J. Inorg. Nucl. Chem. 1956, 3, 194–197. [Google Scholar] [CrossRef]
- Raje, S.; Angamuthu, R. Solvent-free synthesis and reactivity of nickel(II) borohydride and nickel(II) hydride. Green Chem. 2019, 21, 2752–2758. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Chernyshov, D.; Cerny, R. Lightest borohydride probed by synchrotron x-ray diffraction: Experiment calls for a new theoretical revision. J. Phys. Chem. C 2008, 112, 10579–10584. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Chernyshov, D. Looking at hydrogen atoms with X-rays: Comprehensive synchrotron diffraction study of LiBH4. Acta Crystallogr. Sect. A Found. Crystallogr. 2007, 63, s240–s241. Available online: http://scripts.iucr.org/cgi-bin/paper?a38061 (accessed on 31 January 2022). [CrossRef]
- Soldate, A.M. Crystal structure of sodium borohydride. J. Am. Chem. Soc. 1947, 69, 987–988. [Google Scholar] [CrossRef]
- Dmitriev, V.; Filinchuk, Y.; Chernyshov, D.; Talyzin, A.V.; Ilewski, A.; Andersson, O.; Sundqvist, B.; Kurnosov, A. Pressure-temperature phase diagram of LiBH4: Synchrotron x-ray diffraction experiments and theoretical analysis. Phys. Rev. B Condens. Matter Mater. Phys. 2008, 77, 174112. [Google Scholar] [CrossRef]
- Ohba, N.; Miwa, K.; Aoki, M.; Noritake, T.; Towata, S.-i.; Nakamori, Y.; Orimo, S.-i.; Züttel, A. First-principles study on the stability of intermediate compounds of LiBH4. Phys. Rev. B 2006, 74, 075110. [Google Scholar] [CrossRef]
- Rude, L.H.; Nielsen, T.K.; Ravnsbæk, D.B.; Bosenberg, U.; Ley, M.B.; Richter, B.; Arnbjerg, L.M.; Dornheim, M.; Filinchuk, Y.; Besenbacher, F.; et al. Tailoring properties of borohydrides for hydrogen storage: A review. Phys. Status Solidi A 2011, 208, 1754–1773. [Google Scholar] [CrossRef]
- Roedern, E.; Jensen, T.R. Ammine-stabilized transition-metal borohydrides of iron, cobalt, and chromium: Synthesis and characterization. Inorg. Chem. 2015, 54, 10477–10482. [Google Scholar] [CrossRef] [PubMed]
- Rude, L.H.; Corno, M.; Ugliengo, P.; Baricco, M.; Lee, Y.-S.; Cho, Y.W.; Besenbacher, F.; Overgaard, J.; Jensen, T.R. Synthesis and Structural Investigation of Zr(BH4)4. J. Phys. Chem. C 2012, 116, 20239–20245. [Google Scholar] [CrossRef]
- Bird, P.H.; Churchill, M.R. The crystal structure of zirconium(IV) borohydride (at –160°). Chem. Commun. (London) 1967, 403. [Google Scholar] [CrossRef]
- Broach, R.W.; Chuang, I.S.; Marks, T.J.; Williams, J.M. Metrical characterization of tridentate tetrahydroborate ligation to a transition-metal ion. Structure and bonding in Hf(BH4)4 by single-crystal neutron diffraction. Inorg. Chem. 1983, 22, 1081–1084. [Google Scholar] [CrossRef]
- Hoekstra, H.R.; Katz, J.J. The Preparation and Properties of the Group IV-B Metal Borohydrides. J. Am. Chem. Soc. 1949, 71, 2488–2492. [Google Scholar] [CrossRef]
- Dunbar, A.C.; Wright, J.C.; Grant, D.J.; Girolami, G.S. X-ray Crystal Structure of Thorium Tetrahydroborate, Th(BH4)4, and Computational Studies of An(BH4)4 (An = Th, U). Inorg. Chem. 2021, 60, 12489–12497. [Google Scholar] [CrossRef]
- Bernstein, E.R.; Hamilton, W.C.; Keiderling, T.A.; La Placa, S.J.; Lippard, S.J.; Mayerle, J.J. 14-Coordinate uranium(IV). Structure of uranium borohydride by single-crystal neutron diffraction. Inorg. Chem. 1972, 11, 3009–3016. [Google Scholar] [CrossRef]
- Charpin, P.; Nierlich, M.; Vigner, D.; Lance, M.; Baudry, D. Structure of the second crystalline form of uranium(IV) tetrahydroborate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1987, 43, 1465–1467. [Google Scholar] [CrossRef]
- Banks, R.H.; Edelstein, N.M.; Spencer, B.; Templeton, D.H.; Zalkin, A. Volatility and molecular structure of neptunium(IV) borohydride. J. Am. Chem. Soc. 1980, 102, 620–623. [Google Scholar] [CrossRef][Green Version]
- Tumanov, N.A.; Safin, D.A.; Richter, B.; Lodziana, Z.; Jensen, T.R.; Garcia, Y.; Filinchuk, Y. Challenges in the synthetic routes to Mn(BH4)2: Insight into intermediate compounds. Dalton Trans. 2015, 44, 6571–6580. [Google Scholar] [CrossRef] [PubMed]
- Ley, M.B.; Jørgensen, M.; Cerny, R.; Filinchuk, Y.; Jensen, T.R. From M(BH4)3 (M = La, Ce) borohydride frameworks to controllable synthesis of porous hydrides and ion conductors. Inorg. Chem. 2016, 55, 9748–9756. [Google Scholar] [CrossRef] [PubMed]
- Humphries, T.D.; Ley, M.B.; Frommen, C.; Munroe, K.T.; Jensen, T.R.; Hauback, B.C. Crystal structure and in situ decomposition of Eu(BH4)2 and Sm(BH4)2. J. Mater. Chem. A 2015, 3, 691–698. [Google Scholar] [CrossRef]
- Olsen, J.E.; Frommen, C.; Jensen, T.R.; Riktor, M.D.; Sorby, M.H.; Hauback, B.C. Structure and thermal properties of composites with RE-borohydrides (RE = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Er, Yb or Lu) and LiBH4. RSC Adv. 2014, 4, 1570–1582. [Google Scholar] [CrossRef]
- Olsen, J.E.; Frommen, C.; Sorby, M.H.; Hauback, B.C. Crystal structures and properties of solvent-free LiYb(BH4)4−xClx, Yb(BH4)3 and Yb(BH4)2−xClx. RSC Adv. 2013, 3, 10764–10774. [Google Scholar] [CrossRef]
- Jaron, T.; Orłowski, P.A.; Wegner, W.; Fijałkowski, K.J.; Leszczynski, P.J.; Grochala, W. Hydrogen storage materials: Room-temperature wet-chemistry approach toward mixed-metal borohydrides. Angew. Chem. Int. Ed. 2015, 54, 1236–1239. [Google Scholar] [CrossRef]
- Jaron, T.; Wegner, W.; Fijałkowski, K.J.; Leszczynski, P.J.; Grochala, W. Facile formation of thermodynamically unstable novel borohydride materials by a wet chemistry route. Chem. Eur. J. 2015, 21, 5689–5692. [Google Scholar] [CrossRef]
- Nickels, E.A.; Jones, M.O.; David, W.I.F.; Johnson, S.R.; Lowton, R.L.; Sommariva, M.; Edwards, P.P. Tuning the decomposition temperature in complex hydrides: Synthesis of a mixed alkali metal borohydride. Angew. Chem. Int. Ed. 2008, 47, 2817–2819. [Google Scholar] [CrossRef] [PubMed]
- Seballos, L.; Zhang, J.Z.; Ronnebro, E.; Herberg, J.L.; Majzoub, E.H. Metastability and crystal structure of the bialkali complex metal borohydride NaK(BH4)2. J. Alloys Compd. 2009, 476, 446–450. [Google Scholar] [CrossRef]
- Semenenko, K.N.; Chavgun, A.P.; Surov, V.N. Interaction of sodium tetrahydroborate with potassium and lithium tetrahydroborates. Russ. J. Inorg. Chem. 1971, 16, 271–273. [Google Scholar]
- Jensen, S.R.H.; Jepsen, L.H.; Skibsted, J.; Jensen, T.R. Phase diagram for the NaBH4–KBH4 system and the stability of a Na1–xKxBH4 Solid Solution. J. Phys. Chem. C 2015, 119, 27919–27929. [Google Scholar] [CrossRef]
- Grinderslev, J.B.; Jepsen, L.H.; Lee, Y.-S.; Møller, K.T.; Cho, Y.W.; Černý, R.; Jensen, T.R. Structural diversity and trends in properties of an array of hydrogen-rich ammonium metal borohydrides. Inorg. Chem. 2020, 59, 12733–12747. [Google Scholar] [CrossRef]
- Černý, R.; Schouwink, P. The crystal chemistry of inorganic metal borohydrides and their relation to metal oxides. Acta Crystallogr. Sect. B 2015, B71, 619–640. [Google Scholar] [CrossRef]
- Schouwink, P.; D’Anna, V.; Ley, M.B.; Daku, L.M.L.; Richter, B.; Jensen, T.R.; Hagemann, H.; Černý, R. Bimetallic borohydrides in the system M(BH4)2–KBH4 (M = Mg, Mn): On the structural diversity. J. Phys. Chem. C 2012, 116, 10829–10840. [Google Scholar] [CrossRef]
- Schouwink, P.; Ley, M.B.; Tissot, A.; Hagemann, H.; Jensen, T.R.; Smrčok, Ľ.; Černý, R. Structure and properties of complex hydride perovskite materials. Nat. Commun. 2014, 5, 5706. [Google Scholar] [CrossRef]
