Stability of Octadecyltrimethoxysilane-Based Coatings on Aluminum Alloy Surface
Abstract
1. Introduction
2. Experimental Part
3. Results and Discussion
3.1. Surface Morphology and Water Repellency of As-Prepared Samples
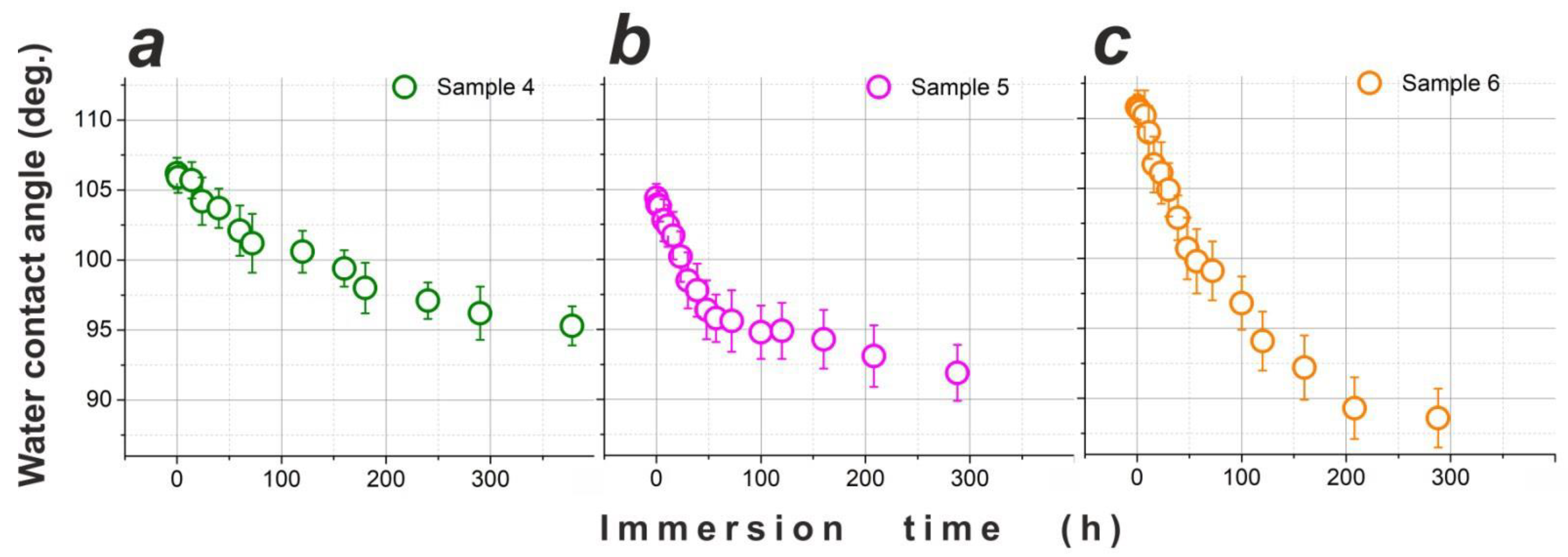
3.2. Long-Term Water Repellency of Samples with One-Layer Coating
3.3. Water Repellency of Two-Layer Samples over Time
3.4. Effect of Ultrasonic Treatment
3.5. Discussion and Comparison with Previous Results
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, R.S.; Jakhar, K.; Shoaib, A.; Antao, D. Elucidating the mechanism of condensation-mediated degradation of organofunctional silane self-assembled monolayer coatings. ACS Appl. Mater. Interfaces 2021, 13, 34923–34934. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Kang, Z.X.; Li, W.; Zhang, J. A facile approach to fabricate a stable superhydrophobic film with switchable water adhesion on titanium surface. Surf. Coat. Technol. 2014, 239, 227–232. [Google Scholar] [CrossRef]
- Ruan, M.; Wang, J.W.; Liu, Q.L.; Ma, F.M.; Yu, Z.L.; Feng, W.; Chen, Y. Superhydrophobic and anti-icing properties of sol-gel prepared alumina coatings. Russ. J. Non-Ferrous Met. 2016, 57, 638–645. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Masson, D.; Du, X.W.; Emelyanenko, A.M. Testing the durability of anti-icing coatings. In Ice Adhesion: Mechanism, Measurement and Mitigation; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 495–519. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Shagieva, F.M.; Domantovsky, A.G.; Boinovich, L.B. Nanosecond laser micro- and nanotexturing for the design of a superhydrophobic coating robust agaist long-term contact with water, cavitation, and abrasion. Appl. Surf. Sci. 2015, 332, 513–517. [Google Scholar] [CrossRef]
