In Vitro Direct and Indirect Cytotoxicity Comparative Analysis of One Pre-Hydrated versus One Dried Acellular Porcine Dermal Matrix
Abstract
:1. Introduction
2. Materials and Methods
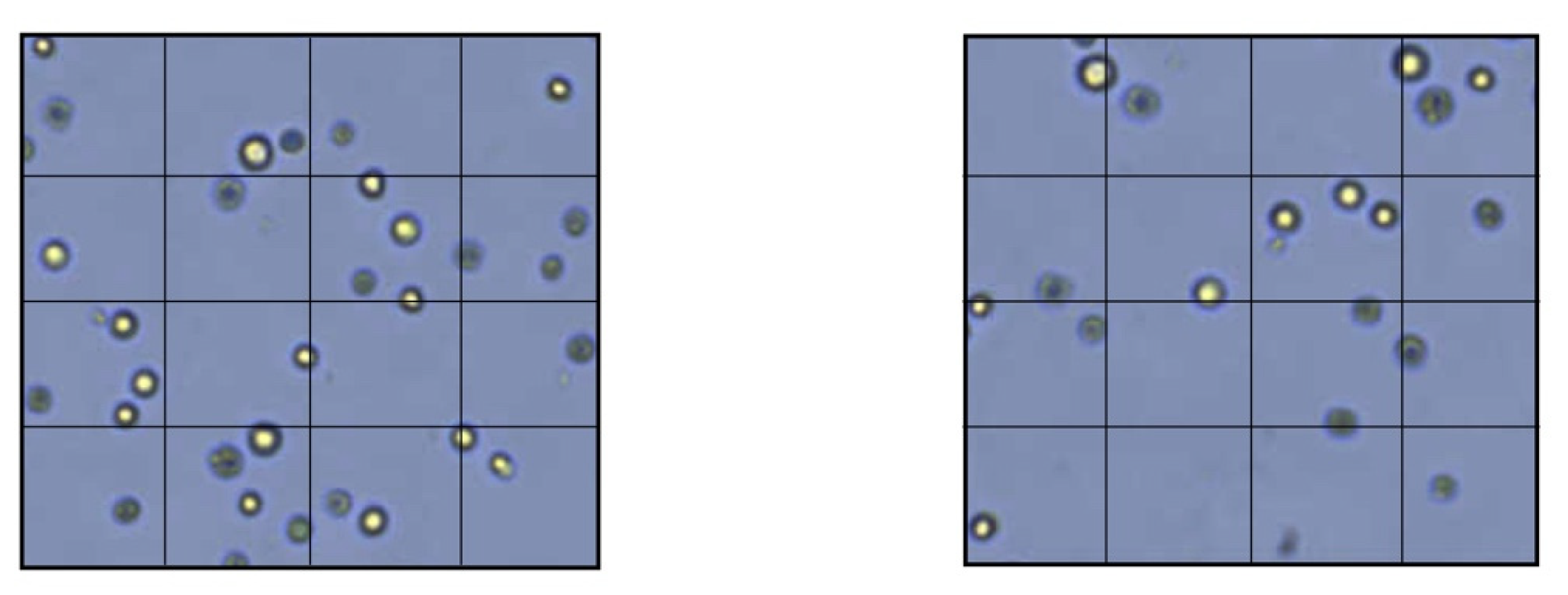
2.1. Direct Cytotoxicity Tests
2.2. Indirect Cytotoxicity Tests
2.3. Statistical Analysis
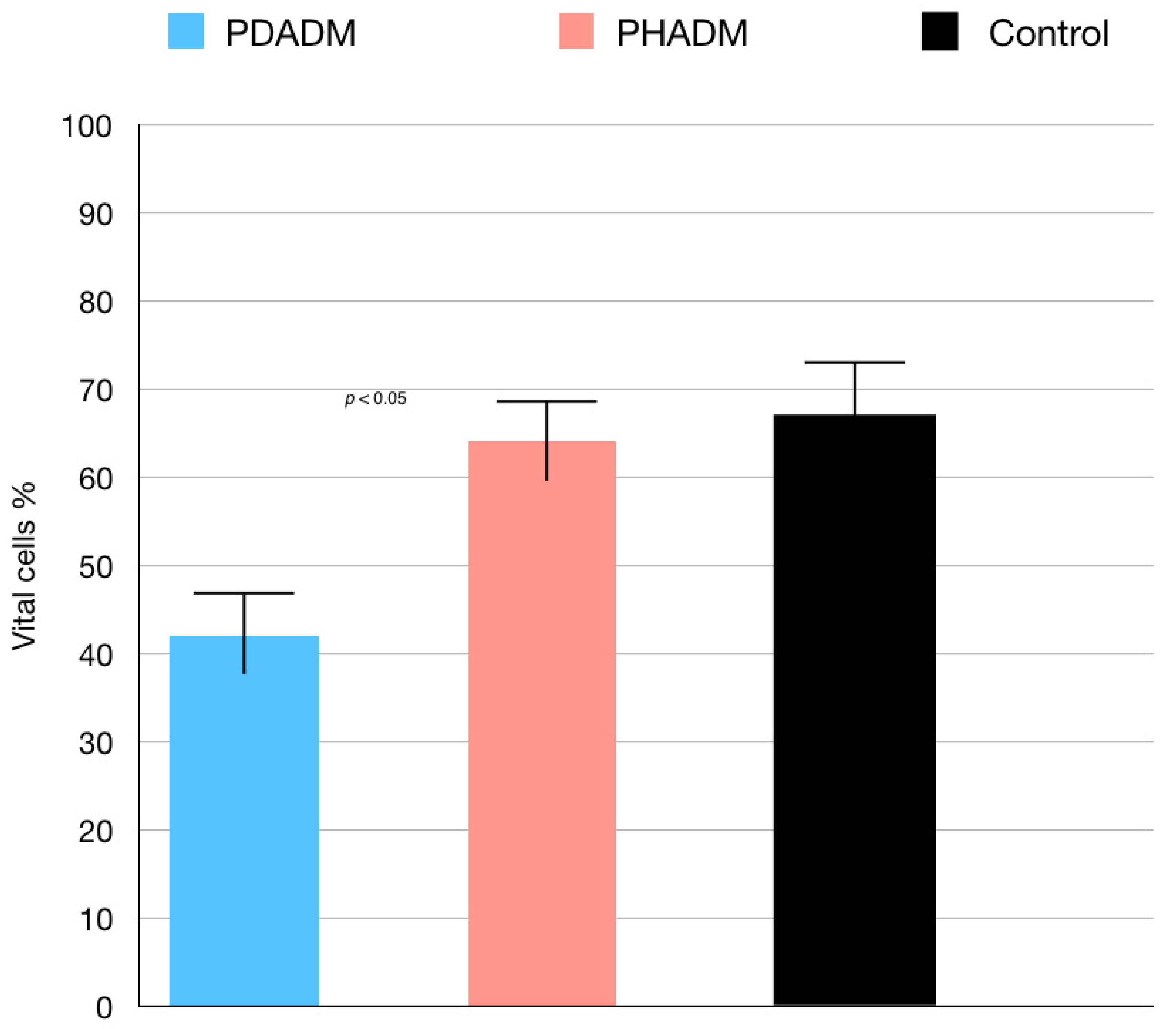
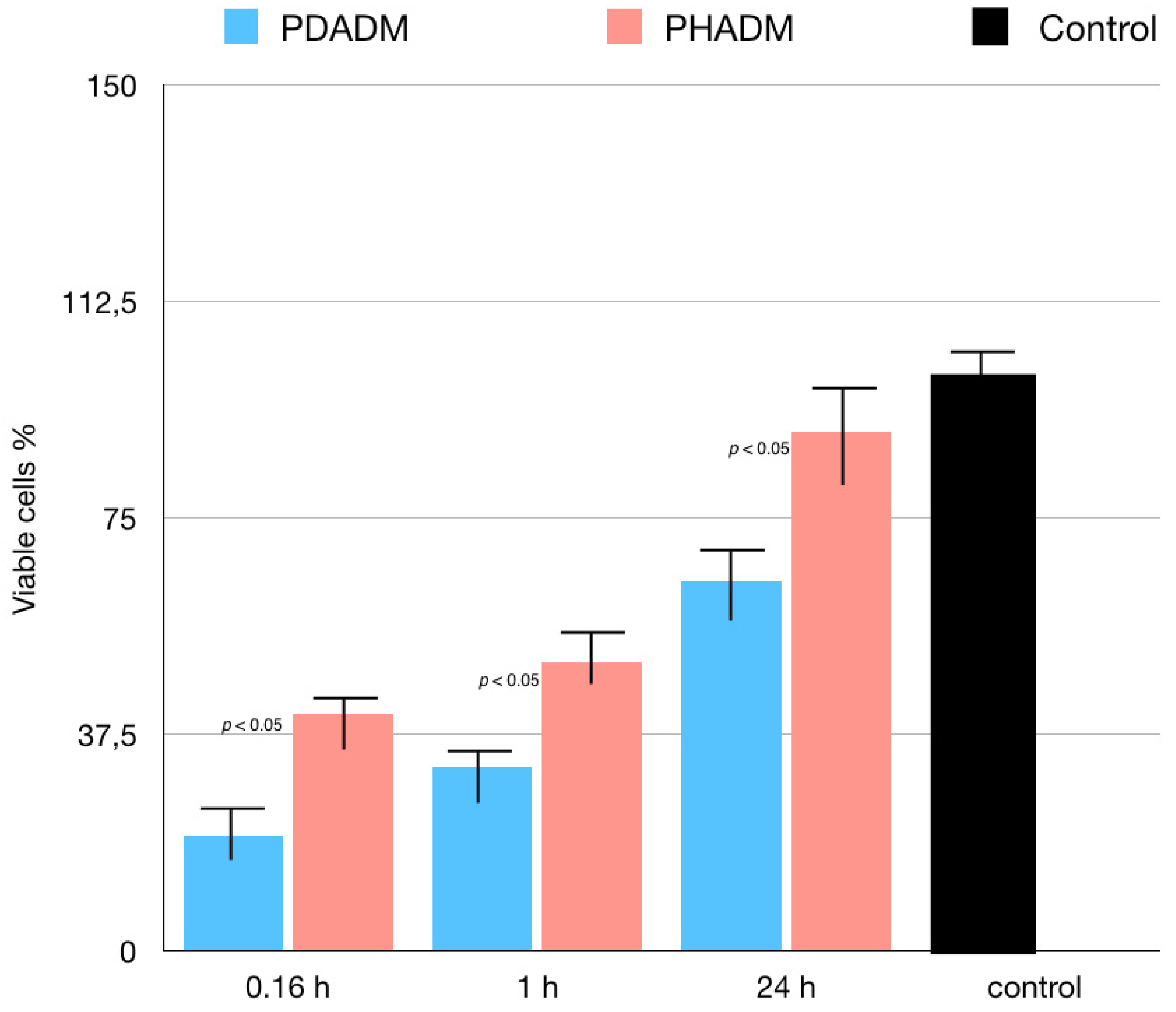
3. Results
Indirect Cytotoxicity Tests: LDH and MTT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Del Amo, F.S.L.; Yu, S.H.; Sammartino, G.; Sculean, A.; Zucchelli, G.; Rasperini, G.; Felice, P.; Pagni, G.; Iorio-Siciliano, V.; Grusovin, M.G.; et al. Peri-implant Soft Tissue Management: Cairo Opinion Consensus Conference. Int. J. Environ. Res. Public Health 2020, 17, 2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herford, A.S.; Akin, L.; Cicciu, M.; Maiorana, C.; Boyne, P.J. Use of a Porcine Collagen Matrix as an Alternative to Autogenous Tissue for Grafting Oral Soft Tissue Defects. J. Oral Maxillofac. Surg. 2010, 68, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W.; Sixma, J.J.; Barnes, M.J.; De Groot, P.G. The role of collagen in thrombosis and hemostasis. J. Thromb. Haemost. 2004, 2, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Schwarz, F.; Sculean, A.; Herten, M.; Scherbaum, W.; Becker, J. Biocompatibility of various collagen membranes in cultures of human PDL fibroblasts and human osteoblast-like cells. Clin. Oral Implant. Res. 2004, 15, 443–449. [Google Scholar] [CrossRef]
- Thibault, M.M.; Hoemann, C.; Buschmann, M. Fibronectin, Vitronectin, and Collagen I Induce Chemotaxis and Haptotaxis of Human and Rabbit Mesenchymal Stem Cells in a Standardized Transmembrane Assay. Stem Cells Dev. 2007, 16, 489–502. [Google Scholar] [CrossRef]
