Impact of Cesium Concentration on Optoelectronic Properties of Metal Halide Perovskites
Abstract
1. Introduction
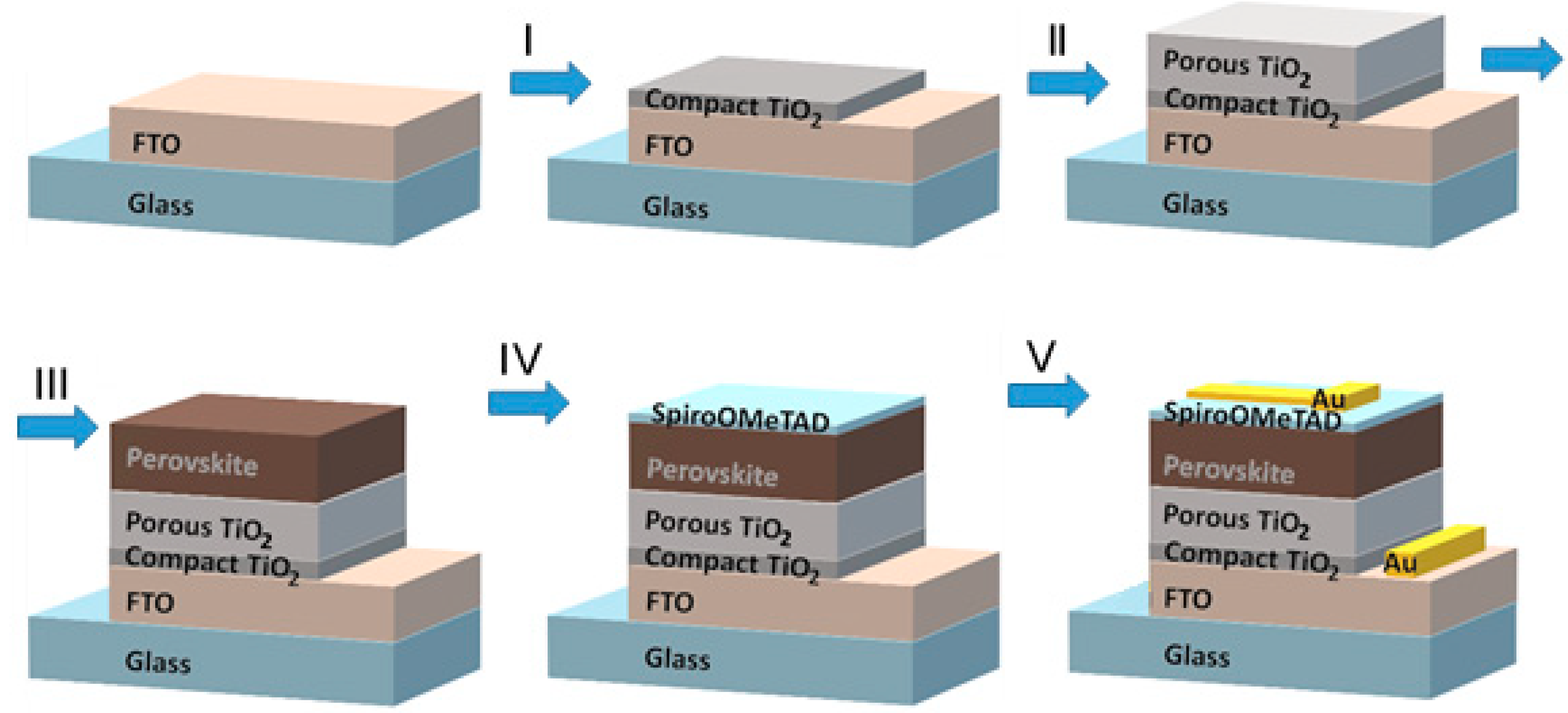
2. Fabrication of Perovskite Films and Their Characterization
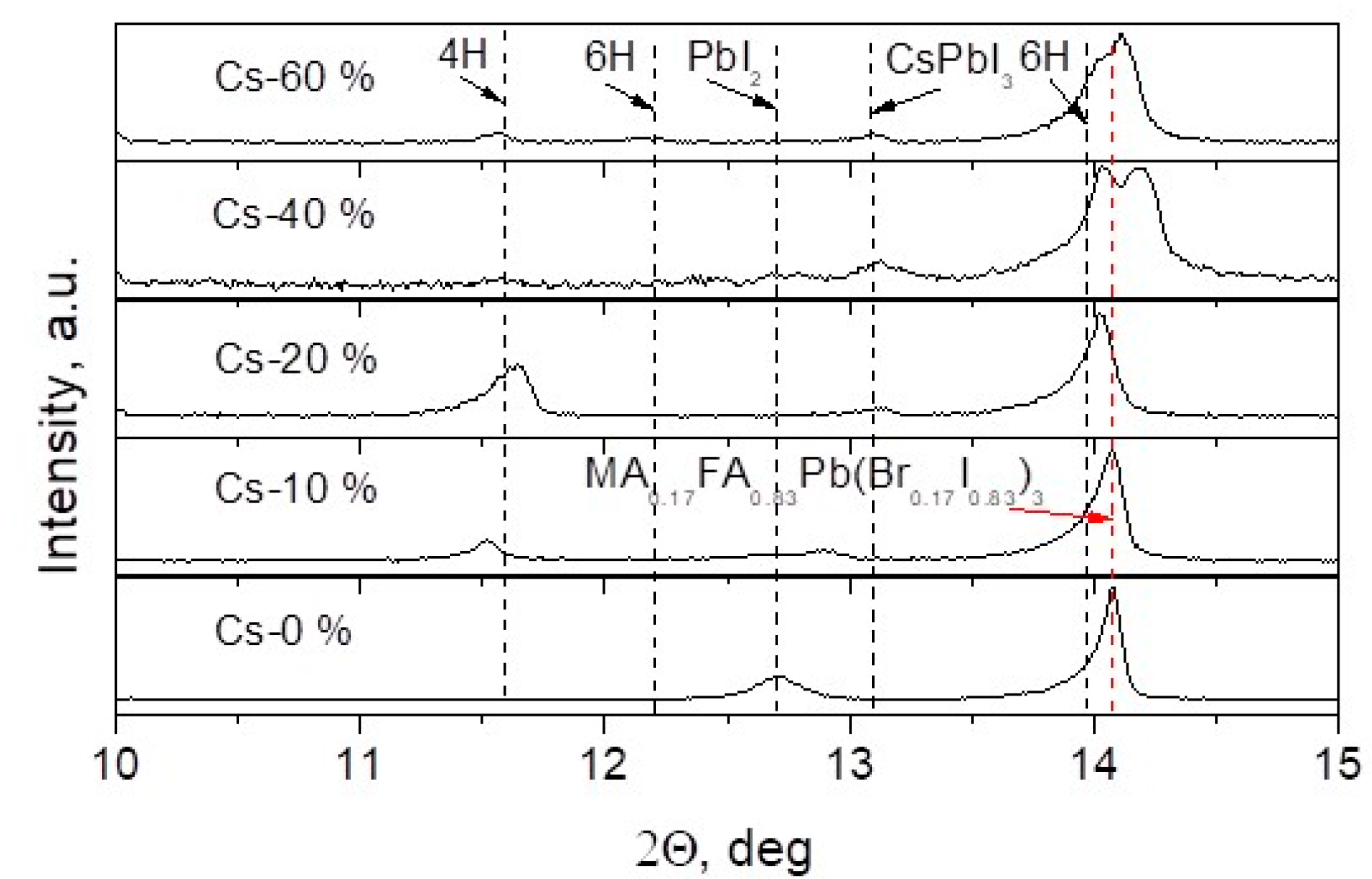
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- Li, T.; Pan, Y.; Wang, Z.; Xia, Y.; Chen, Y.; Huang, W. Additive engineering for highly efficient organic-inorganic halide perovskite solar cells: Recent advances and perspectives. J. Mater. Chem. A 2017, 5, 12602–12652. [Google Scholar] [CrossRef]
- Gao, X.-X.; Xue, D.-J.; Gao, D.; Han, Q.; Ge, Q.-Q.; Ma, J.-Y.; Ding, J.; Zhang, W.; Zhang, B.; Feng, Y.; et al. High-mobility hydrophobic conjugated polymer as effective interlayer for air-stable efficient perovskite solar cells. Sol. RRL 2018, 3, 1800232. [Google Scholar] [CrossRef]
- Shi, Z.; Jayatissa, A.H. Perovskites-based solar cells: A review of recent progress, materials and processing methods. Materials 2018, 11, 729. [Google Scholar] [CrossRef]
- Mesquita, I.; Andrade, L.; Mendes, A. Perovskite solar cells: Materials, configuration and stability. Renew. Sustain. Energy Rev. 2018, 82, 2471–2489. [Google Scholar] [CrossRef]
- Laalioui, S.; Alaoui, K.B.; Dads, H.A.; Assali, K.E.; Ikken, B.; Outzourhit, A. Progress in perovskite based solar cells: Scientific and engineering state of the art. Rev. Adv. Mater. Sci. 2020, 59, 10–25. [Google Scholar] [CrossRef]
- Liu, F.; Zeng, Q.; Li, J.; Hao, X.; Ho-Baillie, A.; Tang, J.; Green, M.A. Emerging inorganic compound thin film photovoltaic materials: Progress, challenges and strategies. Mater. Today 2020, 41, 120–142. [Google Scholar] [CrossRef]
- Jeong, M.; Choi, I.W.; Go, E.M.; Kim, M.; Lee, B.; Jeong, S.; Jo, Y.; Choi, H.W.; Lee, J.; Bae, J.-H.; et al. Stable perovskite solar cells with efficiency exceeding 24.8% and 0.3-V voltage loss. Science 2020, 369, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High efficiency perovskite solar cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Yadavalli, S.K.; Chen, M.; Abbaspourtamijani, A.; Qi, Y.; Padture, N.P. Interfacial toughening with self-assembled monolayers enhances perovskite solar cell reliability. Science 2021, 372, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Yang, Y.M.; You, J.; Hong, Z.; Chang, W.-H.; Li, G.; Yang, Y. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef]
- Dong, R.; Fang, Y.; Chae, J.; Dai, J.; Xiao, Z.; Dong, Q.; Yuan, Y.; Centrone, A.; Zeng, X.C.; Huang, J. High-gain and low-driving-voltage photodetectors based on organolead triiodide perovskites. Adv. Mater. 2015, 27, 1912–1918. [Google Scholar] [CrossRef]
- Dong, Y.; Zou, Y.; Song, J.; Song, X.; Zeng, H. Recent progress of metal halide perovskite photodetectors. J. Mater. Chem. C 2017, 5, 11369–11394. [Google Scholar] [CrossRef]