- Boinovich, L.; Emelyanenko, A. A wetting experiment as a tool to study the physicochemical processes accompanying the contact of hydrophobic and superhydrophobic materials with aqueous media. Adv. Colloid. Interface Sci. 2012, 179–182, 133–141. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chuang, S.I.; Lo, Y.H.; Cheng, H.M.; Duh, J.G.; Chen, P.Y. Stalagmite-line self-cleaning surfaces prepared by silanization of plasma-assisted metal-oxide nanostructures. J. Mater. Chem. A 2016, 4, 3406–3414. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Honda, M.; Zhu, A.L.; Rozhin, A.G.; Du, X.W. The icephobic performance of alkyl-grafted aluminum surfaces. Soft Matter 2015, 11, 856–861. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farzaneh, M. Effect of contact angle hysteresis on water droplet evaporation from super-hydrophobic surfaces. Appl. Surf. Sci. 2009, 255, 4056–4060. [Google Scholar] [CrossRef]
- Zhizhchenko, A.; Kuchmizhak, A.; Vitrik, O.; Kulchin, Y.; Juodkazis, S. On-demand concentration of an analyte on laser-printed polytetrafluoroethylene. Nanoscale 2018, 10, 21414–21424. [Google Scholar] [CrossRef]
- Maccarini, M.; Himmelhaus, M.; Stoycheva, S.; Grunze, M. Characterisation and stability of hydrophobic surfaces in water. Appl. Surf. Sci. 2005, 252, 1941–1946. [Google Scholar] [CrossRef]
- Pavliuk, G.; Pavlov, D.; Mitsai, E.; Vitrik, O.; Mironenko, A.; Zakharenko, A.; Kulinich, S.A.; Juodkazis, S.; Bratskaya, S.; Zhizhchenko, A.; et al. Ultrasensitive SERS-based plasmonic sensor with analyte enrichment system produced by direct laser writing. Nanomaterials 2020, 10, 49. [Google Scholar] [CrossRef]
- Psarski, M.; Pawlak, D.; Grobelny, J.; Celichowski, G. Relationships between surface chemistry, nanotepography, wettability and ice adhesion in epoxy and SU-8 modified with fluoroalkylsilanes from the vapor phase. Appl. Surf. Sci. 2019, 479, 489–498. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farhadi, S.; Nose, K.; Du, X.W. Superhydrophobic surfaces: Are they really ice-repellent? Langmuir 2011, 27, 25–29. [Google Scholar] [CrossRef]
