Use of Xenogenic Collagen Matrices in Peri-Implant Soft Tissue Volume Augmentation: A Critical Review on the Current Evidence and New Technique Presentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Assessments of the Risk of Bias
2.2. Statistical Analysis
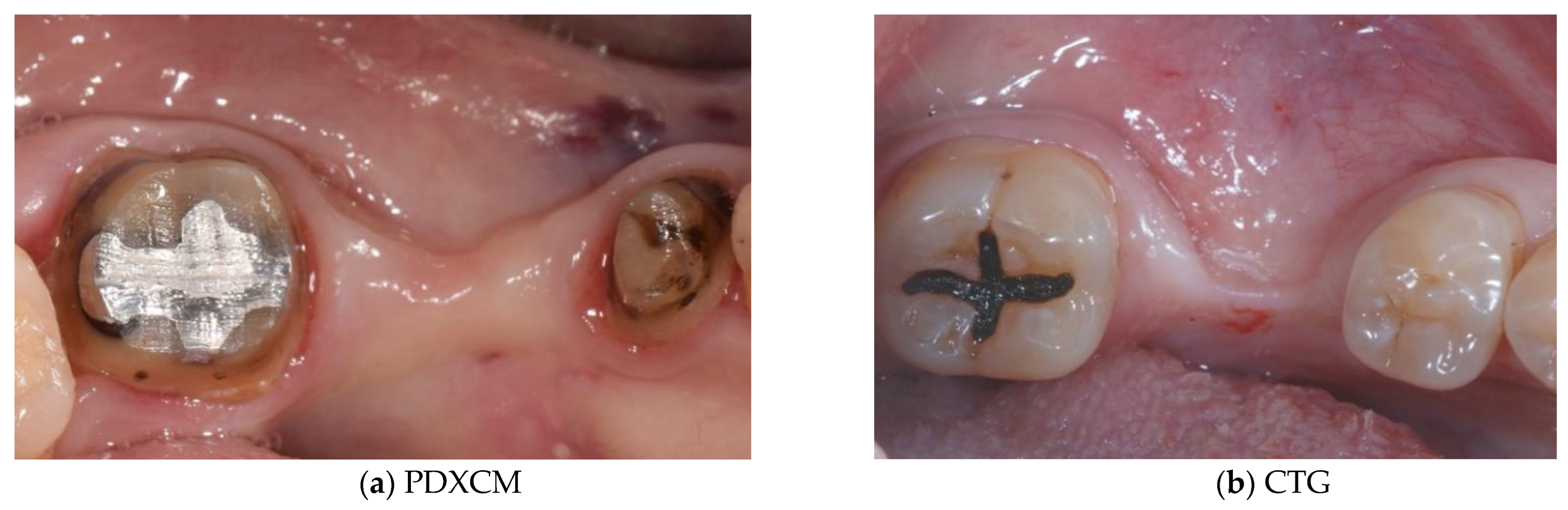
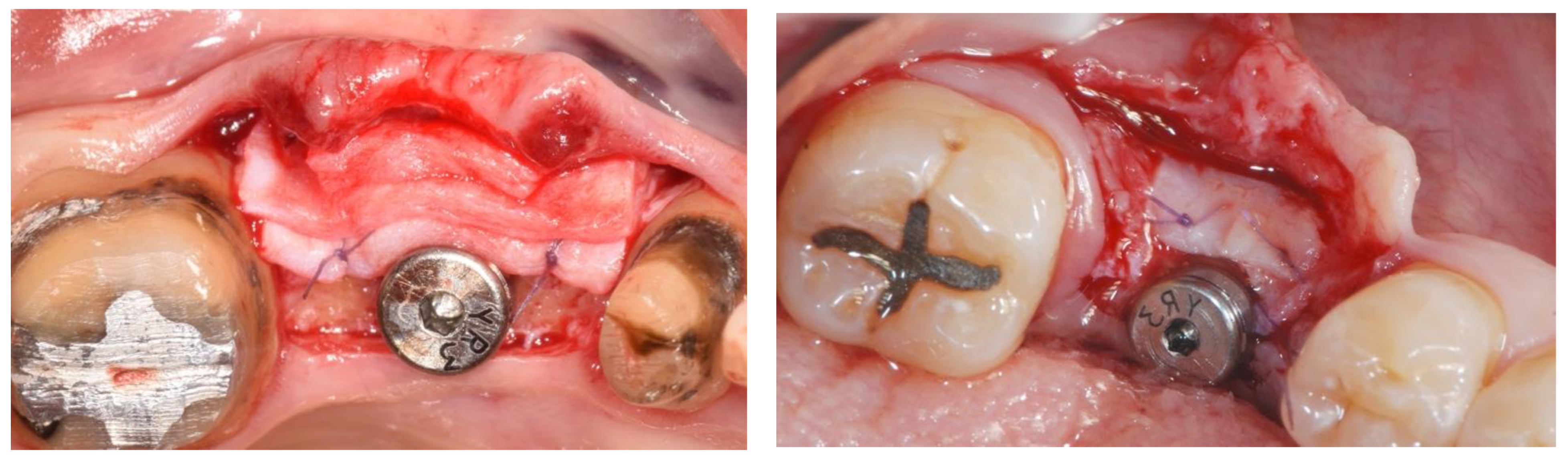
3. Results
| General Overview of the Results of RCT Which Compared XCMs (Test) Versus CTGs (Control) | ||||
|---|---|---|---|---|
| Study | PAS (VAS on a 0 to 100) | Changes in PKMW between Baseline and Final Follow-Up (mm) | Changes in PD between Baseline and Final Follow-Up (mm) | Comments by Authors |
| Sanz et al. [20] | N.R | CTG 2.6 ± 0.96 XCM 2.5 ± 0.7 | N.R. | The XCM was as effective and predictable as the CTG for attaining a band of keratinized tissue, but its use was associated with a significantly lower patient morbidity. |
| Lorenzo et al. [21] | N.R | CTG 2.33 ± 1.03 XCM 2.3 ± 0.47 | CTG 0 ± 1.03 XCM 0.4 ± 0.62 | The results of the study demonstrate that the use of XCM presented similar results to the CTG for the KM band gain. |
| Thoma et al. [22] | 0–10 | CTG 0.8 ± 1.8 o. 0.8 ± 2.2 b. 1.6 ± 2.6 a. XCM 1.4 ± 1.4 o. 1.1 ± 1.4 b. 0.9 ± 1.9 a | N.R | The XCM was as effective and predictable as the CTG for attaining a band of keratinized tissue |
| Zeitner et al. [23] | 0–10 | CTG 4.2 ± 1.9 o. 4.1 ± 2.0 b. 3.4 ± 1.8 a. XCM 3.4 ± 1.0 o. 2.9 ± 1.5 b. 2.6 ± 2.3 a. | N.R | The use of XCM and the subepithelial connective tissue graft for soft tissue augmentation at implant sites rendered a similar gain in soft tissue volume |
| Cairo et al. [24] | CTG 90 ± 9.0 XCM 90 ± 8.0 | CTG 0.9 ± 1.6 XC 1.2 ± 1.2 | CTG 2.9 ± 0.3 XCM 2.8 ± 0.2 | Similar gain in keratinized tissue and in the peri-implant soft tissue thickness |
| Puzio et al. [25] | N R | (change) CTG 1.52 ± 1.0 XCM 0.89 ± 0.6 | N R | Both XCM and CTG increase the keratinized tissue but higher values were noted using CTG |
| Huber et al. [26] | N R | CTG 3.2 ± 0.8 XCM 2.1 ± 1.2 | N R | The buccal peri-implant soft tissue dimensions at implant sites revealed only minimal changes without relevant differences between sites that had previously been grafted with XCM or CTG. |
| General overview of the results of prospective studies which investigated XCMs. | ||||
| Papi & Pompa [27] | NR | XCM 4.32 ± 1.22 | 0.38 ± 0.21 | With XCM, the keratinized tissue width can be augmented, and the width remains stable for the assessment period of 12 months. |
| Schallhorn et al. [28] | 90 ± 20 | XCM 2.1 ± 1.0 | 3.0 ± 1.6 | XCM demonstrated the potential to increase KMW and GT around existing dental implants. |
4. Discussion
| Study | Sample | Study Design | Follow-Up Time | Keratinized Mucosa Width Gain (mm) | Soft Tissue Thickness Gain (mm) |
|---|---|---|---|---|---|
| Papi et al. [27] | 12 patients | Prospective cohort study | 12 months | N R | PDXCM: 1.25 |
| Zafiropoulos et al. [38] | 27 patients | Prospective, randomized examiner-blinded controlled clinical study | 6 months | N R | PDXCM: 1.06 |
