Bioactive Glasses in Periodontal Regeneration: Existing Strategies and Future Prospects—A Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary and Secondary Outcomes
- The changes in human periodontal ligament cells (hPDLCs), human periodontal ligament stem/progenitor cells (HPDLC), and human bone marrow stromal cells (hBMSC) as cell viability, cell proliferation, cell differentiation, enhanced mineralized tissue formation.
- The changes in fibroblast/osteocyte cell lines (RAT primary osteoblastic cells—RPO; pre-osteoblasts murine cells—MC3T3-E1; human osteosarcoma cells—MG63; odontoblast-like cells—MDPC-23; oral mucosa fibroblasts cells—MM1; osteoblast-like cells—Saos-2) as cell viability, cell proliferation, cell differentiation.
- Detecting of cell factors, promoting wound healing response.
- BG antibacterial activity.
- Detecting change in levels of inflammatory modulating molecules.
- Regarding the in vivo tests these involved: (a) dissolution of BGs in implanted liquids/tissues, (b) resorption of material particles based on size, (c) replacement of particles with new bone, (d) new tissue regenerated in the defects considered.
2.2. Inclusion/Exclusion Criteria
- Clinical studies carried out on patients diagnosed with moderate to severe (chronic or aggressive) periodontitis, and at least presenting one periodontal intrabony defect grafted with BG (in particulate form, scaffold, composite, etc.) and treated by periodontal regenerative surgery.
- In vitro and in vivo studies considering the secondary outcomes of interest.
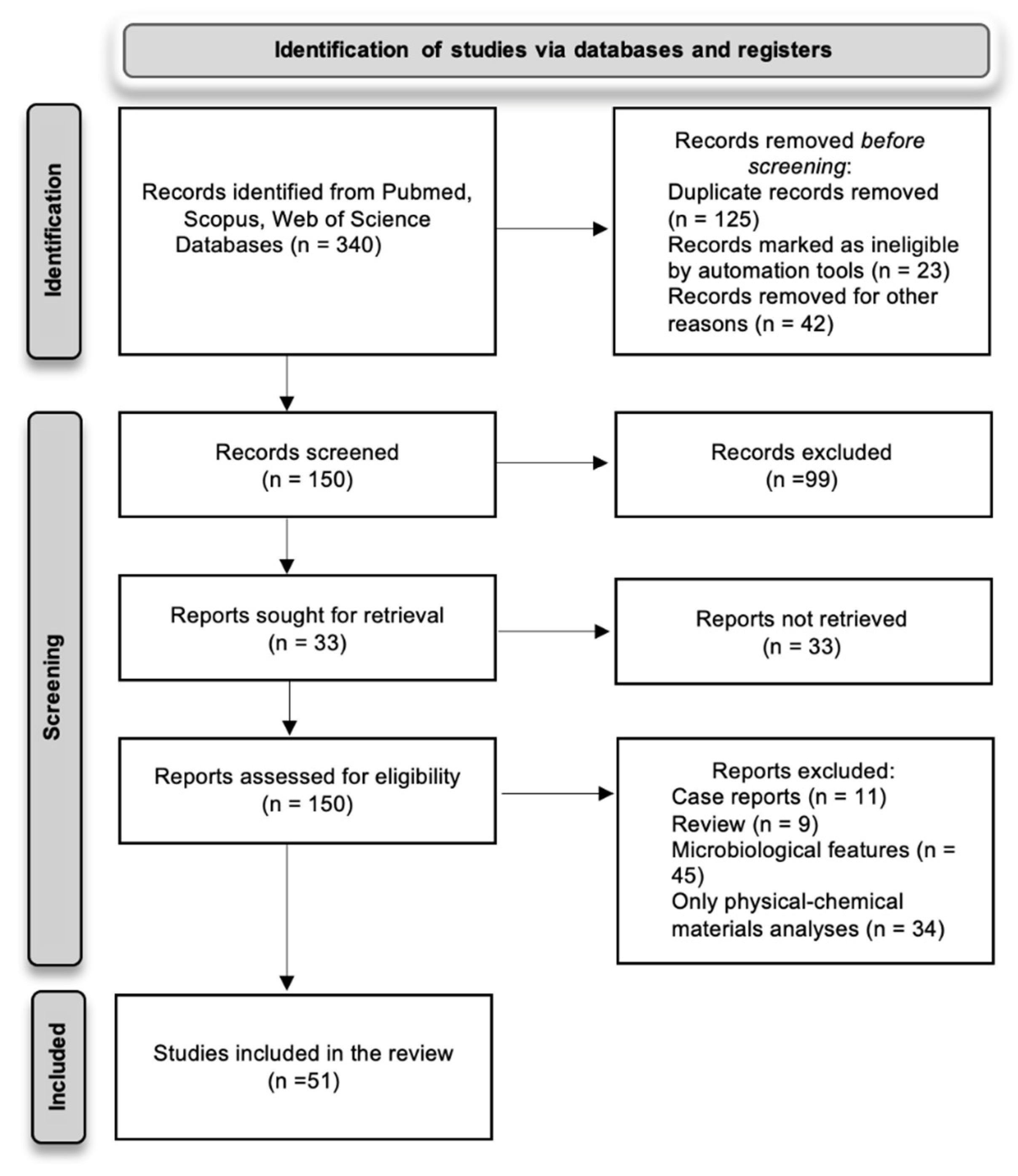
2.3. Search Methods
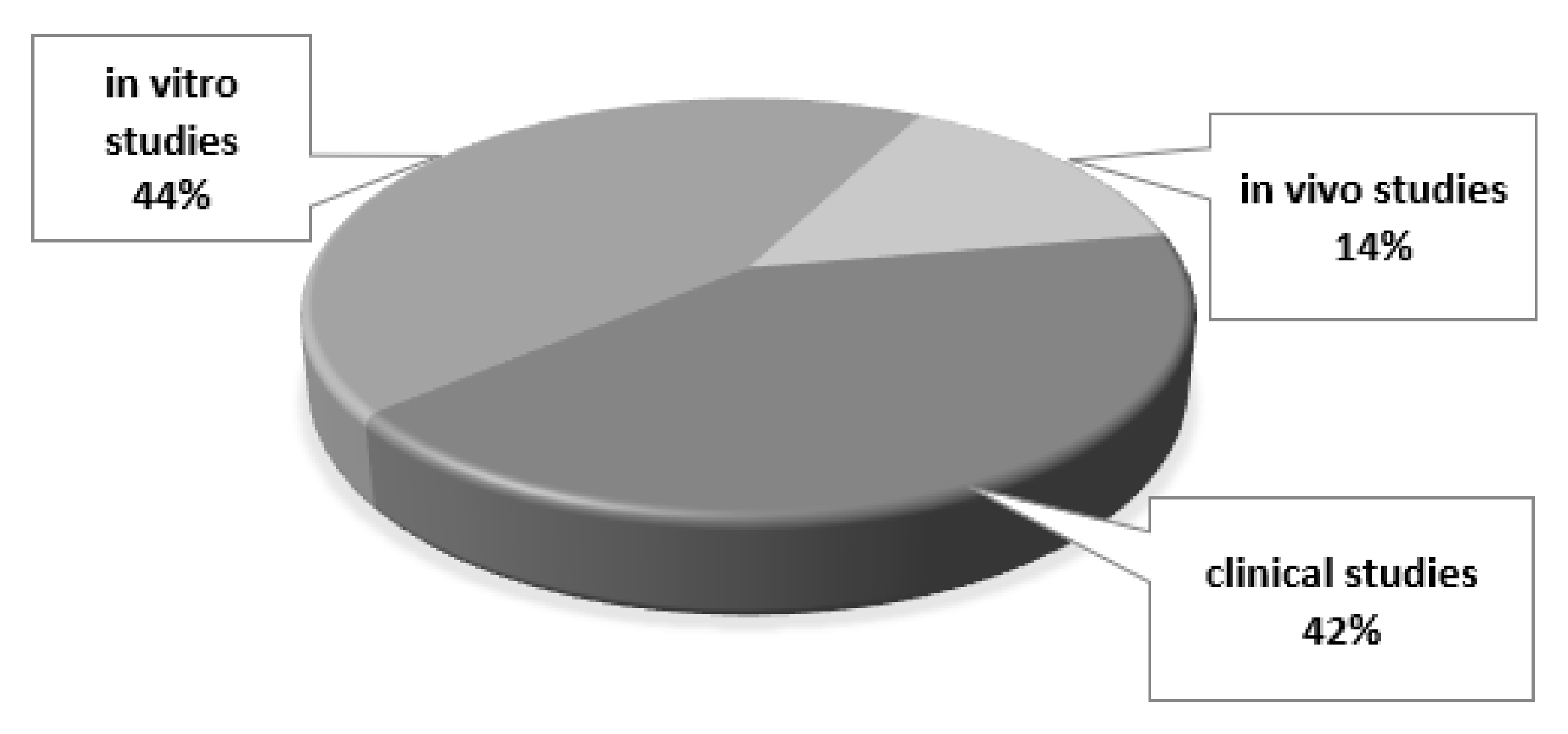
3. Results
3.1. In Vitro Studies
3.1.1. BG Microparticles
3.1.2. BG Nanoparticles and Mesoporous BGs
3.1.3. Scaffolds and Composites
3.1.4. Bulk BG
| References | Materials | Type of Cells and Tests |
|---|---|---|
| Balamurugan et al. [52] | Sol-gel BG containing silver | E. coli (MG1655) Simulated body fluid (SBF) test Antimicrobial activity |
| Varanasi et al. [53] | (i) commercial 45S5 and (ii) an experimental BG | Human periodontal ligament fibroblasts (hPDLF) Cell proliferation assay Osteocalcin and alkaline phosphatase gene expression (quantitative PCR) Protein expression assays (BCA assay) Mineralization Assay (Alizarin Red) |
| Casarrubios et al. [54] | mesoporous BG nanospheres | MC3T3-E1 pre-osteoblasts NanoMBG incorporation (flow cytometry) Cell viability, Cell-Cycle Analysis, apoptosis detection (flow cytometry) Intracellular Reactive Oxygen Species (ROS) and Intracellular Calcium Content (flow cytometer) Alkaline phosphatase (ALP) activity Mineralization Assay (Alizarin Red) Oxidative stress (Interleukin 6 detection) |
| Bai et al. [55] | Boron-containing mesoporous bioactive glass nanospheres (with average size of 60 nm) Boron from 5 to 20 mol.% | Human periodontal ligament cells (hPDLCs) Cell viability [CCK-8] Simulated body fluid (SBF) test |
| Wu et al. [37] | Strontium containing mesoporous BG scaffolds | Human periodontal ligament cells (hPDLCs) Simulated body fluid (SBF) test Alkaline phosphatase (ALP) activity Protein expression assays (BCA assay) Collagen type I (COL1), osteopontin (OPN), runt-related transcription factor 2 (RUNX2) and cementum protein 1 (CEMP1) gene expression |
| Jia et al. [56] | Porous mesoporous BG scaffolds, containing strontium (Sr 5 mol.) | Periodontal ligament stem cells (PDLCs) Epigenetic mechanism evaluation |
| Carvalho et al. [57] | Bioactive glass nanoparticles (BGNP) | Osteoblasts rat calvaria Gingival fibroblasts wistar rats Cementoblast wistar rats Cell viability (Trypan blue assay)Mitochondrial activity [MTT assay] Cell proliferation (BrdU assay) Alkaline phosphatase (ALP) activity Mineralization nodules (Von Kossa staining) Protein expression (Western blot analysis and reverse transcription polymerase chain reaction (RT-PCR) |
| Sowmya et al. [58] | Nanocomposite scaffold based on chitin hydrogel and bioactive glass ceramic particles (nBGC) | Human osteosarcom a cell line (MG63) Human primary osteoblasts cells (POB) Simulated body fluid (SBF) test Cell viability (Alamar blue assay) Cell adhesion (by SEM) Cell proliferation (DAPI staining) POB maturation and mineralization |
| Sowmya et al. [59] | Three-layer nanocomposite scaffold consisting of: (i) chitin—PLGA/nanobioactive glass ceramic (nBGC)/cementum protein 1, (ii) chitin—PLGA/fibroblasts growth factor 2 and (iii) chitin—PLGA/nBGC/platelet-rich plasma-derived growth factors | Human dental follicle stem cells (hDFCs) Cementogenic, fibrogenic, osteogenic, differentiation (by flow cytometry) Alkaline phosphatase (ALP) activity Biomineralization (SEM) |
| Uskoković et al. [60] | Nanocomposites: niobium- and zinc-doped bioglass-ceramic particles and chitosan | Odontoblast-like MDPC-23 cells Cell viability (CellTiter-Blue assay) |