- Gao, M.; Yan, X.W.; Lu, Z.Y.; Xiang, T. Phonon-mediated high-temperature superconductivity in the ternary borohydride KB2H8 under pressure near 12 GPa. Phys. Rev. B 2021, 104, L100504. [Google Scholar] [CrossRef]
- Møller, K.T.; Jørgensen, M.; Fogh, A.S.; Jensen, T.R. Perovskite alkali metal samarium borohydrides: Crystal structures and thermal decomposition. Dalton Trans. 2017, 46, 11905–11912. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Takamura, H.; Maekawa, H.; Li, H.-W.; Orimo, S.-I. Stabilization of lithium superionic conduction phase and enhancement of conductivity of LiBH4 by LiCl addition. Appl. Phys. Lett. 2009, 94, 084103. [Google Scholar] [CrossRef]
- D’Anna, V.; Daku, L.M.L.; Hagemann, H.; Kubel, F. Ionic layered BaFCl and Ba1−xSrxFCl compounds: Physical- and chemical-pressure effects. Phys. Rev. B 2010, 82, 024108. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Arnbjerg, L.M.; Ravnsbæk, D.B.; Filinchuk, Y.; Vang, R.T.; Cerenius, Y.; Besenbacher, F.; Jørgensen, J.-E.; Jakobsen, H.J.; Jensen, T.R. Structure and dynamics for LiBH4−LiCl solid solutions. Chem. Mater. 2009, 21, 5772–5782. [Google Scholar] [CrossRef]
- Blanchard, D.; Maronsson, J.B.; Riktor, M.D.; Kheres, J.; Sveinbjörnsson, D.; Bardají, E.G.; Léon, A.; Juranyi, F.; Wuttke, J.; Lefmann, K.; et al. Hindered rotational energy barriers of BH4− tetrahedra in β-Mg(BH4)2 from quasielastic neutron scattering and DFT calculations. J. Phys. Chem. C 2012, 116, 2013–2023. [Google Scholar] [CrossRef]
- Rude, L.H.; Zavorotynska, O.; Arnbjerg, L.M.; Ravnsbæk, D.B.; Malmkjær, R.A.; Grove, H.; Hauback, B.C.; Baricco, M.; Filinchuk, Y.; Besenbacher, F.; et al. Bromide substitution in lithium borohydride, LiBH4–LiBr. Int. J. Hydrogen Energy 2011, 36, 15664–15672. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Rude, L.H.; Jensen, T.R. Chloride substitution in sodium borohydride. J. Solid State Chem. 2011, 184, 1858–1866. [Google Scholar] [CrossRef]
- Llamas-Jansa, I.; Aliouane, N.; Deledda, S.; Fonneløp, J.E.; Frommen, C.; Humphries, T.; Lieutenant, K.; Sartori, S.; Sørby, M.H.; Hauback, B.C. Chloride substitution induced by mechano-chemical reactions between NaBH4 and transition metal chlorides. J. Alloys Compd. 2012, 530, 186–192. [Google Scholar] [CrossRef]
- Olsen, J.E.; Sørby, M.H.; Hauback, B.C. Chloride-substitution in sodium borohydride. J. Alloys Compd. 2011, 509, L228–L231. [Google Scholar] [CrossRef]
- Rude, L.H.; Groppo, E.; Arnbjerg, L.M.; Ravnsbæk, D.B.; Malmkjær, R.A.; Filinchuk, Y.; Baricco, M.; Besenbacher, F.; Jensen, T.R. Iodide substitution in lithium borohydride, LiBH4–LiI. J. Alloys Compd. 2011, 509, 8299–8305. [Google Scholar] [CrossRef]
- Borgschulte, A.; Gremaud, R.; Kato, S.; Stadie, N.P.; Remhof, A.; Züttel, A.; Matsuo, M.; Orimo, S.-I. Anharmonicity in LiBH4–LiI induced by anion exchange and temperature. Appl. Phys. Lett. 2010, 97, 031916. [Google Scholar] [CrossRef]
- Custelcean, R.; Jackson, J.E. Dihydrogen bonding: Structures, energetics, and dynamics. Chem. Rev. 2001, 101, 1963–1980. [Google Scholar] [CrossRef]
- Noritake, T.; Aoki, M.; Matsumoto, M.; Miwa, K.; Towata, S.; Li, H.W.; Orimo, S. Crystal structure and charge density analysis of Ca(BH4)2. J. Alloys Compd. 2010, 491, 57–62. [Google Scholar] [CrossRef]
- Grinderslev, J.B.; Ley, M.B.; Lee, Y.-S.; Jepsen, L.H.; Jørgensen, M.; Cho, Y.W.; Skibsted, J.; Jensen, T.R. Ammine lanthanum and cerium borohydrides, M (BH4)3·NH3; trends in synthesis, structures, and thermal properties. Inorg. Chem. 2020, 59, 7768–7778. [Google Scholar] [CrossRef]
- Grinderslev, J.B.; Jensen, T.R. Trends in the series of ammine rare-earth-metal borohydrides: Relating structural and thermal properties. Inorg. Chem. 2021, 60, 2573–2589. [Google Scholar] [CrossRef]
- Johnson, S.R.; David, W.I.F.; Royse, D.M.; Sommariva, M.; Tang, C.Y.; Fabbiani, F.P.A.; Jones, M.O.; Edwards, P.P. The monoammoniate of lithium borohydride, Li(NH3)BH4: An effective ammonia storage compound. Chem. Asian J. 2009, 4, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, L.H.; Ley, M.B.; Filinchuk, Y.; Besenbacher, F.; Jensen, T.R. Tailoring the properties of ammine metal borohydrides for solid-state hydrogen storage. ChemSusChem 2015, 8, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Soloveichik, G.; Her, J.-H.; Stephens, P.W.; Gao, Y.; Rijssenbeek, J.; Andrus, M.; Zhao, J.C. Ammine magnesium borohydride complex as a new material for hydrogen storage: Structure and properties of Mg(BH4)2·2NH3. Inorg. Chem. 2008, 47, 4290–4298. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Li, Y.; Gao, M.; Pan, H. Synthesis and thermal decomposition behaviors of magnesium borohydride ammoniates with controllable composition as hydrogen storage materials. Chem. Asian J. 2013, 8, 476–481. [Google Scholar] [CrossRef]
- Huang, J.; Tan, Y.; Su, J.; Gu, Q.; Cerny, R.; Ouyang, L.; Sun, D.; Yu, X.; Zhu, M. Synthesis, structure and dehydrogenation of zirconium borohydride octaammoniate. Chem. Commun. 2015, 51, 2794–2797. [Google Scholar] [CrossRef]
- Konoplev, V.N.; Silina, T.A. Ammonia derivatives of magnesium borohydride. Zh. Neorg. Khim. 1985, 30, 1125–1128. [Google Scholar]
- Chu, H.; Wu, G.; Xiong, Z.; Guo, J.; He, T.; Chen, P. Structure and hydrogen storage properties of calcium borohydride diammoniate. Chem. Mater. 2010, 22, 6021–6028. [Google Scholar] [CrossRef]
- He, T.; Wu, H.; Wu, G.; Wang, J.; Zhou, W.; Xiong, Z.; Chen, J.; Zhang, T.; Chen, P. Borohydride hydrazinates: High hydrogen content materials for hydrogen storage. Energy Environ. Sci. 2012, 5, 5686–5689. [Google Scholar] [CrossRef]
- He, T.; Wu, H.; Chen, J.; Zhou, W.; Wu, G.; Xiong, Z.; Zhang, T.; Chen, P. Alkali and alkaline-earth metal borohydride hydrazinates: Synthesis, structures and dehydrogenation. Phys. Chem. Chem. Phys. 2013, 15, 10487–10493. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, T.; Wu, G.; Ju, X.; Chen, P. The synthesis, structure and dehydrogenation of calcium borohydride hydrazinates. Int. J. Hydrogen Energy 2015, 40, 5333–5339. [Google Scholar] [CrossRef]
- Shriver, D.; Weller, M.; Overton, T.; Rouke, J.; Armstrong, F. Inorganic Chemistry, 6th ed.; Oxford University Press: Oxford, UK, 2014; ISBN-10: 1–4292–9906–1. [Google Scholar]
- Unemoto, A.; Matsuo, M.; Orimo, S. Complex hydrides for electrochemical energy storage. Adv. Funct. Mater. 2014, 24, 2267–2279. [Google Scholar] [CrossRef]
- Møller, K.T.; Sheppard, D.; Ravnsbæk, D.B.; Buckley, C.E.; Akiba, E.; Li, H.-W.; Jensen, T.R. Complex metal hydrides for hydrogen, thermal and electrochemical energy storage. Energies 2017, 10, 1645. [Google Scholar] [CrossRef]
- Matsuo, M.; Nakamori, Y.; Orimo, S.; Maekawa, H.; Takamura, H. Lithium superionic conduction in lithium borohydride accompanied by structural transition. Appl. Phys. Lett. 2007, 91, 224103. [Google Scholar] [CrossRef]
- Tang, W.S.; Unemoto, A.; Zhou, W.; Stavila, V.; Matsuo, M.; Wu, H.; Orimo, S.-I.; Udovic, T.J. Unparalleled lithium and sodium superionic conduction in solid electrolytes with large monovalent cage-like anions. Energy Environ. Sci. 2015, 8, 3637–3645. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Ciucci, F. Metal Borohydrides as Electrolytes for Solid-State Li, Na, Mg, and Ca Batteries: A First-Principles Study. Chem. Mater. 2017, 29, 9308–9319. [Google Scholar] [CrossRef]