- Feng, J.; Gong, C.; Gao, H.; Wen, W.; Gong, Y.; Jiang, X.; Zhang, B.; Wu, Y.; Wu, Y.; Fu, H.; et al. Single-crystalline layered metal-halide perovskite nanowires for ultrasensitive photodetectors. Nat. Electron. 2018, 1, 404–410. [Google Scholar] [CrossRef]
- Wu, H.; Si, H.; Zhang, Z.; Kang, Z.; Wu, P.; Zhou, L.; Zhang, S.; Zhang, Z.; Liao, Q.; Zhang, Y. All-inorganic perovskite quantum dot-monolayer MoS2 ultrasensitive photodetector. Adv. Sci. 2018, 5, 1801219. [Google Scholar] [CrossRef]
- Gegevičius, R.; Franckevičius, M.; Pakštas, V.; Augulis, R.; Gulbinas, V. High-speed, sensitive planar perovskite photodetector based on interdigitated Au electrodes. Phys. Status Solidi A 2018, 215, 1700822. [Google Scholar] [CrossRef]
- Mei, F.; Sun, D.; Mei, S.; Feng, J.; Zhou, Y.; Xu, J.; Xiao, X. Recent progress perovskite-based photodetectors: The design of materials and structures. Adv. Phys. X 2019, 4, 1592709. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Li, T.; Tang, X.; Zhao, H.; Li, J.; Ma, C.; Yao, J. High-performance self-powered perovskite photodetector for visible light communication. Appl. Phys. A 2020, 126, 869. [Google Scholar] [CrossRef]
- Zhang, Y.; Lyu, M.; Qiu, T.; Han, E.; Kim, I.K.; Jung, M.-C.; Ng, Y.H.; Yun, J.-H.; Wang, L. Halide perovskite single crystals: Optoelectronic applications and strategical approaches. Energies 2020, 13, 4250. [Google Scholar] [CrossRef]
- Wu, D.; Li, W.; Liu, H.; Xiao, X.; Shi, K.; Tang, H.; Shan, C.; Wang, K.; Sun, X.W.; Kyaw, A.K.K. Universal strategy for improving perovskite photodiode performance: Interfacial built-in electric field manipulated by unintentional doping. Adv. Sci. 2021, 202101729. [Google Scholar] [CrossRef]
- Tan, Z.-K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright light-emitting diodes on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yeh, Y.-W.; Tran, N.L.; Wu, F.; Xiao, Z.; Kerner, R.A.; Lin, Y.L.; Scholes, G.D.; Yao, N.; Rand, B.P. In situ preparation of metal halide perovskite nanocrystal thin films for improved light-emitting devices. ACS Nano 2017, 11, 3957–3964. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Lin, H.; Ye, F.; Qin, M.; Burkhartsmeyer, J.M.; Zhang, H.; Lu, X.; Wong, K.S.; Choy, W.C.H. All-perovskite emission architecture for white light-emitting diodes. ACS Nano 2018, 12, 10486–10492. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Hoye, R.L.Z.; Di, D.; Friend, R.H.; Deschler, F. The physics of light emission in halide perovskite devices. Adv. Mater. 2018, 1, 1803336. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, S.; Jo, S.H.; Lee, T.-W. Metal halide perovskites: From crystal formations to light-emitting-diode applications. Small Methods 2018, 2, 1800093. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Yang, Y.; Xue, Q.; Yip, H.-L.; Cao, Y. Modulation of recombination zone position for quasi-two-dimensional blue perovskite light-emitting-diodes with efficiency exceeding 5%. Nat. Commun. 2019, 10, 1027. [Google Scholar] [CrossRef]
