Recent Advances on Innovative Materials from Biowaste Recycling for the Removal of Environmental Estrogens from Water and Soil
Abstract
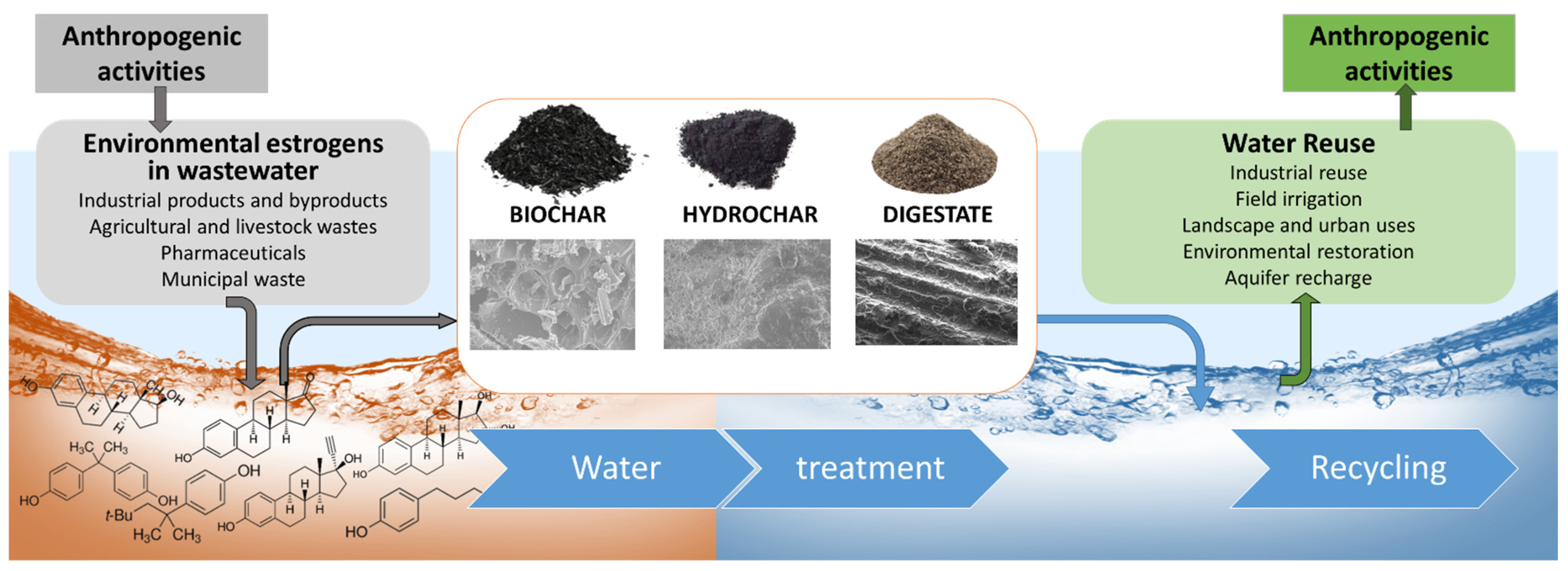
:1. Introduction
2. Biosorbents from Biowaste Recycling
2.1. Biochar
2.2. Hydrochar
2.3. Digestate
3. Characterization of Biosorbents
4. Environmental Estrogens
4.1. Phenolic Estrogens
4.2. Steroidal Estrogens
5. Technologies for the Removal of Estrogens
6. Sorptive Removal of Estrogens by Biosorbents
6.1. Adsorption Kinetics and Equilibrium Isotherms: Type of Interaction and Data Modeling
| Ref. | Model | Equation | Parameters |
|---|---|---|---|
| Adsorption Kinetics Models | |||
| [103] | Pseudo-first order | qt = qe (1 − exp−k1t) | qe and qt are the amount of solute adsorbed per mass unit of sorbent (mg g−1) at equilibrium and at time t, respectively; k1 (h−1) and k2 (kg mg−1 h−1) are the rate constants of sorption |
| [105] | Pseudo-second order | ||
| [106] | Elovich | e−αq | q is the amount of sorbate adsorbed at time t, a is the desorption constant and α is the initial adsorption rate |
| [108] | Intraparticle diffusion | qt = kid t1/2 + C | qt is the amount of solute adsorbed per mass unit of sorbent (mg g−1) at time t, kid (mg g−1 min−1/2) is the particle diffusion rate constant and C (mg g−1) is the intercept |
| Adsorption Isotherm Models | |||
| [109] | Freundlich | qe = KF Ce1/n | qe (mg g−1) is the amount of solute adsorbed per unit of substrate; Ce (mg mL−1) is the equilibrium concentration of the sorbate in solution. 1/n indicates the degree of nonlinearity between solution concentration and amount adsorbed, while the reciprocal n is the sorption intensity, KF is the Freundlich adsorption constant, b (mg g−1) is the maximum monolayer adsorption, KL (mL mg−1) is the Langmuir constant which expresses the energy of adsorption and Kd (mL g−1) is the distribution coefficient |
| [110] | Langmuir | qe = (KLCeb)/(1 + KLCe) | |
| [111] | Henry | qe = Kd Ce | |
| [112] | Temkin | qe = B ln(AT Ce) | qe (mg g−1) is the amount of solute adsorbed per unit of substrate, Ce (mg mL−1) is the equilibrium concentration of sorbate in solution, AT is the Temkin isotherm equilibrium binding constant (mL mg−1) and B (J mol−1) expresses the enthalpy of adsorption. B = RT/bT, where bT is a constant related to the heat of adsorption, T is the absolute temperature (K) and R is the universal gas constant (8.314 J mol−1 K−1) |
| [113] | Dubinin–Radushkevich | qe = (qs) exp (−kad2) | qs (mg g−1) is the maximum sorbate adsorption; kad is the Dubinin–Radushkevich constant (mol2/kJ2). kad = A/E, where E (kJ/mol) is the energy associated |
6.2. Removal from Water and Soil
6.2.1. Biochar
| EE | Feedstock | Process T (°C) | Residence Time (h) | Activation | Kinetics Model | Isotherm Model | Adsorption Capacity (mg g−1) * | Ref. |
|---|---|---|---|---|---|---|---|---|
| BPA | Wheat straw | 400 | 2–7 | - | n. e. | F | 9.33 | [52] |
| BPA | Poultry litter | 400 | 2–7 | - | n. e. | F | 57.54 | [52] |
| BPA | Pine chips | 800 | 2 | - | n. e. | F | 9.22 | [116] |
| BPA | Eucalyptus wood | 600 | 2 | oH3PO4 | PSO | L | 47.65 | [43] |
| BPA | Alfalfa | 650 | 2 | - | PSO | F | 24.30 | [117] |
| BPA | Grapefruit peel | 400 | 2 | - | PSO | L | 123.83 | [45] |
| BPA | Grapefruit peel | 400 | 2 | γ-Fe2O3 | PSO | L | 342.47 | [45] |
| BPA | Wheat straw | 550, 700 | 0.75 | CO2 | n. e. | L | 17.5, 14.2 | [121] |
| BPA | Wheat straw | 700 | 2 | NaOH | PSO | L | 71.42 | [40] |
| BPA | Sawdust | 800 | 1 | - | PSO | L | 5.08 | [46] |
| BPA | Sawdust | Various (from 500 to 900) | 1 | Fe and N2 | PSO | L | From 0.9 to 54.0 | [46] |
| OP | Red spruce wood pellet | 550 | 3 | - | PSO | L | 1.79 | [120] |
| OP | Sawdust | 450, 650, 850 | 1 | - | n. e. | F | 0.56, 1.05, 0.63 | [118] |
| NP | Sawdust | 450, 650, 850 | 1 | - | n. e. | F | 1.50, 2.07, 1.07 | [118] |
| E1 | Swine solids | Various (from 250 to 600) | 2 | - | n. e. | F | From 1.71 to 665.18 | [53] |
| E1 | Poultry litter | Various (from 250 to 600) | 2 | - | n. e. | F | From 4.77 to 460.47 | [53] |
| E1 | Eucalyptus wood | 600 | 2 | oH3PO4 | PSO | L | 75.88 | [43] |
| E1 | Lychee fruits | 650 | 2 | - | PSO | F | 0.65 | [91] |
| E2 | Pine chips | 800 | 2 | - | n. e. | F | 30.20 | [116] |
| E2 | Red spruce wood | 550 | 3 | - | PSO | F | 3.385 | [120] |
| E2 | Rice straw | Various (from 400 to 600) | 2 | - | PSO | F | From 6.86 to 13.95 | [101] |
| E2 | Eucalyptus wood | 600 | 2 | oH3PO4 | PSO | L | 51.26 | [43] |
| E2 | Lotus seedpod | 650 | 2 | - | PSO | L | 135.74 | [44] |
| E2 | Lotus seedpod | 650 | 2 | KOH | PSO | L | 150.10 | [44] |
| E3 | Eucalyptus wood | 400 | 2 | oH3PO4 | PSO | L | 42.17 | [43] |
| EE2 | Wheat straw | 400 | 2–7 | - | n. e. | F | 29.51 | [52] |
| EE2 | Poultry litter | 400 | 2–7 | - | n. e. | F | 8.32 | [52] |
| EE2 | Peanut shell | 550 | 2 | - | n. e. | F | 80.44 | [119] |
| EE2 | Eucalyptus wood | 400 | 2 | oH3PO4 | PSO | L | 51.16 | [43] |
6.2.2. Hydrochar
6.2.3. Digestate
7. Conclusions
Funding
Conflicts of Interest
References
- Schimmelpfennig, S.; Müller, C.; Grünhage, L.; Koch, C.; Kammann, C. Biochar, hydrochar and uncarbonized feedstock application to permanent grassland—Effects on greenhouse gas emissions and plant growth. Agric. Ecosyst. Environ. 2014, 191, 39–52. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Ok, Y.S.; Usman, A.R.A.; Al-Wabel, M.I.; Oleszczuk, P.; Lee, S.S. The effects of biochar amendment on soil fertility. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Guo, M., He, Z., Uchimiya, S.M., Eds.; Soil Science Social America: Madison, WI, USA, 2016; pp. 123–144. [Google Scholar] [CrossRef]
- Peng, W.; Pivato, A. Sustainable Management of Digestate from the Organic Fraction of Municipal Solid Waste and Food Waste Under the Concepts of Back to Earth Alternatives and Circular Economy. Waste Biomass Valoriz. 2017, 10, 465–481. [Google Scholar] [CrossRef]