- Udovic, T.J.; Matsuo, M.; Unemoto, A.; Verdal, N.; Stavila, V.; Skripov, A.V.; Rush, J.J.; Takamura, H.; Orimo, S.-I. Sodium superionic conduction in Na2B12H12. Chem. Commun. 2014, 50, 3750–3752. [Google Scholar] [CrossRef]
- Das, S.; Ngene, P.; Norby, P.; Vegge, T.; De Jongh, P.E.; Blanchard, D. All-Solid-State Lithium-Sulfur Battery Based on a Nanoconfined LiBH4 Electrolyte. J. Electrochem. Soc. 2016, 163, A2029–A2034. [Google Scholar] [CrossRef]
- Unemoto, A.; Ikeshoji, T.; Yasaku, S.; Matsuo, M.; Stavila, V.; Udovic, T.J.; Orimo, S.-I. Stable Interface Formation between TiS2 and LiBH4 in Bulk-Type All-Solid-State Lithium Batteries. Chem. Mater. 2015, 27, 5407–5416. [Google Scholar] [CrossRef]
- Matsuo, M.; Orimo, S. Lithium fast-ionic conduction in complex hydrides: Review and prospects. Adv. Energy Mater. 2011, 1, 161–172. [Google Scholar] [CrossRef]
- Guzik, M.N.; Mohtadi, R.; Sartori, S. Lightweight complex metal hydrides for Li-, Na-, and Mg-based batteries. J. Mater. Res. 2019, 34, 877–904. [Google Scholar] [CrossRef]
- Takano, A.; Oikawa, I.; Kamegawa, A.; Takamura, H. Enhancement of the lithium-ion conductivity of LiBH4 by hydration. Solid State Ion. 2016, 285, 47–50. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Song, T.; Miyaoka, H.; Shinzato, K.; Miyaoka, H.; Ichikawa, T.; Shi, S.; Zhang, X.; Isobe, S.; et al. Ammonia, a switch for controlling high ionic conductivity in lithium borohydride ammoniates. Joule 2018, 2, 1522–1533. [Google Scholar] [CrossRef]
- Yan, Y.; Grinderslev, J.B.; Lee, Y.-S.; Lee, M.; Cho, Y.W.; Černý, R.; Jensen, T.R. Ammonia-assisted fast Li-Ion conductivity in a new hemiammine lithium borohydride, LiBH4·1/2NH3. Chem. Commun. 2020, 56, 3971–3974. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, R.; Li, H.; Zhang, Y.; Wang, Y.; Wu, C.; Yan, Y.; Chen, Y. Li-Ion conductivity enhancement of LiBH4·xNH3 with in situ formed Li2O nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 31635–31641. [Google Scholar] [CrossRef]
- Liu, H.; Ren, Z.; Zhang, X.; Hu, J.; Gao, M.; Pan, H.; Liu, Y. Incorporation of ammonia borane groups in the lithium borohydride structure enables ultrafast lithium ion conductivity at room temperature for solid-state batteries. Chem. Mater. 2020, 32, 671–678. [Google Scholar] [CrossRef]
- Mohtadi, R.; Matsui, M.; Arthur, T.S.; Hwang, S.-J. Magnesium borohydride: From hydrogen storage to magnesium battery. Angew. Chem. Int. Ed. Engl. 2012, 51, 9780–9783. [Google Scholar] [CrossRef] [PubMed]
- Higashi, S.; Miwa, K.; Aoki, M.; Takechi, K. A novel inorganic solid state ion conductor for rechargeable mg batteries. Chem. Commun. 2014, 50, 1320–1322. [Google Scholar] [CrossRef]
- Le Ruyet, R.; Fleutot, B.; Berthelot, R.; Benabed, Y.; Hautier, G.; Filinchuk, Y.; Janot, R. Mg3(BH4)4(NH2)2 as inorganic solid electrolyte with high Mg2+ ionic conductivity. ACS Appl. Energy Mater. 2020, 3, 6093–6097. [Google Scholar] [CrossRef]
- Roedern, E.; Kühnel, R.-S.; Remhof, A.; Battaglia, C. Magnesium ethylenediamine borohydride as solid-state electrolyte for magnesium batteries. Sci. Rep. 2017, 7, 46189. [Google Scholar] [CrossRef]
- Burankova, T.; Roedern, E.; Maniadaki, A.E.; Hagemann, H.; Rentsch, D.; Lodziana, Z.; Battaglia, C.; Remhof, A.; Embs, J.P. Dynamics of the coordination complexes in a solid-state Mg electrolyte. J. Phys. Chem. Lett. 2018, 9, 6450–6455. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Dononelli, W.; Jørgensen, M.; Grinderslev, J.B.; Lee, Y.-S.; Cho, Y.W.; Černý, R.; Hammer, B.; Jensen, T.R. The mechanism of Mg2+ conduction in ammine magnesium borohydride promoted by a neutral molecule. Phys. Chem. Chem. Phys. 2020, 22, 9204–9209. [Google Scholar] [CrossRef]
- Yan, Y.; Grinderslev, J.B.; Jørgensen, M.; Skov, L.N.; Skibsted, J.; Jensen, T.R. Ammine magnesium borohydride nanocomposites for all-solid-state magnesium batteries. ACS Appl. Energy Mater. 2020, 3, 9264–9270. [Google Scholar] [CrossRef]
- Kisu, K.; Kim, S.; Inukai, M.; Oguchi, H.; Takagi, S.; Orimo, S. Magnesium borohydride ammonia borane as a magnesium ionic conductor. ACS Appl. Energy Mater. 2020, 3, 3174–3179. [Google Scholar] [CrossRef]
- Kisu, K.; Kim, S.; Shinohara, T.; Zhao, K.; Züttel, A.; Orimo, S. Monocarborane cluster as a stable fluorine-free calcium battery electrolyte. Sci. Rep. 2021, 11, 7563. [Google Scholar] [CrossRef]
- Luo, X.X.; Rawal, A.; Salman, M.S.; Aguey-Zinsou, K.F. Core-Shell NaBH4@Na2B12H12 Nanoparticles as Fast Ionic Conductors for Sodium-Ion Batteries. ACS Appl. Nano Mater. 2022, 5, 373–379. [Google Scholar] [CrossRef]
- Bao, K.; Pang, Y.; Yang, J.; Sun, D.L.; Fang, F.; Zheng, S.Y. Modulating composite polymer electrolyte by lithium closo-borohydride achieves highly stable solid-state battery at 25°C. Sci. China Mater. 2022, 65, 95–104. [Google Scholar] [CrossRef]
- Tran, B.L.; Allen, T.N.; Bowden, M.E.; Autrey, T.; Jensen, C.M. Effects of Glymes on the Distribution of Mg(B10H10) and Mg(B12H12) from the Thermolysis of Mg(BH4)2. Inorganics 2021, 9, 41. [Google Scholar] [CrossRef]
- Dobbins, T.A. Overview of the Structure-Dynamics-Function Relationships in Borohydrides for Use as Solid-State Electrolytes in Battery Applications. Molecules 2021, 26, 3239. [Google Scholar] [CrossRef] [PubMed]
- Marks, S.; Heck, J.G.; Habicht, M.H.; Oña-Burgos, P.; Feldmann, C.; Roesky, P.W. [Ln (BH4)2(THF)2] (Ln = Eu, Yb)—A highly luminescent material. synthesis, properties, reactivity, and NMR studies. J. Am. Chem. Soc. 2012, 134, 16983–16986. [Google Scholar] [CrossRef] [PubMed]
- Drozdov, A.P.; Eremets, M.I.; Troyan, I.A.; Ksenofontov, V.; Shylin, S.I. Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system. Nature 2015, 525, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Somayazulu, M.; Ahart, M.; Mishra, A.K.; Geballe, Z.M.; Baldini, M.; Meng, Y.; Struzhkin, V.V.; Hemley, R.J. Evidence for superconductivity above 260 K in lanthanum superhydride at megabar pressures. Phys. Rev. Lett. 2019, 122, 027001. [Google Scholar] [CrossRef] [PubMed]
- Drozdov, A.P.; Kong, P.P.; Minkov, V.S.; Besedin, S.P.; Kuzovnikov, M.A.; Mozaffari, S.; Balicas, L.; Balakirev, F.F.; Graf, D.E.; Prakapenka, V.B.; et al. Superconductivity at 250 K in lanthanum hydride under high pressures. Nature 2019, 569, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Dovgaliuk, I.; Hagemann, H.; Leyssens, T.; Devillers, M.; Filinchuk, Y. CO2-promoted hydrolysis of KBH4 for efficient hydrogen co-generation. Int. J. Hydrogen Energy 2014, 39, 19603–19608. [Google Scholar] [CrossRef]
- Lombardo, L.; Ko, Y.; Zhao, K.; Yang, H.; Züttel, A. Direct CO2 capture and reduction to high-end chemicals with tetraalkylammonium borohydrides. Angew. Chem. 2021, 133, 9666–9675. [Google Scholar] [CrossRef]
- Knopf, I.; Cummins, C.C. Revisiting CO2 reduction with NaBH4 under aprotic conditions: Synthesis and characterization of sodium triformatoborohydride. Organometallics 2015, 34, 1601–1603. [Google Scholar] [CrossRef]
