Antibacterial and Antibiofilm Properties of Three Resin-Based Dental Composites against Streptococcus mutans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Realization of Composite Disks
2.2. Saliva Collection
2.3. Microbial Strain
2.4. Experimental Design
- (i).
- Total mass amount by measuring the planktonic optical density (OD600nm);
- (ii).
- Planktonic CFU count of the bacterial cells (CFU/mL);
- (iii).
- Planktonic bacterial metabolic activity by MTT assay;
- (iv).
- Planktonic bacterial viability assay by live/dead staining.
- (i).
- Adherent CFU count for the quantification of cultivable cells;
- (ii).
- Adherent OD for biofilm biomass evaluation, using Hucker’s crystal violet staining method (OD570nm);
- (iii).
- Adherent biofilm metabolic activity by MTT assay;
- (iv).
- Extracellular polymeric substances (EPS) of the biofilm matrix by the Concanavalin A assay;
- (v).
- Biofilm morphology by SEM evaluation.
2.5. Planktonic Optical Density Detection
2.6. Planktonic CFU Count
2.7. Planktonic MTT Assay
2.8. Planktonic Bacterial Viability Assay
2.9. Adherent Bacteria CFU Count
2.10. Biomass Quantification by Optical Density (OD570nm)
2.11. Adherent Bacteria MTT Assay
2.12. Concanavalin Assay
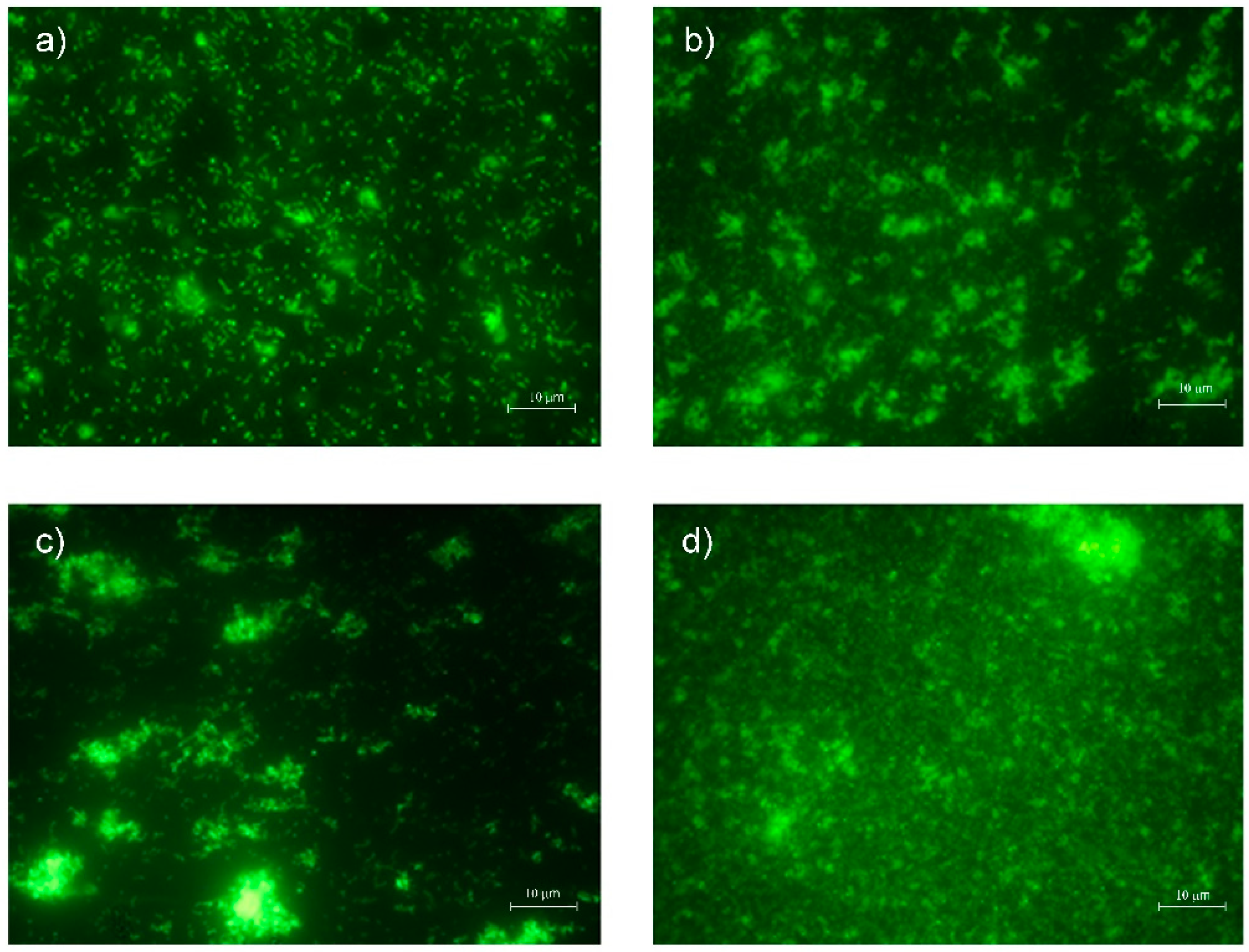
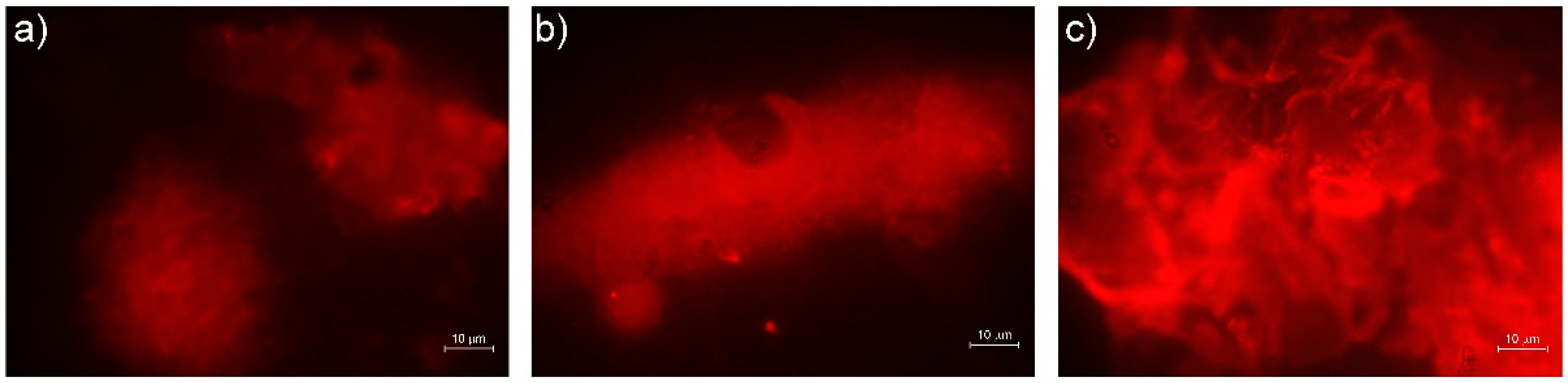
2.13. Scanning Electron Microscope (SEM) Analysis
2.14. Statistical Analysis
3. Results
3.1. Planktonic Optical Density Detection
3.2. Planktonic CFU Count
3.3. Planktonic MTT Assay
3.4. Planktonic Bacterial Viability Assay
3.5. Adherent Bacteria CFU Count
3.6. Biomass Quantification by Optical Density (OD570nm)
3.7. Adherent Bacteria MTT Assay
3.8. Concanavalin Assay
3.9. SEM Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomaidis, S.; Kakaboura, A.; Mueller, W.D.; Zinelis, S. Mechanical properties of contemporary composite resins and their interrelations. Dent. Mater. 2013, 29, e132–e141. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; D’Arcangelo, C.; Maliskova, N.; Vanini, L.; Vadini, M. Wear properties of different additive restorative materials used for onlay/overlay posterior restorations. Oper. Dent. 2020, 45, E156–E166. [Google Scholar] [CrossRef] [PubMed]
- Gurgan, S.; Koc Vural, U.; Miletic, I. Comparison of mechanical and optical properties of a newly marketed universal composite resin with contemporary universal composite resins: An in vitro study. Microsc. Res. Tech. 2022, 85, 1171–1179. [Google Scholar] [CrossRef]