- Rombolà, A.G.; Torri, C.; Vassura, I.; Venturini, E.; Reggiani, R.; Fabbri, D. Effect of biochar amendment on organic matter and dissolved organic matter composition of agricultural soils from a two-year field experiment. Sci. Total Environ. 2021, 812, 151422. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; Zhuang, X.; Li, B.; Xu, X.; Wei, Z.; Song, Y.; Jiang, E. Comparison of characterization and adsorption of biochars produced from hydrothermal carbonization and pyrolysis. Environ. Technol. Innov. 2018, 10, 27–35. [Google Scholar] [CrossRef]
- Cesaro, A. The valorization of the anaerobic digestate from the organic fractions of municipal solid waste: Challenges and perspectives. J. Environ. Manag. 2021, 280, 111742. [Google Scholar] [CrossRef]
- Wang, Z.; Han, L.; Sun, K.; Jin, J.; Ro, K.; Libra, J.; Liu, X.; Xing, B. Sorption of four hydrophobic organic contaminants by biochars derived from maize straw, wood dust and swine manure at different pyrolytic temperatures. Chemosphere 2016, 144, 285–291. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Kan, T.; Al Shoaibi, A.; Srinivasakannan, C.; Strezov, V. Characterization of Food Waste and Its Digestate as Feedstock for Thermochemical Processing. Energy Fuels 2015, 30, 1589–1597. [Google Scholar] [CrossRef]
- Sharma, H.B.; Sarmah, A.K.; Dubey, B. Hydrothermal carbonization of renewable waste biomass for solid biofuel production: A discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, C.; Liu, B.; Liu, G.; Zhao, M.; Xu, G.; Luo, X.; Li, F.; Xing, B. Biochar for water and soil remediation: Production, characterization, and application. In A New Paradigm for Environmental Chemistry and Toxicology; Jiang, G.B., Li, X.D., Eds.; Springer: Singapore, 2020; pp. 153–196. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Loffredo, E.; Carnimeo, C.; Silletti, R.; Summo, C. Use of the Solid By-Product of Anaerobic Digestion of Biomass to Remove Anthropogenic Organic Pollutants with Endocrine Disruptive Activity. Processes 2021, 9, 2018. [Google Scholar] [CrossRef]
- Wu, S.; Wu, H. Incorporating Biochar into Wastewater Eco-treatment Systems: Popularity, Reality, and Complexity. Environ. Sci. Technol. 2019, 53, 3345–3346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- European Commission (EC). Endocrine Disruptors. 2020. Available online: https://ec.europa.eu/environment/chemicals/endocrine/index_en.htm (accessed on 10 January 2022).
- Cho, S.H.; Choi, Y.; Kim, S.H.; Kim, S.J.; Chang, J. Urinary bisphenol A versus serum bisphenol A concentration and ovarian reproductive outcomes among IVF patients: Which is a better biomarker of BPA exposure? Mol. Cell. Toxicol. 2017, 13, 351–359. [Google Scholar] [CrossRef]
- Gao, Q.; Niu, Y.; Wang, B.; Liu, J.; Zhao, Y.; Zhang, J.; Wang, Y.; Shao, B. Estimation of lactating mothers’ daily intakes of bisphenol A using breast milk. Environ. Pollut. 2021, 286, 117545. [Google Scholar] [CrossRef]
- Ougier, E.; Zeman, F.; Antignac, J.-P.; Rousselle, C.; Lange, R.; Kolossa-Gehring, M.; Apel, P. Human biomonitoring initiative (HBM4EU): Human biomonitoring guidance values (HBM-GVs) derived for bisphenol A. Environ. Int. 2021, 154, 106563. [Google Scholar] [CrossRef]
- Lei, K.; Pan, H.-Y.; Zhu, Y.; Chen, W.; Lin, C.-Y. Pollution characteristics and mixture risk prediction of phenolic environmental estrogens in rivers of the Beijing–Tianjin–Hebei urban agglomeration, China. Sci. Total Environ. 2021, 787, 147646. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Huang, Z.; Zhang, Q.-Q.; Ying-He, L.; Wang, T.-T.; Yang, Y.-Y.; Ying, G.-G. Distribution and mass loads of xenoestrogens bisphenol a, 4-nonylphenol, and 4-tert-octylphenol in rainfall runoff from highly urbanized regions: A comparison with point sources of wastewater. J. Hazard. Mater. 2020, 401, 123747. [Google Scholar] [CrossRef]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose-Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef] [Green Version]
- Staples, C.; van der Hoeven, N.; Clark, K.; Mihaich, E.; Woelz, J.; Hentges, S. Distributions of concentrations of bisphenol A in North American and European surface waters and sediments determined from 19 years of monitoring data. Chemosphere 2018, 201, 448–458. [Google Scholar] [CrossRef]
- Russo, G.; Laneri, S.; Di Lorenzo, R.; Ferrara, L.; Grumetto, L. The occurrence of selected endocrine-disrupting chemicals in water and sediments from an urban lagoon in Southern Italy. Water Environ. Res. 2021, 93, 1944–1958. [Google Scholar] [CrossRef]
- Wan, Y.-P.; Chai, B.-W.; Wei, Q.; Hayat, W.; Dang, Z.; Liu, Z.-H. 17α-ethynylestradiol and its two main conjugates in seven municipal wastewater treatment plants: Analytical method, their occurrence, removal and risk evaluation. Sci. Total Environ. 2021, 812, 152489. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, S.; Chen, H.; Luo, S.; Xiong, Y.; Wang, X.; Xu, B.; Zheng, C.; Wang, K.-J. The comparative toxicities of BPA, BPB, BPS, BPF, and BPAF on the reproductive neuroendocrine system of zebrafish embryos and its mechanisms. J. Hazard. Mater. 2021, 406, 124303. [Google Scholar] [CrossRef] [PubMed]
- Ben, W.; Zhu, B.; Yuan, X.; Zhang, Y.; Yang, M.; Qiang, Z. Occurrence, removal and risk of organic micropollutants in wastewater treatment plants across China: Comparison of wastewater treatment processes. Water Res. 2018, 130, 38–46. [Google Scholar] [CrossRef] [PubMed]
- De Luna, Y.; Bensalah, N. Review on the electrochemical oxidation of endocrine-disrupting chemicals using BDD anodes. Curr. Opin. Electrochem. 2021, 32, 100900. [Google Scholar] [CrossRef]
- Gao, X.; Kang, S.; Xiong, R.; Chen, M. Environment-Friendly Removal Methods for Endocrine Disrupting Chemicals. Sustainability 2020, 12, 7615. [Google Scholar] [CrossRef]
- Sousa, J.C.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.; Silva, A. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef]
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- International Biochar Initiative (IBI). Standardized Product Definition and Product Testing Guidelines for Biochar that Is Used in Soil-Version 2.1. Published Online by International Biochar Initiative. 2015. Available online: https://www.biochar-international.org/wp-content/uploads/2018/04/IBI_Biochar_Standards_V2.1_Final.pdf (accessed on 5 December 2021).
