Formation and Growth of Intermetallic Compounds in Lead-Free Solder Joints: A Review
Abstract
1. Introduction
2. Effect of Alloying Additions
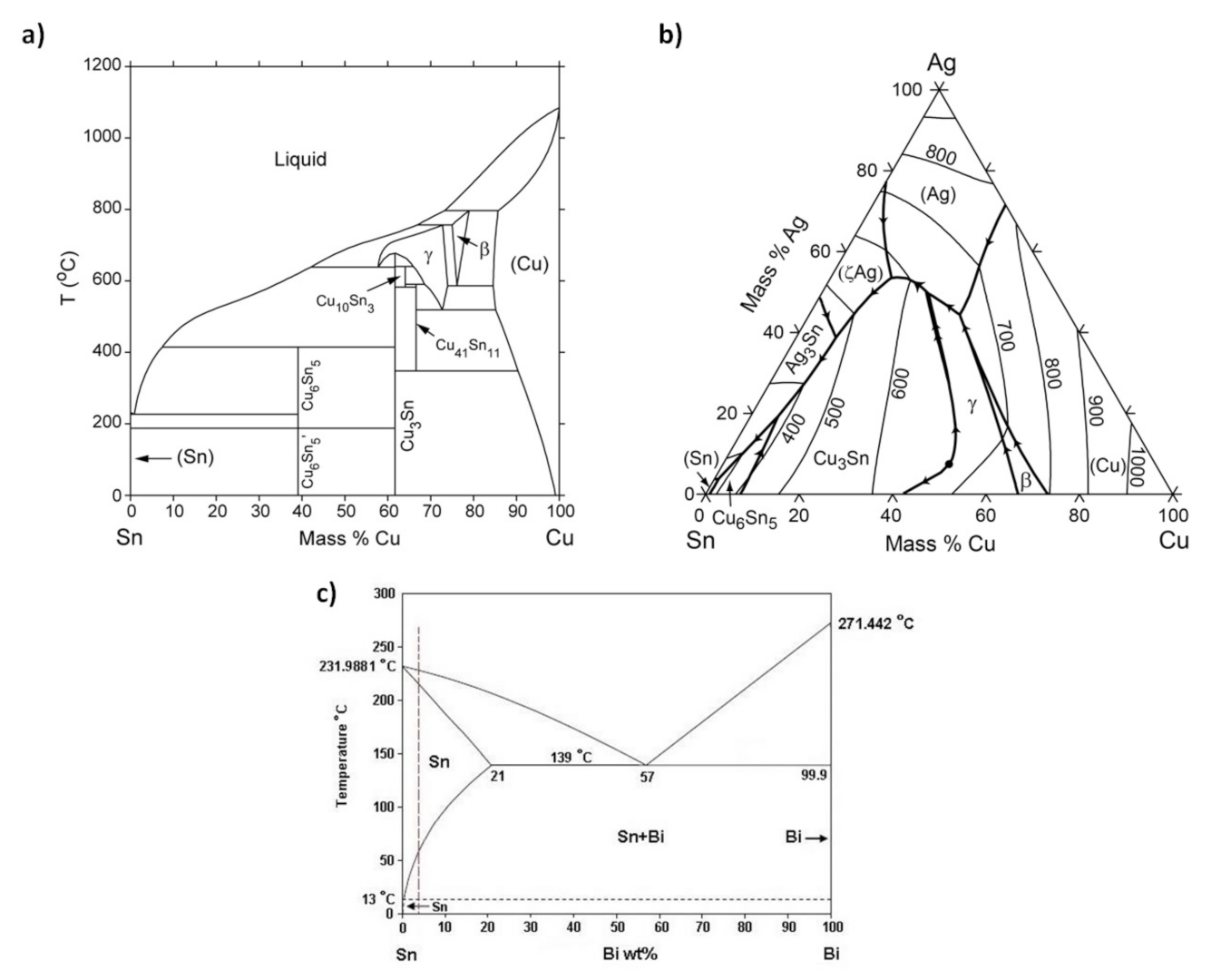
2.1. Formation and Growth of IMC in the Bulk Solder
2.2. Formation and Growth of IMC at the Solder-Substrate Interface
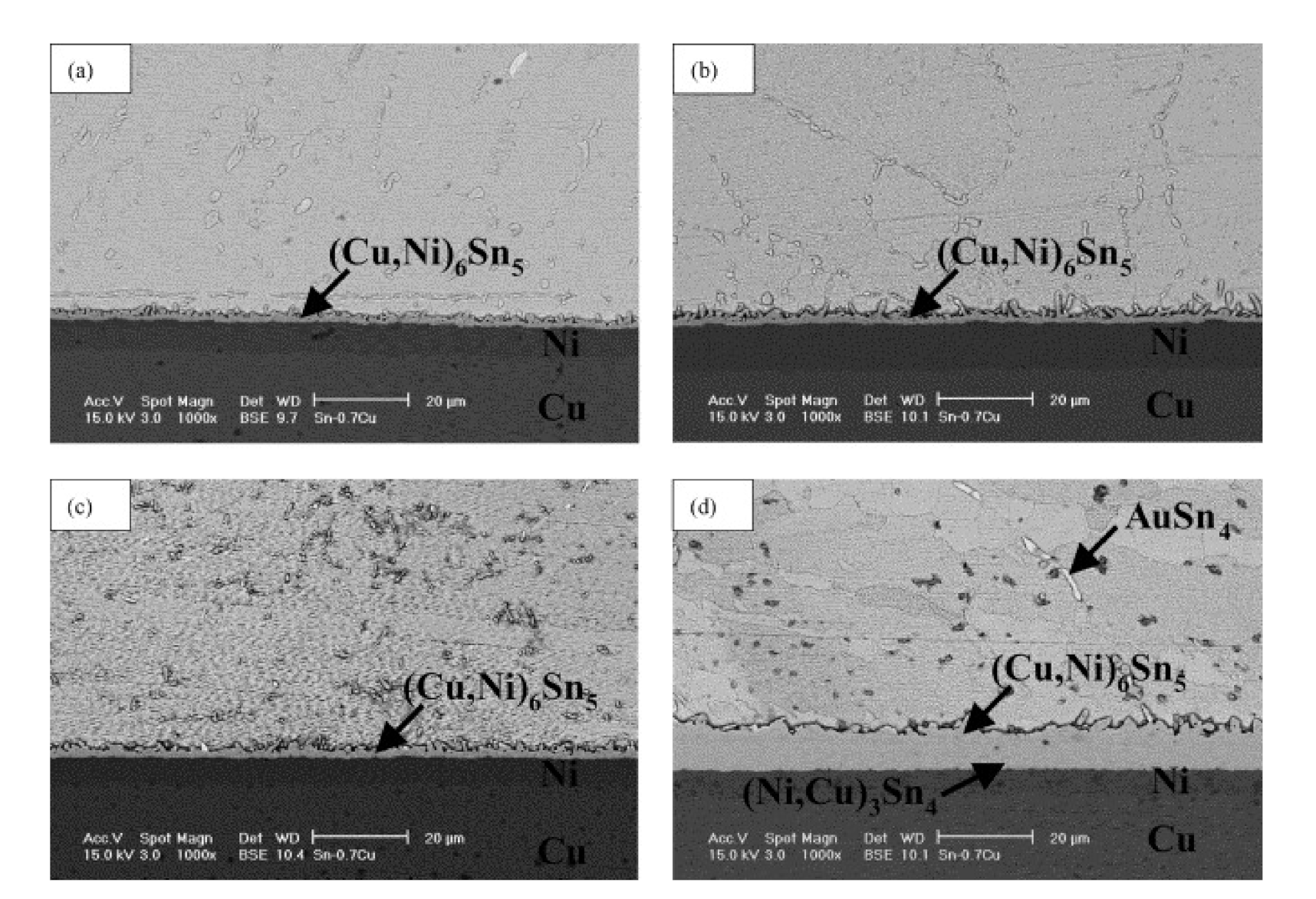
3. Effect of Surface Finish
4. Effect of Aging Time and Temperature
5. Effect of Solder Volume
6. Conclusions
- (1)
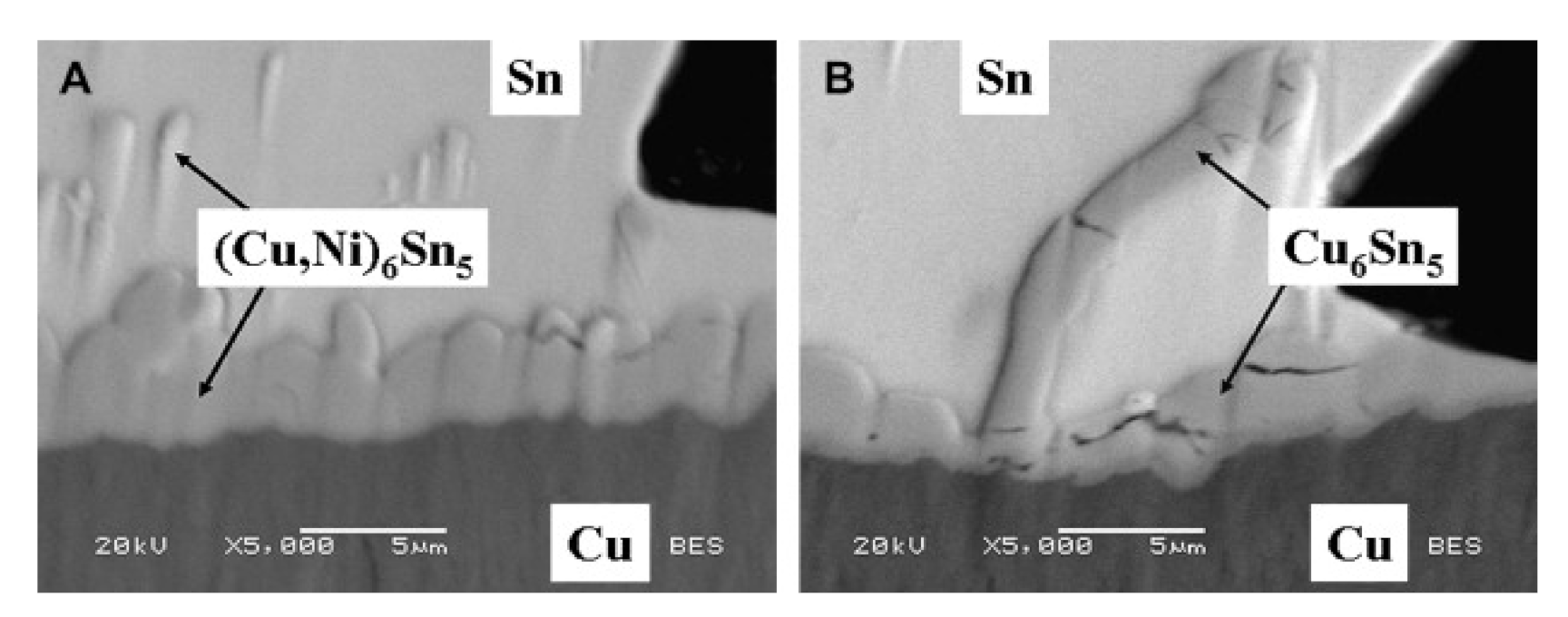
- The effect of minor alloying elements on the primary intermetallic and interfacial IMC can be substantial. The microstructure of eutectic alloy may be changed by the addition of a small amount of a given alloying element to the bulk microstructure. The alloying