- Grice, K.A.; Groenenboom, M.C.; Manuel, J.D.A.; Sovereign, M.A.; Keith, J.A. Examining the selectivity of borohydride for carbon dioxide and bicarbonate reduction in protic conditions. Fuel 2015, 150, 139–145. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, J.; Wang, L.; Teng, Y.-L.; Dong, B.-X. Mechanochemical reactions of alkali borohydride with CO2 under ambient temperature. J. Solid State Chem. 2019, 277, 828–832. [Google Scholar] [CrossRef]
- Picasso, C.V.; Safin, D.A.; Dovgaliuk, I.; Devred, F.; Debecker, D.; Li, H.-W.; Proost, J.; Filinchuk, Y. Reduction of CO2 with KBH4 in solvent-free conditions. Int. J. Hydrogen Energy 2016, 41, 14377–14386. [Google Scholar] [CrossRef]
- Laversenne, L.; Goutaudier, C.; Chiriac, R.; Sigala, C.; Bonnetot, B. Hydrogen storage in borohydrides comparison of hydrolysis conditions of LiBH4, NaBH4 and KBH4. J. Therm. Anal. Calorim. 2008, 94, 785–790. [Google Scholar] [CrossRef]
- Vitillo, J.G.; Groppo, E.; Bardají, E.G.; Baricco, M.; Bordiga, S. Fast carbon dioxide recycling by reaction with γ-Mg(BH4)2. Phys. Chem. Chem. Phys. 2014, 16, 22482–22486. [Google Scholar] [CrossRef] [PubMed]
- Zuttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.; Sudan, P.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloys Compd. 2003, 356, 515–520. [Google Scholar] [CrossRef]
- Nakamori, Y.; Miwa, K.; Ninomiya, A.; Li, H.; Ohba, N.; Towata, S.-I.; Züttel, A.; Orimo, S.-I. Correlation between thermodynamical stabilities of metal borohydrides and cation electronegativites: First-principles calculations and experiments. Phys. Rev. B 2006, 74, 045126. [Google Scholar] [CrossRef]
- Kharbachi, E.A.; Pinatel, E.; Nuta, I.; Baricco, M. A thermodynamic assessment of LiBH4. Calphad Comput. Coupling Phase Diagrams Thermochem. 2012, 39, 80–90. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Pinatel, E.R.; Corno, M.; Jensen, T.R.; Baricco, M. Phase diagrams of the LiBH4-NaBH4-KBH4 system. Phys. Chem. Chem. Phys. 2017, 19, 25071–25079. [Google Scholar] [CrossRef] [PubMed]
- Pinatel, E.R.; Albanese, E.; Civalleri, B.; Baricco, M. Thermodynamic modelling of Mg(BH4)2. J. Alloys Compd. 2015, 645 (Suppl. 1), S64–S68. [Google Scholar] [CrossRef]
- Błoński, P.; Łodziana, Z. Correlation between the ionic potential and thermal stability of metal borohydrides: First-principles investigations. Phys. Rev. B 2014, 90, 054114. [Google Scholar] [CrossRef]
- Blanchard, D.; Zatti, M.; Vegge, T. Analysis of the decomposition gases from α and β-Cd(BH4)2 synthesized by temperature controlled mechanical milling. J. Alloys Compd. 2013, 547, 76–80. [Google Scholar] [CrossRef][Green Version]
- Mulliken, R.S. A new electroaffinity scale; together with data on valence states and on valence ionization potentials and electron affinities. J. Chem. Phys. 1934, 2, 782–793. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Kang, X.; Wang, P.; Cheng, H. Advantage of TiF3 over TiCl3 as a dopant precursor to improve the thermodynamic property of Na3AlH6. Scr. Mater. 2007, 56, 361–364. [Google Scholar] [CrossRef]
- Yin, L.C.; Wang, P.; Fang, Z.; Cheng, H.M. Thermodynamically tuning LiBH4 by fluorine anion doping for hydrogen storage: A density functional study. Chem. Phys. Lett. 2008, 450, 318–321. [Google Scholar] [CrossRef]
- Sveinbjornsson, D.; Blanchard, D.; Myrdal, J.S.G.; Younesi, R.; Viskinde, R.; Riktor, M.D.; Norby, P.; Vegge, T. Ionic conductivity and the formation of cubic CaH2 in the LiBH4-Ca(BH4)2 composite. J. Solid State Chem. 2014, 211, 81–89. [Google Scholar] [CrossRef][Green Version]
- Ozolins, V.; Majzoub, E.H.; Wolverton, C. First-principles prediction of thermodynamically reversible hydrogen storage reactions in the Li-Mg-Ca-B-H system. J. Am. Chem. Soc. 2009, 131, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.T.; Hu, J.J.; Wu, G.T.; Chen, P.; Luo, W.F.; Gross, K.; Wang, J. Thermodynamic and kinetic investigations of the hydrogen storage in the Li–Mg–N–H system. J. Alloys Compd. 2005, 398, 235–239. [Google Scholar] [CrossRef]
- Gibb, B.C. The centenary (maybe) of the hydrogen bond. Nat. Chem. 2020, 12, 665–667. [Google Scholar] [CrossRef]
- Chu, H.; Wu, G.; Zhang, Y.; Xiong, Z.; Guo, J.; He, T.; Chen, P. Improved dehydrogenation properties of calcium borohydride combined with alkaline-earth metal amides. J. Phys. Chem. C 2011, 115, 18035–18041. [Google Scholar] [CrossRef]
- Chater, P.A.; David, W.I.F.; Anderson, P.A. Synthesis and structure of the new complex hydride Li2BH4NH2. Chem. Commun. 2007, 45, 4770–4772. [Google Scholar] [CrossRef]
- Somer, M.; Acar, S.; Koz, C.; Kokal, I.; Hohn, P.; Cardoso-Gil, R.; Aydemir, U.; Akselrud, L. α- and β-Na2[BH4][NH2]: Two modifications of a complex hydride in the system NaNH2–NaBH4; syntheses, crystal structures, thermal analyses, mass and vibrational spectra. J. Alloys Compd. 2010, 491, 98–105. [Google Scholar] [CrossRef]
- Chu, H.L.; Xiong, Z.T.; Wu, G.T.; Guo, J.P.; Zheng, X.L.; He, T.; Wu, C.Z.; Chen, P. Hydrogen Storage Properties of Ca(BH4)2–LiNH2 System. Chem. Asian J. 2010, 5, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Poonyayant, N.; Stavila, V.; Majzoub, E.H.; Klebanoff, L.E.; Behrens, R.; Angboonpong, N.; Ulutagay-Kartin, M.; Pakawatpanurut, P.; Hecht, E.S.; Breit, J.S. An investigation into the hydrogen storage characteristics of Ca(BH4)2/LiNH2 and Ca(BH4)2/NaNH2: Evidence of intramolecular destabilization. J. Phys. Chem. C 2014, 118, 14759–14769. [Google Scholar] [CrossRef]
- Aoki, M.; Miwa, K.; Noritake, T.; Kitahara, G.; Nakamori, Y.; Orimo, S.; Towata, S. Destabilization of LiBH4 by mixing with LiNH2. Appl. Phys. A Mater. Sci. Process. 2005, 80, 1409–1412. [Google Scholar] [CrossRef]
- Grube, E.; Jensen, S.R.H.; Nielsen, U.G.; Jensen, T.R. Reactivity of magnesium borohydride—Metal hydride composites, γ-Mg(BH4)2-MHx, M = Li, Na, Mg, Ca. J. Alloys Compd. 2019, 770, 1155–1163. [Google Scholar] [CrossRef]
- Ibikunle, A.A.; Goudy, A.J. Kinetics and modeling study of a Mg(BH4)2/Ca(BH4)2 destabilized system. Int. J. Hydrogen Energy 2012, 37, 12420–12424. [Google Scholar] [CrossRef]
- Durojaiye, T.; Ibikunle, A.; Goudy, A.J. Hydrogen storage in destabilized borohydride materials. Int. J. Hydrogen Energy 2010, 35, 10329–10333. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Baricco, M. Hydrogen desorption in Mg(BH4)2-Ca(BH4)2 system. Energies 2019, 12, 3230. [Google Scholar] [CrossRef]
- Au, M.; Jurgensen, A. Modified lithium borohydrides for reversible hydrogen storage. J. Phys. Chem. B 2006, 110, 7062–7067. [Google Scholar] [CrossRef] [PubMed]
- Au, M.; Jurgensen, A.; Zeigler, K. Modified lithium borohydrides for reversible hydrogen storage (2). J. Phys. Chem. B 2006, 110, 26482–26487. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Havre, L.; Mathey, F.; Petit, V.I.; Bensoam, J. Production of Hydrogen. U.S. Patent 4193978 A, 18 March 1980. [Google Scholar]
- Mosegaard, L.; Møller, B.; Jørgensen, J.E.; Bosenberg, U.; Dornheim, M.; Hanson, J.C.; Cerenius, Y.; Walker, G.S.; Jakobsen, H.J.; Besenbacher, F.; et al. Intermediate phases observed during decomposition of LiBH4. J. Alloys Compd. 2007, 446, 301–305. [Google Scholar] [CrossRef]
- Her, J.H.; Yousufuddin, M.; Zhou, W.; Jalisatgi, S.S.; Kulleck, J.G.; Zan, J.A.; Hwang, S.J.; Bowman, R.C., Jr.; Udovic, T.J. Crystal structure of Li2B12H12: A possible intermediate species in the decomposition of LiBH4. Inorg. Chem. 2008, 47, 9757–9759. [Google Scholar] [CrossRef] [PubMed]