- Bao, C.; Muhammad, M.; Ma, X.; Wang, Z.; Liu, Y.; Cnen, P.; Chen, S.; Liu, B.; Wang, J.; Duan, Y. Hybrid perovskite charge generation layer for highly efficient tandem organic light-emitting diodes. Org. Electron. 2019, 73, 299–303. [Google Scholar] [CrossRef]
- Chen, S.; Chen, V.; Bao, C.; Mujahid, M.; Li, Y.; Chen, P.; Duan, Y. White light-emitting devices based on inorganic perovskite and organic materials. Molecules 2019, 24, 800. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ding, H.; Li, S.; Liu, M.; Yang, J.; Zhao, Y.; Pan, N.; Wang, X. Improving electron injectionin all-inorganic perovskite light-emitting diode via electron transport layer modulation. Optik 2019, 191, 68–74. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Hopper, T.R.; Bakulin, A.A.; Yip, H.-L. Materials, photophysics and device engineering of perovskite light-emitting-diodes. Rep. Prog. Phys. 2021, 84, 046401. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Xu, X.; Li, J.; Song, J.; Lan, S.; Liu, S.; Cai, B.; Han, B.; Precht, J.T.; et al. Efficient and bright light-emitting-diodes based on single-layer heterophase halide perovskite. Nat. Photonics 2021, 15, 238–244. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Miller, A.M.; Zhu, H.; Egap, E. Lead halide perovskite nanocrystals as photocatalysts for PET-RAFT polymerization under visible and near-infrared irradiation. ACS Macro Lett. 2020, 9, 725–730. [Google Scholar] [CrossRef]
- Kar, M.R.; Chakraborty, R.; Patel, U.; Chakraborty, R.; Ray, S.; Acharija, T.K.; Coswami, C.; Bhaumik, S. Impact of Zn-doping on composition, stability, luminescence properties of silica coated all-inorganic cesium lead bromide nanocrystals and their biocompatibility. Mater. Today Chem. 2022, 23, 100753. [Google Scholar] [CrossRef]
- De Wolf, S.; Holovsky, J.; Moon, S.-J.; Löper, P.; Niesen, B.; Ledinsky, M.; Haug, F.-J.; Yum, J.-H.; Ballif, C. Organometallic halide perovskites: Sharp optical absorption edge and its relation to photovoltaic performance. J. Phys. Chem. Lett. 2014, 5, 1035–1039. [Google Scholar] [CrossRef]
- Quan, L.N.; Rand, B.P.; Friend, R.H.; Mhaisalkar, S.G.; Lee, T.W.; Sargent, E.H. Perovskites for next-generation optical sources. Chem. Rev. 2019, 119, 7444–7478. [Google Scholar] [CrossRef]
- Kothandaraman, R.K.; Jiang, Y.; Feurer, T.; Tiwari, A.N.; Fu, F. Near-infrared-transparent perovskite solar cells and perovskite-based tandem photovoltaics. Small Methods 2020, 4, 2000395. [Google Scholar] [CrossRef]
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. High carrier mobilities and lifetimes in organolead trihalide perovskites. Adv. Mater. 2014, 26, 1584–11589. [Google Scholar] [CrossRef]