- Taskin, E.; Bueno, C.D.C.; Allegretta, I.; Terzano, R.; Rosa, A.H.; Loffredo, E. Multianalytical characterization of biochar and hydrochar produced from waste biomasses for environmental and agricultural applications. Chemosphere 2019, 233, 422–430. [Google Scholar] [CrossRef]
- Singh, L.; Kalia, V.C. Waste Biomass Management—A Holistic Approach; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Igalavithana, A.D.; Mandal, S.; Niazi, N.K.; Vithanage, M.; Parikh, S.J.; Mukome, F.N.D.; Rizwan, M.; Oleszczuk, P.; Al-Wabel, M.; Bolan, N.; et al. Advances and future directions of biochar characterization methods and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2275–2330. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Bogomolova, I.; Glaser, B. Biochar stability in soil: Decomposition during eight years and transformation as assessed by compound-specific 14C analysis. Soil Biol. Biochem. 2014, 70, 229–236. [Google Scholar] [CrossRef]
- Jiang, B.; Lin, Y.; Mbog, J.C. Biochar derived from swine manure digestate and applied on the removals of heavy metals and antibiotics. Bioresour. Technol. 2018, 270, 603–611. [Google Scholar] [CrossRef]
- Loffredo, E.; Parlavecchia, M. Use of plant-based sorbents and mycodegradation for the elimination of endocrine disrupting chemicals from soil: A novel facile and low-cost method. Environ. Technol. Innov. 2021, 21, 101358. [Google Scholar] [CrossRef]
- Yang, X.; Igalavithana, A.D.; Oh, S.-E.; Nam, H.; Zhang, M.; Wang, C.-H.; Kwon, E.E.; Tsang, D.; Ok, Y.S. Characterization of bioenergy biochar and its utilization for metal/metalloid immobilization in contaminated soil. Sci. Total Environ. 2018, 640–641, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Gascó, G.; Paz-Ferreiro, J.; Álvarez, M.; Saa, A.; Méndez, A. Biochars and hydrochars prepared by pyrolysis and hydrothermal carbonisation of pig manure. Waste Manag. 2018, 79, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, Y.; Zhan, L.; Wu, D.; Zhang, S.; Pang, R.; Xie, B. Removal of emerging contaminants (bisphenol A and antibiotics) from kitchen wastewater by alkali-modified biochar. Sci. Total Environ. 2021, 805, 150158. [Google Scholar] [CrossRef]
- Hung, C.-Y.; Tsai, W.-T.; Chen, J.-W.; Lin, Y.-Q.; Chang, Y.-M. Characterization of biochar prepared from biogas digestate. Waste Manag. 2017, 66, 53–60. [Google Scholar] [CrossRef]
- Qian, F.; Zhu, X.; Liu, Y.; Hao, S.; Ren, Z.J.; Gao, B.; Zong, R.; Zhang, S.; Chen, J. Synthesis, characterization and adsorption capacity of magnetic carbon composites activated by CO2: Implication for the catalytic mechanisms of iron salts. J. Mater. Chem. A 2016, 4, 18942–18951. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Johir, A.H.; Sornalingam, K. Sorptive removal of phenolic endocrine disruptors by functionalized biochar: Competitive interaction mechanism, removal efficacy and application in wastewater. Chem. Eng. J. 2018, 335, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Liu, Y.; Zeng, G.; Gong, J.; Tan, X.; Wen, J.; Liu, S.; Jiang, L.; Li, M.; Yin, Z. Adsorption of 17β-estradiol from aqueous solution by raw and direct/pre/post-KOH treated lotus seedpod biochar. J. Environ. Sci. 2020, 87, 10–23. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M. Adsorption Characteristics and Mechanism of Bisphenol A by Magnetic Biochar. Int. J. Environ. Res. Public Health 2020, 17, 1075. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wu, C.; Chai, C.; Cao, S.; Bai, X.; Ma, K.; Jin, X.; Shi, X.; Jin, P. Adsorption of micropollutants from wastewater using iron and nitrogen co-doped biochar: Performance, kinetics and mechanism studies. J. Hazard. Mater. 2021, 424, 127606. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.S.; Pereira, D.; Sousa, Érika; Otero, M.; Esteves, V.I.; Calisto, V. Recent advances on the development and application of magnetic activated carbon and char for the removal of pharmaceutical compounds from waters: A review. Sci. Total Environ. 2020, 718, 137272. [Google Scholar] [CrossRef] [PubMed]
- Reguyal, F.; Sarmah, A.K. Adsorption of sulfamethoxazole by magnetic biochar: Effects of pH, ionic strength, natural organic matter and 17α-ethinylestradiol. Sci. Total Environ. 2018, 628–629, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, D.; Huang, Y.; Zhu, W.; Ding, Y.; Guo, C. Preparation of sludge-based hydrochar at different temperatures and adsorption of BPA. Water Sci. Technol. 2020, 82, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhu, Z.; Zhang, H.; Di, G.; Qiu, Y.; Yin, D.; Wang, S. Hydrochars from pinewood for adsorption and nonradical catalysis of bisphenols. J. Hazard. Mater. 2020, 385, 121548. [Google Scholar] [CrossRef]
- Parlavecchia, M.; Carnimeo, C.; Loffredo, E. Soil Amendment with Biochar, Hydrochar and Compost Mitigates the Accumulation of Emerging Pollutants in Rocket Salad Plants. Water Air Soil Pollut. 2020, 231, 1–12. [Google Scholar] [CrossRef]
- Sun, K.; Ro, K.; Guo, M.; Novak, J.; Mashayekhi, H.; Xing, B. Sorption of bisphenol A, 17α-ethinyl estradiol and phenanthrene on thermally and hydrothermally produced biochars. Bioresour. Technol. 2011, 102, 5757–5763. [Google Scholar] [CrossRef]
- Han, L.; Ro, K.S.; Sun, K.; Sun, H.; Wang, Z.; Libra, J.A.; Xing, B. New Evidence for High Sorption Capacity of Hydrochar for Hydrophobic Organic Pollutants. Environ. Sci. Technol. 2016, 50, 13274–13282. [Google Scholar] [CrossRef]
- Fang, J.; Gao, B.; Zimmerman, A.R.; Ro, K.S.; Chen, J. Physically (CO2) activated hydrochars from hickory and peanut hull: Preparation, characterization, and sorption of methylene blue, lead, copper, and cadmium. RSC Adv. 2016, 6, 24906–24911. [Google Scholar] [CrossRef]
- Fang, J.; Gao, B.; Mosa, A.; Zhan, L. Chemical activation of hickory and peanut hull hydrochars for removal of lead and methylene blue from aqueous solutions. Chem. Speciat. Bioavailab. 2017, 29, 197–204. [Google Scholar] [CrossRef] [Green Version]
- de Lima, H.H.C.; Llop, M.E.G.; dos Santos Maniezzo, R.; Moisés, M.P.; Janeiro, V.; Arroyo, P.A.; Guilherme, M.R.; Rinaldi, A.W. Enhanced removal of bisphenol A using pine-fruit shell-derived hydrochars: Adsorption mechanisms and reusability. J. Hazard. Mater. 2021, 416, 126167. [Google Scholar] [CrossRef]
- Tian, S.-R.; Liu, Y.-G.; Liu, S.-B.; Zeng, G.-M.; Jiang, L.-H.; Tan, X.-F.; Huang, X.-X.; Yin, Z.-H.; Liu, N.; Li, J. Hydrothermal synthesis of montmorillonite/hydrochar nanocomposites and application for 17β-estradiol and 17α-ethynylestradiol removal. RSC Adv. 2018, 8, 4273–4283. [Google Scholar] [CrossRef] [Green Version]
- Ning, Q.; Liu, Y.; Liu, S.; Jiang, L.; Zeng, G.; Zeng, Z.; Wang, X.; Li, J.; Kare, Z. Fabrication of hydrochar functionalized Fe–Mn binary oxide nanocomposites: Characterization and 17β-estradiol removal. RSC Adv. 2017, 7, 37122–37129. [Google Scholar] [CrossRef] [Green Version]