- Kumar, V.A.; Taylor, N.L.; Jalan, A.A.; Hwang, L.K.; Wang, B.K.; Hartgerink, J.D. A Nanostructured Synthetic Collagen Mimic for Hemostasis. Biomacromolecules 2014, 15, 1484–1490. [Google Scholar] [CrossRef]
- Asparuhova, M.B.; Stähli, A.; Guldener, K.; Sculean, A. A Novel Volume-Stable Collagen Matrix Induces Changes in the Behavior of Primary Human Oral Fibroblasts, Periodontal Ligament, and Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 4051. [Google Scholar] [CrossRef]
- Twardowski, T.; Fertala, A.; Orgel, J.; Antonio, J.S. Type I Collagen and Collagen Mimetics as Angiogenesis Promoting Superpolymers. Curr. Pharm. Des. 2007, 13, 3608–3621. [Google Scholar] [CrossRef]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.-L.; Giannobile, W.V. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef]
- Caballé-Serrano, J.; Zhang, S.; Ferrantino, L.; Simion, M.; Chappuis, V.; Bosshardt, D.D. Tissue Response to a Porous Collagen Matrix Used for Soft Tissue Augmentation. Materials 2019, 12, 3721. [Google Scholar] [CrossRef] [Green Version]
- Imber, J.C.; Bosshardt, D.D.; Stähli, A.; Saulacic, N.; Deschner, J.; Sculean, A. Pre-clinical evaluation of the effect of a volume-stable collagen matrix on periodontal regeneration in two-wall intrabony defects. J. Clin. Periodontol. 2021, 48, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Caballé-Serrano, J.; Zhang, S.; Sculean, A.; Staehli, A.; Bosshardt, D.D. Tissue Integration and Degradation of a Porous Collagen-Based Scaffold Used for Soft Tissue Augmentation. Materials 2020, 13, 2420. [Google Scholar] [CrossRef] [PubMed]