- Zimmermann, J.; Artus, G.R.J.; Seeger, S. Long term studies on the chemical stability of a superhydrophobic silicone nanofilament coating. Appl. Surf. Sci. 2007, 253, 5972–5979. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farzaneh, M. On ice-releasing properties of rough hydrophobic coatings. Cold Reg. Sci. Technol. 2011, 65, 60–64. [Google Scholar] [CrossRef]
- Schwartz, D.K.; Steinberg, S.; Israelachvili, J.; Zasadzinski, J.A.N. Growth of a self-assembled monolayer by fractal aggregation. Phys. Rev. Lett. 1992, 69, 3354–3357. [Google Scholar] [CrossRef]
- Carraro, C.; Yauw, O.W.; Sung, M.M.; Maboudian, R. Observation of three mechanisms in self-assembled monolayers. J. Phys. Chem. B 1998, 102, 4441–4445. [Google Scholar] [CrossRef]
- Vallant, T.; Brunner, H.; Mayer, U.; Hoffmann, H.; Leitner, T.; Resch, R.; Friedbacher, G. Formation of self-assembled octadecylsiloxane monolayers on mica and silicon surfaces studied by atomic force microscopy and infrared spectroscopy. J. Phys. Chem. B. 1998, 102, 7190–7197. [Google Scholar] [CrossRef]
- Zucchi, F.; Grassi, V.; Frignani, A.; Monticelli, C.; Trabanelli, G. Influence of a silane treatment on the corrosion resistance of a WE43 magnesium alloy. Surf. Coat. Technol. 2006, 200, 4136–4143. [Google Scholar] [CrossRef]
- Frignani, A.; Zucchi, F.; Trabanelli, G.; Grassi, V. Protective action towards aluminium corrosion by silanes with a long aliphatic chain. Corros. Sci. 2006, 48, 2258–2273. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Nazarov, A.P.; Mikhailovski, Y.N. Formation mechanism and anticorrosive properties of thin siloxane films on metal surfaces. J. Electrochem. Soc. 1996, 143, 251–257. [Google Scholar] [CrossRef]