- Braguglia, C.M.; Gallipoli, A.; Gianico, A.; Pagliaccia, P. Anaerobic bioconversion of food waste into energy: A critical review. Bioresour. Technol. 2018, 248, 37–56. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J. Valorization of anaerobic digestion digestate: A prospect review. Bioresour. Technol. 2021, 323, 124626. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Weihermüller, L.; Tappe, W.; Hofmann, D.; Köppchen, S.; Laabs, V.; Vereecken, H.; Burauel, P. Sorption–desorption behaviour of bentazone, boscalid and pyrimethanil in biochar and digestate based soil mixtures for biopurification systems. Sci. Total Environ. 2016, 559, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Fabbricino, M.; Race, M.; Ferraro, A.; Pontoni, L.; Aimone, O.; Chen, Y. Study of the Digestate as an Innovative and Low-Cost Adsorbent for the Removal of Dyes in Wastewater. Processes 2020, 8, 852. [Google Scholar] [CrossRef]
- Abdel-Fattah, T.M.; Mahmoud, M.E.; Ahmed, S.B.; Huff, M.D.; Lee, J.W.; Kumar, S. Biochar from woody biomass for removing metal contaminants and carbon sequestration. J. Ind. Eng. Chem. 2015, 22, 103–109. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, B.; Budai, A.; Jeng, A.; Hao, X.; Wei, D.; Zhang, Y.; Rasse, D. Study of Biochar Properties by Scanning Electron Microscope—Energy Dispersive X-Ray Spectroscopy (SEM-EDX). Commun. Soil Sci. Plant Anal. 2016, 47, 593–601. [Google Scholar] [CrossRef]
- Missaoui, A.; Bostyn, S.; Belandria, V.; Cagnon, B.; Sarh, B.; Gökalp, I. Hydrothermal carbonization of dried olive pomace: Energy potential and process performances. J. Anal. Appl. Pyrolysis 2017, 128, 281–290. [Google Scholar] [CrossRef]
- Ruan, X.; Liu, Y.; Wang, G.; Frost, R.L.; Qian, G.; Tsang, D.C. Transformation of functional groups and environmentally persistent free radicals in hydrothermal carbonisation of lignin. Bioresour. Technol. 2018, 270, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Zbair, M.; Bottlinger, M.; Ainassaari, K.; Ojala, S.; Stein, O.; Keiski, R.L.; Bensitel, M.; Brahmi, R. Hydrothermal Carbonization of Argan Nut Shell: Functional Mesoporous Carbon with Excellent Performance in the Adsorption of Bisphenol A and Diuron. Waste Biomass Valoriz. 2020, 11, 1565–1584. [Google Scholar] [CrossRef] [Green Version]
- Fierro, J.; Martinez, E.J.; Rosas, J.G.; Fernández, R.A.; López, R.; Gomez, X. Co-Digestion of Swine Manure and Crude Glycerine: Increasing Glycerine Ratio Results in Preferential Degradation of Labile Compounds. Water Air Soil Pollut. 2016, 227, 1–13. [Google Scholar] [CrossRef]
- Suman, S.; Panwar, D.S.; Gautam, S. Surface morphology properties of biochars obtained from different biomass waste. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 37, 1007–1012. [Google Scholar] [CrossRef]
- Senesi, N.; Loffredo, E. The chemistry of soil organic matter. In Soil Physical Chemistry, 2nd ed.; Sparks, D.L., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 239–370. [Google Scholar] [CrossRef]
- Senesi, N.; Loffredo, E.; D’Orazio, V.; Brunetti, G.; Miano, T.M.; La Cava, P. Adsorption of pesticides by humic acids from organic amendments and soils. In Humic Substances and Chemical Contaminants; Clapp, C.E., Hayes, M.H.B., Senesi, N., Bloom, P.R., Jardine, P.M., Eds.; Soil Science Social America: Madison, WI, USA, 2015; pp. 129–153. [Google Scholar] [CrossRef]
- Marra, R.; Vinale, F.; Cesarano, G.; Lombardi, N.; D’Errico, G.; Crasto, A.; Mazzei, P.; Piccolo, A.; Incerti, G.; Woo, S.L.; et al. Biochars from olive mill waste have contrasting effects on plants, fungi and phytoparasitic nematodes. PLoS ONE 2018, 13, e0198728. [Google Scholar] [CrossRef]
- Metcalfe, C.; Bayen, S.; Desrosiers, M.; Muñoz, G.; Sauvé, S.; Yargeau, V. An introduction to the sources, fate, occurrence and effects of endocrine disrupting chemicals released into the environment. Environ. Res. 2022, 207, 112658. [Google Scholar] [CrossRef]
- Boonnorat, J.; Treesubsuntorn, C.; Phattarapattamawong, S.; Cherdchoosilapa, N.; Seemuang-On, S.; Somjit, M.; Huadprom, C.; Rojviroon, T.; Jutakanoke, R.; Prachanurak, P. Effect of leachate effluent water reuse on the phytotoxicity and micropollutants accumulation in agricultural crops. J. Environ. Chem. Eng. 2021, 9, 106639. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. PubChem Open Chemistry Database at the National Institutes of Health (NIH). Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 10 January 2022).
- Huang, R.-P.; Liu, Z.-H.; Yuan, S.-F.; Yin, H.; Dang, Z.; Wu, P.-X. Worldwide human daily intakes of bisphenol A (BPA) estimated from global urinary concentration data (2000–2016) and its risk analysis. Environ. Pollut. 2017, 230, 143–152. [Google Scholar] [CrossRef]
- Diao, P.; Chen, Q.; Wang, R.; Sun, D.; Cai, Z.; Wu, H.; Duan, S. Phenolic endocrine-disrupting compounds in the Pearl River Estuary: Occurrence, bioaccumulation and risk assessment. Sci. Total Environ. 2017, 584–585, 1100–1107. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Shan, B. The effects of urbanization and rainfall on the distribution of, and risks from, phenolic environmental estrogens in river sediment. Environ. Pollut. 2019, 250, 1010–1018. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.; He, J.; Yue, X.; Pan, J.; Wang, Z. Steroidal and phenolic endocrine disrupting chemicals (EDCs) in surface water of Bahe River, China: Distribution, bioaccumulation, risk assessment and estrogenic effect on Hemiculter leucisculus. Environ. Pollut. 2018, 243, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yu, J.; Hu, X.; Yin, D. Characteristics of the alkylphenol and bisphenol A distributions in marine organisms and implications for human health: A case study of the East China Sea. Sci. Total Environ. 2016, 539, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Olaniyan, L.W.B.; Okoh, O.O.; Mkwetshana, N.T.; Okoh, A.I. Environmental Water Pollution, Endocrine Interference and Ecotoxicity of 4-tert-Octylphenol: A Review. Rev. Environ. Contam. Toxicol. 2018, 248, 81–109. [Google Scholar] [CrossRef]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navas, C.; Björklund, E.; Halling-Sørensen, B.; Hansen, M. Biogas final digestive byproduct applied to croplands as fertilizer contains high levels of steroid hormones. Environ. Pollut. 2013, 180, 368–371. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Z.-H.; Wang, H.; Dang, Z.; Liu, Y. Occurrence and removal of 17α-ethynylestradiol (EE2) in municipal wastewater treatment plants: Current status and challenges. Chemosphere 2021, 271, 129551. [Google Scholar] [CrossRef] [PubMed]
- Jarošová, B.; Erseková, A.; Hilscherová, K.; Loos, R.; Gawlik, B.M.; Giesy, J.P.; Bláha, L. Europe-wide survey of estrogenicity in wastewater treatment plant effluents: The need for the effect-based monitoring. Environ. Sci. Pollut. Res. 2014, 21, 10970–10982. [Google Scholar] [CrossRef]
- Yu, W.; Du, B.; Yang, L.; Zhang, Z.; Yang, C.; Yuan, S.; Zhang, M. Occurrence, sorption, and transformation of free and conjugated natural steroid estrogens in the environment. Environ. Sci. Pollut. Res. 2019, 26, 9443–9468. [Google Scholar] [CrossRef]
- UNESCO. The United Nations World Water Development Report 2018: Nature-Based Solutions for Water. 2018. Available online: https://www.unwater.org/publications/world-water-development-report-2018/ (accessed on 11 January 2022).