| Srinivasan et al. [61] | Composite scaffold: alginate and nanobioactive glass ceramic particles (nBGC, CaO–SiO2–P2O5 ternary system) | Human periodontal ligament fibroblast (hPDLF) Human osteosarcoma cell line (MG-63) Protein adsorption studies (bicinchoninic acid assay-BCA) Biomineralization (simulated body fluid—SBF) Cell viability assay (Alamar blue) Alkaline phosphatase (ALP) activity Cell proliferation (DAPI staining) |
| Esfahanizadeh et al. [63] | BG doped with zinc compared to 45S5 | Antibiofilm activity |
| Caridade et al. [64] | Composite membranes: poly (d,l-lactic acid) PDLL and Bioglass® | Saos-2 cells Cell viability (MTS assay) Cell proliferation (PicoGreen test) SEM morphological evaluation |
| Moonesi et al. [65] | Bilayer membranes (of cellulose acetate) containing BG nanoparticles modified with boron | Human dental pulp stem cells (hDPSCs) Dissolution (by inductively coupled plasma mass spectrometry—ICP-MS) Simulated body fluid (SBF) test SEM morphological evaluation Cell viability (Alamar blue assay) Mineralization Assay (Alizarin Red) Alkaline phosphatase (ALP) activity Cell migration (Confocal laser scanning microscopy-CLSM) |
| Mota et al. [66] | Membrane: chitosan with bioactive glass nanoparticles (BG-NP) | Human periodontal ligament cells (hPDL) and human bone marrow stromal cells (hBMSC). Cell viability (Alamar Blue test) Cell proliferation Evaluation of calcium content |
| Ruiz-Clavijo et al. [67] | Binary glasses (CaO-SiO2) | Human osteosarcoma cell line (MG63) Cell viability assay (MTT test) |
| Shah et al. [68] | Three-layered functionally graded membranes, with various concentrations of BG nanoparticles | Murine pre-osteoblasts cell line (MC3T3-E1) Cell viability (Alamar blue assay) Cell adhesion (by SEM) |
| Sunandhakumari et al. [69] | Membranes: polycaprolactone (PCL) and BG particles | Murine fibroblast cell line (L-929 Cell viability (XTT assay)) |
| Beketova et al. [70] | BG/ceramic dental composite | Periodontal ligament fibroblasts (PDLFs) Human gingival fibroblasts (HGFs) Saos-2 osteoblasts Simulated body fluid (SBF) test Cell viability test (MTT) |
| Granel et al. [71] | BG and PCL | Rat primary osteoblastic (RPO) cells Cell viability assay (XTT test) Cell proliferation (CyQUANT NF assay) SEM morphological evaluation Cell signaling (immunoassay for Runx2, FAK, phospho-FAK (Y397), GAPDH) Alkaline phosphatase activity assay |
| Meneses et al. [72] | Gutta-percha/niobium phosphate glass | Human periodontal ligament fibroblasts (hPDLF) Cell viability (AlamarBlue) Gene expression of type I collagen and cement protein by quantitative reverse transcription polymerase chain reaction |
| Theocharidou et al. [73] | BG/ceramic composite scaffolds | Human periodontal ligament fibroblasts (hPDLF) Cell attachment (by SEM) |
| Carvalho et al. [74] | BG by sol-gel | Cementoblasts (from the molars extracted from Wistar male rats) Osteoblasts (from calvaria of neonatal Wistar rats) Neonatal fibroblasts (from hearts of Wistar rats) Cellular viability (Trypan Blue assay, MTT assay) |
| Wen et al. [75] | BG based on the xSiO2-CaO-P2O5 system | Human periodontal ligament cells (hPDLCs) Simulated body fluid [SBF] test Cell viability (CCK-8) |
3.2. In Vivo Studies
| References | Materials | Animal and Follow-Up |
|---|---|---|
| Carvalho et al. [76] | (i) BG (Perioglas®, 90–710 µm); (ii) plasma rich in platelets (PRP); (iii) BG and PRP; (iv) control | 9 mongrel dogs Follow-up: 90 days |
| Felipe et al. [77] | (i) membrane and Perioglas® (particles size 90 to 710 microns); (ii) membrane and BioGran (particles size 300 to 355 microns); (iii) membrane alone; (iv) negative control | 6 dogs Follow-up: 90 days |
| Lee et al. [78] | (i) an amorphous calcium phosphate glass cement with collagen membrane (CM), (ii) biphasic calcium phosphate with CM, (iii) CM alone and (iv) surgical flap operation only (control group, not grafted) | 5 beagle dogs Follow-up: 60 days |
| Sowmya et al. [59] | Nanocomposite scaffold (chitin hydrogel and bioactive glass ceramic particles—nBGC) | 12 rabbits Follow up: 30 and 90 days |
| Zhang et al. [79] | (i) unfilled defects as control, (i) a BG scaffold (BG: CaO-P2O5-SiO2) and (iii) a scaffold made of BG also containing strontium | 15 osteoporotic rats Follow-up: 28 days |
| Granel et al. [71] | Bioactive glass and polycaprolactone (PCL) | 14 rats (calvaria) Follow-up: 30, 60, 90 days |
| Shah et al. [68] | Three-layered functionally graded membranes: lower layer with 50% wt. bioactive glass nanoparticles (BG-NP), middle layer 25% wt. BG-NP and upper layer no BG-NPs | 8 wistar rats Follow-up: 35 days |
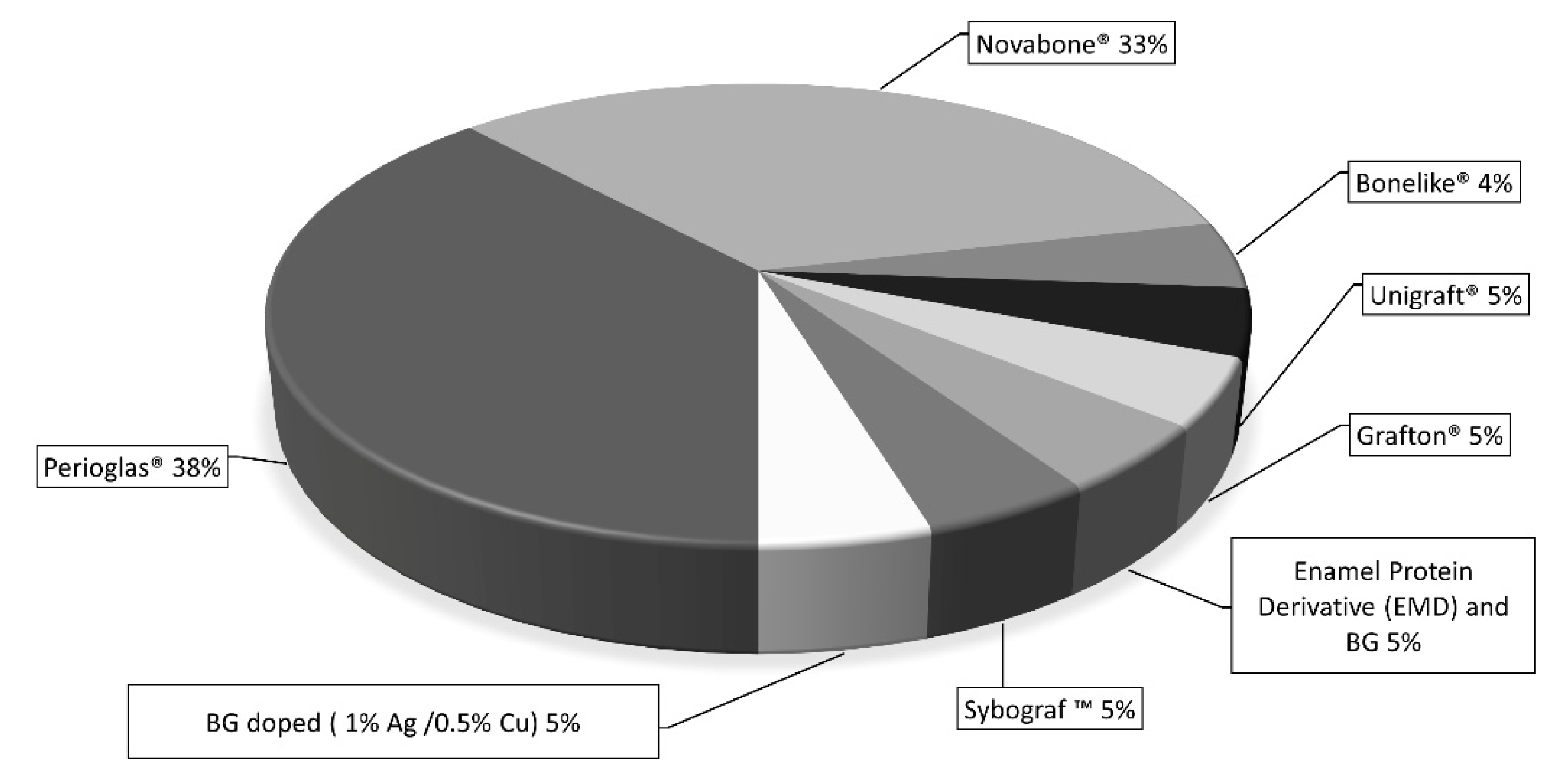
3.3. Clinical Studies
| References | Materials | Number of Patients and Follow-Up |
|---|---|---|
| Humagain et al. [80] | PerioGlas® | 16 patients 6 months |
| Keles et al. [81] | PerioGlas® | 15 patients 6 months |
| Demir et al. [82] | Unigraft® (200–420 μm) | 29 patients 9 months |
| Cetinkaya et al. [83] | PerioGlas® | 11 patients 60 months |
| Kaur et al. [84] | PerioGlas® | 10 patients 6 months |
| Katuri et al. [85] | PerioGlas®, Grafton® | 10 patients 12 months |