- Zucchi, F.; Grassi, V.; Frignani, A.; Trabanelli, G. Inhibition of copper corrosion by silane coatings. Corros. Sci. 2004, 46, 2853–2865. [Google Scholar] [CrossRef]
- Van Schaftinghen, T.; Le Pen, C.; Terryn, H.; Hörzenberger, F. Investigation of the barrier properties of silanes on cold rolled steel. Electrochim. Acta 2004, 49, 2997–3004. [Google Scholar] [CrossRef]
- Van, O.; Zhu, D.Q.; Prasad, G.; Jayaseelan, S.; Fu, Y.; Teredesai, N. Silane based chromate replacements for corrosion control, paint adhesion, and rubber bonding. Surf. Eng. 2000, 16, 386–396. [Google Scholar]
- Van Ooij, W.J.; Zhu, D.; Stacy, M.; Seth, A.; Mugada, T.; Gandhi, J.; Puomi, P. Corrosion protection properties of organofunctional silanes—An overview. Tsinghua Sci. Technol. 2005, 10, 639–664. [Google Scholar] [CrossRef]
- Calistri-Yeh, M.; Kramer, E.J.; Sharma, R.; Zhao, W.; Rafailovich, M.H.; Sokolov, J.; Brock, J.D. Thermal stability of self-assembled monolayers from alkylchlorosilanes. Langmuir 1996, 12, 2747–2755. [Google Scholar] [CrossRef]
- Kluth, G.J.; Sung, M.M.; Maboudian, R. Thermal behavior of alkylsiloxane self-assembled monolayers on the oxidized Si(100) surface. Langmuir 1997, 13, 3775–3780. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Mirji, S.A.; Mandale, A.B.; Vijayamohanan, K.P. Thermal stability of self-assembled octadecyltrichlorosilane monolayers on planar and curved silica surfaces. Thin Solid Film. 2006, 496, 420–425. [Google Scholar] [CrossRef]
- Mirji, S.A.; Halligudi, S.B.; Sawant, D.P.; Jacob, N.E.; Patil, K.R.; Gaikwad, A.B.; Pradhan, S.D. Adsorption of octadecyltrichlorosilane on mesoporous SBA-15. Appl. Surf. Sci. 2006, 252, 4097–4103. [Google Scholar] [CrossRef]