- Javaid, R.; Qazi, U.Y.; Ikhlaq, A.; Zahid, M.; Alazmi, A. Subcritical and supercritical water oxidation for dye decomposition. J. Environ. Manag. 2021, 290, 112605. [Google Scholar] [CrossRef]
- Morillo, E.; Villaverde, J. Advanced technologies for the remediation of pesticide-contaminated soils. Sci. Total Environ. 2017, 586, 576–597. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, L.; Shu, Y.; Jiang, S.; Huang, R.; Jia, Z.; Wei, D. Effect of ageing process on bisphenol A sorption and retention in agricultural soils amended with biochar. Environ. Sci. Pollut. Res. 2020, 27, 17401–17411. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.-Y.; Ge, H.; Shi, J.; Liu, X.; Guo, W.; Zhang, M.; Meng, Y.; Li, X.-Y. The characteristics of oestrone mobility in water and soil by the addition of Ca-biochar and Fe–Mn-biochar derived from Litchi chinensis Sonn. Environ. Geochem. Health 2020, 42, 1601–1615. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhang, B.; Tan, G.; Li, J.; Wang, H. Influence of biochar on sorption, leaching and dissipation of bisphenol A and 17α-ethynylestradiol in soil. Environ. Sci. Process. Impacts 2015, 17, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, J.; Tan, G.; Xu, N. Influence of biochar on the sorption of bisphenol A and 17α-ethynylestradiol in soil. Chin. J. Environ. Eng. 2016, 10, 5255–5261. [Google Scholar] [CrossRef]
- Loffredo, E.; Picca, G.; Parlavecchia, M. Single and combined use of Cannabis sativa L. and carbon-rich materials for the removal of pesticides and endocrine-disrupting chemicals from water and soil. Environ. Sci. Pollut. Res. 2021, 28, 3601–3616. [Google Scholar] [CrossRef]
- Altenburger, R.; Scholze, M.; Busch, W.; Escher, B.I.; Jakobs, G.; Krauss, M.; Krüger, J.; Neale, P.; Ait-Aissa, S.; Almeida, A.; et al. Mixture effects in samples of multiple contaminants—An inter-laboratory study with manifold bioassays. Environ. Int. 2018, 114, 95–106. [Google Scholar] [CrossRef]
- Hom-Diaz, A.; Llorca, M.; Rodriguez-Mozaz, S.; Vicent, T.; Barceló, D.; Blánquez, P. Microalgae cultivation on wastewater digestate: β-estradiol and 17α-ethynylestradiol degradation and transformation products identification. J. Environ. Manag. 2015, 155, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Castellana, G.; Loffredo, E. Simultaneous Removal of Endocrine Disruptors from a Wastewater Using White Rot Fungi and Various Adsorbents. Water Air Soil Pollut. 2014, 225, 1872. [Google Scholar] [CrossRef]
- Madadi, R.; Bester, K. Fungi and biochar applications in bioremediation of organic micropollutants from aquatic media. Mar. Pollut. Bull. 2021, 166, 112247. [Google Scholar] [CrossRef]
- Alves, T.C.; Mota, J.A.X.; Pinheiro, A. Biosorption of organic micropollutants onto lignocellulosic-based material. Water Sci. Technol. 2020, 82, 427–439. [Google Scholar] [CrossRef]
- Peiris, C.; Nawalage, S.; Wewalwela, J.J.; Gunatilake, S.R.; Vithanage, M. Biochar based sorptive remediation of steroidal estrogen contaminated aqueous systems: A critical review. Environ. Res. 2020, 191, 110183. [Google Scholar] [CrossRef]
- Wang, X.; Liu, N.; Liu, Y.; Jiang, L.; Zeng, G.; Tan, X.; Liu, S.; Yin, Z.; Tian, S.; Li, J. Adsorption Removal of 17β-Estradiol from Water by Rice Straw-Derived Biochar with Special Attention to Pyrolysis Temperature and Background Chemistry. Int. J. Environ. Res. Public Health 2017, 14, 1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antero, R.V.P.; Alves, A.C.F.; Sales, P.D.T.F.; De Oliveira, S.B.; Ojala, S.A.; Brum, S.S. A new approach to obtain mesoporous-activated carbon via hydrothermal carbonization of Brazilian Cerrado biomass combined with physical activation for bisphenol-A removal. Chem. Eng. Commun. 2019, 206, 1498–1514. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption gelöster stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Kumar, K.V. Linear and non-linear regression analysis for the sorption kinetics of methylene blue onto activated carbon. J. Hazard. Mater. 2006, 137, 1538–1544. [Google Scholar] [CrossRef]
- Ho, Y.-S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]
- McLintock, I.S. The Elovich Equation in Chemisorption Kinetics. Nature 1967, 216, 1204–1205. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Emik, S.; Öngen, A.H.; Özcan, K.; Aydın, S. Modelling of adsorption kinetic processes—Errors, theory and application. In Advanced Sorption Process Applications; Edebali, S., Ed.; IntechOpen: London, UK, 2018; Available online: https://www.intechopen.com/chapters/63161 (accessed on 10 January 2022). [CrossRef] [Green Version]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Barrer, R.M.; Rees, L.V.C. Henry’s law adsorption constants. Trans. Faraday Soc. 1961, 57, 999–1007. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Badjah, A.Y.; Ali, I. Facile synthesis and characterization of multi walled carbon nanotubes for fast and effective removal of 4-tert-octylphenol endocrine disruptor in water. J. Mol. Liq. 2019, 275, 41–48. [Google Scholar] [CrossRef]