- Karakus, M.; Jensen, S.A.; D’Angelo, F.; Turchinovich, D.; Bonn, M.; Cánovas, E. Phonon-electron scattering limits free charge mobility in methylammonium lead iodide perovskites. J. Phys. Chem. Lett. 2015, 6, 4991–4996. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcicer, M.J.P.; Leijens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an Organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Cao, L.; Huang, J. Electron-hole diffusion lengths >175 µm in solution-grown CH3NH3PbI3 single crystals. Sci. Express 2015, 6225. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.; Hörantner, M.T.; Snaith, H.J. Metal halide perovskite tandem and multiple-junction photovoltaics. Nat. Rev. Chem. 2017, 1, 0095. [Google Scholar] [CrossRef]
- Unger, E.L.; Kegelman, L.; Suchan, K.; Sörell, D.; Korte, L.; Albrecht, S. Roadmap and roadblocks for the band gap tunability of metal halide perovskites. J. Mater. Chem. A 2017, 5, 11401–11409. [Google Scholar] [CrossRef]
- Wang, L.; Shahiduzzaman, M.; Muslih, Y.M.; Nakano, M.; Karakawa, M.; Takahachi, K.; Tomita, K.; Ninzi, J.M.; Taima, T. Double-layer CsI intercalation into MAPbI3 framework for efficient and stable perovskite solar cells. Nano Energy 2021, 86, 106135. [Google Scholar] [CrossRef]
- Correa-Baena, J.P.; Steier, L.; Tress, W.; Saliba, M.; Neutzner, S.; Matsui, T.; Giordano, F.; Jacobsson, T.J.; Kandada, A.R.S.; Zakeeruddin, S.M.; et al. Highly efficient planar perovskite solar cells through band alignment engineering. Energy Environ. Sci. 2015, 8, 2928–2934. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domaski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Correa-Baena, J.P.; Saliba, M.; Buonassisi, T.; Grätzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and challenges of perovskite solar cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef]
- Christians, J.A.; Schulz, P.; Tinkham, J.; Schloemer, T.H.; Harvey, S.P.; Tremolet de Villers, B.J.; Sellinger, A.; Berry, J.J.; Luther, J.M. Taylored interfaces of unencapsulated perovskite solar cells for >1000 hour operational stability. Nat. Energy 2018, 3, 68–74. [Google Scholar] [CrossRef]
- Shen, H.; Omelchenko, S.T.; Jacobs, D.A.; Yalamanchili, S.; Wan, Y.; Yan, D.; Phang, P.; Duong, T.; Wu, Y.; Yin, Y.; et al. In situ recombination junction between p-Si and TiO2 enables high-efficiency monolithic perovskite/Si tandem cells. Sci. Adv. 2018, 4, eaau9711. [Google Scholar] [CrossRef] [PubMed]