- Khaskhoussi, A.; Calabrese, L.; Proverbio, E. Superhydrophobic self-assembled silane monolayers on hierarchical 6082 aluminum alloy for anti-corrosion applications. Appl. Sci. 2020, 10, 2656. [Google Scholar] [CrossRef]
- Meth, S.; Sukenik, C.N. Siloxane-anchored thin films on silicon dioxide-modified stainless steel. Thin Solid Film. 2003, 425, 49–58. [Google Scholar] [CrossRef]
- Thomsen, L.; Watts, B.; Cotton, D.V.; Quinton, J.S.; Dastoor, P.S. Adsorption and orientation kinetics of self-assembled films of octadecyltrimethoxysilane on aluminium oxide surfaces. Surf. Interface Anal. 2005, 37, 472–477. [Google Scholar] [CrossRef]
- Lee, J.J.; Bong, J.H.; Ha, Y.G.; Park, S.Y.; Ju, S.H. Durability of self-assembled monolayers on aluminum oxide surface for determining surface wettability. Appl. Surf. Sci. 2015, 330, 445–448. [Google Scholar] [CrossRef]
- Somlo, B.; Gupta, V. A hydrophobic self-assembled monolayer with improved adhesion to aluminum for deicing application. Mech. Mater. 2010, 33, 471–480. [Google Scholar] [CrossRef]
- Han, S.W.; Jeong, J.; Lee, D.H. Ice-phobic behavior of superhydrophobic Al surface under various etching conditions. J. Electroceram. 2014, 33, 82–88. [Google Scholar] [CrossRef]
- Habib, S.; Shakoor, R.A.; Kahraman, R. A focused review on smart carriers tailored for corrosion protection: Developments, applications, and challenges. Prog. Org. Coat. 2021, 154, 106218. [Google Scholar] [CrossRef]
- Franquet, A.; de Laet, J.; Schram, T.; Terryn, H.; Subramanian, V.; van Ooij, W.J.; Vereecken, J. Determination of the thickness of thin silane films on aluminium surfaces by means of spectroscopic ellipsometry. Thin Solid Film. 2001, 384, 37–45. [Google Scholar] [CrossRef]