- Her, J.H.; Wu, H.; Verdal, N.; Zhou, W.; Stavila, V.; Udovic, T.J. Structures of the strontium and barium dodecahydro-closo-dodecaborates. J. Alloys Compd. 2012, 514, 71–75. [Google Scholar] [CrossRef]
- Friedrichs, O.; Remhof, A.; Hwang, S.; Zuttel, A. Role of Li2B12H12 for the formation and decomposition of LiBH4. Chem. Mater. 2010, 22, 3265–3268. [Google Scholar] [CrossRef]
- Mauron, P.; Buchter, F.; Friedrichs, O.; Remhof, A.; Bielmann, M.; Zwicky, C.N.; Zuttel, A. Stability and reversibility of LiBH4. J. Phys. Chem. B 2008, 112, 906–910. [Google Scholar] [CrossRef]
- Orimo, S.I.; Nakamori, Y.; Kitahara, G.; Miwa, K.; Ohba, N.; Towata, S.; Zuttel, A. Dehydriding and rehydriding reactions of LiBH4. J. Alloys Compd. 2005, 404, 427–430. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Ohba, N.; Miwa, K.; Aoki, M.; Towata, S.; Zuttel, A. Experimental studies on intermediate compound of LiBH4. Appl. Phys. Lett. 2006, 89, 021920. [Google Scholar] [CrossRef]
- Hwang, S.J.; Bowman, R.C., Jr.; Reiter, J.W.; Rijssenbeck, J.; Soloveichik, G.L.; Zhao, J.C.; Kabbour, H.; Ahn, C.C. NMR confirmation for formation of [B12H12]2− complexes during hydrogen desorption from metal borohydrides. J. Phys. Chem. C 2008, 112, 3164–3169. [Google Scholar] [CrossRef]
- Kato, S.; Bielmann, M.; Borgschulte, A.; Zakaznova-Herzog, V.; Remhof, A.; Orimo, S.; Zuttel, A. Effect of the surface oxidation of LiBH4 on the hydrogen desorption mechanism. Phys. Chem. Chem. Phys. 2010, 12, 10950–10955. [Google Scholar] [CrossRef] [PubMed]
- Zuttel, A.; Borgschulte, A.; Orimo, S.I. Tetrahydroborates as new hydrogen storage materials. Scr. Mater. 2007, 56, 823–828. [Google Scholar] [CrossRef]
- Schlapbach, L.; Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Orimo, S.I.; Nakamori, Y.; Eliseo, J.R.; Zuttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Palade, P.; Comanescu, C.; Mercioniu, I. Improvements of hydrogen desorption of lithium borohydride by impregnation onto MSH-H carbon replica. J. Ovonic Res. 2012, 8, 155–160. [Google Scholar]
- Comanescu, C.; Guran, C.; Palade, P. Improvements of kinetic properties of LiBH4 by supporting on MSU-H type mesoporous silica. Optoelectron. Adv. Mater. Rapid Commun. 2010, 4, 705–708. [Google Scholar]
- Cahen, S.; Eymery, J.B.; Janot, R.; Tarascon, J.M. Improvement of the LiBH4 hydrogen desorption by inclusion into mesoporous carbons. J. Power Sources 2009, 189, 902–908. [Google Scholar] [CrossRef]
- Kruk, M.; Dufour, B.; Celer, E.B.; Kowalewski, T.; Jaroniec, M.; Matyjaszewski, K. Synthesis of mesoporous carbons using ordered and disordered mesoporous silica templates and polyacrylonitrile as carbon precursor. J. Phys. Chem. B 2005, 109, 9216–9225. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Kruk, M.; Jaroniec, M. Ordered mesoporous carbons. Adv. Mater. 2001, 13, 677–681. [Google Scholar] [CrossRef]
- Ngene, P.; Adelhelm, P.; Beale, A.M.; de Jong, K.P.; de Jongh, P.E. LiBH4/SBA-15 Nanocomposites prepared by melt infiltration under hydrogen pressure: Synthesis and hydrogen sorption properties. J. Phys. Chem. C 2010, 114, 6163–6168. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.Z.; Fan, M.Q.; Liu, S.S.; Chu, H.L.; Zhang, Y.H.; Gao, X.Y.; Sun, L.X. Enhanced hydrogen storage performance of LiBH4−SiO2−TiF3 composite. J. Phys. Chem. C 2008, 112, 4005–4010. [Google Scholar] [CrossRef]
- Gross, A.F.; Vajo, J.J.; Van Atta, S.L.; Olson, G.L. Enhanced hydrogen storage kinetics of LiBH4 in nanoporous carbon scaffolds. J. Phys. Chem. C 2008, 112, 5651–5657. [Google Scholar] [CrossRef]
- David, R.L. Handbook of Chemistry and Physics, 90th ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Fang, Z.Z.; Wang, P.; Rufford, T.E.; Kang, X.D.; Lu, G.Q.; Cheng, H.M. Kinetic- and thermodynamic-based improvements of lithium borohydride incorporated into activated carbon. Acta Mater. 2008, 56, 6257–6263. [Google Scholar] [CrossRef]
- Ozawa, T. A New method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetic analysis of derivative curves in thermal analysis. J. Therm. Anal. 1970, 2, 301–324. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Bosenberg, U.; Gosalawit, R.; Dornheim, M.; Cerenius, Y.; Besenbacher, F.; Jensen, T.R. A Reversible nanoconfined chemical reaction. ACS Nano 2010, 4, 3903–3908. [Google Scholar] [CrossRef]
- Au, M.; Spencer, W.; Jurgensen, A.; Zeigler, C. Hydrogen storage properties of modified lithium borohydrides. J. Alloys Compd. 2008, 462, 1–2. [Google Scholar] [CrossRef]
- Faivre, C.; Bellet, D.; Dolino, G. Phase transitions of fluids confined in porous silicon: A differential calorimetry investigation. Eur. Phys. J. B 1999, 7, 19–36. [Google Scholar] [CrossRef]
- Petrov, O.; Furo, I. Curvature-dependent metastability of the solid phase and the freezing-melting hysteresis in pores. Phys. Rev. E 2006, 73, 011608. [Google Scholar] [CrossRef]
- Verdal, N.; Udovic, T.J.; Rush, J.J.; Liu, X.; Majzoub, E.H.; Vajo, J.J.; Gross, A.F. Dynamical perturbations of tetrahydroborate anions in LiBH4 due to nanoconfinement in controlled-pore carbon scaffolds. J. Phys. Chem. C 2013, 117, 17983–17995. [Google Scholar] [CrossRef]
- Ngene, P.; Nale, A.; Eggenhuisen, T.M.; Oschatz, M.; Embs, J.P.; Remhof, A.; de Jongh, P.E. Confinement effects for lithium borohydride: Comparing silica and carbon scaffolds. J. Phys. Chem. C 2017, 121, 4197–4205. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Zhang, W.; Ren, Z.; Huang, Z.; Hu, J.; Gao, M.; Pan, H.; Liu, Y. Nano-synergy enables highly reversible storage of 9.2 wt% hydrogen at mild conditions with lithium borohydride. Nano Energy 2021, 83, 105839. [Google Scholar] [CrossRef]
- Grochala, W.; Edwards, P.P. Thermal decomposition of the non-interstitial hydrides for the storage and production of hydrogen. Chem. Rev. 2004, 104, 1283–1316. [Google Scholar] [CrossRef] [PubMed]
- Zaluska, A.; Zaluski, L.; Ström-Olsen, J.O. Structure, catalysis and atomic reactions on the nano-scale: A systematic approach to metal hydrides for hydrogen storage. Appl. Phys. A 2001, 72, 783. [Google Scholar] [CrossRef]
- Imamura, H.; Masanari, K.; Kusuhara, M.; Katsumoto, H.; Sumi, T.; Sakata, Y. High hydrogen storage capacity of nanosized magnesium synthesized by high energy ball-milling. J. Alloys Compd. 2005, 386, 211–216. [Google Scholar] [CrossRef]
- Zaluski, L.; Zaluska, A.; Ström-Olsen, J.O. Nanocrystalline metal hydrides. J. Alloys Compd. 1997, 253, 70–79. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, H.; Ouyang, L.Z.; Zeng, M.Q. Composite structure and hydrogen storage properties in Mg-base alloys. Int. J. Hydrogen Energy 2006, 31, 251–257. [Google Scholar] [CrossRef]
- Wiswall, R. Hydrogen Storage in Metals. In Hydrogen Metals II; Springer: Berlin, Germany, 1978; Volume 29, pp. 201–242. [Google Scholar] [CrossRef]
- Fukai, Y. The Metal–Hydrogen System, Basic Bulk Properties; Springer Series in Materials Science; Springer: Berlin, Germany, 1993; ISBN 978-3-540-28883-1. [Google Scholar] [CrossRef]
- Bogdanovic, B.; Bohmhamme, K.; Christ, B.; Reiser, A.; Schlichte, K.; Vehlen, R.; Wolf, U. Thermodynamic investigation of the magnesium–hydrogen system. J. Alloys Compd. 1999, 282, 84–92. [Google Scholar] [CrossRef]