- Pabst, A.M.; Happe, A.; Callaway, A.; Ziebart, T.; Stratul, S.-I.; Ackermann, M.; Konerding, M.A.; Willershausen, B.; Kasaj, A. In vitro and in vivo characterization of porcine acellular dermal matrix for gingival augmentation procedures. J. Periodontal Res. 2013, 49, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Pabst, A.M.; Ackermann, M.; Moergel, M.; Jung, J.; Kasaj, A. Biofunctionalization of Porcine-Derived Collagen Matrix Using Enamel Matrix Derivative and Platelet-Rich Fibrin: Influence on Mature Endothelial Cell Characteristics In Vitro. Clin. Oral Investig. 2018, 22, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Blatt, S.; Burkhardt, V.; Kämmerer, P.W.; Pabst, A.M.; Sagheb, K.; Heller, M.; Al-Nawas, B.; Schiegnitz, E. Biofunctionalization of porcine-derived collagen matrices with platelet rich fibrin: Influence on angiogenesis in vitro and in vivo. Clin. Oral Investig. 2020, 24, 3425–3436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Nica, C.; Sculean, A.; Asparuhova, M.B. Positive Effects of Three-Dimensional Collagen-Based Matrices on the Behavior of Osteoprogenitors. Front. Bioeng. Biotechnol. 2021, 9, 708830. [Google Scholar] [CrossRef]
- Del Amo, F.S.-L.; Rodriguez, J.C.; Asa’Ad, F.; Wang, H.-L. Comparison of two soft tissue substitutes for the treatment of gingival recession defects: An animal histological study. J. Appl. Oral Sci. 2019, 27, e20180584. [Google Scholar] [CrossRef]
- Lima, R.S.; Peruzzo, D.C.; Napimoga, M.H.; Saba-Chujfi, E.; Dos Santos-Pereira, S.A.; Martinez, E.F. Evaluation of the Biological Behavior of Mucograft® in Human Gingival Fibroblasts: An In Vitro Study. Braz. Dent. J. 2015, 26, 602–606. [Google Scholar] [CrossRef] [Green Version]
- Nica, C.; Lin, Z.; Sculean, A.; Asparuhova, M.B. Adsorption and Release of Growth Factors from Four Different Porcine-Derived Collagen Matrices. Materials 2020, 13, 2635. [Google Scholar] [CrossRef]
- Vallecillo, C.; Toledano-Osorio, M.; Vallecillo-Rivas, M.; Toledano, M.; Osorio, R. In Vitro Biodegradation Pattern of Collagen Matrices for Soft Tissue Augmentation. Polymers 2021, 13, 2633. [Google Scholar] [CrossRef]