- Subramanian, V.; van Ooij, W.J. Silane based metal pretreatments as alternatives to chromating. Surf. Eng. 1999, 15, 168–172. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Akhtar, A.S.; Wong, P.C.; Wong, K.C.; Mitchell, K.A.R. Growth of permanganate conversion coating on 2024-Al alloy. Thin Solid Film. 2007, 515, 8386–8392. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Akhtar, A.S. On conversion coating treatments to replace chromating for Al alloys: Recent developments and possible future directions. Rus. J. Non-Ferrous Met. 2012, 53, 176–203. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farzaneh, M.; Du, X.W. Growth of corrosion-resistant manganese oxide coatings on an aluminum alloy. Inorg. Mater. 2007, 43, 956–963. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Akhtar, A.S.; Susac, D.; Wong, P.C.; Wong, K.C.; Mitchell, K.A.R. On the growth of conversion chromate coatings on aluminium 2024-T3 alloy. Appl. Surf. Sci. 2007, 253, 3144–3153. [Google Scholar] [CrossRef]
- Ruan, M.; Xu, J.J.; Lu, L.L.; Chen, Y.; Zuo, X.H.; Wang, B.S. Theoretical study of perfluorodecyltrimethoxysilane and polyethylene glycol adsorption/dissociation reactions on dry and hydrated Al2O3 surface. Comput. Theor. Chem. 2020, 1191, 113027. [Google Scholar] [CrossRef]
- Britt, D.W.; Hlady, V. An AFM study of the effects of silanization temperature, hydration, and annealing on the nucleation and aggregation of condensed OTS domains on mica. J. Colloid Interface Sci. 1996, 178, 775–784. [Google Scholar] [CrossRef][Green Version]
- Rozlosnik, N.; Gerstenberg, M.C.; Larsen, N.B. Effect of solvents and concentration on the formation of a self-assembled monolayer of octadecylsiloxane on silicon (001). Langmuir 2003, 19, 1182–1188. [Google Scholar] [CrossRef]
- Zimmermann, J.; Reifler, F.A.; Schrade, U.; Artus, G.R.J.; Seeger, S. Long term environmental durability of a superhydrophobic silicone nanofilament coating. Colloid. Surf. A 2007, 302, 234–240. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Grant, R.P.; Hlava, P.F.; Mckenzie, B.; Zender, G.L. Local dissolution phenomena associated with S phase (Al2CuMg) particles in aluminum alloy 2024-T3. J. Electrochem. Soc. 1997, 144, 2621–2628. [Google Scholar] [CrossRef]