- Reule, H.; Hirscher, M.; Weißhardt, A.; Krönmuller, H. Hydrogen desorption properties of mechanically alloyed MgH2 composite materials. J. Alloys Compd. 2000, 305, 246–252. [Google Scholar] [CrossRef]
- Vajo, J.J.; Skeith, S.L.; Mertens, F. Reversible storage of hydrogen in destabilized LiBH4. J. Phys. Chem. B 2005, 109, 3719–3722. [Google Scholar] [CrossRef]
- Bosenberg, U.; Doppiu, S.; Mosegaard, L.; Barkhordarian, G.; Eigen, N.; Borgschulte, A.; Jensen, T.R.; Cerenius, Y.; Gutfleisch, O.; Klassen, T.; et al. Hydrogen sorption properties of MgH2–LiBH4 composites. Acta Mater. 2007, 55, 3951–3958. [Google Scholar] [CrossRef]
- Pinkerton, F.E.; Meyer, M.S.; Meisner, G.P.; Balogh, M.P.; Vajo, J.J. Phase boundaries and reversibility of LiBH4/MgH2 hydrogen storage material. J. Phys. Chem. C 2007, 111, 12881–12885. [Google Scholar] [CrossRef]
- Walker, G.S.; Grant, D.M.; Price, T.C.; Yu, X.; Legrand, V. High capacity multicomponent hydrogen storage materials: Investigation of the effect of stoichiometry and decomposition conditions on the cycling behaviour of LiBH4–MgH2. J. Power Sources 2009, 194, 1128–1134. [Google Scholar] [CrossRef]
- Bosenberg, U.; Kim, J.W.; Gosslar, D.; Eigen, N.; Jensen, T.R.; von Colbe, J.M.B.; Zhou, Y.; Dahms, M.; Kim, D.H.; Gunther, R.; et al. Role of additives in LiBH4–MgH2 reactive hydride composites for sorption kinetics. Acta Mater. 2010, 58, 3381–3389. [Google Scholar] [CrossRef]
- Wan, X.F.; Markmaitree, T.; Osborn, W.; Shaw, L.L. Nanoengineering-enabled solid-state hydrogen uptake and release in the LiBH4 Plus MgH2 System. J. Phys. Chem. C 2008, 112, 18232–18243. [Google Scholar] [CrossRef]
- Bosenberg, U.; Ravnsbæk, D.B.; Hagemann, H.; D’Anna, V.; Minella, C.B.; Pistidda, C.; van Beek, W.; Jensen, T.R.; Bormann, R.; Dornheim, M. Pressure and temperature influence on the desorption pathway of the LiBH4−MgH2 composite system. J. Phys. Chem. C 2010, 114, 15212–15217. [Google Scholar] [CrossRef]
- Price, T.E.C.; Grant, D.M.; Legrand, V.; Walker, G.S. Enhanced kinetics for the LiBH4:MgH2 multi-component hydrogen storage system—The effects of stoichiometry and decomposition environment on cycling behaviour. Int. J. Hydrogen Energy 2010, 35, 4154–4161. [Google Scholar] [CrossRef]
- Martelli, P.; Caputo, R.; Remhof, A.; Mauron, P.; Borgschulte, A.; Zuttel, A. Stability and decomposition of NaBH4. J. Phys. Chem. C 2010, 114, 7173–7177. [Google Scholar] [CrossRef]
- Ugale, A.D.; Ghodke, N.P.; Kang, G.S.; Nam, K.B.; Bhoraskar, S.V.; Mathe, V.L.; Yoo, J.B. Cost-effective synthesis of carbon loaded Co3O4 for controlled hydrogen generation via NaBH4 hydrolysis. Int. J. Hydrogen Energy 2022, 47, 16–29. [Google Scholar] [CrossRef]
- Kaya, S.; Yilmaz, Y.; Er, O.F.; Alpaslan, D.; Ulas, B.; Dudu, T.E.; Kivrak, H. Highly Active RuPd Bimetallic Catalysts for Sodium Borohydride Electrooxidation and Hydrolysis. J. Electron. Mater. 2022, 51, 403–411. [Google Scholar] [CrossRef]
- Yu, S.S.; Lee, T.H.; Oh, T.H. Ag-Ni nanoparticles supported on multiwalled carbon nanotubes as a cathode electrocatalyst for direct borohydride-hydrogen peroxide fuel cells. Fuel 2022, 315, 123151. [Google Scholar] [CrossRef]
- Oh, T.H. Effect of cathode conditions on performance of direct borohydride-hydrogen peroxide fuel cell system for space exploration. Renew. Energy 2021, 178, 1156–1164. [Google Scholar] [CrossRef]
- Schlesinger, H.J.; Brown, H.C.; Abraham, B.; Bond, A.C.; Davidson, N.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.; Horvitz, L.; Hyde, E.K.; et al. New Developments in the chemistry of diborane and the borohydrides. I. General SUmmary. J. Am. Chem. Soc. 1953, 75, 186–190. [Google Scholar] [CrossRef]
- Broja, G.; Schlabacher, W. Process for the Production of Alkali Metal Borohydrides. DE Patent DE 000001108670 A, 15 June 1961. [Google Scholar]
- Schubert, F.; Lang, K.; Schlabacher, W. Process for the Production of Borohydrides. DE Patent DE 000001067005 A, 15 October 1959. [Google Scholar]
- Haga, T.; Kojima, Y. Method for Manufacturing Metal Borohydride. JP Patent P 2002-241109, 28 August 2002. [Google Scholar]
- Kojima, Y.; Haga, T. Recycling process of sodium metaborate to sodium borohydride. Int. J. Hydrogen Energy 2003, 28, 989–993. [Google Scholar] [CrossRef]
- Li, Z.P.; Morigazaki, N.; Liu, B.H.; Suda, S. Preparation of sodium borohydride by the reaction of MgH2 with dehydrated borax through ball milling at room temperature. J. Alloys Compd. 2003, 349, 232–236. [Google Scholar] [CrossRef]
- Ozolins, V.; Majzoub, E.H.; Wolverton, C. First-principles prediction of a ground state crystal structure of magnesium borohydride. Phys. Rev. Lett. 2008, 100, 135501. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, M.; Zhao-Karger, Z.; Hu, J.; Roth, A.; Weidler, P. The kinetic properties of Mg(BH4)2 infiltrated in activated carbon. Nanotechnology 2009, 20, 204029. [Google Scholar] [CrossRef]
- Sartori, S.; Knudsen, K.D.; Zhao-Karger, Z.; Bardaij, E.G.; Fichtner, M.; Hauback, B.C. Small-angle scattering investigations of Mg-borohydride infiltrated in activated carbon. Nanotechnology 2009, 20, 505702. [Google Scholar] [CrossRef]
- George, L.; Saxena, S.K. Structural stability of metal hydrides, alanates and borohydrides of alkali and alkali-earth elements: A review. Int. J. Hydrogen Energy 2010, 35, 5454–5470. [Google Scholar] [CrossRef]
- Matsunaga, T.; Buchter, F.; Mauron, P.; Bielman, A.; Nakamori, Y.; Orimo, S.; Ohba, N.; Miwa, K.; Towata, S.; Zuttel, A. Hydrogen storage properties of Mg[BH4]2. J. Alloys Compd. 2008, 459, 583–588. [Google Scholar] [CrossRef]
- Severa, G.; Ronnebro, E.; Jensen, C.M. Direct hydrogenation of magnesium boride to magnesium borohydride: Demonstration of >11 weight percent reversible hydrogenstorage. Chem. Commun. 2010, 46, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.; Kikuchi, K.; Nakamori, Y.; Ohba, N.; Miwa, K.; Towata, S.; Orimo, S. Dehydriding and rehydriding processes of well-crystallized Mg(BH4)2 accompanying with formation of intermediate compounds. Acta Mater. 2008, 56, 1342–1347. [Google Scholar] [CrossRef]
- Van Setten, M.J.; de Wijs, G.A.; Fichtner, M.; Brocks, G. A Density Functional Study of α-Mg(BH4)2. Chem. Mater. 2008, 20, 4952–4956. [Google Scholar] [CrossRef]
- Cansizoglu, M.F.; Karabacak, T. Enhanced hydrogen storage properties of magnesium nanotrees with nanoleaves. Mat. Res. Soc. Symp. Proc. 2009, 1216, 503. [Google Scholar] [CrossRef]
- Bayca, S.U.; Cansizoglu, M.F.; Biris, A.S.; Watanabe, F.; Karabacak, T. Enhanced oxidation resistance of magnesium nanorods grown by glancing angle deposition. Int. J. Hydrogen Energy 2011, 36, 5998–6004. [Google Scholar] [CrossRef]
- Zavorotynska, O.; Deledda, S.; Vitillo, J.; Saldan, I.; Guzik, M.; Baricco, M.; Walmsley, J.C.; Muller, J.; Hauback, B.C. Combined X-ray and raman studies on the effect of cobalt additives on the decomposition of magnesium borohydride. Energies 2015, 8, 9173–9190. [Google Scholar] [CrossRef]
- Zavorotynska, O.; Saldan, I.; Hino, S.; Humphries, T.D.; Deledda, S.; Hauback, B.C. Hydrogen cycling in γ-Mg(BH4)2 with cobalt-based additives. J. Mater. Chem. A 2015, 3, 6592–6602. [Google Scholar] [CrossRef]