| Stefanini et al. [39] | 10 patients | Case series | 12 months | PDXCM 0.65 ± 0.41 | PDXCM: 1.2 ± 0.18 |
| Papi and Pompa 12 [40] | 12 patients | Prospective pilot cohort study | 12 months | PDXCM: 4.32 | N R |
| Schmitt et al. [41] | 14 patients | Controlled clinical trial | 6 months | N R | PDXCM: 0.30 ± 0.16 |
| Verardi et al. [42] | 24 patients 24 implants | Prospective study | 6 months | PDXCM 1.33 ± 0.71 | N R |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salvi, G.E.; Bosshardt, D.D.; Lang, N.P.; Abrahamsson, I.; Berglundh, T.; Lindhe, J.; Ivanovski, S.; Donos, N. Temporal sequence of hard and soft tissue healing around titanium dental implants. Periodontology 2000 2015, 68, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.I.; Barootchi, S.; Tavelli, L.; Wang, H.L. The peri-implant phenotype and implant esthetic complications. Contemporary overview. J. Esthet. Restor. Dent. 2021, 33, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018, 45, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Gobbato, L.; Avila-Ortiz, G.; Sohrabi, K.; Wang, C.-W.; Karimbux, N. The effect of keratinized mucosa width on peri-implant health: A systematic review. Int. J. Oral Maxillofac. Implants 2013, 28, 1536–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila-Ortiz, G.; Gonzalez-Martin, O.; Couso-Queiruga, E.; Wang, H.L. The peri-implant phenotype. J. Periodontol. 2020, 91, 283–288. [Google Scholar] [CrossRef]
- Giannobile, W.V.; Jung, R.E.; Schwarz, F. Evidence-based knowledge on the aesthetics and maintenance of peri-implant soft tissues: Osteology foundation consensus report part 1—Effects of soft tissue augmentation procedures on the maintenance of peri-implant soft tissue health. Clin. Oral Implants Res. 2018, 29, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.H.; Chan, H.L.; Wang, H.L. The significance of keratinized mucosa on implant health: A systematic review. J. Periodontol. 2013, 84, 1755–1767. [Google Scholar] [CrossRef]
- Wennström, J.L.; Derks, J. Is there a need for keratinized mucosa around implants to maintain health and tissue stability? Clin. Oral Implants Res. 2012, 23, 136–146. [Google Scholar] [CrossRef]
- Adibrad, M.; Shahabuei, M.; Sahabi, M. Significance of the width of keratinized mucosa on the health status of the supporting tissue around implants supporting overdentures. J. Oral Implantol. 2009, 35, 232–237. [Google Scholar] [CrossRef]
- Perussolo, J.; Souza, A.B.; Matarazzo, F.; Oliveira, R.P.; Araújo, M.G. Influence of the keratinized mucosa on the stability of peri-implant tissues and brushing discomfort: A 4-year follow-up study. Clin. Oral Implants Res. 2018, 29, 1177–1185. [Google Scholar] [CrossRef]