- D’Arcangelo, C.; Vanini, L.; Rondoni, G.D.; Pirani, M.; Vadini, M.; Gattone, M.; De Angelis, F. Wear properties of a novel resin composite compared to human enamel and other restorative materials. Oper. Dent. 2014, 39, 612–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Arcangelo, C.; Vadini, M.; Buonvivere, M.; De Angelis, F. Safe clinical technique for increasing the occlusal vertical dimension in case of erosive wear and missing teeth. Clin. Case Rep. 2021, 9, e04747. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, C.; Vanini, L.; Casinelli, M.; Frascaria, M.; De Angelis, F.; Vadini, M.; D’Amario, M. Adhesive cementation of indirect composite inlays and onlays: A literature review. Compend. Contin. Educ. Dent. 2015, 36, 570–577; quiz 78. [Google Scholar]
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Cadenaro, M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review. Dent. Mater. 2016, 32, e41–e53. [Google Scholar] [CrossRef]
- Kusuma Yulianto, H.D.; Rinastiti, M.; Cune, M.S.; de Haan-Visser, W.; Atema-Smit, J.; Busscher, H.J.; van der Mei, H.C. Biofilm composition and composite degradation during intra-oral wear. Dent. Mater. 2019, 35, 740–750. [Google Scholar] [CrossRef]
- Auschill, T.M.; Arweiler, N.B.; Brecx, M.; Reich, E.; Sculean, A.; Netuschil, L. The effect of dental restorative materials on dental biofilm. Eur. J. Oral Sci. 2002, 110, 48–53. [Google Scholar] [CrossRef]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus mutans, caries and simulation models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef] [Green Version]
- Hannig, M. Transmission electron microscopy of early plaque formation on dental materials in vivo. Eur. J. Oral Sci. 1999, 107, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Hahnel, S.; Henrich, A.; Rosentritt, M.; Handel, G.; Bürgers, R. Influence of artificial ageing on surface properties and streptococcus mutans adhesion to dental composite materials. J. Mater. Sci. Mater. Med. 2010, 21, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Jung, M.; Wehlen, O.; Klimek, J. Finishing and polishing of indirect composite and ceramic inlays in-vivo: Occlusal surfaces. Oper. Dent. 2004, 29, 131–141. [Google Scholar]
- Lutz, F.; Setcos, J.C.; Phillips, R.W. New finishing instruments for composite resins. J. Am. Dent. Assoc. 1983, 107, 575–580. [Google Scholar] [CrossRef]
- Kurt, A.; Cilingir, A.; Bilmenoglu, C.; Topcuoglu, N.; Kulekci, G. Effect of different polishing techniques for composite resin materials on surface properties and bacterial biofilm formation. J. Dent. 2019, 90, 103199. [Google Scholar] [CrossRef]
- Cazzaniga, G.; Ottobelli, M.; Ionescu, A.C.; Paolone, G.; Gherlone, E.; Ferracane, J.L.; Brambilla, E. In vitro biofilm formation on resin-based composites after different finishing and polishing procedures. J. Dent. 2017, 67, 43–52. [Google Scholar] [CrossRef]