- Nguyen, C.; Do, D. The Dubinin–Radushkevich equation and the underlying microscopic adsorption description. Carbon 2001, 39, 1327–1336. [Google Scholar] [CrossRef]
- Eibisch, N.; Schroll, R.; Fuß, R.; Mikutta, R.; Helfrich, M.; Flessa, H. Pyrochars and hydrochars differently alter the sorption of the herbicide isoproturon in an agricultural soil. Chemosphere 2015, 119, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Parlavecchia, M.; D’Orazio, V.; Loffredo, E. Wood biochars and vermicomposts from digestate modulate the extent of adsorption-desorption of the fungicide metalaxyl-m in a silty soil. Environ. Sci. Pollut. Res. 2019, 26, 35924–35934. [Google Scholar] [CrossRef]
- Kim, E.; Jung, C.; Han, J.; Her, N.; Park, C.M.; Jang, M.; Son, A.; Yoon, Y. Sorptive removal of selected emerging contaminants using biochar in aqueous solution. J. Ind. Eng. Chem. 2016, 36, 364–371. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Kan, E. Effects of pyrolysis temperature on the physicochemical properties of alfalfa-derived biochar for the adsorption of bisphenol A and sulfamethoxazole in water. Chemosphere 2019, 218, 741–748. [Google Scholar] [CrossRef]
- Del Bubba, M.; Anichini, B.; Bakari, Z.; Bruzzoniti, M.C.; Camisa, R.; Caprini, C.; Checchini, L.; Fibbi, D.; El Ghadraoui, A.; Liguori, F.; et al. Physicochemical properties and sorption capacities of sawdust-based biochars and commercial activated carbons towards ethoxylated alkylphenols and their phenolic metabolites in effluent wastewater from a textile district. Sci. Total Environ. 2020, 708, 135217. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, H.; Huang, W.; Arocena, J.M.; Ge, S. Sorption of Atrazine, 17α-Estradiol, and Phenanthrene on Wheat Straw and Peanut Shell Biochars. Water Air Soil Pollut. 2016, 227, 1–13. [Google Scholar] [CrossRef]
- Loffredo, E.; Taskin, E. Adsorptive removal of ascertained and suspected endocrine disruptors from aqueous solution using plant-derived materials. Environ. Sci. Pollut. Res. 2017, 24, 19159–19166. [Google Scholar] [CrossRef]
- Kozyatnyk, I.; Oesterle, P.; Wurzer, C.; Mašek, O.; Jansson, S. Removal of contaminants of emerging concern from multicomponent systems using carbon dioxide activated biochar from lignocellulosic feedstocks. Bioresour. Technol. 2021, 340, 125561. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yan, C.; Nie, M.; Ding, M. Bisphenol A adsorption behavior on soil and biochar: Impact of dissolved organic matter. Environ. Sci. Pollut. Res. 2021, 28, 32434–32445. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Qi, Z.; Prasher, S.O.; Li, L.; Gui, D.; Jiang, Q. Effect of biochar amendment on soil’s retention capacity for estro-genic hormones from poultry manure treatment. Front. Agric. Sci. Eng. 2017, 4, 208–219. [Google Scholar] [CrossRef]
- Selvaraj, P.S.; Periasamy, K.; Suganya, K.; Ramadass, K.; Muthusamy, S.; Ramesh, P.; Bush, R.; Vincent, S.G.T.; Palanisami, T. Novel resources recovery from anaerobic digestates: Current trends and future perspectives. Crit. Rev. Environ. Sci. Technol. 2020, in press. [Google Scholar] [CrossRef]
- Wang, N.; Huang, D.; Shao, M.; Sun, R.; Xu, Q. Use of activated carbon to reduce ammonia emissions and accelerate humification in composting digestate from food waste. Bioresour. Technol. 2022, 347, 126701. [Google Scholar] [CrossRef] [PubMed]
- Le Pera, A.; Sellaro, M.; Bencivenni, E.; D’Amico, F. Environmental sustainability of an integrate anaerobic digestion-composting treatment of food waste: Analysis of an Italian plant in the circular bioeconomy strategy. Waste Manag. 2022, 139, 341–351. [Google Scholar] [CrossRef]
| Biochar | Hydrochar | Digestate | |||
|---|---|---|---|---|---|
| Type of biomass conversion | Thermochemical | Thermochemical | Biochemical | ||
| Process | Slow pyrolysis | Fast pyrolysis | Gasification | Hydrothermal carbonization | Anaerobic digestion |
| Type of feedstock | Agricultural residues Woody residues | Agricultural residues OFMSW | Agricultural residues Livestock wastes Sewage sludge OFMSW | ||
| Feedstock moisture | Dry | Wet | 80–90% | ||
| Temperature (°C) | 300–650 | 500–650 | 800–900 | 180–260 | Psychrophilic (20–25) Mesophilic (35–37) Thermophilic (>55) |
| Residence time | 1–12 h | <2 s | 10–20 s | 1–12 h | 14–30 days (mesophilic) 14–16 (thermophilic) |
| Pressure | - | - | - | Autogenous (2–10 MPa) | - |
| Product yield (%) | |||||
| Solid | 25–35 | 12 | <10 | 50–80 | - |