- Liang, J.J. Theoretical insight on tailoring energetics of Mg hydrogen absorption/desorption through nano-engineering. Appl. Phys. A Mater. Sci. Process. 2005, 80, 173–178. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jin, S.-A.; Shim, J.-H.; Cho, Y.W. Reversible hydrogen storage in calcium borohydride Ca(BH4)2. Scr. Mater. 2008, 58, 481–483. [Google Scholar] [CrossRef]
- Aoki, M.; Miwa, K.; Noritake, T.; Ohba, N.; Matsumoto, M.; Li, H.W.; Nakamori, Y.; Towata, S.; Orimo, S. Structural and dehydriding properties of Ca(BH4)2. Appl. Phys. A 2008, 92, 601–605. [Google Scholar] [CrossRef]
- Kim, J.H.; Jin, S.A.; Shim, J.H.; Cho, Y.W. Thermal decomposition behavior of calcium borohydride Ca(BH4)2. J. Alloys Compd. 2008, 461, L20–L22. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, J.H.; Cho, Y.W. On the reversibility of hydrogen storage in Ti- and Nb-catalyzed Ca(BH4)2. J. Power Sources 2008, 181, 140–143. [Google Scholar] [CrossRef]
- Manchester, F.D.; San-Martin, A.; Pitre, J.M. The H-Pd (hydrogen-palladium) System. J. Phase Equilibria JPE 1994, 15, 62–83. [Google Scholar] [CrossRef]
- Parry, R.W.; Schultz, D.R.; Girardot, P.R. The Preparation and properties of hexamminecobalt(III) borohydride, hexamminechromium(III) borohydride and ammonium borhydride. J. Am. Chem. Soc. 1958, 80, 1–3. [Google Scholar] [CrossRef]
- Crabtree, R.H. A New Type of Hydrogen Bond. Science 1998, 282, 2000–2001. [Google Scholar] [CrossRef]
- Sit, V.; Geanangel, R.A.; Wendlandt, W.W. The thermal dissociation of NH3BH3. Thermochim. Acta 1987, 113, 379–382. [Google Scholar] [CrossRef]
- Li, L.; Yao, X.; Sun, C.; Du, A.; Cheng, L.; Zhu, Z.; Yu, C.; Zou, J.; Smith, S.C.; Wang, P.; et al. Lithium-catalyzed dehydrogenation of ammonia borane within mesoporous carbon framework for chemical hydrogen storage. Adv. Funct. Mater. 2009, 19, 265–271. [Google Scholar] [CrossRef]
- Sepehri, S.; Feaver, A.; Shaw, W.J.; Howard, C.J.; Zhang, Q.; Autrey, T.; Cao, G. Spectroscopic studies of dehydrogenation of ammonia borane in carbon cryogel. J. Phys. Chem. B 2007, 111, 14285–14289. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Karkamkar, A.; Autrey, T.; Chupas, P.; Proffen, T. Determination of structure and phase transition of light element nanocomposites in mesoporous silica: Case study of NH3BH3 in MCM-41. J. Am. Chem. Soc. 2009, 131, 13749–13755. [Google Scholar] [CrossRef] [PubMed]
- Paolone, A.; Palumbo, O.; Rispoli, P.; Cantelli, R.; Autrey, T.; Karkamkar, A. Absence of the structural phase transition in ammonia borane dispersed in mesoporous silica: Evidence of novel thermodynamic properties. J. Phys. Chem. C 2009, 113, 10319–10321. [Google Scholar] [CrossRef]
- Wolf, G.; Baumann, J.; Baitalow, F.; Hoffmann, F.P. Calorimetric process monitoring of thermal decomposition of B–N–H compounds. Thermochim. Acta 2000, 343, 19–25. [Google Scholar] [CrossRef]
- Lai, S.-W.; Lin, H.-L.; Yu, T.L.; Lee, L.-P.; Weng, B.-J. Hydrogen release from ammonia borane embedded in mesoporous silica scaffolds: SBA-15 and MCM-41. Int. J. Hydrogen Energy 2012, 37, 14393–14404. [Google Scholar] [CrossRef]
- Feaver, A.; Sepehri, S.; Shamberger, P.; Stowe, A.; Autrey, T.; Cao, G. Coherent carbon cryogel−ammonia borane nanocomposites for H2 storage. J. Phys. Chem. B 2007, 111, 7469–7472. [Google Scholar] [CrossRef] [PubMed]
- Moussa, G.; Bernard, S.; Demirci, U.B.; Chiriac, R.; Miele, P. Room-temperature hydrogen release from activated carbon-confined ammonia borane. Int. J. Hydrogen Energy 2012, 37, 13437–13445. [Google Scholar] [CrossRef]
- Srinivas, G.; Travis, W.; Ford, J.; Wu, H.; Guo, Z.-X.; Yildirim, T. Nanoconfined ammonia borane in a flexible metal–organic framework Fe–MIL-53: Clean hydrogen release with fast kinetics. J. Mater. Chem. A 2013, 1, 4167–4172. [Google Scholar] [CrossRef]
- Chung, J.-Y.; Liao, C.-W.; Chang, Y.-W.; Chang, B.K.; Wang, H.; Li, J.; Wang, C.-Y. Influence of metal–organic framework porosity on hydrogen generation from nanoconfined ammonia borane. J. Phys. Chem. C 2017, 121, 27369–27378. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Raju, B.C.; Gagare, P.D. One-pot synthesis of ammonia-borane and trialkylamine-boranes from trimethyl borate. Org. Lett. 2012, 14, 6119–6121. [Google Scholar] [CrossRef] [PubMed]
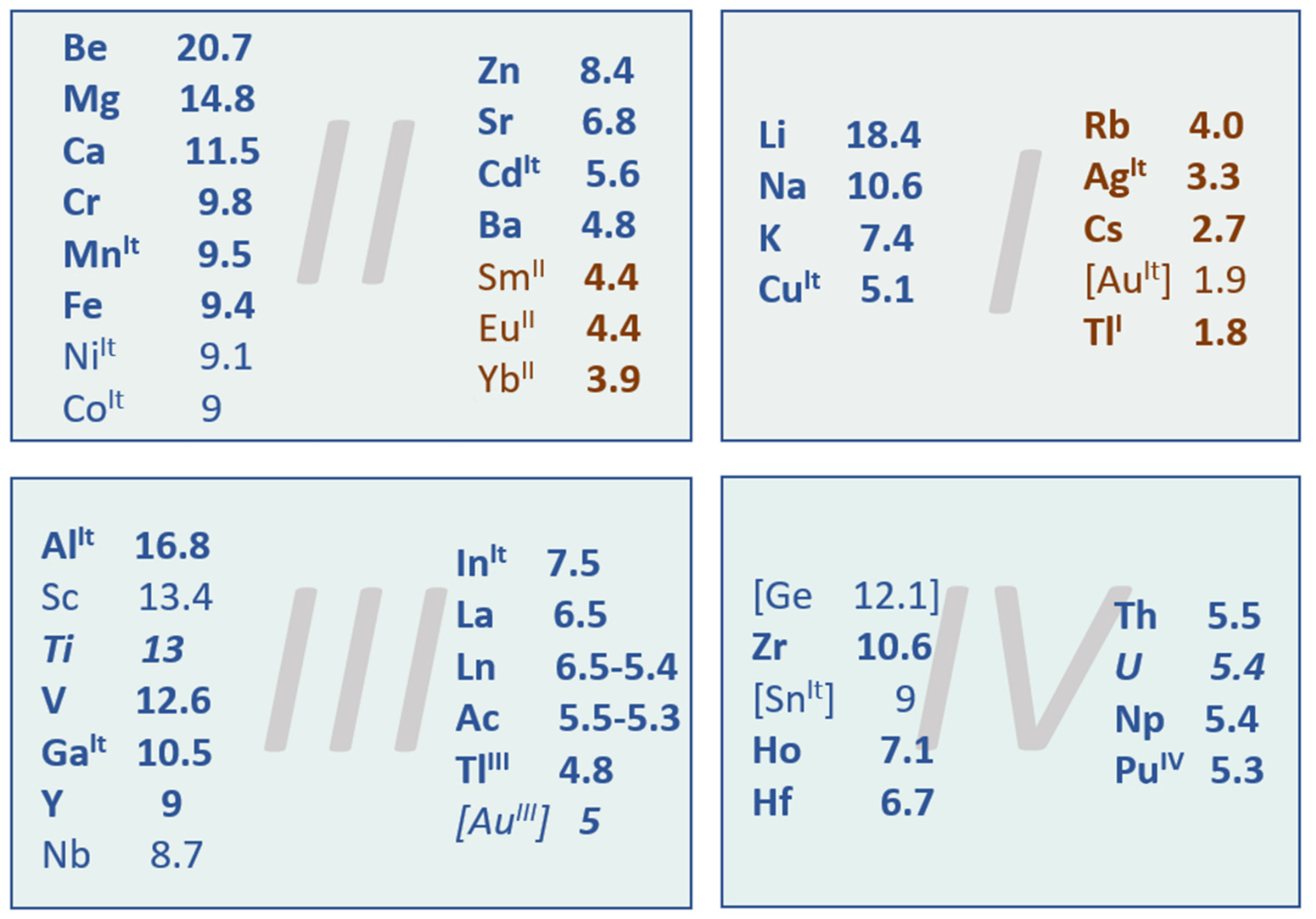
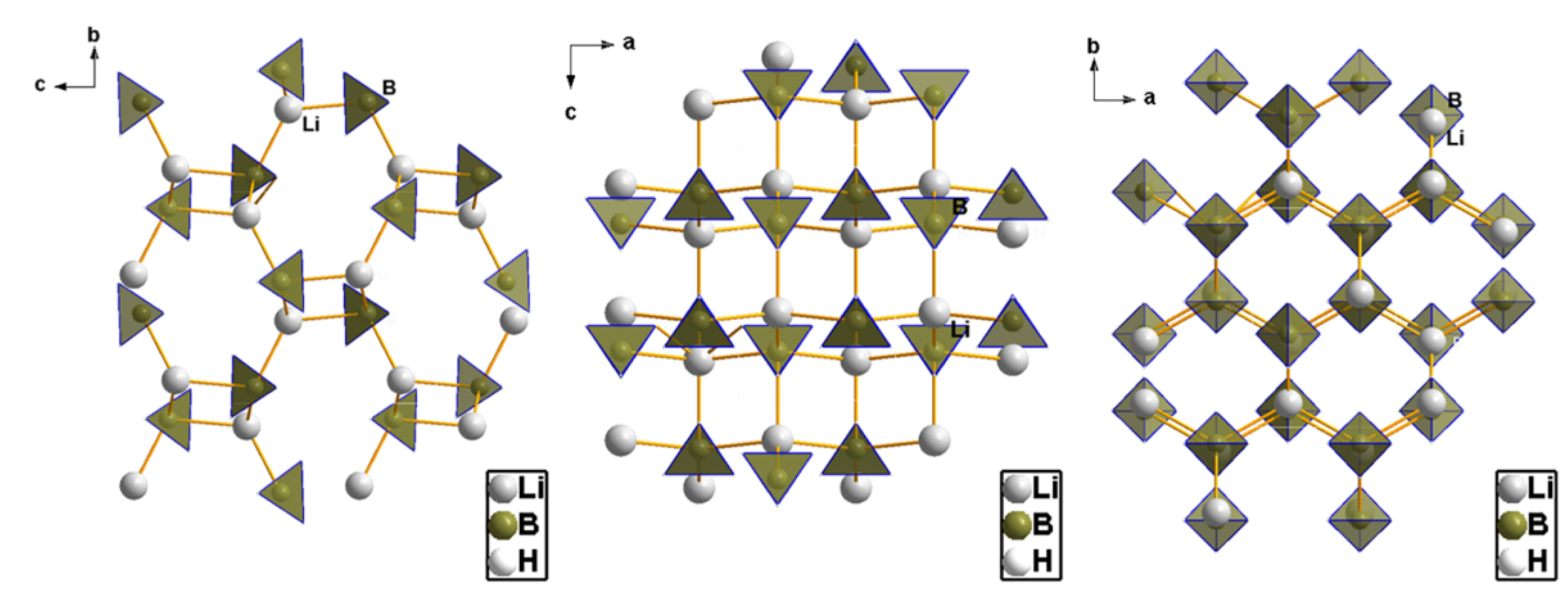
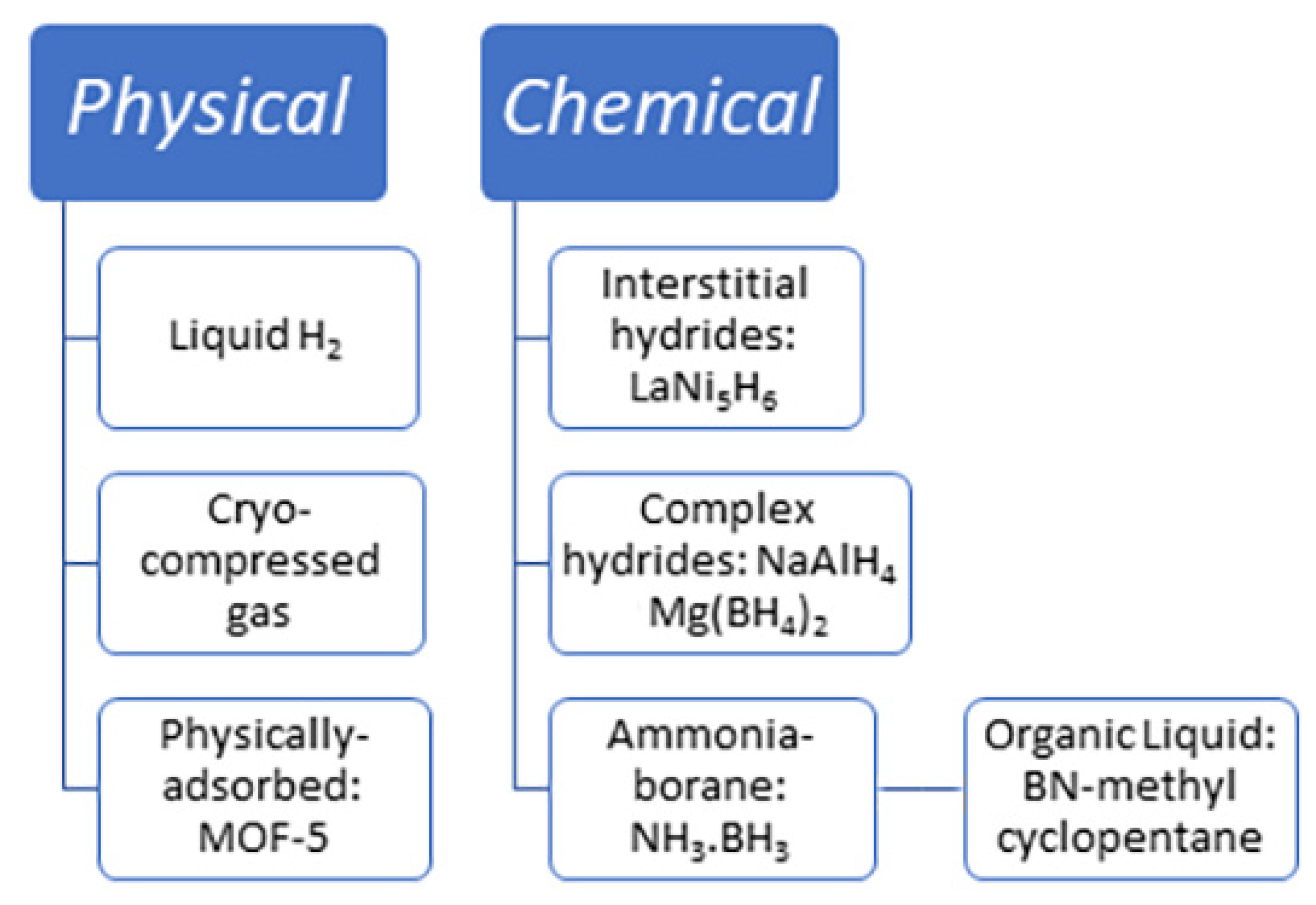
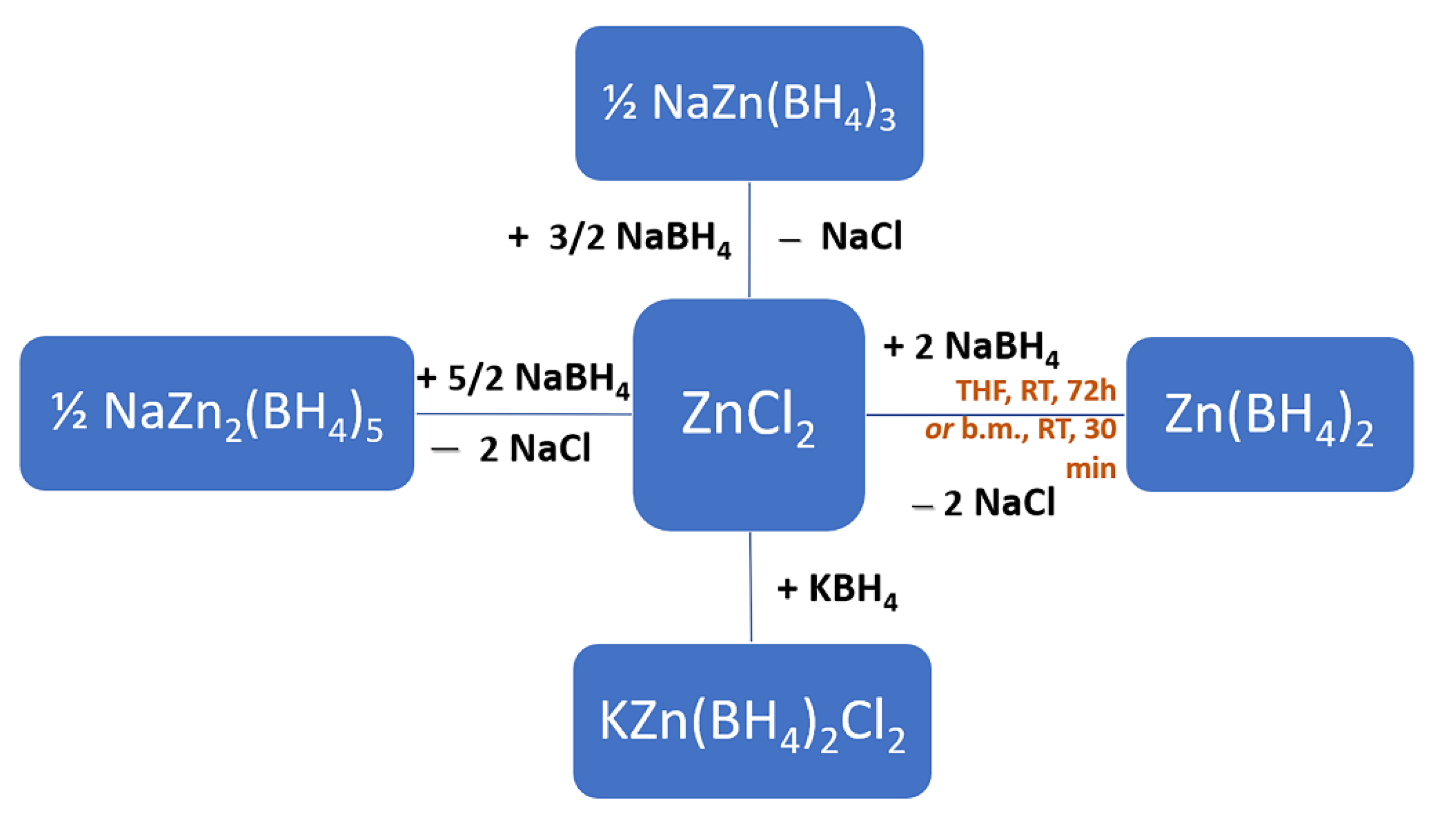

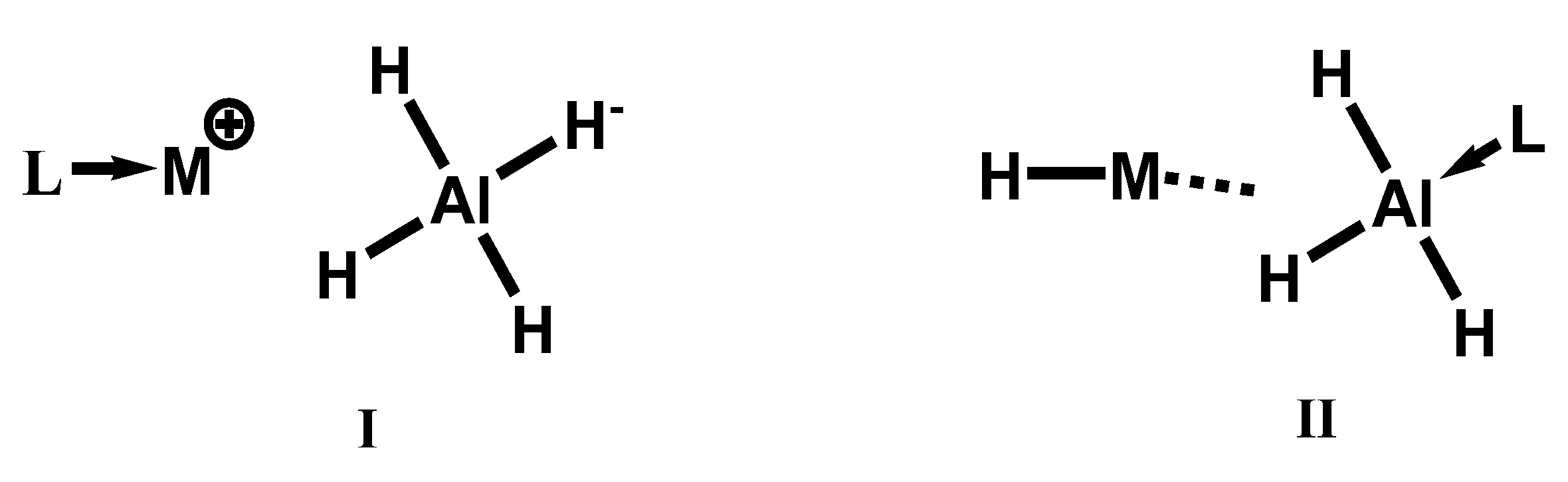
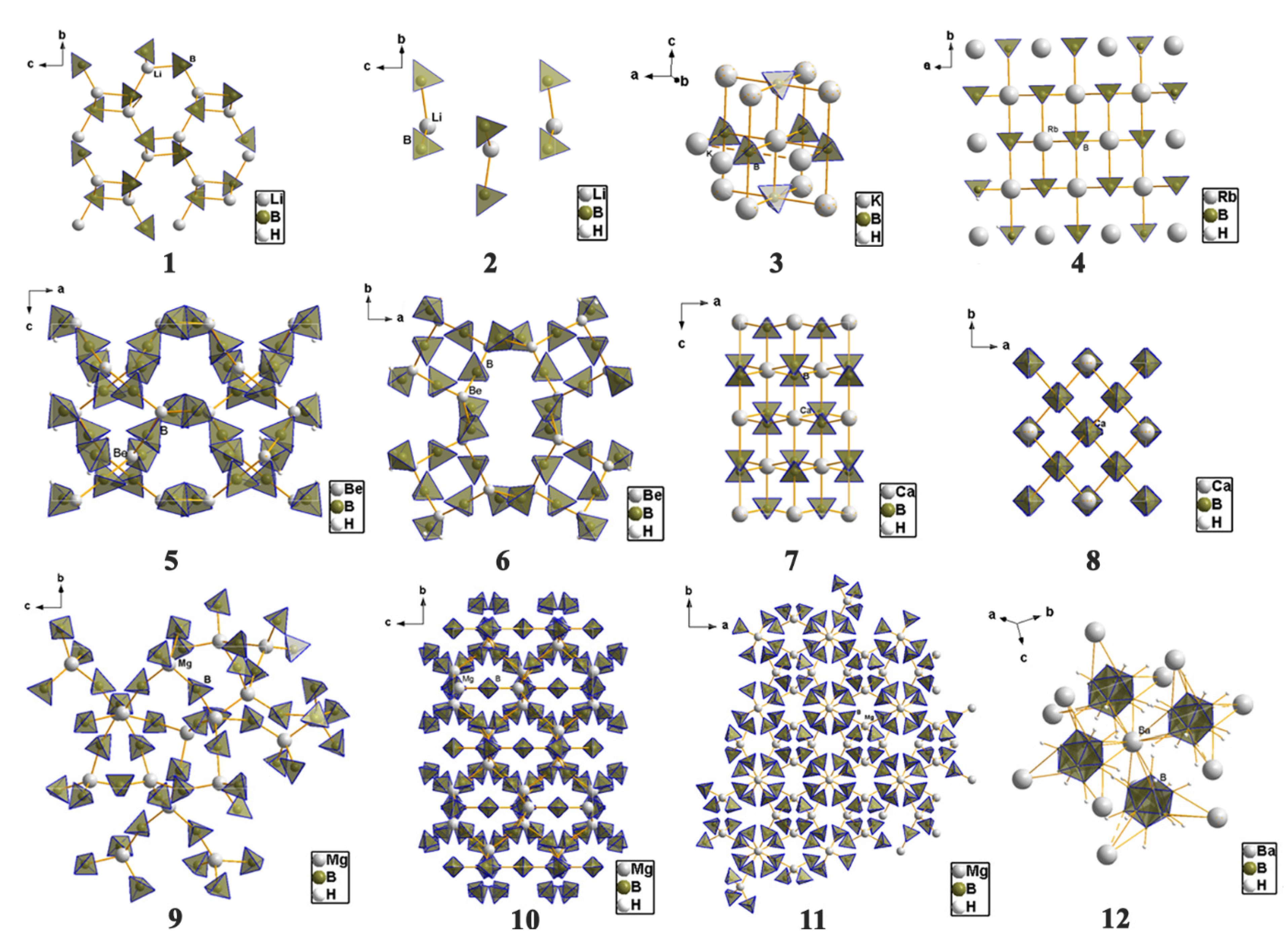
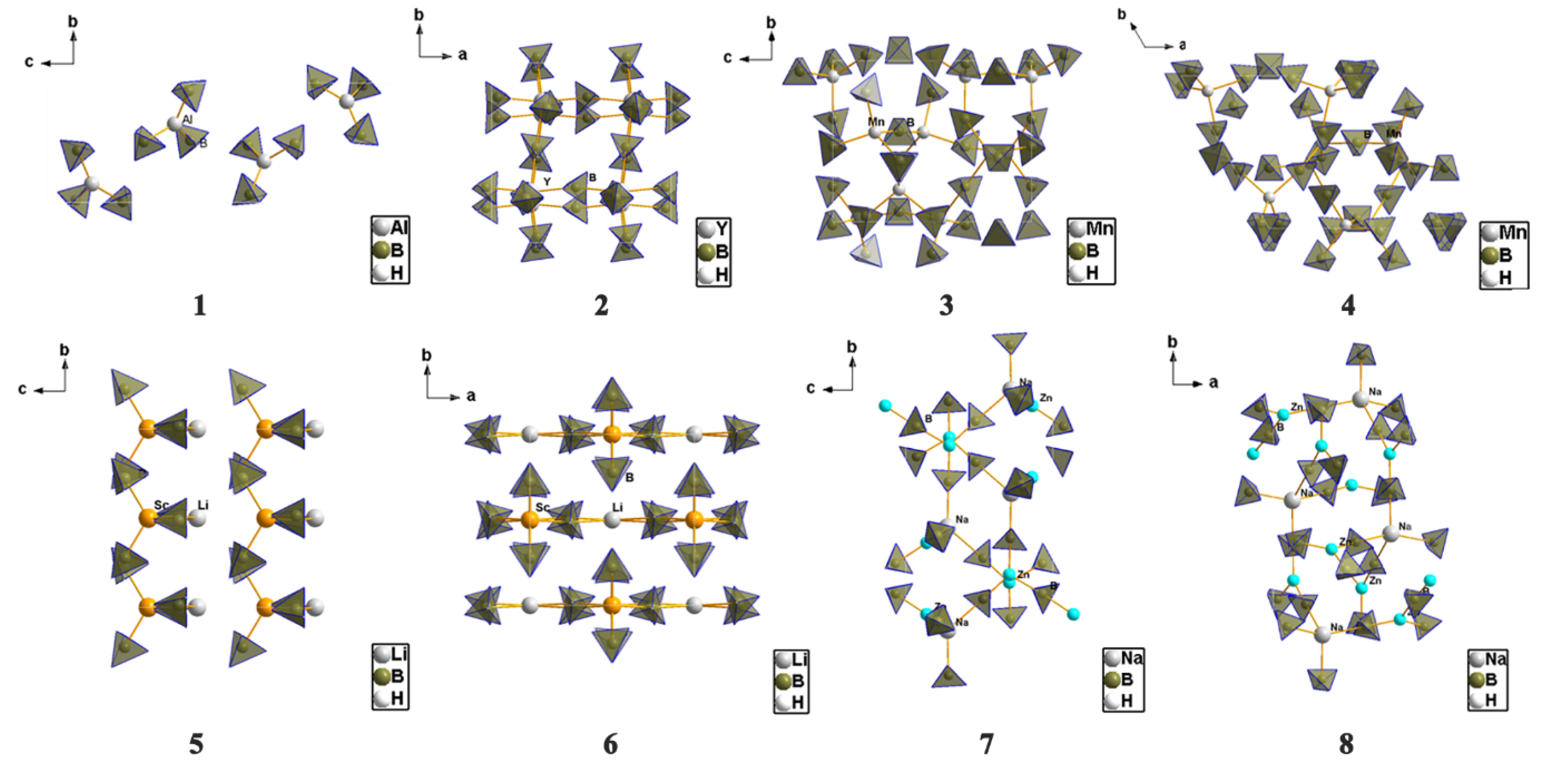
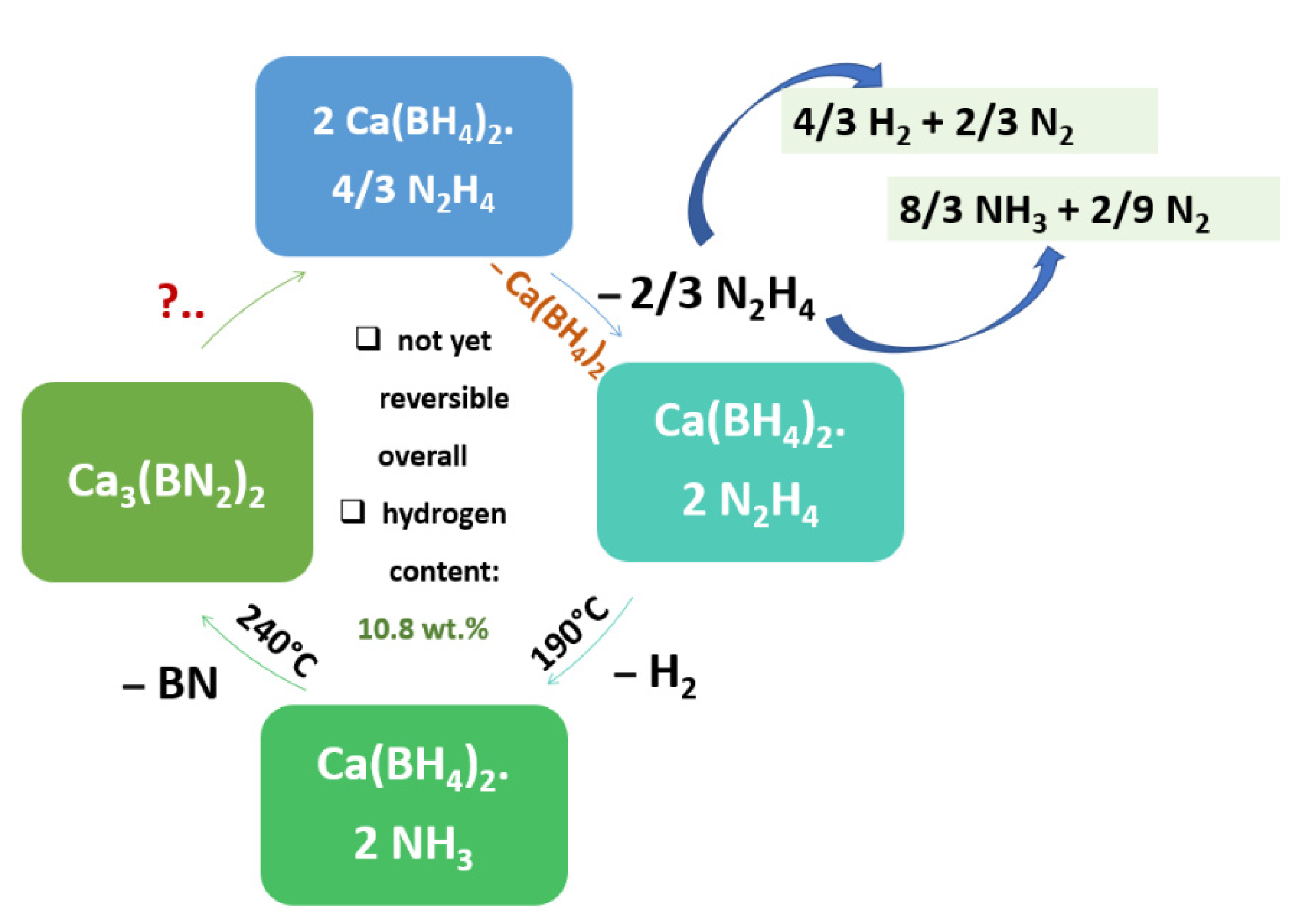
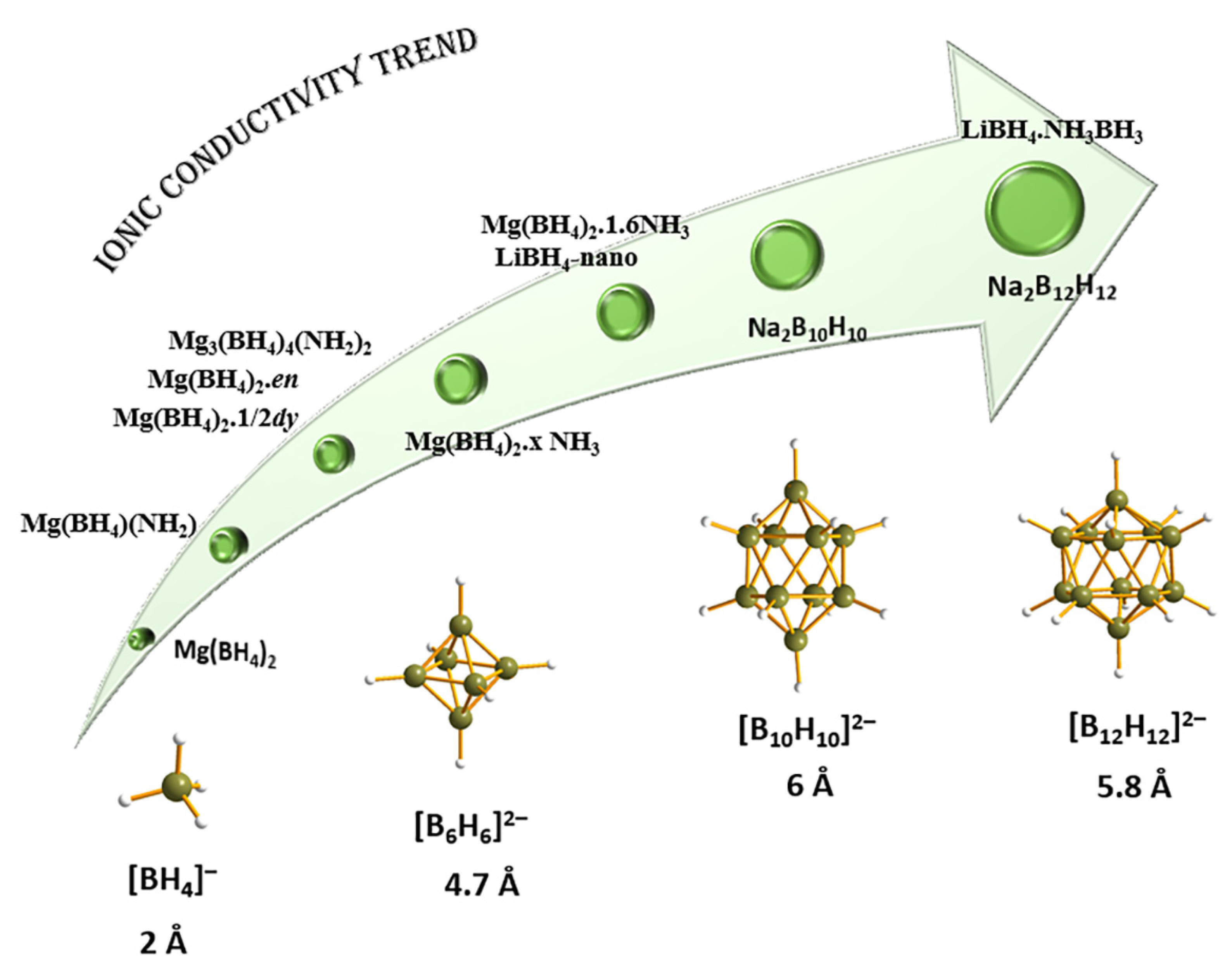
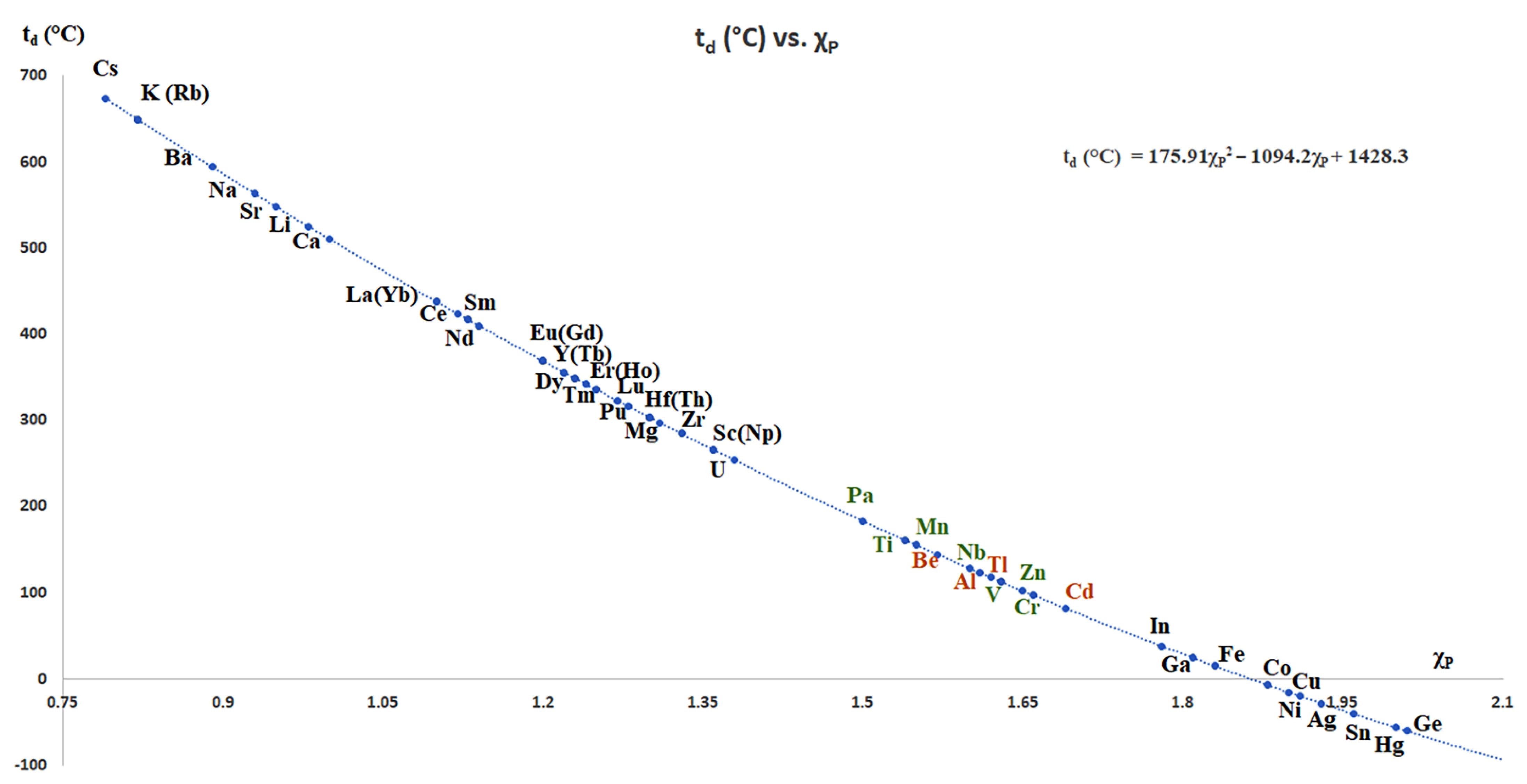

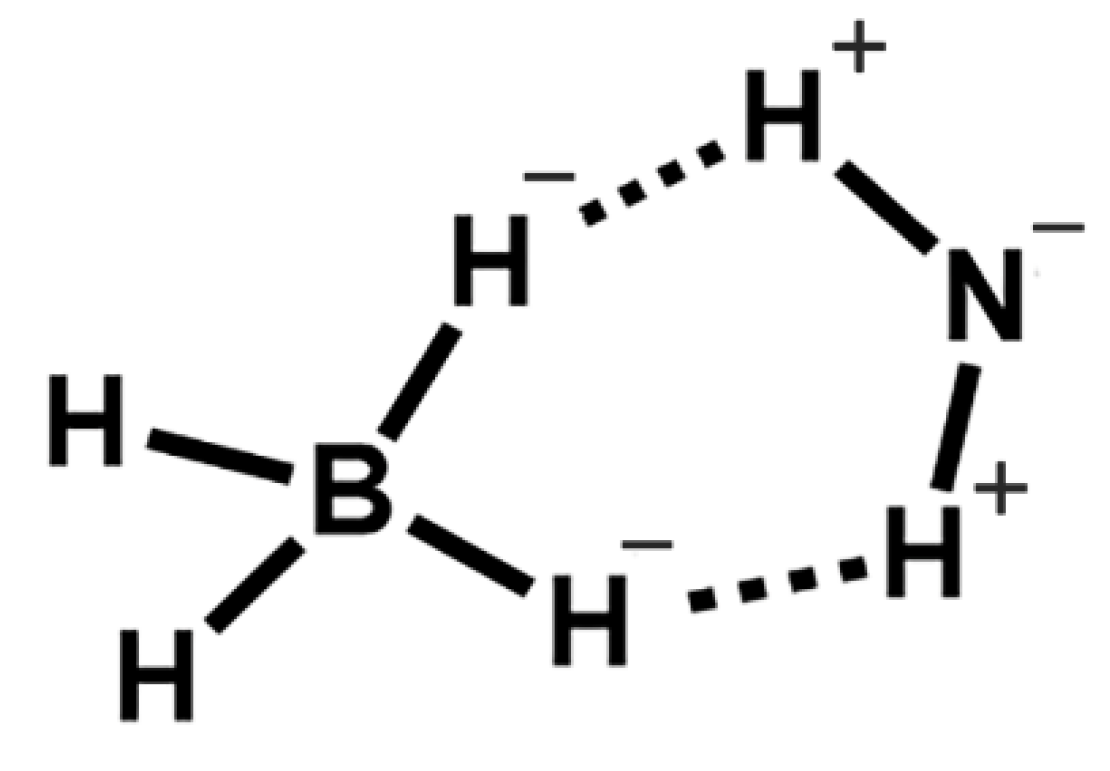
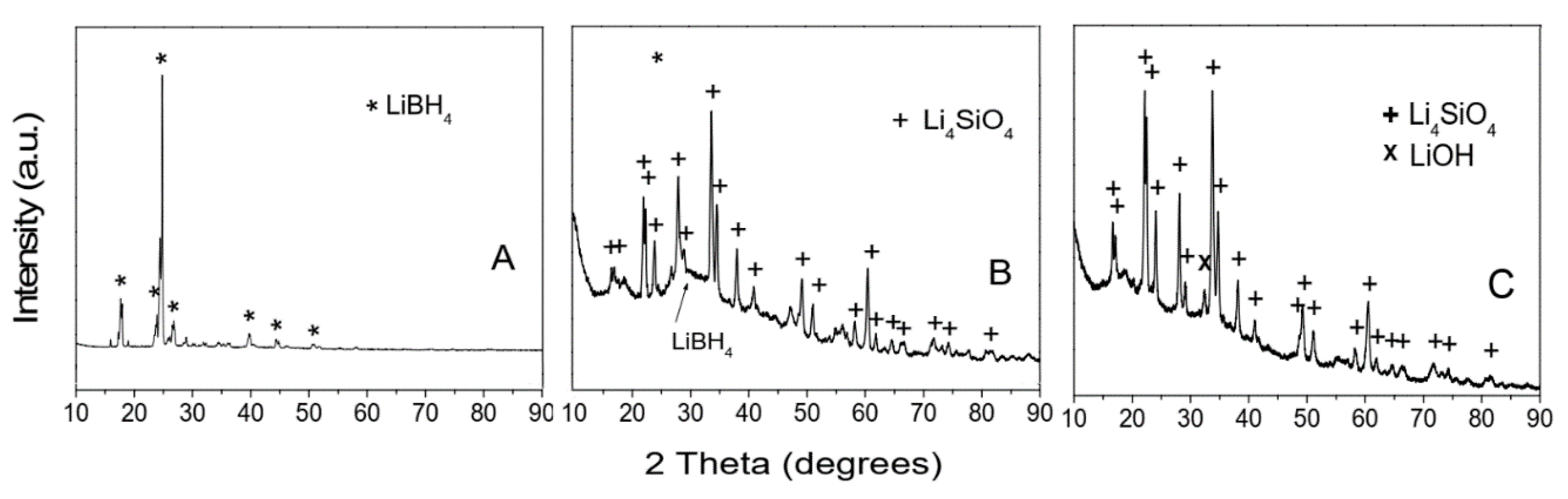
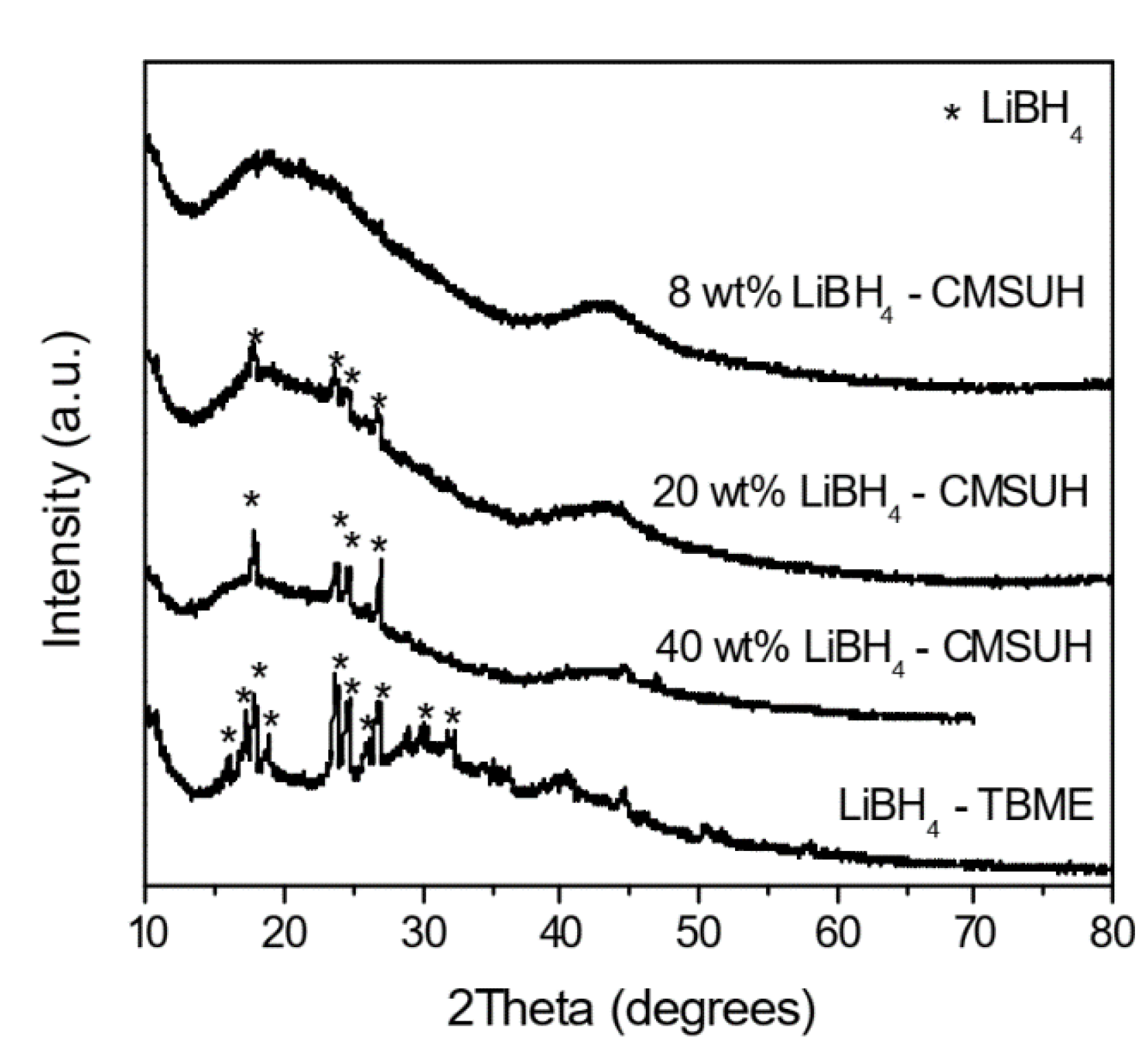
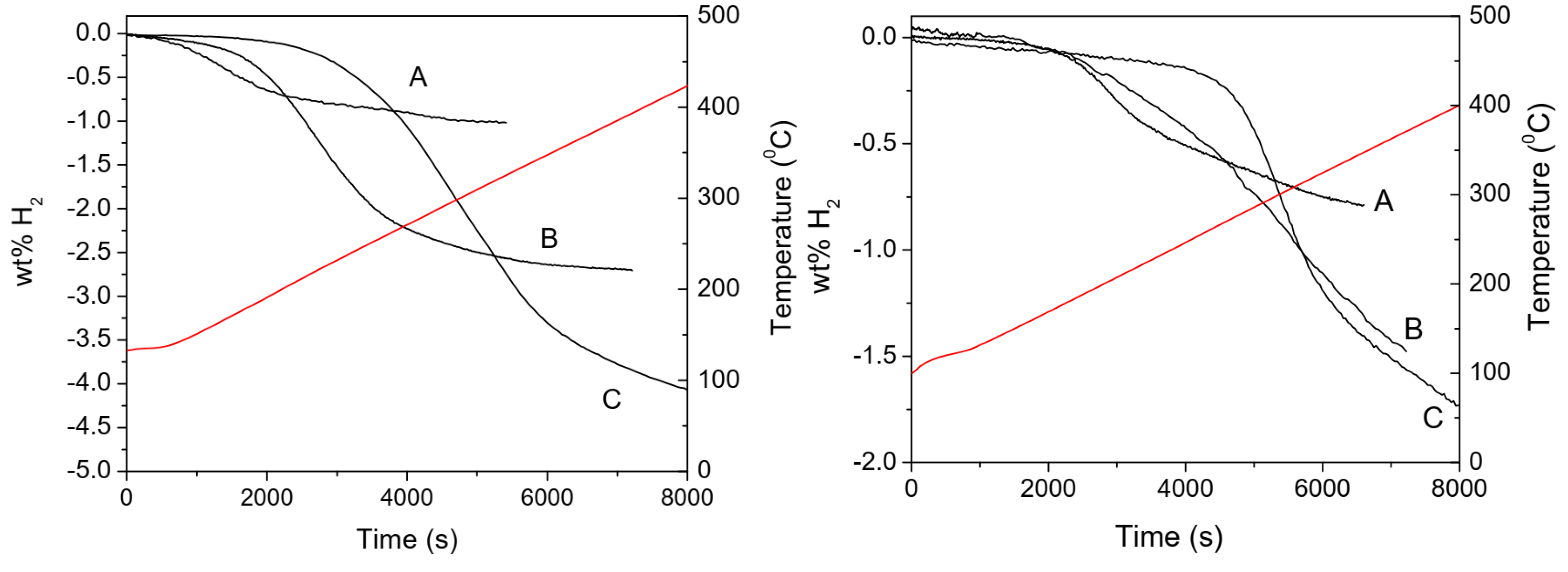
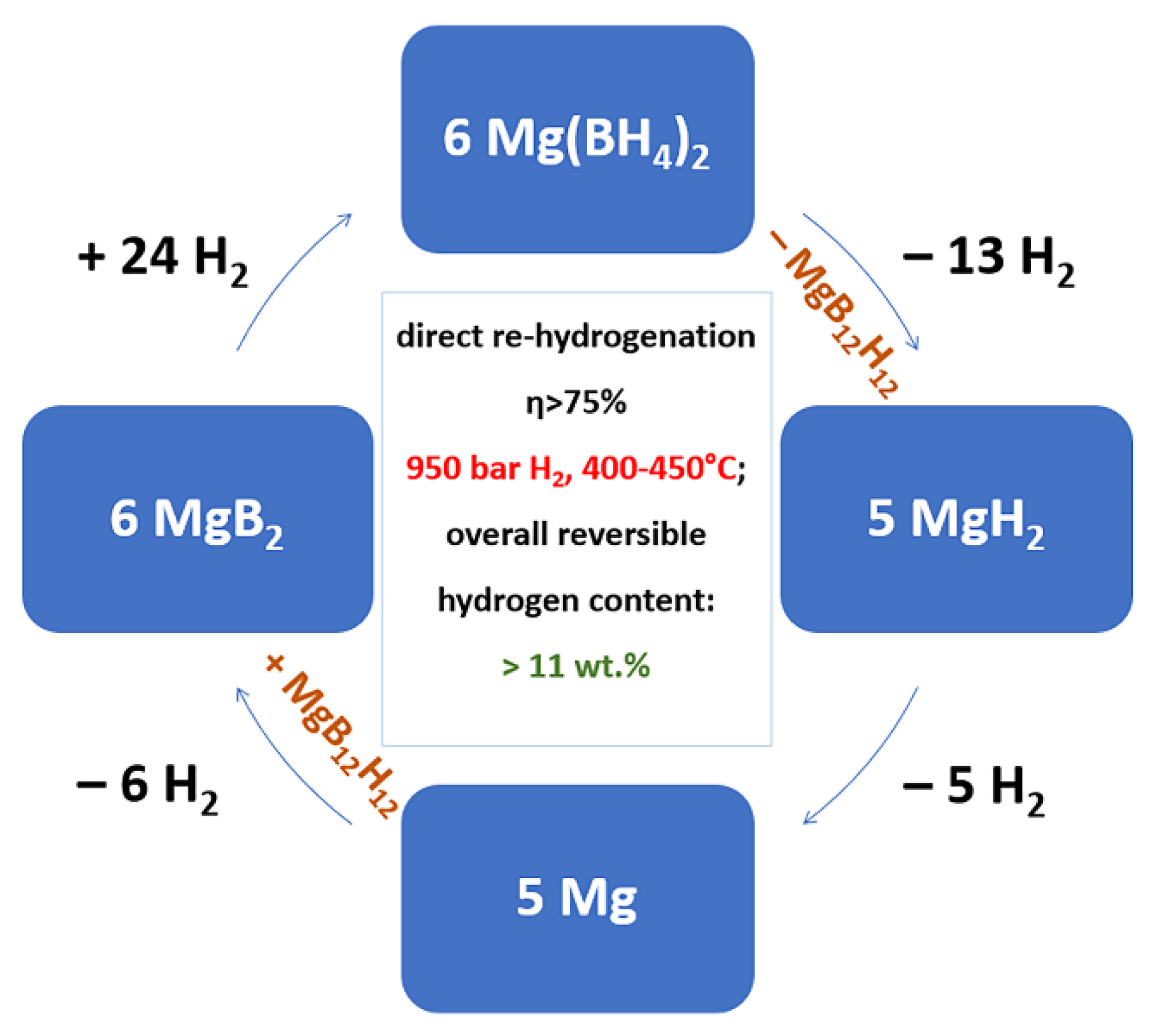
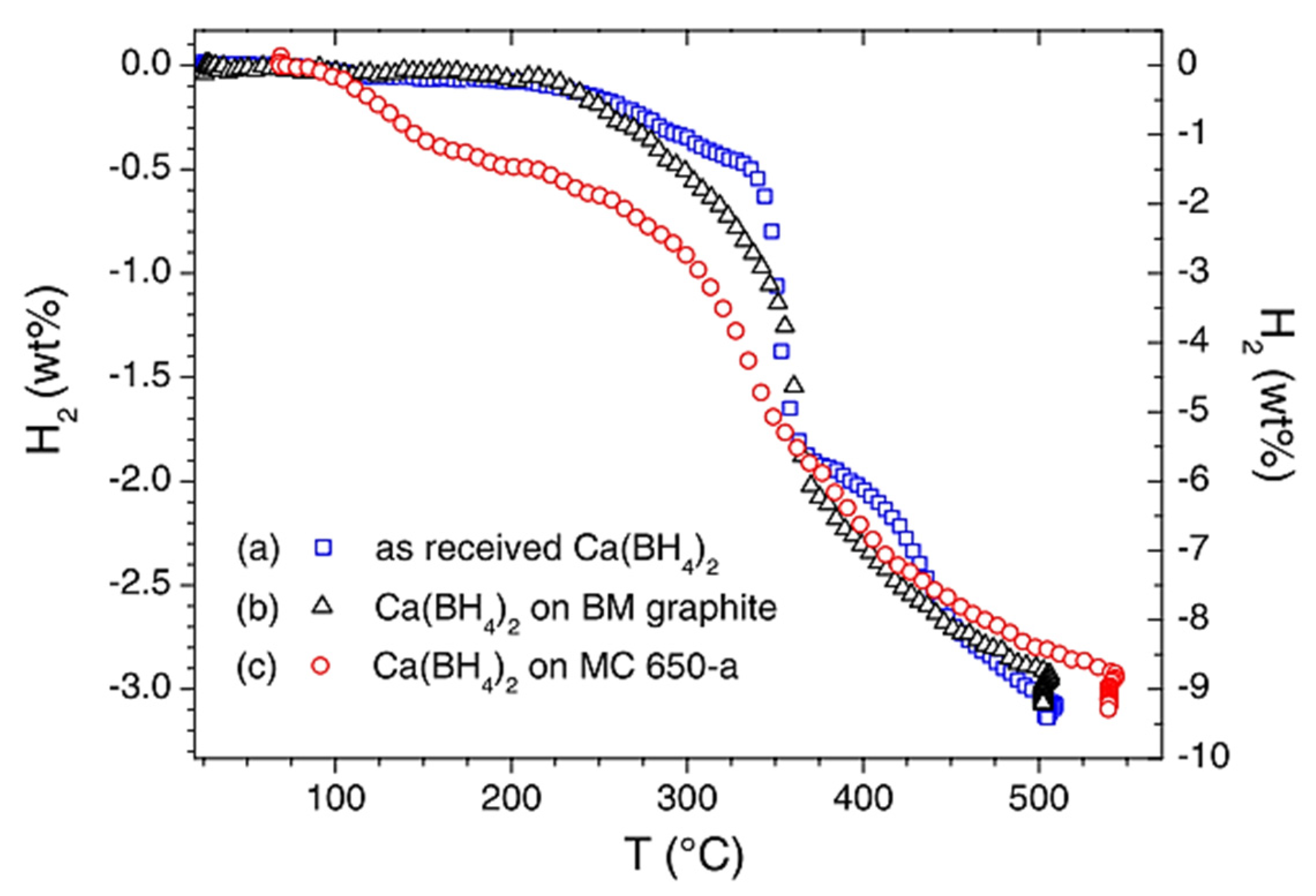
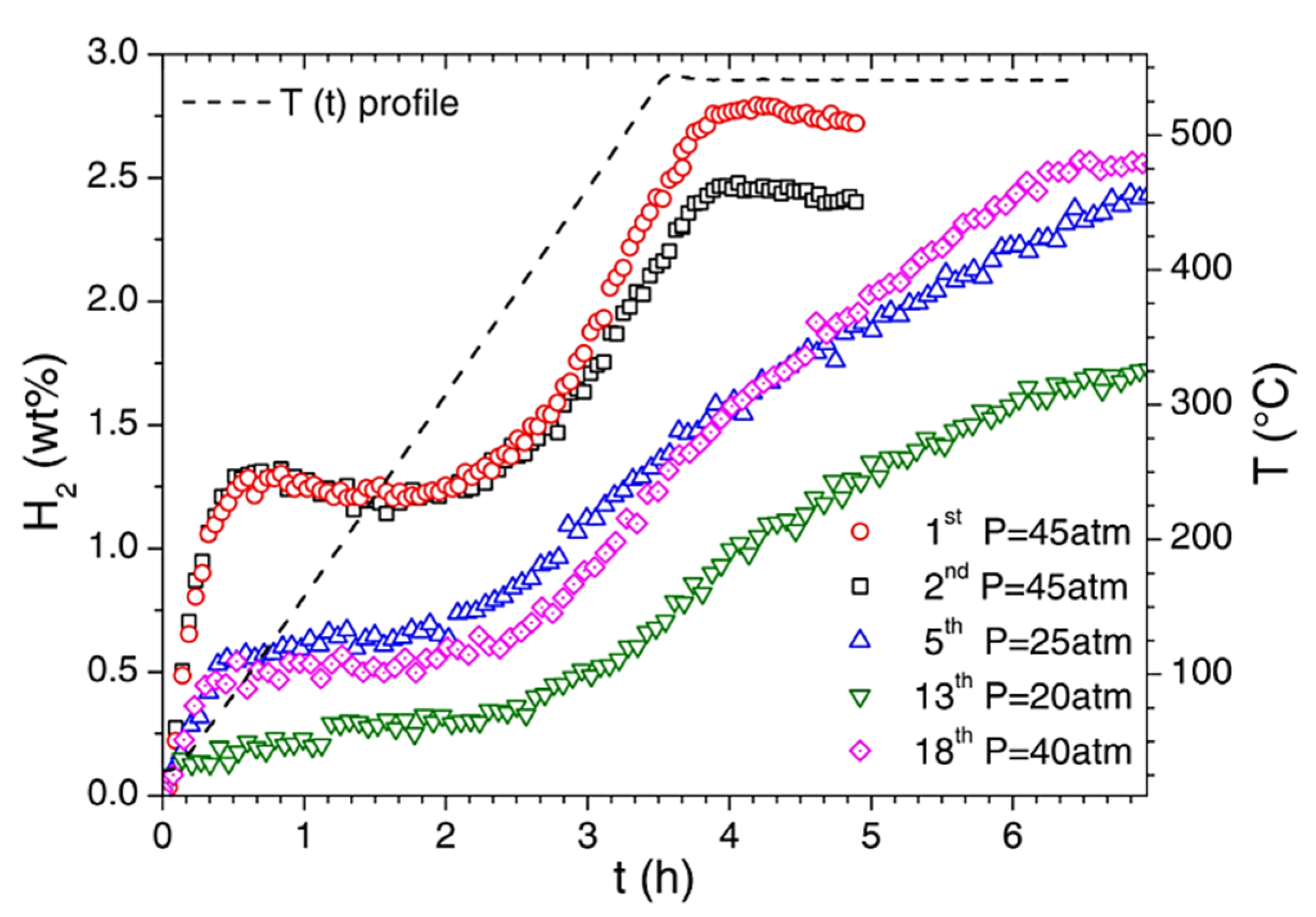
| Metal Borohydride | Decomposition Temperature (°C) | Rehydrogenation Temperature (°C) | Space Group | Crystal System | Reference |
|---|---|---|---|---|---|
| o-LiBH4 | (mp = 277); | 600 | Pnma | Orthorhombic | [30,31,32,33,34,35,36,37,38,164] |
| h-LiBH4 | (107) | 600 | P63mc | Hexagonal | [30,31,32,33,34,35,36,37,38] |
| α-NaBH4 | 535 | 270–400 (2NaH/ MgH2) | Cubic | [165,166,167,168,169] | |
| Be(BH4)2 | (mp = 91.3) td = 123 | Explosive decomp. in air/moisture | I41/cd | Tetragonal; helical polymeric chains BeH2BH2BeH2BH2 and terminal bidentate BH4 groups | [170] |
| α-Mg(BH4)2 (6 known polymorphs) | ~300 | (390 °C, 90-bar H2, 72 h) (400 °C, 80-bar D2) RT stable | P6122 | Hexagonal | [106,171] |
| β-Mg(BH4)2 | ~300 | HT polymorph; RT metastable | Fddd | Orthorhombic | [172,173] |
| γ-Mg(BH4)2 | ~300 | RT metastable | Ia3d | Cubic; 3D network of interpenetrated channels (1st borohydride with permanent porosity, S = 1505 m2/g) | [158] |
| α-Ca(BH4)2 (4 known polymorphs) | 347–387 °C, 397–497 °C (2-step process) | RT stable | F2dd | Orthorhombic | [146,147,148,149,150,151] |
| β-Ca(BH4)2 | β-Ca(BH4)2 | RT, metastable, HT polymorph | Tetragonal | [146,147,148,149,150,151,157] | |
| α-Al(BH4)3 | <100 | −123 | C2/c | Monoclinic | [83,84,85,86,87,88] |
| β-Al(BH4)3 | <100 | −78 | Pna21 | Orthorhombic | [83,84,85,86,87,88] |
| α-Y(BH4)3 (β-Y(BH4)3 is the HT polymorph) | 190, 270 | 180 °C (α-to-β phase transition) | Cubic | [67,68,69,70,71,72,73] | |
| α-Mn(BH4)2 (4 known polymorphs) | 130 | RT stable | P3112 | Hexagonal | [174,175,176,177,178,179,180] |
| LiSc(BH4)4 | 400 | (400), irreversible hydrogen storage in Li-Sc-B-H system | 2c | Tetragonal | [181,182,183] |
| NaZn(BH4)3 | >85 °C (at 110 °C NaBH4 reacts with Na2ZnCl4 to form Zn and NaCl) | stable RT | P21c | Monoclinic | [41,184] |
| NaZn2(BH4)5 | unstable, converts at low temp. to NaZn(BH4)3 | unstable at RT or −32 °C | P21c | Monoclinic | [41,184] |
| Compound | Experimental Conditions/Observations | Reference | |
|---|---|---|---|
| LiBH4 | 2 ∙ 10−3 | 107 °C | [261] |
| LiBH4·H2O | 4.89 ∙ 10−4 | t = 45 °C | [264] |
| LiBH4·x NH3 | 2.21 ∙ 10−3 | ; | [265,266] |
| LiBH4·x NH3@Li2O | 5.4 ∙ 10−4 | 0.67 < x < 0.8; 20 °C; max for 78 wt.% Li2O | [267] |
| LiBH4·x NH3BH3 | 1.47 ∙ 10−5–4.04 ∙ 10−4 | ½ < x < 1; highest for x = 1 | [268] |