- Zigdon, H.; Machtei, E.E. The dimensions of keratinized mucosa around implants affect clinical and immunological parameters. Clin. Oral Implants Res. 2008, 19, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Grischke, J.; Karch, A.; Wenzlaff, A.; Foitzik, M.M.; Stiesch, M.; Eberhard, J. Keratinized mucosa width is associated with severity of peri-implant mucositis. A cross-sectional study. Clin. Oral Implants Res. 2019, 30, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Becker, J.; Civale, S.; Sahin, D.; Iglhaut, T.; Iglhaut, G. Influence of the width of keratinized tissue on the development and resolution of experimental peri-implant mucositis lesions in humans. Clin. Oral Implants Res. 2018, 29, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Penarrocha-Oltra, D.; Covani, U.; Botticelli, D.; Serino, G.; Penarrocha, M. Clinical and microbiological findings in patients with peri-implantitis: A cross-sectional study. Clin. Oral Implants Res. 2016, 27, 376–382. [Google Scholar] [CrossRef]
- Park, S.E.; Da Silva, J.D.; Weber, H.P.; Ishikawa-Nagai, S. Optical phenomenon of peri-implant soft tissue. Part I. Spectrophotometric assessment of natural tooth gingiva and peri-implant mucosa. Clin. Oral Implants Res. 2007, 18, 569–574. [Google Scholar] [CrossRef]
- Bressan, E.; Paniz, G.; Lops, D.; Corazza, B.; Romeo, E.; Favero, G. Influence of abutment material on the gingival color of implant-supported all-ceramic restorations: A prospective multicenter study. Clin. Oral Implants Res. 2011, 22, 631–637. [Google Scholar] [CrossRef]
- Jung, R.E.; Holderegger, C.; Sailer, I.; Khraisat, A.; Suter, A.; Hämmerle, C.H. The effect of all-ceramic and porcelain-fused-to-metal restorations on marginal peri-implant soft tissue color: A randomized controlled clinical trial. Int. J. Periodontics Restor. Dent. 2008, 28, 357–365. [Google Scholar]
- Kim, A.; Campbell, S.D.; Viana, M.A.; Knoernschild, K.L. Abutment material effect on peri-implant soft tissue color and perceived esthetics. J. Prosthodont. 2016, 25, 634–640. [Google Scholar] [CrossRef]
- Del Amo, F.S.L.; Yu, S.H.; Sammartino, G.; Sculean, A.; Zucchelli, G.; Rasperini, G.; Felice, P.; Pagni, G.; Iorio-Siciliano, V.; Grusovin, M.G.; et al. Peri-implant Soft Tissue Management: Cairo Opinion Consensus Conference. Int. J. Environ. Res. Public Health 2020, 17, 2281. [Google Scholar] [CrossRef] [Green Version]
- Sanz, M.; Lorenzo, R.; Aranda, J.J.; Martin, C.; Orsini, M. Clinical evaluation of a new collagen matrix (Mucograft prototype) to enhance the width of keratinized tissue in patients with fixed prosthetic restorations: A randomized prospective clinical trial. J. Clin. Periodontol. 2009, 36, 868–876. [Google Scholar] [CrossRef]
- Lorenzo, R.; García, V.; Orsini, M.; Martin, C.; Sanz, M. Clinical efficacy of a xenogeneic collagen matrix in augmenting keratinized mucosa around implants: A randomized controlled prospective clinical trial. Clin. Oral Implants Res. 2012, 23, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Zeltner, M.; Hilbe, M.; Hämmerle, C.H.; Hüsler, J.; Jung, R.E. Randomized controlled clinical study evaluating effec-tiveness and safety of a volume-stable collagen matrix compared to autogenous connective tissue grafts for soft tissue aug-mentation at implant sites. J. Clin. Periodontol. 2016, 43, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Zeltner, M.; Jung, R.E.; Hämmerle, C.H.; Hüsler, J.; Thoma, D.S. Randomized controlled clinical study comparing a volume-stable collagen matrix to autogenous connective tissue grafts for soft tissue augmentation at implant sites: Linear volumetric soft tissue changes up to 3 months. J. Clin. Periodontol. 