| Liquid | 20–30 | 75 | <5 | 5–20 | - |
| Gases | 25–35 | 13 | >85 | 2–5 | 60–70 (fresh biomass) |
| Sample | Feedstock | Process T (°C) | Elemental Analysis | SEM, SEM- EDX | BET | XRF | FTIR | TG, DTG | NMR | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| BC | Poultry litter and wheat straw | 400 | • | • | • | • | [52] | |||
| BC | Pinewood | 500 | • | • | • | • | • | [63] | ||
| BC | Corncob | 700 | • | • | [64] | |||||
| BC | Swine solids and poultry litter | 250, 450, 600 | • | • | • | • | [53] | |||
| BC | Eucalyptus wood | 600 | • | • | • | [43] | ||||
| BC | Digestate | Various (from 300 to 900) | • | • | [41] | |||||
| BC | Dairy manure and sorghum | 600 | • | • | • | • | [37] | |||
| BC | Algae and sorghum | 500 | • | • | • | • | [38] | |||
| BC | Red spruce and grapevine wood | 550 | • | • | • | • | • | [32] | ||
| BC | Lotus seedpod | 650 | • | • | • | • | [44] | |||
| BC | Wheat straw | 700 | • | • | • | [40] | ||||
| HC | Orange peels | 200 | • | • | • | • | [64] | |||
| HC | Swine solids and poultry litter | 250 | • | • | • | • | [53] | |||
| HC | Corncob | 230 | • | • | [64] | |||||
| HC | Olive pomace | 180–250 | • | • | • | [65] | ||||
| HC | Lignin | 240, 300 | • | • | [66] | |||||
| HC | Rice husk | 200 | • | • | • | [58] | ||||
| HC | Rice husk | 180 | • | • | • | • | • | [57] | ||
| HC | Urban pruning and OFMW | 180–210 | • | • | • | • | • | [32] | ||
| HC | Sewage sludge | 160, 190, 250 | • | • | • | • | [49] | |||
| HC | Argan nut shells | 180, 200 | • | • | • | • | [67] | |||
| HC | Pine fruit shells | 190 | • | • | • | [56] | ||||
| DG | Swine manure | 34 | • | • | • | • | [68] | |||
| DG | Food waste | - | • | • | • | [8] | ||||
| DG | Swine manure | - | • | • | [41] | |||||
| DG | Mixed residues and olive pomace | 20–45 | • | • | [12] |
| Process | Feedstock | Process T (°C) | Residence Time | Elemental Composition a (%) | pH b | EC b (dS m−1) | Ash (%) | Surface Area c (m2 g−1) | Avg. Pore Size (nm) | Pore Volume (cm3 g−1) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | ||||||||||||
| BC | Py | Digestate from swine manure | 800 | 1.3 h | -- | -- | -- | -- | -- | -- | -- | 101.9 | 3.04 | 0.08 | [41] |
| BC | Py | Algae | 500 | 2 h | 24.6 | 1.3 | 3.2 | 11.4 | 10.2 | 10.70 | 59.7 | 0.5 | 1.88 | 0.16 | [38] |
| BC | Py | Sorghum | 500 | 2 h | 46.7 | 3.0 | 0.0 | 13.0 | 7.4 | 5.95 | 29.4 | 4.1 | 13.22 | 13.27 | |
| BC | Py | Sorghum | 600 | 2 h | 47.4 | 2.3 | 0.0 | 9.8 | 9.6 | 5.92 | 45.1 | 4.1 | 13.29 | 12.99 | |
| BC | Py | Red spruce wood | 550 | 3 h | 84.0 | 1.5 | 0.2 | n.d. | 9.1 | 0.39 | 4.7 | -- | -- | -- | [32] |
| BC | Py | Grapevine pruning | 550 | 3 h | 75.5 | 1.3 | 0.5 | n.d. | 9.9 | 2.23 | 9.9 | -- | -- | -- | |
| BC | Py | Lotus seedpod | 650 | 2 h | 69.9 | 2.1 | 1.1 | 15.6 | -- | -- | -- | 25.2 | 2.55 | 0.03 | [44] |
| KOH- BC | Py | Lotus seedpod | 650 | 2 h | 78.8 | 2.4 | 1.3 | 15.1 | -- | -- | -- | 306.2 | 1.90 | 0.13 | |
| BC | Py | Wheat straw | 700 | 2 h | -- | -- | -- | -- | -- | -- | -- | 57.2 | 3.70 | 0.05 | [40] |
| NaOH-BC | Py | Wheat straw | 700 | 2 h | -- | -- | -- | -- | -- | -- | -- | 254.9 | 1.92 | 0.12 | |
| HCl- BC | Py | Wheat straw | 700 | 2 h | -- | -- | -- | -- | -- | -- | -- | 197.2 | 2.86 | 0.14 | |
| HC | HTC | Pig manure | 200 | 2 h | 33.8 | 4.2 | 2.5 | 15.0 | 8.3 | 19.86 | 44.0 | -- | -- | 2.10 | [39] |
| HC | HTC | Pig manure | 240 | 2 h | 25.8 | 3.0 | 1.9 | 10.4 | 7.8 | 10.93 | 58.6 | -- | -- | 2.80 | |
| HC | HTC | Urban pruning | 210 | 8 h | 61.5 | 6.2 | 1.7 | -- | 6.6 | 1.03 | 12.5 | -- | -- | -- | [32] |
| HC | HTC | OFMSW | 210 | 8 h | 62.6 | 6.0 | 1.7 | -- | 7.7 | 1.09 | 15.7 | -- | -- | -- | |
| HC | HTC | Sewage sludge | 160 | 4 h | 30.8 | 4.9 | 3.2 | 14.0 | 5.1 | 6.10 | 46.5 | 9.5 | -- | -- | [49] |
| HC | HTC | Sewage sludge | 190 | 4 h | 30.0 | 4.3 | 2.4 | 11.4 | 5.7 | 8.44 | 51.3 | 11.9 | -- | -- | |
| HC | HTC | Sewage sludge | 250 | 4 h | 31.0 | 4.1 | 2.4 | 8.00 | 6.6 | 16.53 | 53.8 | 2.9 | -- | -- | |
| DG | AD | Food waste | -- | -- | 42.1 | 5.2 | 5.8 | 21.3 | -- | -- | 25.6 | -- | -- | 0.32 | [8] |
| DG | AD | Mixed residues | -- | 30 d | 40.0 d | -- | 6.5 | -- | 8.7 | -- | -- | 3.10 | -- | -- | [61] |
| DG | AD | Swine manure | -- | -- | 37.2 | 5.5 | 4.6 | 31.9 | -- | -- | 23.0 | -- | -- | -- | [41] |
| DG | AD | Mixed residues | 30 | 40 d | 50.5 d | - | - | - | 8.7 | 1.36 | 12.8 | -- | -- | -- | [12] |
| EE | Chemical Structure | Molecular Weight (g mol−1) | Water Solubility (mg L−1 at 25 °C) | LogKow | pKa | Vapor Pressure (mm Hg at 25 °C) |
|---|---|---|---|---|---|---|
| BPA |  | 228.29 | 300 | 3.32 | 9.6 | 4.00 × 10−8 |
| 4-tert-OP | 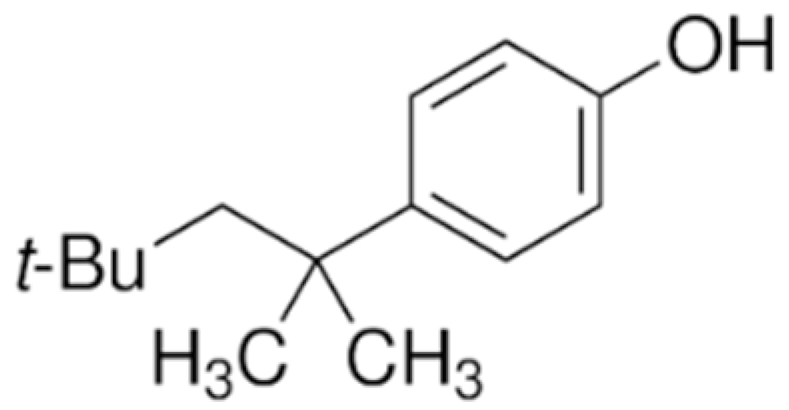 | 206.32 | 5.1 | 5.25 | 10.33 | 4.80 × 10−4 |