- Schmutz, P.; Frankel, G.S. Corrosion study of AA2024-T3 by scanning Kelvin probe force microscopy and in situ atomic force microscopy scratching. J. Electrochem. Soc. 1998, 145, 2295–2306. [Google Scholar] [CrossRef]
- Teo, M.; Kim, J.; Wong, P.C.; Wong, K.C.; Mitchell, K.A.R. Pre-treatments applied to oxidized aluminum surfaces to modify the interfacial bonding with bis-1,2-(triethoxysilyl)ethane (BTSE)—Part, I. High-purity Al with native oxide. Appl. Surf. Sci. 2005, 252, 1293–1304. [Google Scholar] [CrossRef]
- Kim, J.; Wong, K.C.; Wong, P.C.; Kulinich, S.A.; Metson, J.B.; Mitchell, K.A.R. Characterization of AZ91 magnesium alloy and organosilane adsorption on its surface. Appl. Surf. Sci. 2007, 253, 4197–4207. [Google Scholar] [CrossRef]
- Nisancioglu, K.; Lunder, O.; Holtan, H. Improving the corrosion resistance of aluminum alloys by cathodic polarization in aqueous media. Corrosion 1985, 41, 247–257. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Li, H.; Zhang, S.; Zhao, R.; Li, G.; Zhang, R.; Sheng, Y.; Cao, S.; Zhao, Y.; et al. Corrosion resistance and biocompatibility of calcium-containing coatings developed in near-neutral solutions containing phytic acid and phosphoric acid on AZ31B alloy. J. Alloys Compd. 2020, 823, 153721. [Google Scholar] [CrossRef]
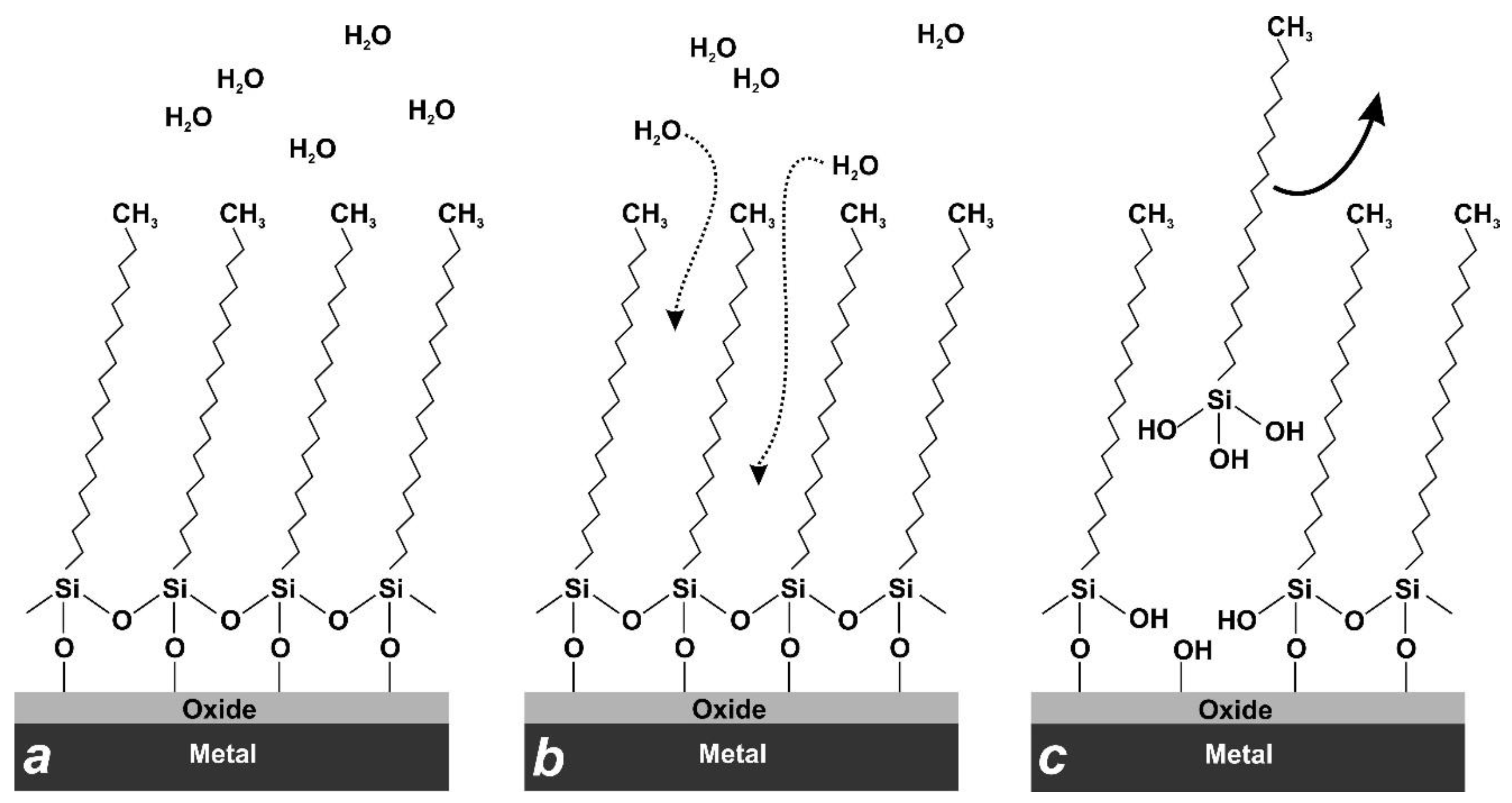
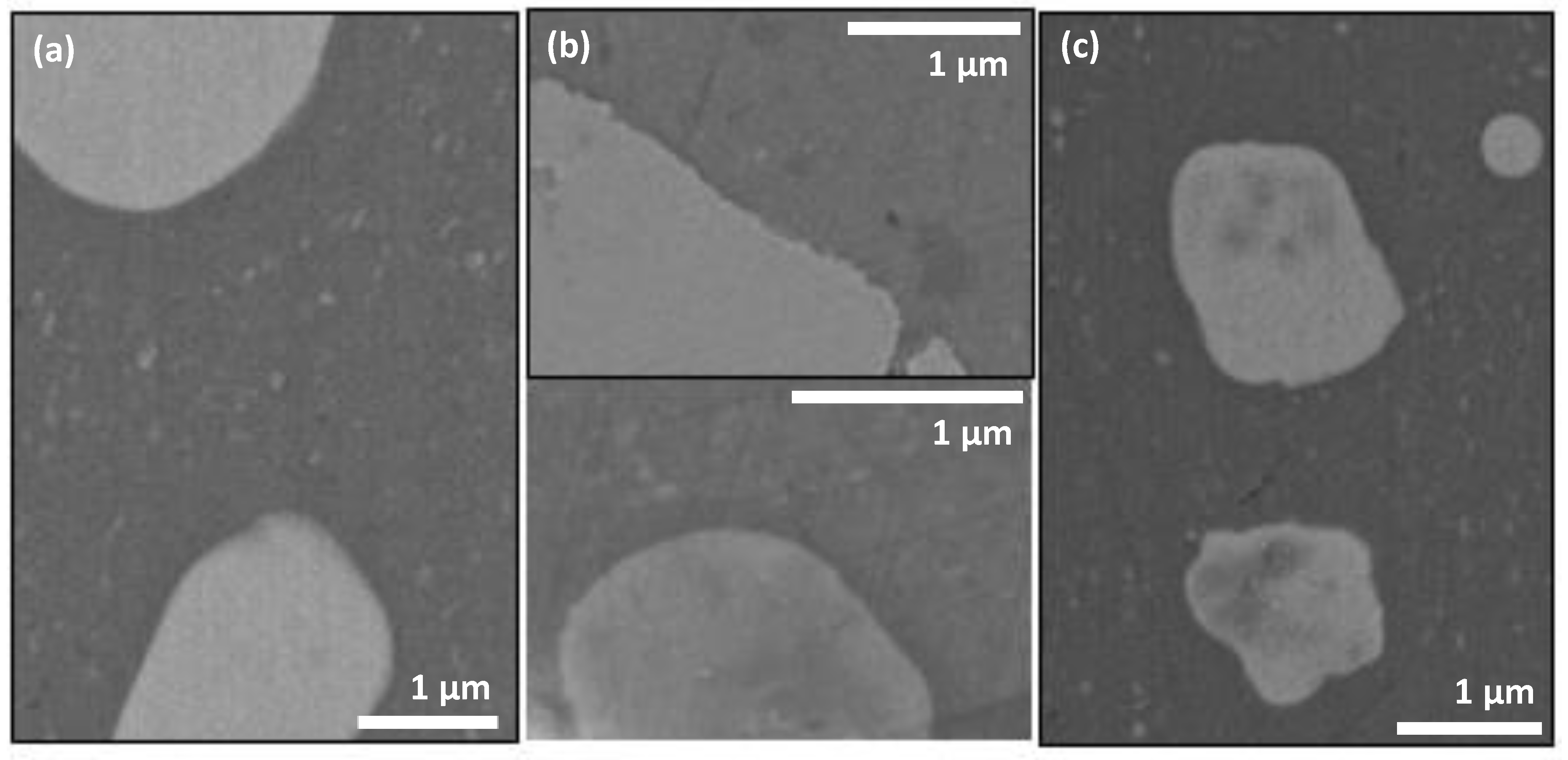
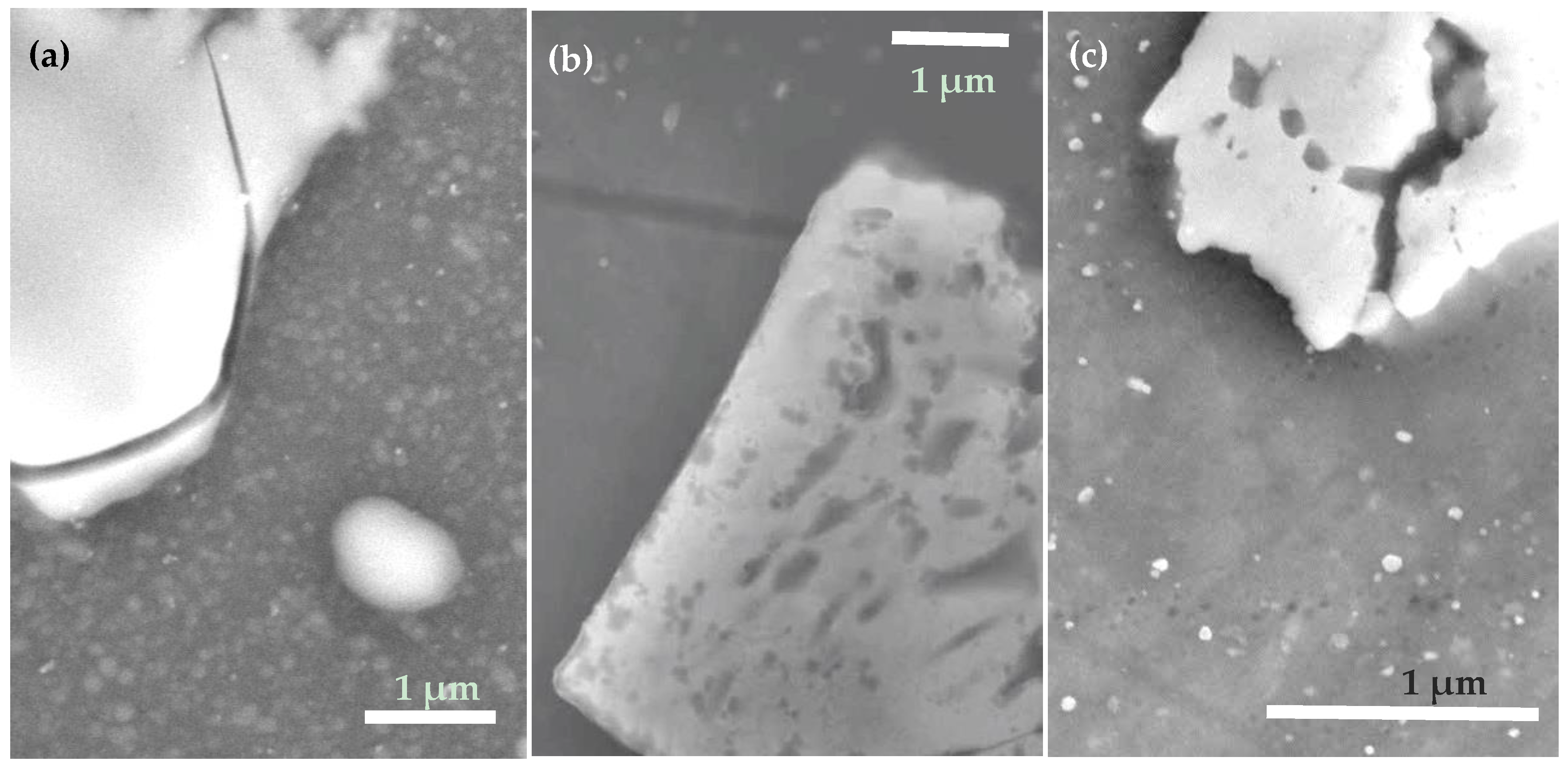
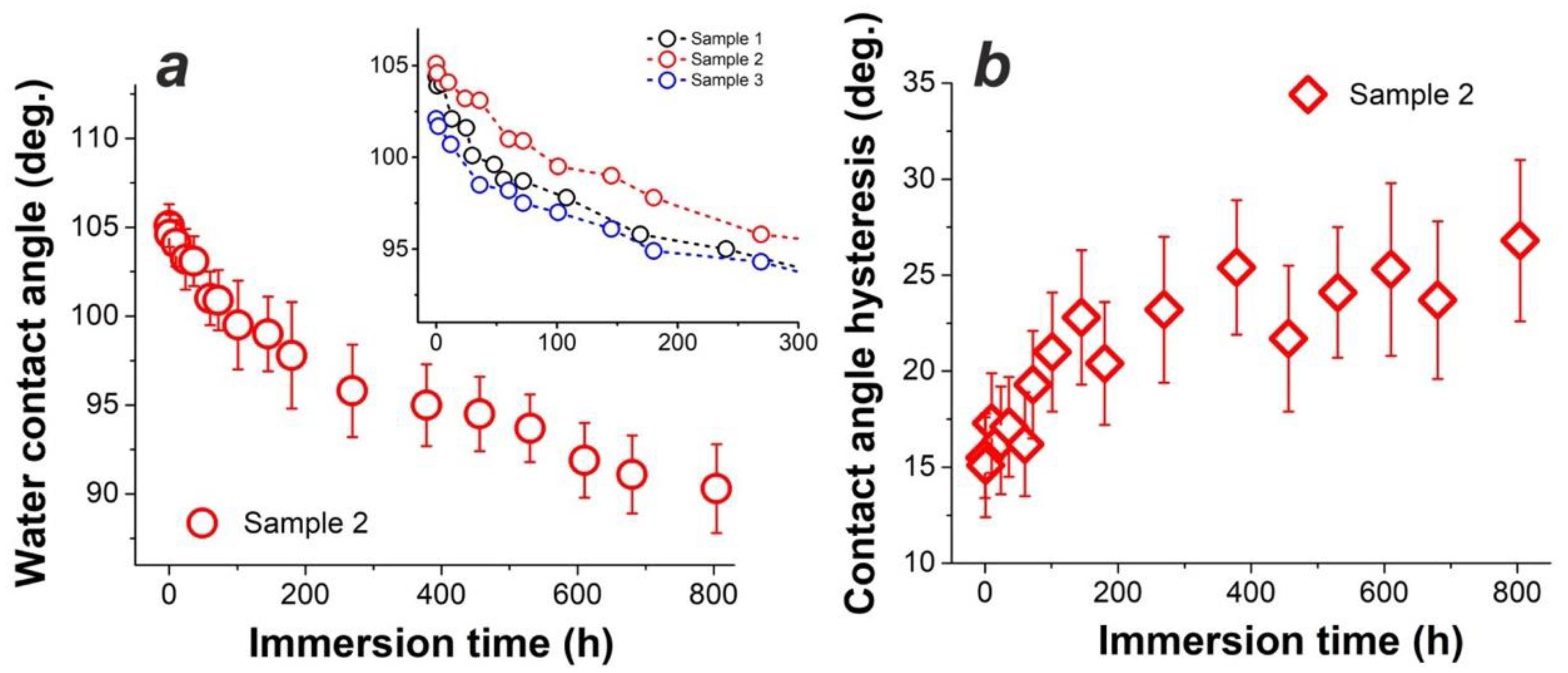
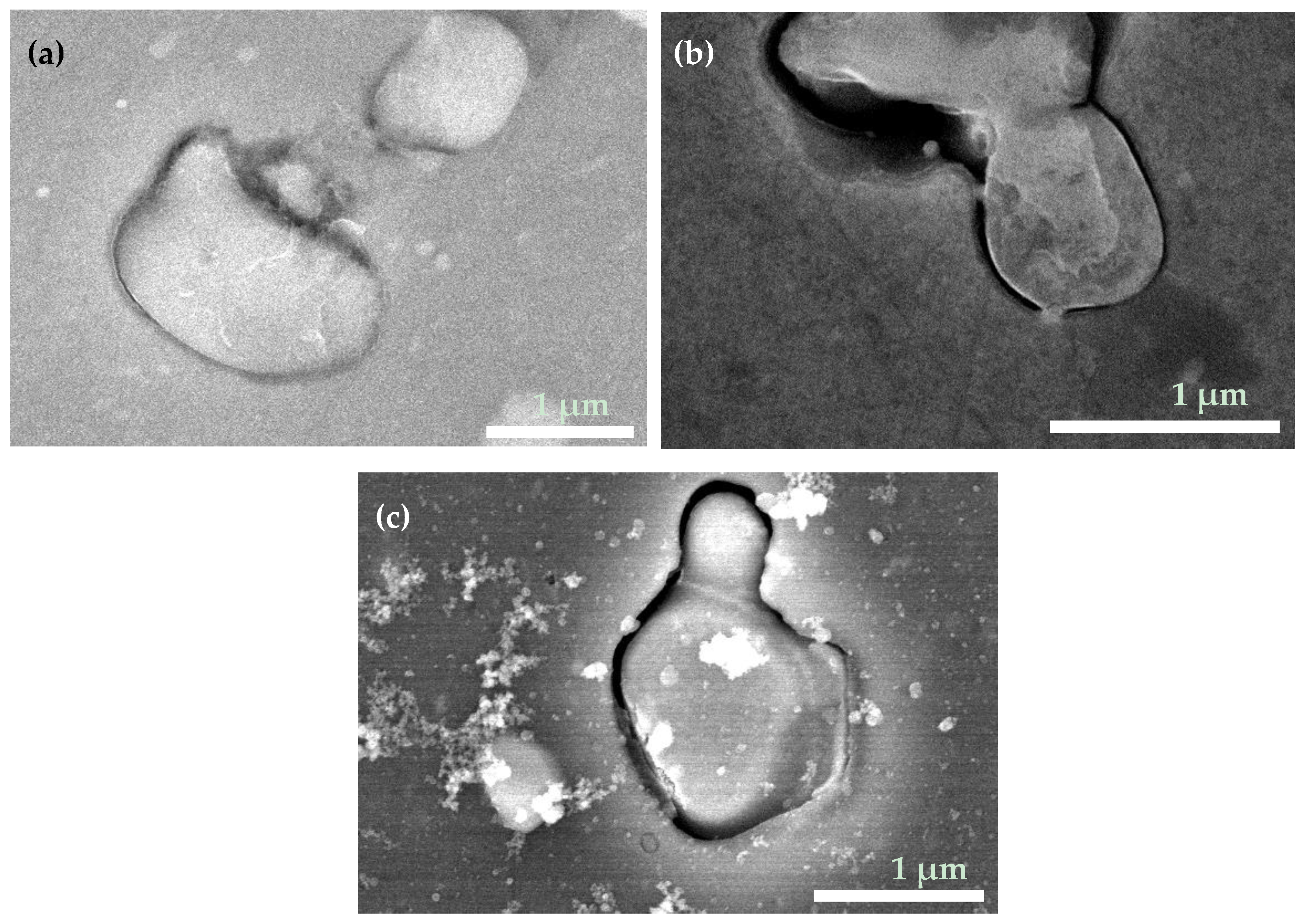
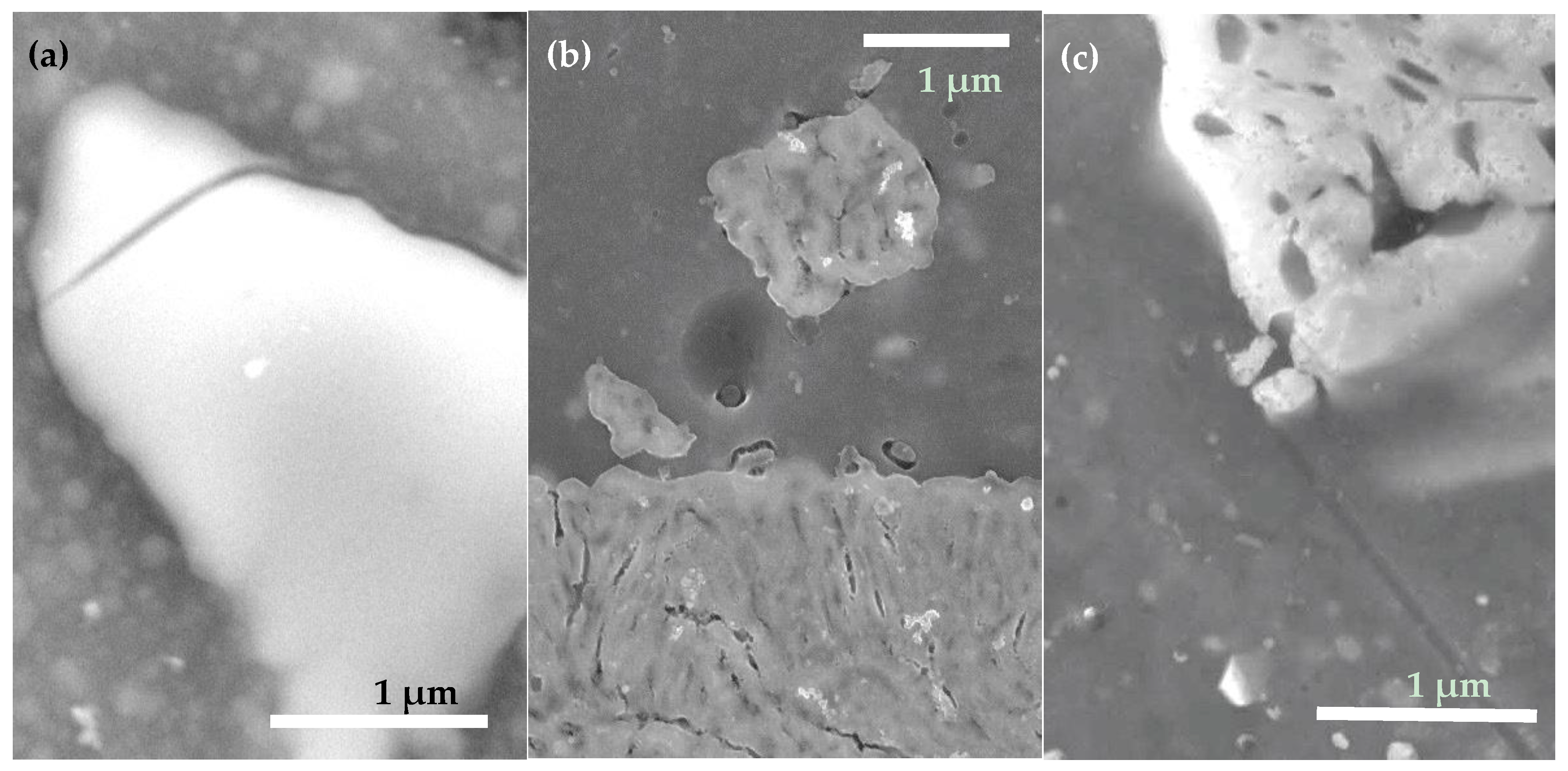
| Sample | Under-Layer | ODTMS Bath Immersion Time (min) | Bath Composition (v/v Ratio) | Initial Water CA Value (°) |