- Mateen, M.; Arain, Z.; Liu, X.; Liu, C.; Yang, Y.; Ding, Y.; Ma, S.; Ren, Y.; Wu, Y.; Tao, Y.; et al. High-performance mixed-cation mixed–halide perovskite solar cells enabled by a facile intermediate engineering technique. J. Power Sources 2020, 448, 227386. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Z.; Qin, C.; He, T.; Li, B.; Ma, L.; Shaheen, K.; Yang, K.; Yang, H.; Liu, H.; et al. High-performance perovskite solar cells based on passivating interfacial and intergranular defects. Sol. Energy Mater. Sol. Cells 2020, 212, 110555. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.S.; Wolf, C.; Kim, Y.-H.; Yun, H.J.; Jeong, S.-H.; Sadhanala, A.; Venugopalan, V.; Choi, J.W.; Lee, C.-L.; et al. High-efficiency plycrystalline perovskite light-emitting diodes based on mixed cations. ACS Nano 2018, 12, 2883–2892. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide perovskite photovoltaic: Background, status, and future prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Hu, Y.; Hutter, E.M.; Rieder, P.; Grill, I.; Hanisch, J.; Aygüler, M.F.; Hufnagel, A.G.; Handloser, M.; Bein, T.; Hartschun, A.; et al. Understanding the role of cesium and rubidium additives in perovskite solar cells: Trap states, charge transport, and recombination. Adv. Energy Mater. 2018, 1703057. [Google Scholar] [CrossRef]
- Ašmontas, S.; Čerškus, A.; Gradauskas, J.; Grigucevičienė, A.; Leinartas, K.; Lučun, A.; Petrauskas, K.; Selskis, A.; Sužiedelis, A.; Širmulis, E.; et al. Cesium-containing triple cation perovskite solar cells. Coatings 2021, 11, 279. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, D.H.; Kim, H.S.; Seo, S.W.; Cho, S.M.; Park, N.G. Formamidinium and cesium hybridization for photo- and moisture-stable perovskite solar cell. Adv. Energy Mater. 2015, 5, 1501310. [Google Scholar] [CrossRef]
- Liu, T.; Zong, Y.; Zhou, Y.; Yang, M.; Li, Z.; Game, O.S.; Zhu, K.; Gong, Q.; Padture, N.P. High-performance formamidinium–based perovskite solar cells via microstructure-mediated δ–to–α phase transformation. Chem. Mater. 2017, 29, 3246–3250. [Google Scholar] [CrossRef]
- Rehman, W.; McMeekin, D.P.; Patel, J.B.; Milot, R.L.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Photovoltaic mixed-cation lead mixed-halide perovskite: Links between crystallinity, photo-stability and electronic properties. Energy Environ. Sci. 2017, 10, 361–369. [Google Scholar] [CrossRef]
- Herz, L.M. Charge-carrier mobilities in metal halide perovskites: Fundamentals mechanisms and limits. ACS Energy Lett. 2017, 2, 1539–1548. [Google Scholar] [CrossRef]
- McMeekin, D.P.; Sadoudhi, G.; Rehman, W.; Eperon, G.E.; Saliba, M.; Hörantner, M.T.; Haghighirad, A.; Sakai, N.; Korte, L.; Rech, B.; et al. A mixed-cation lead mixed-halide perovskite absorber for perovskite solar cells. Science 2016, 351, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; Niesen, B.; Ballif, C. Perovskite/silicon tandem solar cells: Marriage of convenience or true love story? An overview. Adv. Mater. Interfaces 2018, 5, 1700731. [Google Scholar] [CrossRef]