- Ionescu, A.; Brambilla, E.; Wastl, D.S.; Giessibl, F.J.; Cazzaniga, G.; Schneider-Feyrer, S.; Hahnel, S. Influence of matrix and filler fraction on biofilm formation on the surface of experimental resin-based composites. J. Mater. Sci. Mater. Med. 2015, 26, 5372. [Google Scholar] [CrossRef]
- Ikeda, M.; Matin, K.; Nikaido, T.; Foxton, R.M.; Tagami, J. Effect of surface characteristics on adherence of S. mutans biofilms to indirect resin composites. Dent. Mater. J. 2007, 26, 915–923. [Google Scholar] [CrossRef] [Green Version]
- Motevasselian, F.; Zibafar, E.; Yassini, E.; Mirzaei, M.; Pourmirhoseni, N. Adherence of streptococcus mutans to microhybrid and nanohybrid resin composites and dental amalgam: An in vitro study. J. Dent. 2017, 14, 337–343. [Google Scholar]
- Pereira, C.A.; Eskelson, E.; Cavalli, V.; Liporoni, P.C.; Jorge, A.O.; do Rego, M.A. Streptococcus mutans biofilm adhesion on composite resin surfaces after different finishing and polishing techniques. Oper. Dent. 2011, 36, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedeljkovic, I.; Yoshihara, K.; De Munck, J.; Teughels, W.; Van Meerbeek, B.; Van Landuyt, K.L. No evidence for the growth-stimulating effect of monomers on cariogenic streptococci. Clin. Oral Investig. 2017, 21, 1861–1869. [Google Scholar] [CrossRef]
- Takahashi, Y.; Imazato, S.; Russell, R.R.; Noiri, Y.; Ebisu, S. Influence of resin monomers on growth of oral streptococci. J. Dent. Res. 2004, 83, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.N.; Lim, B.S.; Ahn, S.J. Urethane dimethacrylate influences the cariogenic properties of streptococcus mutans. Materials 2021, 14, 1015. [Google Scholar] [CrossRef] [PubMed]
- Khalichi, P.; Cvitkovitch, D.C.; Santerre, J.P. Effect of composite resin biodegradation products on oral streptococcal growth. Biomaterials 2004, 25, 5467–5472. [Google Scholar] [CrossRef]
- Petrini, M.; Giuliani, A.; Di Campli, E.; Di Lodovico, S.; Iezzi, G.; Piattelli, A.; D’Ercole, S. The bacterial anti-adhesive activity of double-etched titanium (dae) as a dental implant surface. Int. J. Mol. Sci. 2020, 21, 8315. [Google Scholar] [CrossRef]
- D’Ercole, S.; Di Giulio, M.; Grande, R.; Di Campli, E.; Di Bartolomeo, S.; Piccolomini, R.; Cellini, L. Effect of 2-hydroxyethyl methacrylate on streptococcus spp. Biofilms. Lett. Appl. Microbiol. 2011, 52, 193–200. [Google Scholar] [CrossRef]
- Yaghmoor, R.B.; Xia, W.; Ashley, P.; Allan, E.; Young, A.M. Effect of novel antibacterial composites on bacterial biofilms. J. Funct. Biomater. 2020, 11, 55. [Google Scholar] [CrossRef]
- Peralta, S.L.; Leles, S.B.; Dutra, A.L.; Guimarães, V.; Piva, E.; Lund, R.G. Evaluation of physical-mechanical properties, antibacterial effect, and cytotoxicity of temporary restorative materials. J. Appl. Oral. Sci. 2018, 26, e20170562. [Google Scholar] [CrossRef]