|---|---|---|---|---|
| 1 | - | 0.5 | 9:1 (EtOH/H2O) | 104.4 |
| 2 | - | 5 | 9:1 (EtOH/H2O) | 105.1 |
| 3 | - | 5 | 9:1 (MeOH/H2O) | 102.1 |
| 4 | PCC | 5 | 9:1 (EtOH/H2O) | 106.2 |
| 5 | BTSE | 5 | 9:1 (EtOH/H2O) | 104.4 |
| 6 | SiOx | 5 | 9:1 (EtOH/H2O) | 110.8 |
| Sample | Detection Time | Element (at. %) | |||
|---|---|---|---|---|---|
| C | O | Si | Al | ||
| 2 | Before immersion | 43.2 | 31.3 | 13.1 | 12.4 |
| After immersion | 27.5 | 38.7 | 4.7 | 29.1 | |
| 4 | Before immersion | 46.7 | 33.6 | 19.7 | 0 |
| After immersion | 47.9 | 39.7 | 12.4 | 0 | |
| 5 | Before immersion | 47.3 | 29.8 | 22.9 | 0 |
| After immersion | 48.8 | 27.7 | 21.8 | 1.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhizhchenko, A.Y.; Shabalina, A.V.; Aljulaih, A.A.; Gurbatov, S.O.; Kuchmizhak, A.A.; Iwamori, S.; Kulinich, S.A. Stability of Octadecyltrimethoxysilane-Based Coatings on Aluminum Alloy Surface. Materials 2022, 15, 1804. https://doi.org/10.3390/ma15051804
Zhizhchenko AY, Shabalina AV, Aljulaih AA, Gurbatov SO, Kuchmizhak AA, Iwamori S, Kulinich SA. Stability of Octadecyltrimethoxysilane-Based Coatings on Aluminum Alloy Surface. Materials. 2022; 15(5):1804. https://doi.org/10.3390/ma15051804
Chicago/Turabian StyleZhizhchenko, Alexey Y., Anastasiia V. Shabalina, Ali A. Aljulaih, Stanislav O. Gurbatov, Aleksandr A. Kuchmizhak, Satoru Iwamori, and Sergei A. Kulinich. 2022. "Stability of Octadecyltrimethoxysilane-Based Coatings on Aluminum Alloy Surface" Materials 15, no. 5: 1804. https://doi.org/10.3390/ma15051804
APA StyleZhizhchenko, A. Y., Shabalina, A. V., Aljulaih, A. A., Gurbatov, S. O., Kuchmizhak, A. A., Iwamori, S., & Kulinich, S. A. (2022). Stability of Octadecyltrimethoxysilane-Based Coatings on Aluminum Alloy Surface. Materials, 15(5), 1804. https://doi.org/10.3390/ma15051804