- Zheng, J.; Lau, C.F.J.; Mehrvarz, H.; Ma, F.J.; Jiang, Y.; Deng, X.; Soeriyadi, A.; Kim, J.; Zhang, M.; Hu, L.; et al. Large area efficient interface layer free monolithic perovskite/homo-junction-silicon tandem solar cell with over 20% efficiency. Energy Environ. Sci. 2018, 11, 2432–2443. [Google Scholar] [CrossRef]
- Jaysankar, M.; Filipič, M.; Zielinski, B.; Schmager, R.; Song, W.; Qiu, W.; Paetzold, U.W.; Aernouts, T.; Debucquoy, M.; Gehlhaar, R.; et al. Perovskite–silicon tandem solar modules with optimized light harvesting. Energy Environ. Sci. 2018, 11, 1489–1498. [Google Scholar] [CrossRef]
- Köhnen, E.; Jošt, M.; Morales-vilches, A.B.; Tockhorn, P.; Al-Ashouri, A.; Macco, B.; Kogelmann, L.; Korte, L.; Rech, B.; Schlatmann, R.; et al. Highly efficient monolithic perovskite silicon solar cells: Analyzing the influence of current mismatch on device performance. Sustain. Energy Fuels 2019, 3, 1995–2005. [Google Scholar] [CrossRef]
- Chen, B.; Yu, Z.; Liu, K.; Zheng, X.; Liu, Y.; Shi, J.; Spronk, D.; Rudd, P.N.; Holman, Z.; Huang, J. Grain engineering for perovskite/silicon monolithic tandem solar cells with efficiency of 25.4%. Joule 2019, 3, 177–190. [Google Scholar] [CrossRef]
- Shen, H.; Walter, D.; Wu, Y.; Fong, K.C.; Jacobs, D.A.; Duong, T.; Peng, J.; Weber, K.; White, T.P.; Catchpole, K.R. Monolithic perovskite/Si solar cells: Pathways to over 30% efficiency. Adv. Energy Mater. 2019, 10, 1902840. [Google Scholar] [CrossRef]
- Duong, T.; Pham, H.; Kho, T.C.; Phang, P.; Fong, K.C.; Yan, D.; Yin, Y.; Peng, J.; Mahmud, M.A.; Charibzadeh, S.; et al. Highly efficiency perovskite-silicon tandem solar cells: Effect of surface coating versus bulk incorporation of 2D perovskite. Adv. Energy Mater. 2020, 10, 1903553. [Google Scholar] [CrossRef]
- Al-Ashouri, A.; Köhnen, E.; Li, B.; Magomedov, A.; Hempel, H.; Caprioglio, P.; Márquez, J.A.; Vilches, A.B.; Kasparavičius, E.; Smith, J.A.; et al. Monolithic perovskite/silicon tandem solar cell with >29% efficiency by enhanced hole extraction. Science 2020, 370, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Yeom, K.M.; Kim, S.U.; Woo, M.Y.; Noh, J.H.; Im, S.H. Recent progress in metal halide perovskite-based tandem solar cell. Adv. Mater. 2020, 32, 2002228. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, X.; Hou, Y.; Wang, K.; Ye, T.; Yoon, J.; Wu, C.; Sanghadasa, M.; Liu, S.; Priya, S. 28.3%-efficiency perovskite/silicon tandem solar cell by optimal transparent electrode for high efficient semitransparent top cell. Nano Energy 2021, 84, 105934. [Google Scholar] [CrossRef]
- Wu, T.; Qin, Z.; Wang, Y.; Wu, Y.; Chen, W.; Zhang, S.; Cai, M.; Dai, S.; Zhang, J.; Liu, J.; et al. The main progress of perovskitesolar cells in 2020–2021. Nano-Micro Lett. 2021, 13, 152. [Google Scholar] [CrossRef]
- Singh, T.; Miyasaka, T. Stabilizing the efficiency beyond 20% with a mixed cation perovskite solar cell fabricated in ambient air under controlled humidity. Adv. Energy Mater. 2018, 8, 1700677. [Google Scholar] [CrossRef]