| Sculean et al. [86] | Enamel matrix protein derivative (EMD) and bioactive glass | 10 patients 12months |
| Kumar et al. [87] | Bonelike® (glass reinforced HA with α and β forms of tricalcium-phosphate) | 10 patients 6 months |
| Satyanarayana et al. [88] | PerioGlas® | 12 patients 12 months |
| Subbaiah and Thomas [89] | PerioGlas® | 8 patients 3, 6, 9 months |
| Mistry et al. [90] | PerioGlas® | 22 patients 6 months |
| Lysienko and Borysenko [91] | BG graft. BG doped with 1% silver and 0.5% copper | 47 patients 6, 12 months |
| Slezák et al. [92] | NovaBone® | 10 patients 3, 6 months |
| Grover et al. [93] | NovaBone® | 12 patients 3, 6 months |
| Asmita et al. [94] | NovaBone Dental Putty® graft or BG (PerioGlas®) | 28 patients 6 months |
| Biswas et al. [95] | NovaBone Dental putty® | 15 patients 3, 6, 9 months |
| Naqvi et al. [96] | NovaBone® | 20 patients 6, 9 months |
| Saravanan et al. [97] | BG putty | 20 patients 6, 9 months |
| Koduru et al. [98] | Nanocrystalline hydroxyapatite (Nc-HA) (Sybograf ™) | 20 patients 3, 6, 9 months |
| Bansal et al. [99] | NovaBone® | 10 patients 6 months |
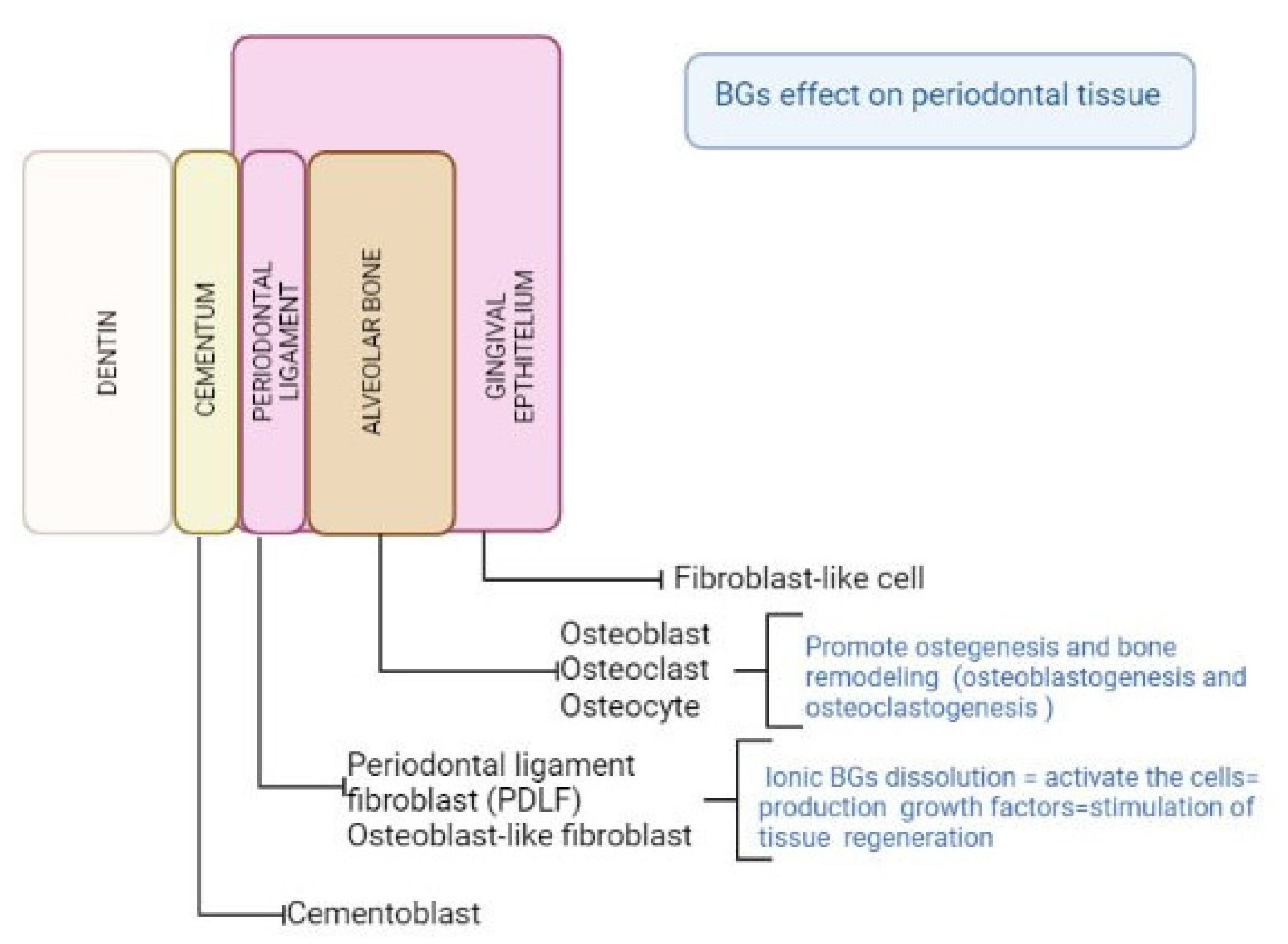
4. Discussion
4.1. In Vitro Tests
4.2. In Vivo Tests
4.3. Clinical Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larry, R.; Hench, L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23, S53–S82. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.G.L.; Serakides, R.; Rosado, I.R.; Pereira, M.M.; Ocarino, N.M.; Oliveira, H.P.; Góes, A.M.; Rezende, C.M.F. Effect of the ionic product of bioglass 60s on osteoblastic activity in canines. BMC Vet. Res. 2015, 11, 247. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taherkhani, S.; Moztarzadeh, F. Influence of strontium on the structure and biological properties of sol–gel-derived mesoporous bioactive glass [MBG] powder. J. Sol-Gel Sci. Technol. 2016, 78, 539–549. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zheng, K.; Boccaccini, A.R. Multi-functional silica-based mesoporous materials for simultaneous delivery of biologically active ions and therapeutic biomolecules. Acta Biomater. 2021, 129, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, T.; Mesgar, A.S.; Mohammadi, Z. Bioactive Glasses: A Promising Therapeutic Ion Release Strategy for Enhancing Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5399–5430. [Google Scholar] [CrossRef]
- Bellucci, D.; Veronesi, E.; Strusi, V.; Petrachi, T.; Murgia, A.; Mastrolia, I.; Dominici, M.; Cannillo, V. Human mesenchymal stem cell combined with a new strontium-enriched bioactive glass: An ex-vivo model for Bone Regeneration. Materials 2019, 12, 3633. [Google Scholar] [CrossRef]
- Bellucci, D.; Veronesi, E.; Dominici, M.; Cannillo, V. A new bioactive glass with extremely high crystallization temperature and outstanding biological performance. Mater. Sci. Eng. C 2020, 110, 110699. [Google Scholar] [CrossRef]
- Bellucci, D.; Cannillo, V. A novel bioactive glass containing strontium and magnesium with ultra-high crystallization temperature. Mater. Lett. 2018, 213, 67–70. [Google Scholar] [CrossRef]
- Jell, G.; Stevens, M.M. Gene activation by bioactive glasses. J. Mater. Sci. Mater. Med. 2006, 17, 997–1002. [Google Scholar] [CrossRef]
- Farano, V.; Maurin, J.C.; Attik, N.; Jackson, P.; Grosgogeat, B.; Gritsch, K. Sol–gel bioglasses in dental and periodontal regeneration: A systematic review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1210–1227. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Ahmed, I.; Pratten, J.; Nazhat, S.N.; Knowles, J.C. Characterisation of antibacterial copper releasing degradable phosphate glass fibres. Biomaterials 2005, 26, 2247–2254. [Google Scholar] [CrossRef]
- Abou, E.A.; Ae, N.; O’Austin, L.; Ae, D.; Ae, M.E.S.; Knowles, J.C. Processing, characterisation, and biocompatibility of zinc modified metaphosphate based glasses for biomedical applications. J. Mater. Sci. Mater. Med. 2008, 19, 1669–1679. [Google Scholar] [CrossRef]
- Valappil, S.P.; Pickup, D.M.; Carroll, D.L.; Hope, C.K.; Pratten, J.; Newport, R.J.; Smith, M.E.; Wilson, M.; Knowles, J.C. Effect of silver content on the structure and antibacterial activity of silver-doped phosphate-based glasses. Antimicrob. Agents Chemother. 2007, 51, 4453–4461. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Chrzanowski, W.; Knowles, J.C. Effect of increasing titanium dioxide content on bulk and surface properties of phosphate-based glasses. Acta Biomater. 2008, 4, 523–534. [Google Scholar] [CrossRef]
- Lakhkar, N.J.; Abou Neel, E.A.; Salih, V.; Knowles, J.C. Strontium oxide doped quaternary glasses: Effect on structure, degradation and cytocompatibility. J. Mater. Sci. Mater. Med. 2009, 20, 1339–1346. [Google Scholar] [CrossRef]