| LiBH4·NH3BH3 | 10−1 | t = 55 °C; record value for Li+ | [263] |
| Li2B12H12 | 10−1 | 110 °C | [257] |
| Li2B10H10 | 3 ∙ 10−2 | 81 °C | [258] |
| Na2B10H10 | 3 ∙ 10−2 | RT | [258] |
| Na2B12H12 | 10−1 | 256 °C | [259] |
| LiBH4-nano | 10−3 | RT; nanoconfined electrolyte | [260] |
| Mg(BH4)2 | <10−12 | t = 30 °C | [269] |
| Mg(BH4)(NH2) | 10−6 | t = 150 °C | [270] |
| Mg3(BH4)4(NH2)2 | 4.1 ∙ 10−5 | 100 °C | [271] |
| Mg(BH4)2·en en = NH2CH2CH2NH2 | 6 ∙ 10−5 | 70 °C, crucial role of [BH4−], Mg(en)2X2 showed σ very low | [268,272] |
| Mg(BH4)2·1/2dy dy = diglyme | 2 ∙ 10−5 | 80 °C; chelating of flexible dy ligand | [273] |
| Mg(BH4)2·x NH3 | 3.3 ∙ 10−4 | x = 1,2,3 6; t = 80 °C; pas-de-deux mechanism proposed | [274] |
| Mg(BH4)2·1.6NH3 | 2.2 ∙ 10−3 | t = 55 °C | [275] |
| Metal (M) | M(BH4)x | td [°C] | Reference | |||
|---|---|---|---|---|---|---|
| Li | LiBH4 | 0.98 | 798.1 | 524.95 | −147.074 | [30,31,32,33,34,35,36,37,38,196,199] |
| Na | NaBH4 | 0.93 | 836.01 | 562.86 | −159.509 | [165,168,169] |
| K | KBH4 | 0.82 | 922.51 | 649.36 | −186.866 | [165,166,168] |
| Cu | CuBH4 | 1.9 | 257.56 | −15.59 | 81.73 | [113] forms at 253 K, dec.at 261–273 K |
| Rb | RbBH4 | 0.82 | 922.51 | 649.36 | −186.866 | [167] |
| Ag | AgBH4 | 1.93 | 244.94 | −28.21 | 89.191 | [114]; forms at 193 K, dec. at 243 K |
| Cs | CsBH4 | 0.79 | 946.84 | 673.69 | −194.327 | [165] |
| AuI | AuBH4 | 2.54 | 57.15 | −216 | 240.898 | [115] * unstable |
| AuIII | Au(BH4)3 | 2.54 | 57.15 | −216 | 240.898 | [116] |
| Be | Be(BH4)2 | 1.57 | 417.2 | 144.05 | −0.341 | [170] |
| Mg | Mg(BH4)2 | 1.31 | 569.96 | 296.81 | −65.003 | [106,158,171,172,173,189,190,191,192,193,194,195] |
| Ca | Ca(BH4)2 | 1 | 783.19 | 510.04 | −142.1 | [146,147,148,149,150,151,157] |
| Cr | Cr(BH4)2 | 1.66 | 369.86 | 96.71 | 22.042 | [28,29] |
| Mn | Mn(BH4)2 | 1.55 | 428.1 | 154.95 | −5.315 | [175,189,190,191,224] |
| Fe | Fe(BH4)2 | 1.83 | 288.22 | 15.07 | 64.321 | [192,193] |
| Ni | Ni(BH4)2 | 1.91 | 253.32 | −19.83 | 84.217 | [195] * as heteroleptic complex |
| Co | Co(BH4)2 | 1.88 | 266.14 | −7.01 | 76.756 | [194] * presumed, but unstable |
| Zn | Zn(BH4)2 | 1.65 | 374.98 | 101.83 | 19.555 | [119,120,121,122] |
| Sr | Sr(BH4)2 | 0.95 | 820.74 | 547.59 | −154.535 | [147,148] |
| Ba | Ba(BH4)2 | 0.89 | 866.97 | 593.82 | −169.457 | [148] |
| Cd | Cd(BH4)2 | 1.69 | 354.71 | 81.56 | 29.503 | [149] |
| Hg | Hg(BH4)2 | 2 | 216.74 | −56.41 | 106.6 | [151] * failed attempts |
| Al | Al(BH4)3 | 1.61 | 395.81 | 122.66 | 9.607 | [83,84,85,86,87,88] |
| Sc | Sc(BH4)3 | 1.36 | 538.74 | 265.59 | −52.568 | [182,183,295,296,297,298,299] |
| TiIII | Ti(BH4)3 | 1.54 | 433.61 | 160.46 | −7.802 | [176,177,178,179,206] * Ti(IV) is reduced in situ to Ti(III) |
| V | V(BH4)3 | 1.63 | 385.32 | 112.17 | 14.581 | [66] |
| Ga | Ga(BH4)3 | 1.81 | 297.29 | 24.14 | 59.347 | [84,85] * as GdH(BH4)2 (volatile, 203K) and GdH2(BH4) (unstable) |
| Y | Y(BH4)3 | 1.22 | 628.38 | 355.23 | −87.386 | [65,66,68,69,70,71,72,73] |
| Nb | Nb(BH4)3 | 1.6 | 401.1 | 127.95 | 7.12 | [180] * no homoleptic form; as complex |
| In | In(BH4)3 | 1.78 | 311.17 | 38.02 | 51.886 | [86] |
| La | La(BH4)3 | 1.1 | 710.71 | 437.56 | −117.23 | [212] |
| Ce | Ce(BH4)3 | 1.12 | 696.64 | 423.49 | −112.256 | [212] |
| Nd | Nd(BH4)3 | 1.14 | 682.7 | 409.55 | −107.282 | [69] |
| SmII | Sm(BH4)2 | 1.13 | 689.65 | 416.5 | −109.769 | [213,214] |
| SmIII | Sm(BH4)3 | 1.13 | 689.65 | 416.5 | −109.769 | [214] |
| Pr | Pr(BH4)3 | 1.13 | 689.65 | 416.5 | −109.769 | [64] |
| EuII | Eu(BH4)2 | 1.2 | 641.75 | 368.6 | −92.36 | [213] |
| EuIII | Eu(BH4)3 | 1.2 | 641.75 | 368.6 | −92.36 | [70,148] |
| Gd | Gd(BH4)3 | 1.2 | 641.75 | 368.6 | −92.36 | [68,71,72,214] |
| Tb | Tb(BH4)3 | 1.22 | 628.38 | 355.23 | −87.386 | [73,214] |
| Dy | Dy(BH4)3 | 1.23 | 621.75 | 348.6 | −84.899 | [68] |
| Ho | Ho(BH4)4 | 1.24 | 615.15 | 342 | −82.412 | [69] |
| Er | Er(BH4)3 | 1.24 | 615.15 | 342 | −82.412 | [214] |
| Tm | Tm(BH4)3 | 1.25 | 608.59 | 335.44 | −79.925 | [73] |
| YbII | Yb(BH4)2 | 1.1 | 710.71 | 437.56 | −117.23 | [215] |
| YbIII | Yb(BH4)3 | 1.1 | 710.71 | 437.56 | −117.23 | [215] |
| Lu | Lu(BH4)3 | 1.27 | 595.57 | 322.42 | −74.951 | [73] |
| Th | Th(BH4)4 | 1.3 | 576.31 | 303.16 | −67.49 | [78,206,207] |
| Pa | Pa(BH4)3 | 1.5 | 455.99 | 182.84 | −17.75 | [78] |
| U | U(BH4)4 | 1.38 | 526.49 | 253.34 | −47.594 | [78,80,208,209] |
| Np | Np(BH4)4 | 1.36 | 538.74 | 265.59 | −52.568 | [79,80,210] |
| PuIII | Pu(BH4)3 | 1.28 | 589.12 | 315.97 | −72.464 | [78,80] |
| PuIV | Pu(BH4)4 | [79] | ||||
| TlI | TlBH4 | 1.62 | 390.55 | 117.4 | 12.094 | [87] |
| TlIII | Tl(BH4)3 | 1.62 | 390.55 | 117.4 | 12.094 | [88] * failed attempts; as TlCl(BH4)2 (dec. at 178 K) |
| Ge | Ge(BH4)4 | 2.01 | 212.86 | −60.29 | 109.087 | no conclusive reports |
| Zr | Zr(BH4)4 | 1.33 | 557.37 | 284.22 | −60.029 | [203,204] |
| Sn | Sn(BH4)4 | 1.96 | 232.65 | −40.5 | 96.652 | no conclusive reports |
| Hf | Hf(BH4)4 | 1.3 | 576.31 | 303.16 | −67.49 | [205] |
| Binary System | Ea (kJ/mol) | A × 10−9 (min−1) | k × 102 (min−1) | Reference |
|---|---|---|---|---|
| Ca(BH4)2-2Mg(NH2)2 | 132.7 | 15 | 1.2 | [309] |
| Ca(BH4)2-2Ca(NH2)2 | 119.3 | 3.2 | 4.3 | [309] |
| Compound | DSC Peak 1; Polymorphic Transformation o-LiBH4→h-LiBH4 | DSC Peak 2; Melting Point | DSC Peak 3; DehyDrogenation (MgH2) | DSC Peak 4; DehyDrogenation (LiBH4) |
|---|---|---|---|---|
| 2LiBH4-MgH2@RF-CA21nm | 113 °C | 267 °C | 332 °C | 351 °C |
| 2LiBH4-MgH2-bulk | 117 °C | 290 °C | 364 °C | 462 °C |
| Substance | Scaffold | Scaffold Pore | Loading (wt.%) | td (°C) | Ea (kJ/mol) | ΔH (kJ/mol) | Ref. |
|---|---|---|---|---|---|---|---|
| NH3BH3 | - | - | 100 | 114 (110), 155 | 184 | −19.6 to −21 | [145,406] |
| NH3BH3 | MCM-41 | 4 nm | 33, 50, 75 (outside pores) | 100, 182 | N/A | 34.09 (2 steps: 30.33 and 3.76) | [404,407] |
| NH3BH3 | SBA-15 | 7.5 nm | 50 | 50–100, 130 | 67 | −1 | [145] |
| NH3BH3 | OMC | 4.5 nm | (50-)95 | −2.1 | [402] | ||
| NH3BH3 | RF-CC (carbon cryogel) | 2–20 (mean 5) nm | 24 | 85–150; 80–90 for CC(exo- peak) | N/A | −120 (CC) | [403,408] |
| NH3BH3 | AC (activated carbon) | 3 nm | 17.6 wt.% (computed from pore volume decrease from 0.36 cm3/g to 0.14 cm3/g for AC@ABTHF) | 3–4; RT(25) | N/A | −1.6 (AC@ABTHF), −3.0 (AC@ABMET) and −5.9 (AC@ABmilled) | [409] |
| NH3BH3 | MOF (Fe–MIL-53) | 11–13 nm | AB:Fe = 6.5% (0.5:1 molar); 13% (1:1 molar) | 80–110 | 130 +/− 7; 135 +/−3 | N/A | [410] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comanescu, C. Complex Metal Borohydrides: From Laboratory Oddities to Prime Candidates in Energy Storage Applications. Materials 2022, 15, 2286. https://doi.org/10.3390/ma15062286
Comanescu C. Complex Metal Borohydrides: From Laboratory Oddities to Prime Candidates in Energy Storage Applications. Materials. 2022; 15(6):2286. https://doi.org/10.3390/ma15062286
Chicago/Turabian StyleComanescu, Cezar. 2022. "Complex Metal Borohydrides: From Laboratory Oddities to Prime Candidates in Energy Storage Applications" Materials 15, no. 6: 2286. https://doi.org/10.3390/ma15062286
APA StyleComanescu, C. (2022). Complex Metal Borohydrides: From Laboratory Oddities to Prime Candidates in Energy Storage Applications. Materials, 15(6), 2286. https://doi.org/10.3390/ma15062286