- Di Giulio, M.; D’Ercole, S.; Zara, S.; Cataldi, A.; Cellini, L. Streptococcus mitis/human gingival fibroblasts co-culture: The best natural association in answer to the 2-hydroxyethyl methacrylate release. Apmis 2012, 120, 139–146. [Google Scholar] [CrossRef] [PubMed]
- D’Ercole, S.; Cellini, L.; Pilato, S.; Di Lodovico, S.; Iezzi, G.; Piattelli, A.; Petrini, M. Material characterization and streptococcus oralis adhesion on polyetheretherketone (peek) and titanium surfaces used in implantology. J. Mater. Sci. Mater. Med. 2020, 31, 84. [Google Scholar] [CrossRef] [PubMed]
- D’Ercole, S.; Di Fermo, P.; Di Giulio, M.; Di Lodovico, S.; Di Campli, E.; Scarano, A.; Tripodi, D.; Cellini, L.; Petrini, M. Near-infrared nir irradiation and sodium hypochlorite: An efficacious association to counteract the enterococcus faecalis biofilm in endodontic infections. J. Photochem. Photobiol. B 2020, 210, 111989. [Google Scholar] [CrossRef] [PubMed]
- Mena Silva, P.A.; Garcia, I.M.; Nunes, J.; Visioli, F.; Leitune, V.C.B.; Melo, M.A.; Collares, F.M. Myristyltrimethylammonium bromide (mytab) as a cationic surface agent to inhibit streptococcus mutans grown over dental resins: An in vitro study. J. Funct. Biomater. 2020, 11, 9. [Google Scholar] [CrossRef] [Green Version]
- Cazzaniga, G.; Ottobelli, M.; Ionescu, A.; Garcia-Godoy, F.; Brambilla, E. Surface properties of resin-based composite materials and biofilm formation: A review of the current literature. Am. J. Dent. 2015, 28, 311–320. [Google Scholar]
- Kim, K.; An, J.S.; Lim, B.S.; Ahn, S.J. Effect of bisphenol a glycol methacrylate on virulent properties of streptococcus mutans ua159. Caries Res. 2019, 53, 84–95. [Google Scholar] [CrossRef]
- Trubiani, O.; Caputi, S.; Di Iorio, D.; D’Amario, M.; Paludi, M.; Giancola, R.; Di Nardo Di Maio, F.; De Angelis, F.; D’Arcangelo, C. The cytotoxic effects of resin-based sealers on dental pulp stem cells. Int. Endod. J. 2010, 43, 646–653. [Google Scholar] [CrossRef]
- Trubiani, O.; Cataldi, A.; De Angelis, F.; D’Arcangelo, C.; Caputi, S. Overexpression of interleukin-6 and -8, cell growth inhibition and morphological changes in 2-hydroxyethyl methacrylate-treated human dental pulp mesenchymal stem cells. Int. Endod. J. 2012, 45, 19–25. [Google Scholar] [CrossRef]
- Kuan, Y.H.; Huang, F.M.; Lee, S.S.; Li, Y.C.; Chang, Y.C. Bisgma stimulates prostaglandin e2 production in macrophages via cyclooxygenase-2, cytosolic phospholipase a2, and mitogen-activated protein kinases family. PLoS ONE 2013, 8, e82942. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.M.; Chang, Y.C.; Lee, S.S.; Yeh, C.H.; Lee, K.G.; Huang, Y.C.; Chen, C.J.; Chen, W.Y.; Pan, P.H.; Kuan, Y.H. Bisgma-induced cytotoxicity and genotoxicity in macrophages are attenuated by wogonin via reduction of intrinsic caspase pathway activation. Environ. Toxicol. 2016, 31, 176–184. [Google Scholar] [CrossRef]