- Domanski, K.; Alharbi, E.A.; Hagfeldt, A.; Grätzel, M.; Tress, W. Systematic investigation of the impact of operation conditions on the degradation behavior of perovskite solar cells. Nat. Energy 2018, 3, 61–67. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Yonezawa, K.; Yamamoto, K.; Ripolles, T.S.; Karakawa, M.; Kuwabara, T.; Takahashi, K.; Hayase, S.; Taima, T. Improved reproducibility and intercalation control of efficient planar inorganic perovskite solar cells by simple alternate vacuum deposition of PbI2 and CsI. ACS Omega 2017, 11, 4464–4469. [Google Scholar] [CrossRef]
- Gratia, P.; Zimmerman, I.; Schouwink, P.; Yum, J.-H.; Audinot, J.-N.; Sivula, K.; Wirtz, T.; Nazeeruddin, M.K. The many faces of mixed ion perovskite: Unraveling and understanding the crystallization process. ACS Energy Lett. 2017, 2, 2686–2693. [Google Scholar] [CrossRef]
- Sutanto, A.A.; Queloz, V.I.E.; Garcia-Benito, I.; Smit, B.; Nazeeruddin, M.K.; Syzgantseva, O.A.; Grancini, G. Pushing the limit of Cs incorporation into FAPbBr3 perovskite to enhance solar cell perfomances. APL Mater. 2019, 7, 041110–041111. [Google Scholar] [CrossRef]
- Paek, S.; Khan, S.B.; Franckevičius, M.; Gegevičius, R.; Syzgantseva, O.A.; Syzgantseva, M.A.; Kinge, S.; Asiri, A.M.; Roldán-Carmona, C.; Nazeeruddin, M.K. Cation optimization for burn-in loss-free perovskite solar devices. J. Mater. Chem. A 2021, 9, 5374–5380. [Google Scholar] [CrossRef]
- Krustok, J.; Collan, H.; Yakushev, M.; Hjelt, K. The role of spatial potential fluctuations in the shape of the PL bands of multinary semiconductor compounds. Phys. Scr. 1999, T79, 179–182. [Google Scholar] [CrossRef]
- Chirvony, V.S.; Martínez-Pastor, J.P. Trap-Limited Dynamics of Excited Carriers and Interpretation of the Photoluminescence Decay Kinetics in Metal Halide Perovskites. J. Phys. Chem. Lett. 2018, 9, 4955–4962. [Google Scholar] [CrossRef] [PubMed]
- Campbella, S.; Qua, Y.; Majorb, J.D.; Lagardec, D.; Labbéd, C.; Maielloa, P.; Barrioza, V.; Beattiea, N.S.; Zoppi, G.J. Direct evidence of causality between chemical purity and band-edge potential fluctuations in nanoparticle ink-based Cu2ZnSn(S,Se)4 solar cells. Phys. D Appl. Phys. 2019, 52, 135102. [Google Scholar] [CrossRef]
- Berberan-Santos, N.M. A luminescence decay function encompassing the stretched exponential and the compressed hyperbola. Chem. Phys. Lett. 2008, 460, 146–150. [Google Scholar] [CrossRef][Green Version]
- Ahrenkiel, R.K.; Johnston, S.W.; Kuciauskas, D.; Tynan, J. Dual-sensor technique for characterization of carrier lifetime decay transients in semiconductors. J. Appl. Phys. 2014, 116, 214510. [Google Scholar] [CrossRef]
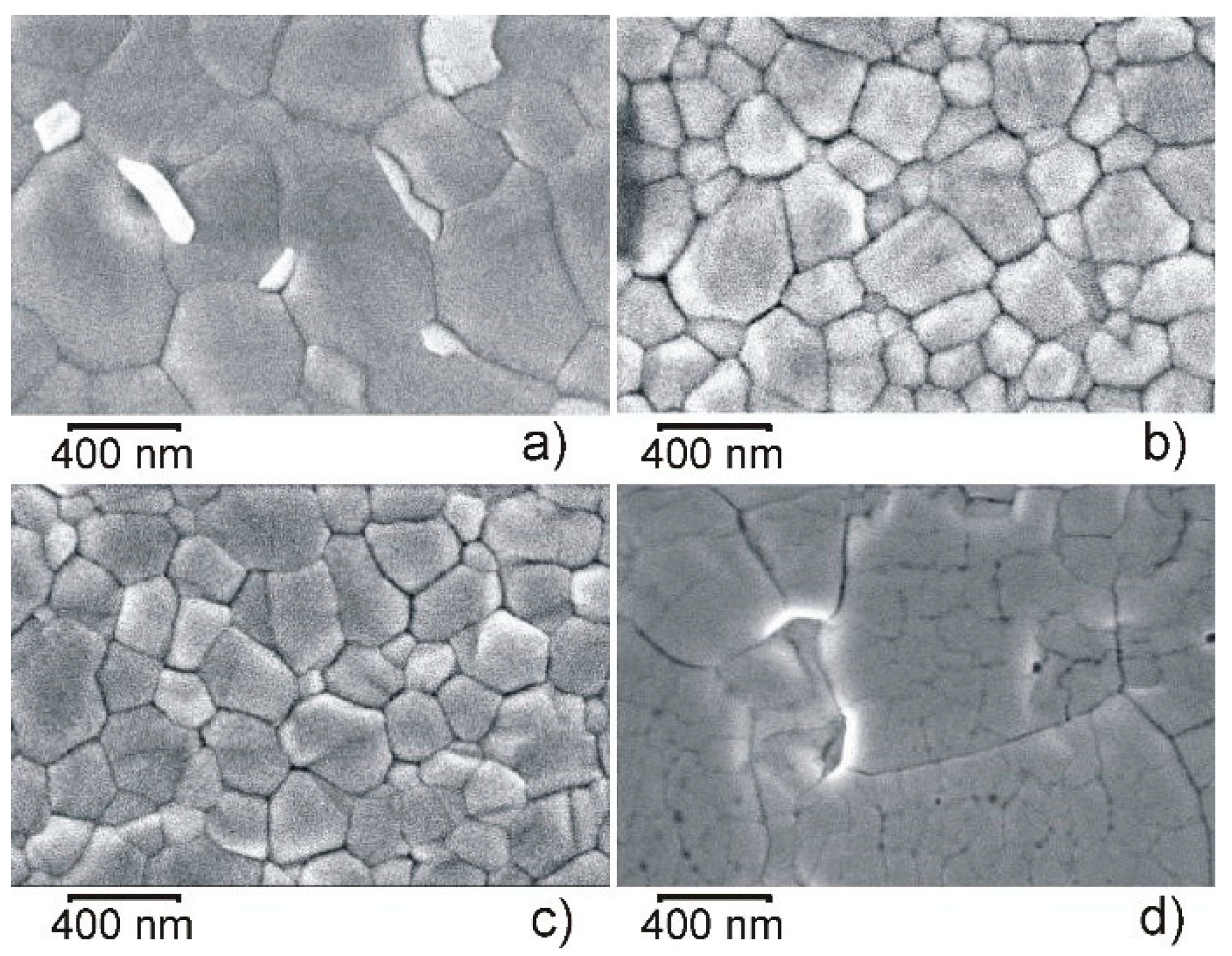

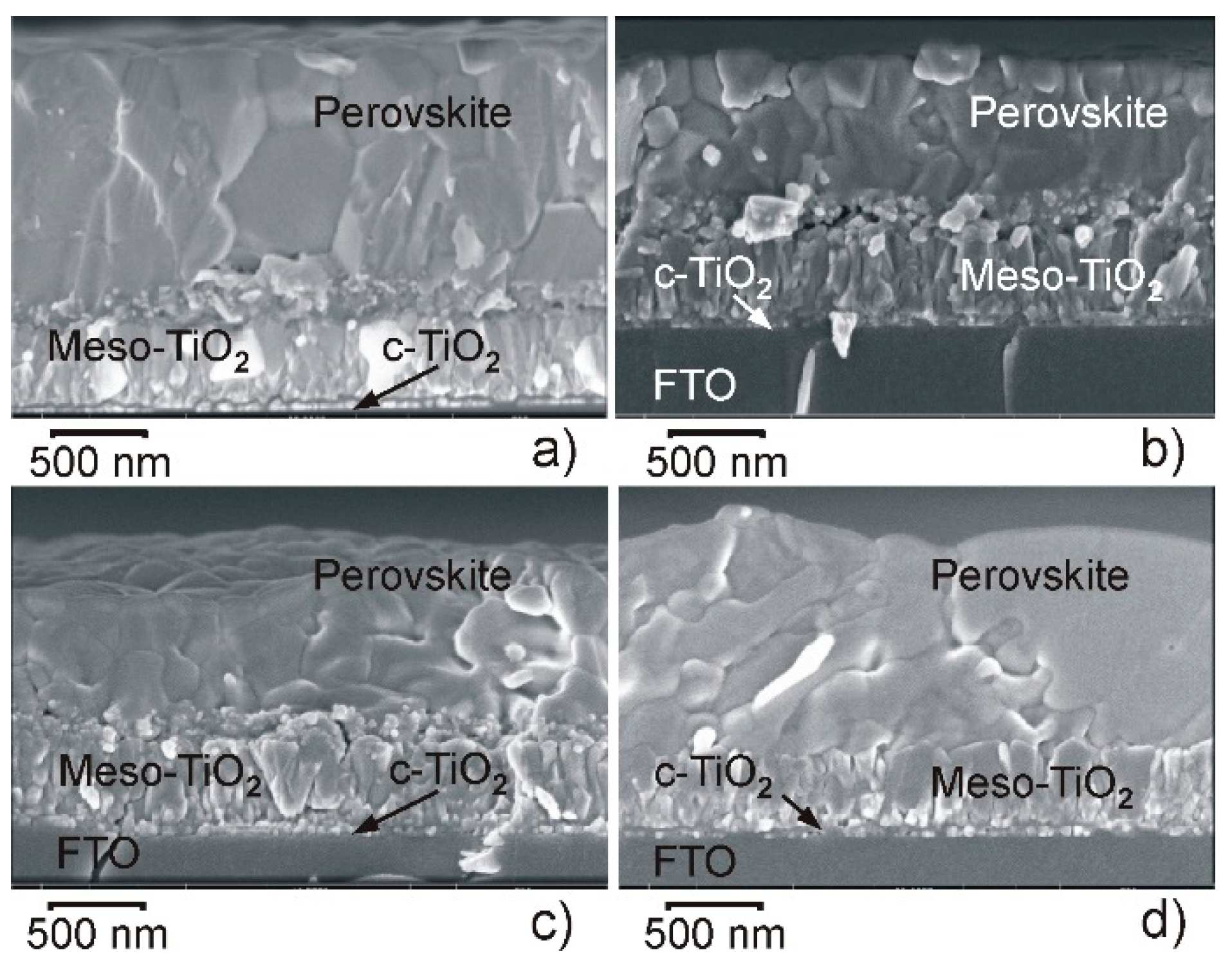
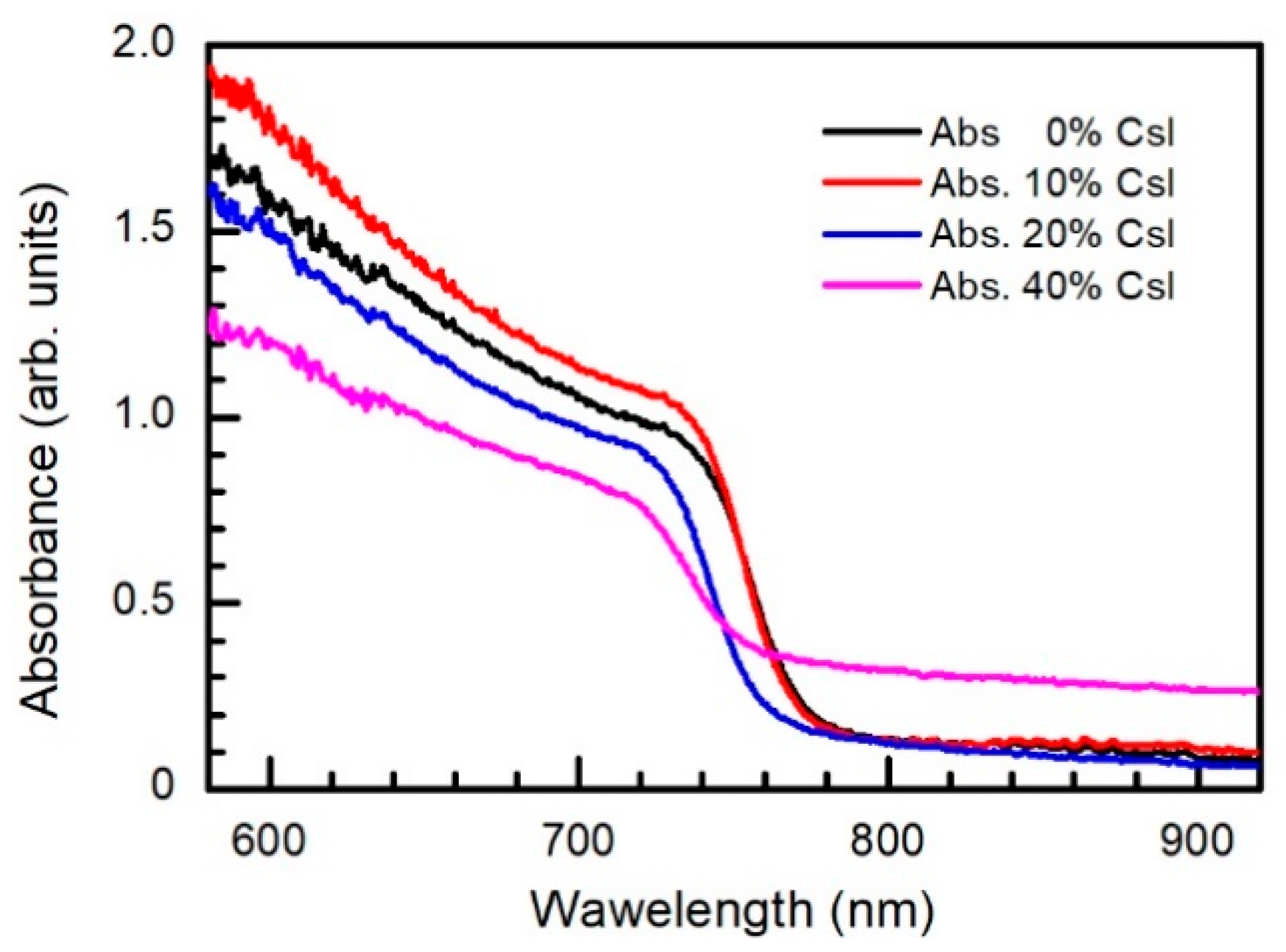


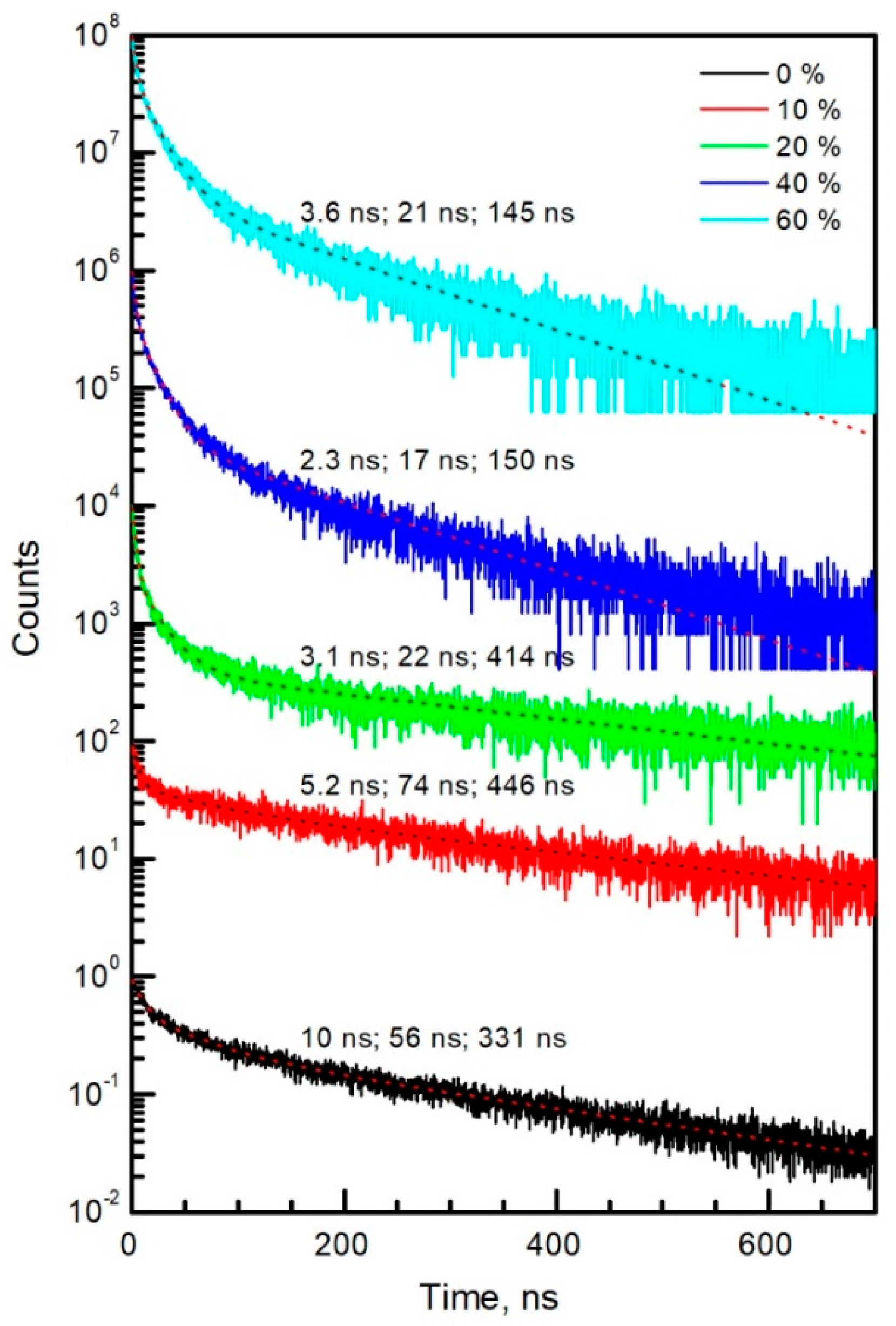