- Islam, M.T.; Felfel, R.M.; Abou Neel, E.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M.Z. Bioactive calcium phosphate–based glasses and ceramics and their biomedical applications: A review. J. Tissue Eng. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Nazhat, S.N.; Abou Neel, E.A.; Kidane, A.; Ahmed, I.; Hope, C.; Kershaw, M.; Lee, P.D.; Stride, E.; Saffari, N.; Knowles, J.C.; et al. Controlled microchannelling in dense collagen scaffolds by soluble phosphate glass fibers. Biomacromolecules 2007, 8, 543–551. [Google Scholar] [CrossRef]
- Baino, F.; Verné, E. Production and characterization of glass-ceramic materials for potential use in dental applications: Thermal and mechanical properties, microstructure, and in vitro bioactivity. Appl. Sci. 2017, 7, 1330. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Salaria, S.K.; Ghuman, S.K.; Kumar, S.; Sharma, G. Management of localized advance loss of periodontal support associated Grade II furcation and intrabony defect in chronic periodontitis patient through amalgamation of platelet-rich fibrin and hydroxyapatite bioactive glass composite granules. Contemp. Clin. Dent. 2016, 7, 405–408. [Google Scholar] [CrossRef]
- Salem, A.M.; Jones, S.J.; Ellis, I.R.; Chadwick, R.G. Investigating the addition of collagen and its integrin binding sequence (RGD) to glass polyalkenoate: In terms of material and cellular properties to explore a more biocompatible method of root caries restoration. J. Dent. 2016, 54, 68–76. [Google Scholar] [CrossRef][Green Version]
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive glass applications: A literature review of human clinical trials. Materials 2021, 14, 5440. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.M.; Mahapatra, C.; Kim, H.W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Bertoldi, C.; Zaffe, D.; Consolo, U. Polylactide/polyglycolide copolymer in bone defect healing in humans. Biomaterials 2008, 29, 1817–1823. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef]
- Cannillo, V.; Sola, A. Different approaches to produce coatings with bioactive glasses: Enamelling vs plasma spraying. J. Eur. Ceram. Soc. 2010, 30, 2031–2039. [Google Scholar] [CrossRef]
- Bolelli, G.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Rauch, J. Microstructural and in vitro characterisation of high-velocity suspension flame sprayed (HVSFS) bioactive glass coatings. J. Eur. Ceram. Soc. 2009, 29, 2249–2257. [Google Scholar] [CrossRef]
- Cannillo, V.; Colmenares-Angulo, J.; Lusvarghi, L.; Pierli, F.; Sampath, S. In vitro characterisation of plasma-sprayed apatite/wollastonite glass-ceramic biocoatings on titanium alloys. J. Eur. Ceram. Soc. 2009, 29, 1665–1677. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Gentile, P.; Ciardelli, G.; Cannillo, V. Biomimetic coating on bioactive glass-derived scaffolds mimicking bone tissue. J. Biomed. Mater. Res. Part A 2012, 100, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Cattini, A.; Bellucci, D.; Sola, A.; Pawłowski, L.; Cannillo, V. Suspension plasma spraying of optimized functionally graded coatings of bioactive glass/hydroxyapatite. Surf. Coat. Technol. 2013, 236, 118–126. [Google Scholar] [CrossRef]
- Cannillo, V.; Montorsi, M.; Siligardi, C.; Sola, A.; de Portu, G.; Micele, L.; Pezzotti, G. Microscale computational simulation and experimental measurement of thermal residual stresses in glass-alumina functionally graded materials. J. Eur. Ceram. Soc. 2006, 26, 1411–1419. [Google Scholar] [CrossRef]
- Cannillo, V.; Leonelli, C.; Boccaccini, A.R. Numerical models for thermal residual stresses in Al2O3 platelets/borosilicate glass matrix composites. Mater. Sci. Eng. A 2002, 323, 246–250. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A review of bioactive glass/natural polymer composites: State of the art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Lin, C.; Chang, J.; Xiao, Y. Strontium-containing mesoporous bioactive glass scaffolds with improved osteogenic/cementogenic differentiation of periodontal ligament cells for periodontal tissue engineering. Acta Biomater. 2012, 8, 3805–3815. [Google Scholar] [CrossRef]
- Zhu, M.; Li, J.; Chen, B.; Mei, L.; Yao, L.; Tian, J.; Li, H. The effect of calcium sodium phosphosilicate on dentin hypersensitivity: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0140176. [Google Scholar] [CrossRef]
- Zamani, D.; Moztarzadeh, F.; Bizari, D. Alginate-bioactive glass containing Zn and Mg composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 137, 1256–1267. [Google Scholar] [CrossRef]
- Lotfi, G.; Shokrgozar, M.A.; Mofid, R.; Abbas, F.M.; Ghanavati, F.; Baghban, A.A.; Yavari, S.K.; Pajoumshariati, S. Biological Evaluation [In Vitro and In Vivo] of Bilayered Collagenous Coated [Nano Electrospun and Solid Wall] Chitosan Membrane for Periodontal Guided Bone Regeneration. Ann. Biomed. Eng. 2016, 44, 2132–2144. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef]