- Lottner, S.; Shehata, M.; Hickel, R.; Reichl, F.X.; Durner, J. Effects of antioxidants on DNA-double strand breaks in human gingival fibroblasts exposed to methacrylate based monomers. Dent. Mater. 2013, 29, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, M.; Petzel, C.; Bolay, C.; Hiller, K.A.; Cataldi, A.; Buchalla, W.; Krifka, S.; Schweikl, H. Activation of the nrf2-regulated antioxidant cell response inhibits hema-induced oxidative stress and supports cell viability. Biomaterials 2015, 56, 114–128. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; Mandatori, D.; Schiavone, V.; Melito, F.P.; Valentinuzzi, S.; Vadini, M.; Di Tomo, P.; Vanini, L.; Pelusi, L.; Pipino, C.; et al. Cytotoxic and genotoxic effects of composite resins on cultured human gingival fibroblasts. Materials 2021, 14, 5225. [Google Scholar] [CrossRef] [PubMed]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-biofilm activity of cannabigerol against streptococcus mutans. Microorganisms 2021, 9, 2031. [Google Scholar] [CrossRef]
- Uçtaşli, M.B.; Arisu, H.D.; Omürlü, H.; Eligüzeloğlu, E.; Ozcan, S.; Ergun, G. The effect of different finishing and polishing systems on the surface roughness of different composite restorative materials. J. Contemp. Dent. Pract. 2007, 8, 89–96. [Google Scholar]
- Ono, M.; Nikaido, T.; Ikeda, M.; Imai, S.; Hanada, N.; Tagami, J.; Matin, K. Surface properties of resin composite materials relative to biofilm formation. Dent. Mater. J. 2007, 26, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Ferracane, J.L. Models of caries formation around dental composite restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Opdam, N.J.; van de Sande, F.H.; Bronkhorst, E.; Cenci, M.S.; Bottenberg, P.; Pallesen, U.; Gaengler, P.; Lindberg, A.; Huysmans, M.C.; van Dijken, J.W. Longevity of posterior composite restorations: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 943–949. [Google Scholar] [CrossRef]
- Svanberg, M.; Mjör, I.A.; Orstavik, D. Mutans streptococci in plaque from margins of amalgam, composite, and glass-ionomer restorations. J. Dent. Res. 1990, 69, 861–864. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Simmonds, R.S.; Tagg, J.R. Dental caries is a preventable infectious disease. Aust. Dent. J. 2000, 45, 235–245. [Google Scholar] [CrossRef]

| Group | Material | Manufacturer | Lot Number | Composition |
|---|---|---|---|---|