2017, 44, 446–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairo, F.; Barbato, L.; Tonelli, P.; Batalocco, G.; Pagavino, G.; Nieri, M. Xenogeneic collagen matrix versus connective tissue graft for buccal soft tissue augmentation at implant site. A randomized, controlled clinical trial. J. Clin. Periodontol. 2017, 44, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Puzio, M.; Błaszczyszyn, A.; Hadzik, J.; Dominiak, M. Ultrasound assessment of soft tissue augmentation around implants in the aesthetic zone using a connective tissue graft and xenogeneic collagen matrix—1-year randomised follow-up. Ann. Anat. 2018, 217, 129–141. [Google Scholar] [CrossRef]
- Huber, S.; Zeltner, M.; Hämmerle, C.H.F.; Jung, R.E.; Thoma, D.S. Non-interventional 1-year follow-up study of peri-implant soft tissues following previous soft tissue augmentation and crown insertion in single-tooth gaps. J. Clin. Periodontol. 2018, 45, 504–512. [Google Scholar] [CrossRef] [Green Version]
- Papi, P.; Pompa, G.G. The use of a novel porcine derived acellular dermal matrix (mucoderm) in peri-implant soft tissue augmentation: Preliminary results of a prospective pilot cohort study. BioMed Res. Int. 2018, 2018, 6406051. [Google Scholar] [CrossRef]
- Schallhorn, R.A.; McClain, P.K.; Charles, A.; Clem, D.; Newman, M.G. Evaluation of a porcine collagen matrix used to augment keratinized tissue and increase soft tissue thickness around existing dental implants. Int. J. Periodontics Restor. Dent. 2015, 35, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, C.M.; Moest, T.; Lutz, R.; Wehrhan, F.; Neukam, F.W.; Schlegel, K.A. Long-term outcomes after vestibuloplasty with a porcine collagen matrix (Mucograft®) versus the free gingival graft: A comparative prospective clinical trial. Clin. Oral Implants Res. 2016, 27, e125–e133. [Google Scholar] [CrossRef]
- Azar, E.L.; Rojas, M.A.; Mandalunis, P.; Gualtieri, A.; Carranza, N. Histological evaluation of subepithelial connective tissue grafts harvested by two different techniques: Preliminary study in humans. Acta Odontol. Latinoam. 2019, 32, 10–16. [Google Scholar]
- Thoma, D.S.; Naenni, N.; Benic, G.I.; Hammerle, C.H.; Jung, R.E. Soft tissue volume augmentation at dental implant sites using a volume stable three-dimensional collagen matrix—Histological outcomes of a preclinical study. J. Clin. Periodontol. 2017, 44, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoma, D.S.; Hammerle, C.H.F.; Cochran, D.L.; Jones, A.A.; Gorlach, C.; Uebersax, L.; Mathes, S.; Graf-Hausner, U.; Jung, R.E. Soft tissue volume augmentation by the use of collagen-based matrices in the dog mandible—A histological analysis. J. Clin. Periodontol. 2011, 38, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.M.; Matta, R.E.; Moest, T.; Humann, J.; Gammel, L.; Neukam, F.W.; Schlegel, K.A. Soft tissue volume alterations after connective tissue grafting at teeth: The subepithelial autologous connective tissue graft versus a porcine collagen matrix—A pre-clinical volumetric analysis. J. Clin. Periodontol. 2016, 43, 609–617, Erratum in J. Clin. Periodontol. 2018, 45, 392. [Google Scholar] [CrossRef] [PubMed]