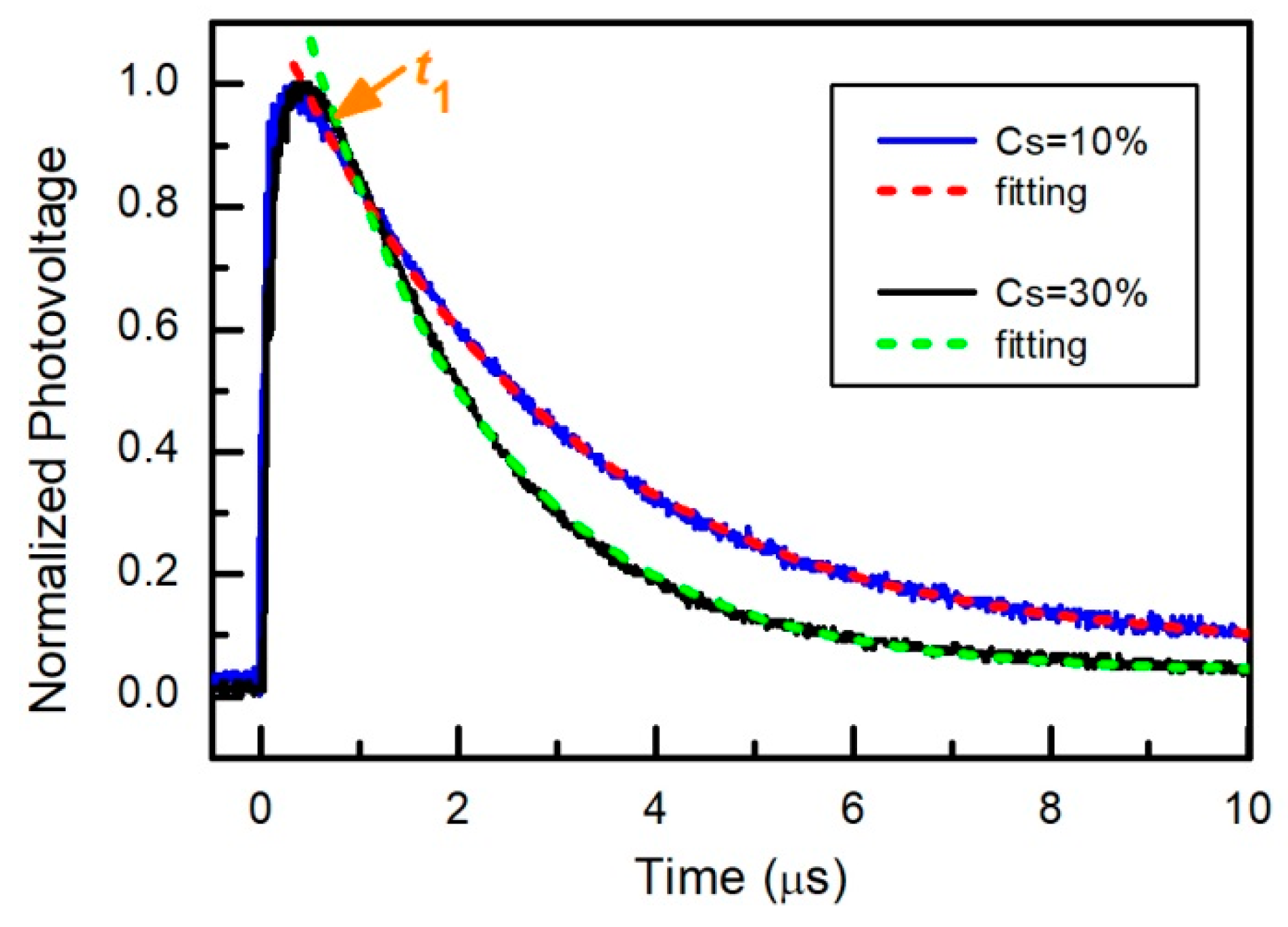


| Point in Figure 3 | Br | Sn | I | Cs | Pb |
|---|---|---|---|---|---|
| 1 | 10.7 | 4.1 | 57.6 | 0.8 | 22.1 |
| 2 | 6.3 | 2.0 | 52.4 | 17.5 | 17.4 |
| Cs Concentration, % | ||||||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | 60 | ||
| Band gap, eV (wavelength, nm) | 1.61 (770) | 1.62 (765) | 1.64 (756) | 1.63 (760) | 1.62 (765) | |
| λmax, nm | 770 | 762 | 752 | 746 | 747 | |
| Gaussian | λc1, nm | 767 | 761 | 752 | 739 | 743 |
| ω1 (FWHM), nm | 11 (26) | 12 (28) | 14 (33) | 37 (87) | 32 (75) | |
| A1, arb. u. | 0.29 | 0.34 | 0.47 | 0.13 | 0.17 | |
| λc2, nm | 775 | 770 | 756 | 746 | 747 | |
| ω2 (FWHM), nm | 23 (54) | 25 (59) | 23 (54) | 18 (42) | 17 (40) | |
| A2, arb. u. | 0.73 | 0.69 | 0.53 | 0.88 | 0.83 | |
| Equation (1) | A, arb. u. | 2.1 | 2.4 | 2.3 | 1.5 | 1.8 |
| λc, nm | 769 | 761 | 752 | 745 | 747 | |
| ω, nm | 19 | 13 | 15 | 31 | 25 | |
| ω1, nm | 10 | 11 | 10 | 10 | 12 | |
| ω2, nm | 15 | 17 | 13 | 11 | 11 | |
| Equations (4) and (5) | A, arb. u. | 0.95 | 0.83 | 0.79 | 0.72 | 0.79 |
| α | 9.5 | 59 | 1.3 | 1.7 | 1.2 | |
| β | 0.37 | 0.35 | 7E-8 | 0.24 | 0.19 | |
| , ns | 7.6 | 0.54 | 2.7 | 4.7 | 6.4 | |
| 698 | 2206 | Inf. | 108 | 117 | ||
| Cs, % | Voc, V | Jsc, mA·cm−2 | FF, % | PCE, % |
|---|---|---|---|---|
| 0 | 1.13 | 20.2 | 64 | 14.6 |
| 10 | 1.11 | 23.6 | 77 | 20.2 |
| 20 | 1.09 | 20.8 | 74 | 16.8 |
| 40 | 1.01 | 18.3 | 71 | 13.1 |
| 60 | 0.96 | 16.0 | 70 | 10.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ašmontas, S.; Čerškus, A.; Gradauskas, J.; Grigucevičienė, A.; Juškėnas, R.; Leinartas, K.; Lučun, A.; Petrauskas, K.; Selskis, A.; Sužiedėlis, A.; et al. Impact of Cesium Concentration on Optoelectronic Properties of Metal Halide Perovskites. Materials 2022, 15, 1936. https://doi.org/10.3390/ma15051936
Ašmontas S, Čerškus A, Gradauskas J, Grigucevičienė A, Juškėnas R, Leinartas K, Lučun A, Petrauskas K, Selskis A, Sužiedėlis A, et al. Impact of Cesium Concentration on Optoelectronic Properties of Metal Halide Perovskites. Materials. 2022; 15(5):1936. https://doi.org/10.3390/ma15051936
Chicago/Turabian StyleAšmontas, Steponas, Aurimas Čerškus, Jonas Gradauskas, Asta Grigucevičienė, Remigijus Juškėnas, Konstantinas Leinartas, Andžej Lučun, Kazimieras Petrauskas, Algirdas Selskis, Algirdas Sužiedėlis, and et al. 2022. "Impact of Cesium Concentration on Optoelectronic Properties of Metal Halide Perovskites" Materials 15, no. 5: 1936. https://doi.org/10.3390/ma15051936
APA StyleAšmontas, S., Čerškus, A., Gradauskas, J., Grigucevičienė, A., Juškėnas, R., Leinartas, K., Lučun, A., Petrauskas, K., Selskis, A., Sužiedėlis, A., & Širmulis, E. (2022). Impact of Cesium Concentration on Optoelectronic Properties of Metal Halide Perovskites. Materials, 15(5), 1936. https://doi.org/10.3390/ma15051936