- Liu, Q.; Humpe, A.; Kletsas, D.; Warnke, F.; Becker, S.T.; Douglas, T.; Sivananthan, S.; Warnke, P.H. Proliferation assessment of primary human mesenchymal stem cells on collagen membranes for guided bone regeneration. Int. J. Oral Maxillofac. Implants 2011, 26, 1004–1010. [Google Scholar] [PubMed]
- Bartmann, C.; Rohde, E.; Schallmoser, K.; Pürstner, P.; Lanzer, G.; Linkesch, W.; Strunk, D. Two steps to functional mesenchymal stromal cells for clinical application. Transfusion 2007, 47, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Yazdanian, M.; Arefi, A.H.; Alam, M.; Abbasi, K.; Tebyaniyan, H.; Tahmasebi, E.; Ranjbar, R.; Seifalian, A.; Rahbar, M. Decellularized and biological scaffolds in dental and craniofacial tissue engineering: A comprehensive overview. J. Mater. Res. Technol. 2021, 15, 1217–1251. [Google Scholar] [CrossRef]
- Lin, Z.; Nica, C.; Sculean, A.; Asparuhova, M.B. Enhanced Wound Healing Potential of Primary Human Oral Fibroblasts and Periodontal Ligament Cells Cultured on Four Different Porcine-Derived Collagen Matrices. Materials 2020, 13, 3819. [Google Scholar] [CrossRef]
- Soundararajan, M.; Kannan, S. Fibroblasts and mesenchymal stem cells: Two sides of the same coin? J. Cell Physiol. 2018, 233, 9099–9109. [Google Scholar] [CrossRef]
- Zupan, J. Mesenchymal Stem/Stromal Cells and Fibroblasts: Their Roles in Tissue Injury and Regeneration, and Age-Related Degeneration. In Fibroblasts—Advances in Inflammation, Autoimmunity and Cancer; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [Green Version]
- Kirsner, R.S.; Eaglstein, W.H. The wound healing process. Dermatol. Clin. 1993, 11, 629–640. [Google Scholar] [CrossRef]