- Annen, B.M.; Ramel, C.F.; Hämmerle, C.H.; Jung, R.E. Use of a new cross-linked collagen membrane for the treatment of peri-implant dehiscence defects: A randomised controlled double-blinded clinical trial. Eur. J. Oral Implantol. 2011, 4, 87–100. [Google Scholar]
- Imber, J.C.; Bosshardt, D.D.; Stähli, A.; Saulacic, N.; Deschner, J.; Sculean, A. Pre-clinical evaluation of the effect of a volume-stable collagen matrix on periodontal regeneration in two-wall intrabony defects. J. Clin. Periodontol. 2021, 48, 560–569. [Google Scholar] [CrossRef]
- Caballé-Serrano, J.; Zhang, S.; Sculean, A.; Staehli, A.; Bosshardt, D.D. Tissue Integration and Degradation of a Porous Col-lagen-Based Scaffold Used for Soft Tissue Augmentation. Materials 2020, 13, 2420. [Google Scholar] [CrossRef]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.-L.; Giannobile, W.V. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef]
- Zafiropoulos, G.G.; Deli, G.; Hoffmann, O.; John, G. Changes of the peri-implant soft tissue thickness after grafting with a collagen matrix. J. Indian Soc. Periodontol. 2016, 20, 441–445. [Google Scholar] [CrossRef]
- Stefanini, M.; Rendon, A.; Zucchelli, G. Porcine-derived acellular dermal matrix for buccal soft tissue augmentation at single implant sites: A 1-year follow-up case series. Int. J. Periodontics Restor. Dent. 2020, 40, 121–128. [Google Scholar] [CrossRef]
- Papi, P.; Penna, D.; Di Murro, B.; Pompa, G. Clinical and volumetric analysis of peri-implant soft tissue augmentation using an acellular dermal matrix: A prospective cohort study. J. Periodontol. 2021, 92, 803–813. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Bruckbauer, P.; Schlegel, K.A.; Buchbender, M.; Adler, W.; Matta, R.E. Volumetric soft tissue alterations in the early healing phase after periimplant soft tissue contour augmentation with a porcine collagen matrix versus the autologous connective tissue graft: A controlled clinical trial. J. Clin. Periodontol. 2021, 48, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Verardi, S.; Orsini, M.; Lombardi, T.; Ausenda, F.; Testori, T.; Pulici, A.; Oreglia, F.; Valente, N.A.; Stacchi, C. Comparison between two different techniques for peri-implant soft tissue augmentation: Porcine dermal matrix graft versus tenting screw. J. Periodontol. 2019, 91, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Papi, P.; Pranno, N.; Di Murro, B.; Pompa, G. Early implant placement and peri-implant augmentation with a porcine-derived acellular dermal matrix and synthetic bone in the aesthetic area: A 2-year follow-up prospective cohort study. Int. J. Oral Maxillofac. Surg. 2021, 50, 258–266. [Google Scholar] [CrossRef]