| VOCO | GrandioSO Shade A2 (Nanohybrid) | Voco GmbH (Cuxhaven, Germany) | 1847313 | 89% (w/w) fillers (1 μm glass ceramic filler, 20–40 nm silicon dioxide fillers), Bis-GMA *, Bis-EMA *, TEGDMA *. |
| VD | Venus DiamondShade A2 (Nanohybrid) | Kulzer GmbH (Hanau, Germany) | K010070 | 80–82% (w/w) fillers (5 nm–20 μm barium aluminum fluoride glass fillers), TCD-UA *, UDMA *, TEGDMA *. |
| ES-2 | Clearfil Majesty ES-2 Classic Shade A2 (Nanohybrid) | Kuraray (Chiyoda, Tokyo, Japan) | 7D008 | 78% (w/w) fillers (0.37 μm–1.5 μm silanated barium glass fille, pre-polymerized organic fillers), Bis-GMA *, hydrophobic aromatic dimethacrylate. |
| Streptococcus Mutans CH02 | VOCO | VD | ES2 | CTRL+ |
|---|---|---|---|---|
| Planktonic OD600nm (SD) | 0.3054 b | 0.2931 b | 0.3117 b | 0.3978 a |
| (0.0567) | (0.0540) | (0.0532) | (0.0491) | |
| Planktonic CFU count (×105 CFU/mL) (SD) | 583.0 b | 281.0 c | 273.0 c | 1900.0 a |
| (53.4) | (26.4) | (14.1) | (353.3) | |
| Planktonic MTT (SD) | 0.451 a | 0.549 a | 0.448 a | 0.442 a |
| (0.094) | (0.136) | (0.085) | (0.120) | |
| Adherent Bacteria CFU count (×103 CFU) (SD) | 150.0 b | 252.0 a | 248.3 a | |
| (29.4) | (39.7) | (47.8) | ||
| Biomass Quantification OD570nm (SD) | 0.4775 b | 0.6364 b | 1.6040 a | |
| (0.1548) | (0.2376) | (0.2075) | ||
| Adherent Bacteria MTT (SD) | 0.003 b | 0.040 b | 0.035 b | 0.367 a |
| (0.004) | (0.013) | (0.021) | (0.274) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ercole, S.; De Angelis, F.; Biferi, V.; Noviello, C.; Tripodi, D.; Di Lodovico, S.; Cellini, L.; D’Arcangelo, C. Antibacterial and Antibiofilm Properties of Three Resin-Based Dental Composites against Streptococcus mutans. Materials 2022, 15, 1891. https://doi.org/10.3390/ma15051891
D’Ercole S, De Angelis F, Biferi V, Noviello C, Tripodi D, Di Lodovico S, Cellini L, D’Arcangelo C. Antibacterial and Antibiofilm Properties of Three Resin-Based Dental Composites against Streptococcus mutans. Materials. 2022; 15(5):1891. https://doi.org/10.3390/ma15051891
Chicago/Turabian StyleD’Ercole, Simonetta, Francesco De Angelis, Virginia Biferi, Chiara Noviello, Domenico Tripodi, Silvia Di Lodovico, Luigina Cellini, and Camillo D’Arcangelo. 2022. "Antibacterial and Antibiofilm Properties of Three Resin-Based Dental Composites against Streptococcus mutans" Materials 15, no. 5: 1891. https://doi.org/10.3390/ma15051891
APA StyleD’Ercole, S., De Angelis, F., Biferi, V., Noviello, C., Tripodi, D., Di Lodovico, S., Cellini, L., & D’Arcangelo, C. (2022). Antibacterial and Antibiofilm Properties of Three Resin-Based Dental Composites against Streptococcus mutans. Materials, 15(5), 1891. https://doi.org/10.3390/ma15051891