- Vagropoulou, G.; Trentsiou, M.; Georgopoulou, A.; Papachristou, E.; Prymak, O.; Kritis, A.; Epple, M.; Chatzinikolaidou, M.; Bakopoulou, A.; Koidis, P. Hybrid chitosan/gelatin/nanohydroxyapatite scaffolds promote odontogenic differentiation of dental pulp stem cells and in vitro biomineralization. Dent. Mater. 2021, 37, e23–e36. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ugawa, Y.; Kawamura, M.; Yamashiro, K.; Kochi, S.; Ideguchi, H.; Takashiba, S. Modulation of microenvironment for controlling the fate of periodontal ligament cells: The role of Rho/ROCK signaling and cytoskeletal dynamics. J. Cell Commun. Signal. 2018, 12, 369–378. [Google Scholar] [CrossRef]
- Aydin, S.; Şahin, F. Stem Cells Derived from Dental Tissues. Adv. Exp. Med. Biol. 2019, 1144, 123–132. [Google Scholar] [CrossRef]
- Hosoya, A.; Shalehin, N.; Takebe, H.; Fujii, S.; Seki, Y.; Mizoguchi, T.; Shimo, T.; Iijima, M.; Irie, K. Stem cell properties of Gli1-positive cells in the periodontal ligament. J. Oral Biosci. 2020, 62, 299–305. [Google Scholar] [CrossRef]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Zheng, K.; Niu, W.; Lei, B.; Boccaccini, A.R. Immunomodulatory bioactive glasses for tissue regeneration. Acta Biomater. 2021, 133, 168–186. [Google Scholar] [CrossRef]
- Bertoldi, C.; Bellei, E.; Pellacani, C.; Ferrari, D.; Lucchi, A.; Cuoghi, A.; Bergamini, S.; Cortellini, P.; Tomasi, A.; Zaffe, D.; et al. Non-bacterial protein expression in periodontal pockets by proteome analysis. J. Clin. Periodontol. 2013, 40, 573–582. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. The nature of periodontal diseases. Ann. Periodontol. 1997, 2, 3–10. [Google Scholar] [CrossRef]
- Meyle, J.; Iain Chapple, I. Molecular aspects of the pathogenesis of periodontitis. Periodontology 2000 2015, 69, 7–17. [Google Scholar] [CrossRef]
- Snyder, H. Literature review as a research methodology: An overview and guidelines. J. Bus. Res. 2019, 104, 333–339. [Google Scholar] [CrossRef]
- Balamurugan, A.; Balossier, G.; Laurent-Maquin, D.; Pina, S.; Rebelo, A.H.S.; Faure, J.; Ferreira, J.M.F. An in vitro biological and anti-bacterial study on a sol–gel derived silver-incorporated bioglass system. Dent. Mater. 2008, 24, 1343–1351. [Google Scholar] [CrossRef]
- Varanasi, V.G.; Owyoung, J.B.; Saiz, E.; Marshall, S.J.; Marshall, G.W.; Loomer, P.M. The ionic products of bioactive glass particle dissolution enhance periodontal ligament fibroblast osteocalcin expression and enhance early mineralized tissue development. J. Biomed. Mater. Res. Part A 2011, 98, 177–184. [Google Scholar] [CrossRef]
- Casarrubios, L.; Gómez-Cerezo, N.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Ipriflavone-loaded mesoporous nanospheres with potential applications for periodontal treatment. Nanomaterials 2020, 10, 2573. [Google Scholar] [CrossRef]
- Bai, N.; Chen, W.; Luo, L.; Tong, W.; Wen, C.; Zhan, X.; Sa, B. Effect of B2O3 on the structural and in vitro biological assessment of mesoporous bioactive glass nanospheres. J. Am. Ceram. Soc. 2021, 104, 3058–3072. [Google Scholar] [CrossRef]
- Jia, X.; Miron, R.J.; Yin, C.; Xu, H.; Luo, T.; Wang, J.; Jia, R.; Wu, M.; Zhang, Y.; Li, Y. HnRNPL inhibits the osteogenic differentiation of PDLCs stimulated by SrCl2 through repressing Setd2. J. Cell. Mol. Med. 2019, 23, 2667–2677. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Oliveira, A.A.R.; Jardim, C.A.; Melo, C.B.S. Characterization and induction of cementoblast cell proliferation by bioactive glass nanoparticles. J. Tissue Eng. Regen. Med. 2012, 6, 813–821. [Google Scholar] [CrossRef]
- Sowmya, S.; Kumar, P.T.S.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Biocompatible β-chitin hydrogel/nanobioactive glass ceramic nanocomposite scaffolds for periodontal bone regeneration. Trends Biomater. Artif. Organs 2011, 25, 1–11. [Google Scholar]
- Sowmya, S.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Tri-Layered Nanocomposite Hydrogel Scaffold for the Concurrent Regeneration of Cementum, Periodontal Ligament, and Alveolar Bone. Adv. Healthc. Mater. 2017, 6, 1601251. [Google Scholar] [CrossRef]
- Uskoković, V.; Abuna, G.; Ferreira, P.; WU, V.M.; Gower, L.; Pires-de-Souza, F.C.P.; Murata, R.M.; Sinhoreti, M.A.C.; Geraldeli, S. Synthesis and characterization of nanoparticulate niobium- and zinc-doped bioglass-ceramic/chitosan hybrids for dental applications. J. Sol-Gel Sci. Technol. 2021, 97, 245–258. [Google Scholar] [CrossRef]
- Srinivasan, S.; Jayasree, R.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Biocompatible alginate/nano bioactive glass ceramic composite scaffolds for periodontal tissue regeneration. Carbohydr. Polym. 2012, 87, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Esfahanizadeh, N.; Nourani, M.R.; Bahador, A.; Akhondi, N.; Montazeri, M. The Anti-biofilm Activity of Nanometric Zinc doped Bioactive Glass against Putative Periodontal Pathogens: An in vitro Study. Biomed. Glasses 2020, 4, 95–107. [Google Scholar] [CrossRef]
- Caridade, S.G.; Merino, E.G.; Martins, G.V.; Luz, G.M.; Alves, N.M.; Mano, J.F. Membranes of poly[dl-lactic acid]/Bioglass® with asymmetric bioactivity for biomedical applications. J. Bioact. Compat. Polym. 2012, 27, 429–440. [Google Scholar] [CrossRef]