| NP | 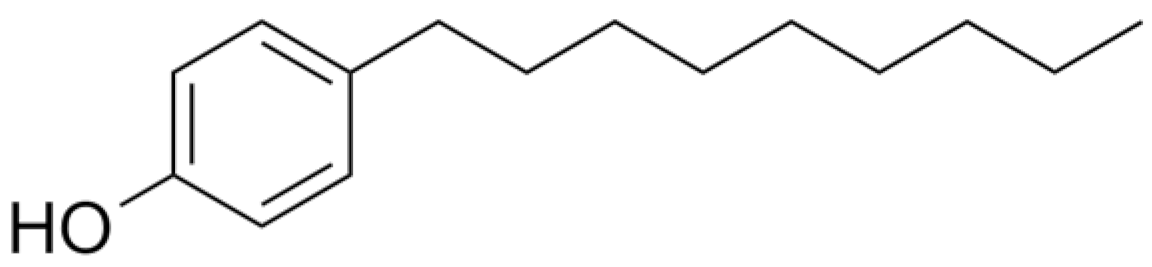 | 220.35 | 7.0 | 5.76 | 10.31 | 8.18 × 10−4 |
| E1 | 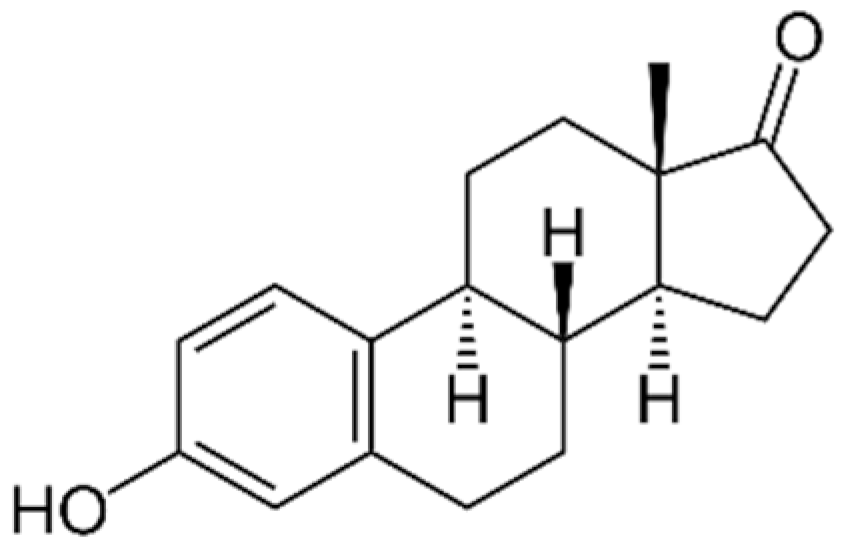 | 270.37 | 30 | 3.13 | 10.40 | 2.49 × 10−10 |
| E2 | 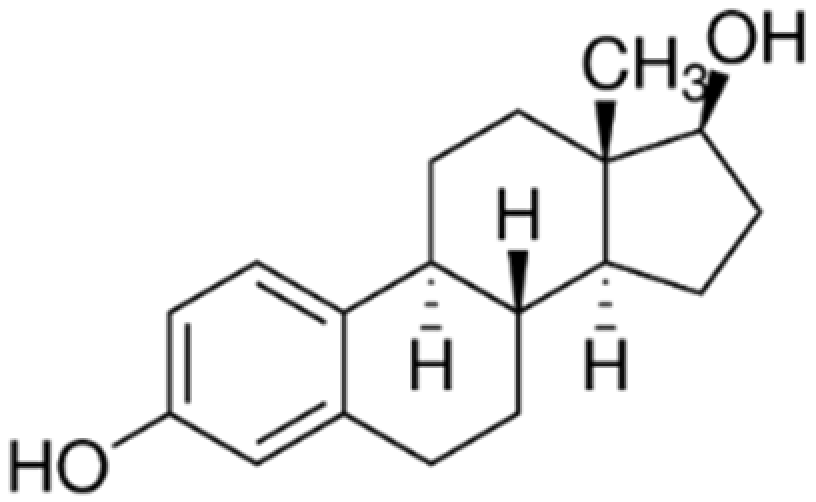 | 272.38 | 3.9 | 4.01 | 10.71 | 6.38 × 10−9 |
| E3 | 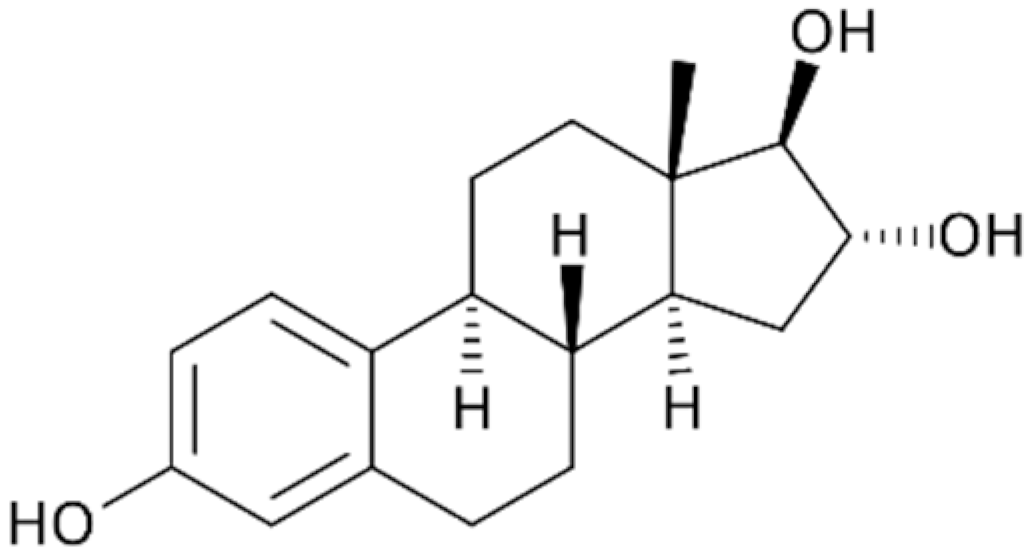 | 288.39 | 27.3 | 2.45 | 10.33 | 9.93 × 10−12 |
| EE2 | 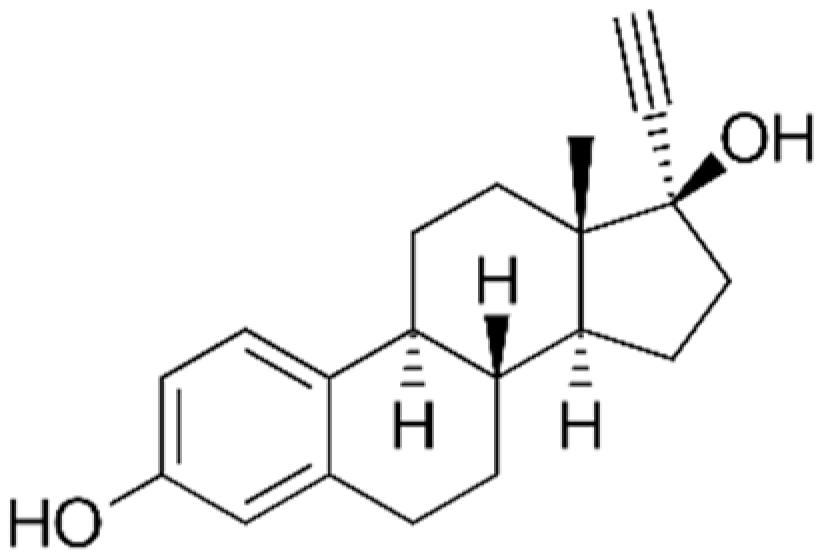 | 296.41 | 11.3 | 3.67 | 10.33 | 1.95 × 10−9 |
| Method | Advantages | Limitations | EE | Ref. |
|---|---|---|---|---|
| Water | ||||
| Immobilization | ||||
| Coagulation/sedimentation |
| BPA, NP, E1, E2, E3 | [26] | |
| Destruction | ||||
| Chemical and Photochemical Treatments | ||||
| Advanced oxidation processes (AOPs) Photocatalysis using catalysts and nanocatalysts (TiO2, ZnO and others) Electrochemical oxidation |
|
| BPA, NP, E1, E2, E3 | [26] [27] [88] |
| Ozonation |
|
| BPA, NP, E1, E2, E3 | [26] |
| Chlorination |
|
| BPA, NP, E1, E2, E3 | [26] |
| UV photolysis |
|
| BPA, NP, E1, E2, E3 | [26] |
| Biological Treatments | ||||
| Activated sludge treatment |
| BPA, NP, E1, E2, E3 | [26] | |
| Membrane bioreactor |
|
| BPA, NP, E1, E2, E3 | [26] |
| Separation | ||||
| Ultrafiltration |
| BPA, NP, E1, E2, E3 | [26] | |
| Adsorption | ||||
| Use of activated carbon |
|
| ||
| Use of recalcitrant carbon-rich biosorbents (biochar, hydrochar, digestate) |
|
| BPA, OP | * |
| Soil | ||||
| Containment | ||||
| Engineering techniques for the isolation of polluted sites and sources of contamination (containment barriers) |
|
| [89] | |
| Immobilization | ||||
| Incorporation of recalcitrant carbon-rich materials, especially carbonaceous adsorbents, as soil amendments (biochar, hydrochar, digestate, compost) |
|
| BPA, OP, E1 EE2 | [90] [51] [91] [92] [93] |
| Destruction | ||||
| Chemical remediation | ||||
| Advanced oxidation processes (AOPs, Fenton processes, TiO2 photo-catalysis, ozonation, electrochemical oxidation |
|
| [89] | |
| Biological remediation | ||||
| Phytoremediation (plants and algae) Assisted phytoremediation (combination of plants and soil amendments) |
|
| [89] [94] | |
| Bioremediation (bacteria and fungi) |
|
| [89] | |
| Separation | ||||
| Adsorption on carbon-rich biosorbents without incorporation in soil (biochar, hydrochar, digestate, compost) |
|
| BPA, OP | [37] [89] |
| Ex situ soil washing (extractants) |
|
| [89] | |
| EE | Feedstock | Process T (°C) | Residence Time (h) | Activation | Kinetics Model | Isotherm Model | Adsorption Capacity (mg g−1) * | Ref. |
|---|---|---|---|---|---|---|---|---|
| Hydrochar | ||||||||
| BPA | Poultry litter | 250 | 20 | - | n. e. | F | 77.62 | [52] |
| BPA | Swine solids | 250 | 20 | - | n. e. | F | 33.11 | [52] |
| BPA | Magonia pubescens wood | 180 | 6 | - | PFO | L | 21.26 | [102] |
| BPA | Sewage sludge | 160, 190, 250 | 4 | - | n. e. | L | From 10.86 to 18.37 | [49] |
| BPA | Argan nut shells | 200 | 6 | - | PSO | L | 1162.79 | [67] |
| BPA | Pine fruit shells | 190 | 24, 48, 72 | NaOH | PSO | L | From 332.52 to 378.77 | [56] |
| E1 | Swine solids | 250 | 8 | - | n. e. | F | 100.48 | [53] |
| E1 | Poultry litter | 250 | 8 | - | n. e. | F | 51.88 | [53] |
| E2 | Rice husk | 200 | 6 | - | n. e. | F | 15.87 | [58] |
| E2 | Rice husk | 200 | 6 | KMnO4 + FeCl2 | PSO | F | 22.31 | [58] |
| E2 | Montmorillonite and rice husk | 180 | 16 | KOH | PSO | L | 138.00 | [57] |
| EE2 | Swine solids | 250 | 20 | - | n. e. | F | 18.62 | [52] |
| EE2 | Poultry litter | 250 | 20 | - | n. e. | F | 181.97 | [52] |
| EE2 | Montmorillonite and rice husk | 180 | 16 | KOH | PSO | L | 69.00 | [57] |
| Digestate | ||||||||
| BPA | Mixed residues and olive pomace | 20–45 | 28 days | - | PFO/PSO | F | 0.13 | [12] |
| OP | Mixed residues and olive pomace | 20–45 | 28 days | - | PFO | F | 1.07 | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loffredo, E. Recent Advances on Innovative Materials from Biowaste Recycling for the Removal of Environmental Estrogens from Water and Soil. Materials 2022, 15, 1894. https://doi.org/10.3390/ma15051894
Loffredo E. Recent Advances on Innovative Materials from Biowaste Recycling for the Removal of Environmental Estrogens from Water and Soil. Materials. 2022; 15(5):1894. https://doi.org/10.3390/ma15051894
Chicago/Turabian StyleLoffredo, Elisabetta. 2022. "Recent Advances on Innovative Materials from Biowaste Recycling for the Removal of Environmental Estrogens from Water and Soil" Materials 15, no. 5: 1894. https://doi.org/10.3390/ma15051894
APA StyleLoffredo, E. (2022). Recent Advances on Innovative Materials from Biowaste Recycling for the Removal of Environmental Estrogens from Water and Soil. Materials, 15(5), 1894. https://doi.org/10.3390/ma15051894