- Aragoneses, J.; Suarez, A.; Rodriguez, C.; Aragoneses, J.M. Clinical and histological differences between guided tissue regeneration with acellular dermal matrix of porcine origin and autologous connective tissue: An animal study. Materials 2021, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Villar, C.C.; Cochran, D.L.; Hammerle, C.H.; Jung, R.E. Tissue integration of collagen-based matrices: An experimental study in mice. Clin. Oral Implants Res. 2012, 23, 1333–1339. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, Z.; Pan, W.L.; Wang, H.L. Impact of timing on soft tissue augmentation during implant treatment: A systematic review and meta-analysis. Clin. Oral Implants Res. 2018, 29, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Chambrone, L.; Pini Prato, G.P. Clinical insights about the evolution of root coverage procedures: The flap, the graft, and the surgery. J. Periodontol. 2018, 90, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Wan, H.; Sandor, M.; Qi, S.; Ervin, F.; Harper, J.R.; Silverman, R.P.; McQuillan, D.J. Host response to human acellular dermal matrix transplantation in a primate model abdominal wall repair. Tissue Eng. Part A 2008, 14, 2009–2019. [Google Scholar] [CrossRef]
- Harper, J.R.; McQuillan, D.J. Extracellular wound matrices: A novel regenerative tissue matrix (RTM) technology for connective tissue reconstruction. Wounds 2007, 19, 163–168. [Google Scholar]
- Palmer, R.M.; Wilson, R.F.; Hasan, A.S.; Scott, D.A. Mechanisms of action of environmental factors: Tobacco smoking. J. Clin. Periodontol. 2005, 32, 180–190. [Google Scholar] [CrossRef]




| Identification of Studies via Databases and Registers | ||||
|---|---|---|---|---|
| Identification | Records identified from PubMed searching: (n = 87) | Records identified from Scopus searching: (n = 77) | Records identified from Cochrane Library: (n = 75) | Records identified from Web of Sciences: (n = 5) |
 |  |  |  | |
| Screening | Records after duplicates removed (n = 133) | |||
 | ||||
| Records screened (n = 133) |  | Records excluded by title and abstract (n = 122) | ||
 | ||||
| Full text assessed for eligibility |  | Full text excluded after full text assessed (n = 4) Reasons for exclusion: no comparison between CTG and XCM | ||
 | ||||
| Included | Studies included in review (n = 7) | |||
| General Overview of RCT Which Compared XCMs Versus CTGs | ||||||
|---|---|---|---|---|---|---|
| Study | Follow-Up | Patients/Implants | Systemic Periodontal Status Smoking | Time of Surgery | Outcomes Measurements | XCM |
| Sanz et al. [20] | 1,3,6 months | P = 14 I = 14 | Systemic, Periodontally healthy FMPI < 20% Smokers < 10 sig.die | After crown placement | PKMW, PPD, CAL, GI, PI, pain, PAS | Mucograft® |
| Lorenzo et al. [21] | 6 months | P = 24 I = 24 | Systemic, Periodontally healthy FMPI < 20% Smokers < 10 sig.die | After crown placement | PKMW, GI, PI, PD, CAL | Mucograft® |
| Thoma et al. [22] | 3 months | P = 20 I = 20 | Systemic, Periodontally healthy FMPI < 20% Smokers < 10 sig.die | After implant placement. From 6 weeks to 6 months before | PKMM, PPD, CAL, BOP, PI | Mucograft® |
| Zeitner et al. [23] | 3 months | P = 20 I = 20 | Systemic, Periodontally healthy FMPI < 20% Smokers < 10 sig.die | After implant placement. From 6 weeks to 6 months before | PKMM, PPD, CAL, BOP, PI | Mucograft® |