- Moonesi Rad, R.; Atila, D.; Evis, Z.; Keskin, D.; Tezcaner, A. Development of a novel functionally graded membrane containing boron-modified bioactive glass nanoparticles for guided bone regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 1331–1345. [Google Scholar] [CrossRef]
- Mota, J.; Yu, N.; Caridade, S.G.; Luz, G.M.; Gomes, M.E.; Reis, R.L.; Jansen, J.A.; Walboomers, X.F.; Mano, J.F. Chitosan/bioactive glass nanoparticle composite membranes for periodontal regeneration. Acta Biomater. 2012, 8, 4173–4180. [Google Scholar] [CrossRef]
- Ruiz-Clavijo, A.; Hurt, A.P.; Kotha, A.K.; Coleman, N.J. Effect of calcium precursor on the bioactivity and biocompatibility of sol–gel-derived glasses. J. Funct. Biomater. 2019, 10, 13. [Google Scholar] [CrossRef]
- Shah, A.T.; Zahid, S.; Ikram, F.; Maqbool, M.; Chaudhry, A.A.; Rahim, M.I.; Schmidt, F.; Goerke, O.; Khan, A.S.; Ur Rehman, I. Tri-layered functionally graded membrane for potential application in periodontal regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109812. [Google Scholar] [CrossRef]
- Sunandhakumari, V.J.; Vidhyadharan, A.K.; Alim, A.; Kumar, D.; Ravindran, J.; Krishna, A.; Prasad, M. Fabrication and in vitro characterization of bioactive glass/nano hydroxyapatite reinforced electrospun poly[ε-caprolactone] composite membranes for guided tissue regeneration. Bioengineering 2018, 5, 54. [Google Scholar] [CrossRef]
- Beketova, A.; Poulakis, N.; Bakopoulou, A.; Zorba, T.; Papadopoulou, L.; Christofilos, D.; Kantiranis, N.; Zachariadis, G.A.; Kontonasaki, E.; Kourouklis, G.A. Inducing bioactivity of dental ceramic/bioactive glass composites by Nd:YAG laser. Dent. Mater. 2016, 32, e284–e296. [Google Scholar] [CrossRef]
- Granel, H.; Bossard, C.; Collignon, A.-M.; Wauquier, F.; Lesieur, J.; Rochefort, G.Y.; Jallot, E.; Lao, J.; Wittrant, Y. Bioactive Glass/Polycaprolactone Hybrid with a Dual Cortical/Trabecular Structure for Bone Regeneration. ACS Appl. Bio Mater. 2019, 2, 3473–3483. [Google Scholar] [CrossRef]
- Meneses, C.C.B.; Olivi, L.T.; Carvalho, C.N.; Gavini, G.; Sipert, C.R. Cytotoxic Effect of Niobium Phosphate Glass–based Gutta-Percha Points on Periodontal Ligament Fibroblasts In Vitro. J. Endod. 2020, 46, 1297–1301. [Google Scholar] [CrossRef]
- Theocharidou, A.; Tsoptsias, K.; Kontonasaki, E.; Papadopoulou, L.; Panayiotou, C.; Paraskevopoulos, K.M.; Koidis, P. SEM observation of composite ceramic scaffolds’ surface during incubation in culture medium with or without human PDL fibroblasts. Key Eng. Mater. 2012, 493–494, 866–871. [Google Scholar] [CrossRef]
- Carvalho, S.; Oliveira, A.; Andrade, V.; De Fatima Leite, M.; Goes, A.; Pereira, M. Comparative Effect of the Ionic Products from Bioactive Glass Dissolution on the Behavior of Cementoblasts, Osteoblasts, and Fibroblasts. Key Eng. Mater. 2008, 396–398, 55–59. [Google Scholar] [CrossRef]
- Wen, C.; Bai, N.; Luo, L.; Ye, J.; Zhan, X.; Zhang, Y.; Sa, B. Structural behavior and in vitro bioactivity evaluation of hydroxyapatite-like bioactive glass based on the SiO2-CaO-P2O5 system. Ceram. Int. 2021, 47, 18094–18104. [Google Scholar] [CrossRef]
- Carvalho, M.D.; Suaid, F.F.; Santamaria, M.P.; Casati, M.Z.; Nociti, F.M., Jr.; Sallum, A.W.; Sallum, E.A. Platelet-rich plasma plus bioactive glass in the treatment of intra-bony defects: A study in dogs. J. Appl. Oral Sci. Rev. FOB 2011, 19, 82–89. [Google Scholar] [CrossRef]
- Felipe, M.E.M.C.; Andrade, P.F.; Novaes, A.B.J.; Grisi, M.F.M.; Souza, S.L.S.; Taba, M.; Palioto, D.B. Potential of bioactive glass particles of different size ranges to affect bone formation in interproximal periodontal defects in dogs. J. Periodontol. 2009, 80, 808–815. [Google Scholar] [CrossRef]
- Lee, S.-B.; Jung, U.-W.; Choi, Y.; Jamiyandorj, O.; Kim, C.S.; Lee, Y.K.; Chai, J.K.; Choi, S.H. Investigation of bone formation using calcium phosphate glass cement in beagle dogs. J. Periodontal Implant. Sci. 2010, 40, 125–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, L.; Wu, C.; Miron, R.J. Periodontal regeneration using strontium-loaded mesoporous bioactive glass scaffolds in osteoporotic rats. PLoS ONE 2014, 9, e104527. [Google Scholar] [CrossRef]
- Humagain, M.; Nayak, D.G.; Uppoor, A.S. A clinical evaluation of bioactive glass particulate in the treatment of mandibular class II furcation defects. Braz. J. Oral Sci. 2007, 6, 1450–1456. [Google Scholar]
- Cayir Keles, G.; Ozkan Cetinkaya, B.; Albayrak, D.; Koprulu, H.; Acikgoz, G. Comparison of platelet pellet and bioactive glass in periodontal regenerative therapy. Acta Odontol. Scand. 2006, 64, 327–333. [Google Scholar] [CrossRef]
- Demir, B.; Sengün, D.; Berberoğlu, A. Clinical evaluation of platelet-rich plasma and bioactive glass in the treatment of intra-bony defects. J. Clin. Periodontol. 2007, 34, 709–715. [Google Scholar] [CrossRef]