- Hess, C.T.; Kirsner, R.S. Orchestrating wound healing: Assessing and preparing the wound bed. Adv. Skin Wound Care 2003, 16, 246–257. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.P.; Kirsner, R.S. Angiogenesis in wound repair: Angiogenic growth factors and the extracellular matrix. Microsc. Res. Tech. 2003, 60, 107–114. [Google Scholar] [CrossRef]
- Wang, A.Y.; Leong, S.; Liang, Y.-C.; Huang, R.C.C.; Chen, C.S.; Yu, S.M. Immobilization of growth factors on collagen scaffolds mediated by polyanionic collagen mimetic peptides and its effect on endothelial cell morphogenesis. Biomacromolecules 2008, 9, 2929–2936. [Google Scholar] [CrossRef]
- Kasaj, A.; Levin, L.; Stratul, S.I.; Götz, H.; Schlee, M.; Rütters, C.B.; Konerding, M.A.; Ackermann, M.; Willershausen, B.; Pabst, A.M. The influence of various rehydration protocols on biomechanical properties of different acellular tissue matrices. Clin. Oral Investig. 2016, 20, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Jose, M.V.; Thomas, V.; Dean, D.R.; Janowski, G.M. Freeze-dried acellular dermal matrix graft: Effects of rehydration on physical, chemical, and mechanical properties. Dent. Mater. 2009, 25, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.; da Veiga Moreira, J.; Jolicoeur, M. Physical forces modulate cell differentiation and proliferation processes. J. Cell Mol. Med. 2018, 22, 738–745. [Google Scholar] [CrossRef] [Green Version]
- Roffay, C.; Molinard, G.; Kim, K.; Urbanska, M.; Andrade, V.; Barbarasa, V.; Nowak, P.; Mercier, V.; García-Calvo, J.; Matile, S.; et al. Passive coupling of membrane tension and cell volume during active response of cells to osmosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2103228118. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Koster, D.; Ruez, R.; Gonnord, P.; Bastiani, M.; Abankwa, D.; Stan, R.V.; Butler-Browne, G.; Vedie, B.; Johannes, L.; et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 2011, 144, 402–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diz-Muñoz, A.; Thurley, K.; Chintamen, S.; Altschuler, S.J.; Wu, L.F.; Fletcher, D.A.; Weiner, O.D. Membrane tension acts through PLD2 and mTORC2 to limit actin network assembly during neutrophil migration. PLoS Biol. 2016, 14, e1002474. [Google Scholar] [CrossRef] [Green Version]
- Erickson, G.R.; Alexopoulos, L.; Guilak, F. Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. J. Biomech. 2001, 34, 1527–1535. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarnieri, R.; Reda, R.; Di Nardo, D.; Miccoli, G.; Zanza, A.; Testarelli, L. In Vitro Direct and Indirect Cytotoxicity Comparative Analysis of One Pre-Hydrated versus One Dried Acellular Porcine Dermal Matrix. Materials 2022, 15, 1937. https://doi.org/10.3390/ma15051937
Guarnieri R, Reda R, Di Nardo D, Miccoli G, Zanza A, Testarelli L. In Vitro Direct and Indirect Cytotoxicity Comparative Analysis of One Pre-Hydrated versus One Dried Acellular Porcine Dermal Matrix. Materials. 2022; 15(5):1937. https://doi.org/10.3390/ma15051937
Chicago/Turabian StyleGuarnieri, Renzo, Rodolfo Reda, Dario Di Nardo, Gabriele Miccoli, Alessio Zanza, and Luca Testarelli. 2022. "In Vitro Direct and Indirect Cytotoxicity Comparative Analysis of One Pre-Hydrated versus One Dried Acellular Porcine Dermal Matrix" Materials 15, no. 5: 1937. https://doi.org/10.3390/ma15051937
APA StyleGuarnieri, R., Reda, R., Di Nardo, D., Miccoli, G., Zanza, A., & Testarelli, L. (2022). In Vitro Direct and Indirect Cytotoxicity Comparative Analysis of One Pre-Hydrated versus One Dried Acellular Porcine Dermal Matrix. Materials, 15(5), 1937. https://doi.org/10.3390/ma15051937