| Cairo et al. [24] | 6 months | P = 60 I = 60 | Systemic, Periodontally healthy FMPI < 15% PPD < 5mm Smokers < 10 sig.die | During second surgery implant uncovering | PKMW, GT, PD, PAS | Mucograft® |
| Puzio et al. [25] | 12 months | P = 22 I = 30 | Systemic, Periodontally healthy PI < 20% FMBS < 15% Smokers < 10 sig.die | During second surgery implant uncovering | PKMW, GT | Mucograft® |
| Huber et al. [26] | 12 months | P = 20 I = 20 | Systemic, Periodontally healthy Smokers < 10 sig.die | During second surgery implant uncovering | PKMW, GT | Mucograft® |
| General overview of prospective studies which investigated XCMs | ||||||
| Pompa & Papi. [27] | 12 months | P = 12 I = 10 | Systemic, Periodontally healthy FMPI < 20% Smokers < 10 sig.die | After crown placement | KMW, PI, PD, BP | Mucoderm® |
| Schallhorn et al. [28] | 6 months | P = 30 I = 32 | Systemic, Periodontally healthy FMPI < 20% Smokers < 10 sig.die | After crown placement | KMW, GT, PD, colour, PAS | Mucograft® |
| Study | Adequate Sequence Generation | Allocation Concealment | Blinding | Incomplete Outcomes Data Addressed | Selective Outcome Reporting | Free of Other Source of Bias | Estimate Potential Source of Bias |
|---|---|---|---|---|---|---|---|
| Sanz et al. [20] | No | Yes | Yes | Yes | Yes | Yes | Low risk |
| Lorenzo et al. [21] | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Thoma et al. [22] | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Zeitner et al. [23] | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Cairo et al. [24] | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Puzio et al. [25] | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Huber et al. [26] | Yes | Yes | Yes | Yes | Yes | Yes | Low risk |
| Advantages | Disadvantages | |
|---|---|---|
| CTG |
|
|
| XCMs/PDXCMs |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Annuntiis, C.; Testarelli, L.; Guarnieri, R. Use of Xenogenic Collagen Matrices in Peri-Implant Soft Tissue Volume Augmentation: A Critical Review on the Current Evidence and New Technique Presentation. Materials 2022, 15, 3937. https://doi.org/10.3390/ma15113937
De Annuntiis C, Testarelli L, Guarnieri R. Use of Xenogenic Collagen Matrices in Peri-Implant Soft Tissue Volume Augmentation: A Critical Review on the Current Evidence and New Technique Presentation. Materials. 2022; 15(11):3937. https://doi.org/10.3390/ma15113937
Chicago/Turabian StyleDe Annuntiis, Carlo, Luca Testarelli, and Renzo Guarnieri. 2022. "Use of Xenogenic Collagen Matrices in Peri-Implant Soft Tissue Volume Augmentation: A Critical Review on the Current Evidence and New Technique Presentation" Materials 15, no. 11: 3937. https://doi.org/10.3390/ma15113937
APA StyleDe Annuntiis, C., Testarelli, L., & Guarnieri, R. (2022). Use of Xenogenic Collagen Matrices in Peri-Implant Soft Tissue Volume Augmentation: A Critical Review on the Current Evidence and New Technique Presentation. Materials, 15(11), 3937. https://doi.org/10.3390/ma15113937