- Cetinkaya, B.O.; Keles, G.C.; Pamuk, F.; Balli, U.; Keles, Z.P. Long-term clinical results on the use of platelet concentrate in the treatment of intrabony periodontal defects. Acta Odontol. Scand. 2014, 72, 92–98. [Google Scholar] [CrossRef]
- Kaur, M.; Ramakrishnan, T.; Amblavanan, N.; Emmadi, P. Effect of platelet-rich plasma and bioactive glass in the treatment of intrabony defects—A split-mouth study in humans. Braz. J. Oral Sci. 2010, 9, 108–114. [Google Scholar]
- Katuri, K.; Kumar, P.; Swarna, C.; Swamy, D.; Arun, K. Evaluation of bioactive glass and demineralized freeze dried bone allograft in the treatment of periodontal intraosseous defects: A comparative clinico-radiographic study. J. Indian Soc. Periodontol. 2013, 17, 367–372. [Google Scholar] [CrossRef]
- Sculean, A.; Pietruska, M.; Arweiler, N.B.; Auschill, T.M.; Nemcovsky, C. Four-year results of a prospective-controlled clinical study evaluating healing of intra-bony defects following treatment with an enamel matrix protein derivative alone or combined with a bioactive glass. J. Clin. Periodontol. 2007, 34, 507–513. [Google Scholar] [CrossRef]
- Kumar, P.G.; Kumar, J.A.; Anumala, N.; Reddy, K.P.; Avula, H.; Hussain, S.N. Volumetric analysis of intrabony defects in aggressive periodontitis patients following use of a novel composite alloplast: A pilot study. Quintessence Int. 2011, 42, 375–384. [Google Scholar]
- Satyanarayana, K.V.; Anuradha, B.R.; Srikanth, G.; Chandra Mohan, P.; Anupama, T.; Durga Prasad, M. Clinical evaluation of intrabony defects in localized aggressive periodontitis patients with and without bioglass—An In-vivo study. Kathmandu Univ. Med. J. 2012, 10, 11–15. [Google Scholar] [CrossRef]
- Subbaiah, R.; Thomas, B. Efficacy of a bioactive alloplast, in the treatment of human periodontal osseous defects-a clinical study. Med. Oral Patol. Oral Y Cir. Bucal 2011, 16, e239–e244. [Google Scholar] [CrossRef]
- Mistry, S.; Kundu, D.; Datta, S.; Basu, D. Effects of bioactive glass, hydroxyapatite and bioactive glass-Hydroxyapatite composite graft particles in the treatment of infrabony defects. J. Indian Soc. Periodontol. 2012, 16, 241–246. [Google Scholar] [CrossRef]
- Lysenko, O.; Borysenko, A. Bioactive Glass-Ceramic Composition In Surgical Management of Periodontal Intrabony Defects. Georgian Med. News 2019, 295, 34–41. [Google Scholar]
- Slezák, R.; Paulusová, V. Use of the NovaBone augmentation material in the treatment of chronic periodontitis. Preliminary communication. Acta Med. [Hradec Králové]/Univ. Carol. Fac. Med. Hradec Králové 2013, 56, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Grover, V.; Kapoor, A.; Malhotra, R.; Uppal, R.S. Evaluation of the efficacy of a bioactive synthetic graft material in the treatment of intrabony periodontal defects. J. Indian Soc. Periodontol. 2013, 17, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Bains, V.K.; Singh, G.P.; Jhingran, R. Clinical and cone beam computed tomography comparison of NovaBone dental putty and perioglas in the treatment of mandibular class II furcations. Indian J. Dent. Res. 2014, 25, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Sambashivaiah, S.; Kulal, R.; Bilichodmath, S.; Kurtzman, G.M. Comparative evaluation of bioactive glass [Putty] and platelet rich fibrin in treating furcation Defects. J. Oral Implantol. 2016, 42, 411–415. [Google Scholar] [CrossRef]
- Naqvi, A.; Gopalakrishnan, D.; Bhasin, M.T.; Sharma, N.; Haider, K.; Martande, S. Comparative evaluation of bioactive glass putty and platelet rich fibrin in the treatment of human periodontal intrabony defects: A randomized control trial. J. Clin. Diagn. Res. 2017, 11, ZC09–ZC13. [Google Scholar] [CrossRef]
- Saravanan, D.; Rethinam, S.; Muthu, K.; Thangapandian, A. The Combined Effect of Bioactive Glass and Platelet-Rich Fibrin in Treating Human Periodontal Intrabony Defects—A Clinicoradiographic Study. Contemp. Clin. Dent. 2019, 10, 110–116. [Google Scholar] [CrossRef]
- Koduru, S.; Aghanashini, S.; Nadiger, S.; Apoorva, S.; Bhat, D.; Puvvalla, B. A clinical and radiographic evaluation of the efficacy of nanohydroxyapatite [SybografTM] versus bioactive calcium phosphosilicate putty [Novabone®] in the treatment of human periodontal infrabony defects: A randomized clinical trial. Contemp. Clin. Dent. 2019, 10, 16–23. [Google Scholar] [CrossRef]
- Bansal, A.; Kulloli, A.; Kathariya, R.; Shetty, S.; Jain, H.; Raikar, S. Comparative Evaluation of Coronally Advanced Flap with and without Bioactive Glass Putty in the Management of Gingival Recession Defects: A Randomized Controlled Clinical Trial. J. Int. Acad. Periodontol. 2016, 18, 7–15. [Google Scholar]
- Allan, I.; Newman, H.; Wilson, M. Particulate Bioglass reduces the viability of bacterial biofilms formed on its surface in an in vitro model. Clin. Oral Implant. Res. 2002, 13, 53–58. [Google Scholar] [CrossRef]
- Zhang, D.; Hupa, M.; Hupa, L. In situ pH within particle beds of bioactive glasses. Acta Biomater. 2008, 4, 1499–1505. [Google Scholar] [CrossRef]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent Evidence on Bioactive Glass Antimicrobial and Antibiofilm Activity: A Mini-Review. Materials 2018, 11, 326. [Google Scholar] [CrossRef]
- Melcher, A.H. Cells of periodontium: Their role in the healing of wounds. Ann. R. Coll. Surg. Engl. 1985, 67, 130–131. [Google Scholar]
- Lusvardi, G.; Zaffe, D.; Menabue, L.; Bertoldi, C.; Malavasi, G.; Consolo, U. In vitro and in vivo behaviour of zinc-doped phosphosilicate glasses. Acta Biomater. 2009, 5, 419–428. [Google Scholar] [CrossRef]
- Samira, J.; Saoudi, M.; Abdelmajid, K.; Hassane, O.; Treq, R.; Hafed, E.; Abdelfatteh, E.; Hassib, K. Accelerated bone ingrowth by local delivery of Zinc from bioactive glass: Oxidative stress status, mechanical property, and microarchitectural characterization in an ovariectomized rat model. Libyan J. Med. 2015, 10, 28572. [Google Scholar] [CrossRef]
- Bertoldi, C.; Venuta, M.; Guaraldi, G.; Lalla, M.; Guaitolini, S.; Generali, L.; Monzani, D.; Cortellini, P.; Zaffe, D. Are periodontal outcomes affected by personality patterns? A 18-month follow-up study. Acta Odontol. Scand. 2018, 76, 48–57. [Google Scholar] [CrossRef]
- Bertoldi, C.; Pradelli, J.M.; Consolo, U.; Zaffe, D. Release of elements from retrieved maxillofacial plates and screws. J. Mater. Sci. Mater. Med. 2005, 16, 857–861. [Google Scholar] [CrossRef]
- Bertoldi, C.; Zaffe, D. In vivo comparison of two bone substitutes in the distal femur of the rabbit. Int. J. Oral Maxillofac. Implant. 2012, 27, 119–127. [Google Scholar]
- Cortellini, P.; Tonetti, M.S. Clinical concepts for regenerative therapy in intrabony defects. Periodontology 2000 2015, 68, 282–307. [Google Scholar] [CrossRef]
- Bertoldi, C.; Ferrari, M.; Giannetti, L. The use of only enamel matrix derivative allows outstanding regeneration results in periodontal intrabony defect treatment: A retrospective study. J. Biol. Regul. Homeost. Agents 2019, 33, 633–636. [Google Scholar]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.L.; Giannobile, W.V. Biologics-based regenerative technologies for periodontal soft tissue engineering. J. Periodontol. 2020, 91, 147–154. [Google Scholar] [CrossRef]
- Chambrone, L.; Ortega, M.A.S.; Sukekava, F.; Rotundo, R.; Kalemaj, Z.; Buti, J.; Pini Prato, G.P. Root coverage procedures for treating single and multiple recession-type defects: An updated Cochrane systematic review. J. Periodontol. 2019, 90, 1399–1422. [Google Scholar] [CrossRef]
- Pellegrini, G.; Pagni, G.; Rasperini, G. Surgical approaches based on biological objectives: GTR versus GBR techniques. Int. J. Dent. 2013, 2013, 521547. [Google Scholar] [CrossRef] [PubMed]
- Mir-Mari, J.; Wui, H.; Jung, R.E.; Hämmerle, C.H.F.; Benic, G.I. Influence of blinded wound closure on the volume stability of different GBR materials: An in vitro cone-beam computed tomographic examination. Clin. Oral Implant. Res. 2016, 27, 258–265. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R. Clinical outcomes with bioactive agents alone or in combination with grafting or guided tissue regeneration. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 117–135. [Google Scholar] [CrossRef]
- Cortellini, P.; Tonetti, M.S. Improved wound stability with a modified minimally invasive surgical technique in the regenerative treatment of isolated interdental intrabony defects. J. Clin. Periodontol. 2009, 36, 157–163. [Google Scholar] [CrossRef]
- Trombelli, L.; Simonelli, A.; Pramstraller, M.; Wikesjö, U.M.E.; Farina, R. Single Flap Approach with and without Guided Tissue Regeneration and a Hydroxyapatite Biomaterial in the Management of Intraosseous Periodontal Defects. J. Periodontol. 2010, 81, 1256–1263. [Google Scholar] [CrossRef]
- Bertoldi, C.; Monari, E.; Cortellini, P.; Gnerali, L.; Lucchi, A.; Spinato, S.; Zaffe, D. Clinical and histological reaction of periodontal tissues to subgingival resin composite restorations. Clin. Oral Investig. 2020, 24, 1001–1011. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannillo, V.; Salvatori, R.; Bergamini, S.; Bellucci, D.; Bertoldi, C. Bioactive Glasses in Periodontal Regeneration: Existing Strategies and Future Prospects—A Literature Review. Materials 2022, 15, 2194. https://doi.org/10.3390/ma15062194
Cannillo V, Salvatori R, Bergamini S, Bellucci D, Bertoldi C. Bioactive Glasses in Periodontal Regeneration: Existing Strategies and Future Prospects—A Literature Review. Materials. 2022; 15(6):2194. https://doi.org/10.3390/ma15062194
Chicago/Turabian StyleCannillo, Valeria, Roberta Salvatori, Stefania Bergamini, Devis Bellucci, and Carlo Bertoldi. 2022. "Bioactive Glasses in Periodontal Regeneration: Existing Strategies and Future Prospects—A Literature Review" Materials 15, no. 6: 2194. https://doi.org/10.3390/ma15062194
APA StyleCannillo, V., Salvatori, R., Bergamini, S., Bellucci, D., & Bertoldi, C. (2022). Bioactive Glasses in Periodontal Regeneration: Existing Strategies and Future Prospects—A Literature Review. Materials, 15(6), 2194. https://doi.org/10.3390/ma15062194