elements added to the solder also can influence the formation and growth of IMCs. This can decrease or increase the IMC’s growth/reaction rate and result in the formation of an extra reaction layer at the interface
- (2)
- The IMC layer is considerably affected by the surface finishes material during soldering. The thickness and composition of IMCs also greatly affected by surface finish layers that formed by a process called dissolution, where some amount of the surface metallization dissolves into the molten solder, and the formation of IMCs differs depending on the surface finish.
- (3)
- Different aging temperatures and time also influenced the thickness of interfacial intermetallic. Higher temperatures and longer aging times increase the IMC growth
- (4)
- The average thickness of the intermetallic for low solder volume is thicker than for high solder volume solders. This was due to the fact the Cu concentration in the solder grew quicker in smaller solder balls than in bigger ones.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, X.; Li, Y.; Liu, Y.; Min, Z. Developments of high strength Bi-containing Sn0.7Cu lead-free solder alloys prepared by directional solidification. J. Alloys Compd. 2015, 625, 241–250. [Google Scholar] [CrossRef]
- Qiu, J.; Peng, Y.; Gao, P.; Li, C. Effect of Cu Content on Performance of Sn-Zn-Cu Lead-Free Solder Alloys Designed by Cluster-Plus-Glue-Atom Model. Materials 2021, 14, 2335. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Ju, G.; Lin, F. Interfacial IMC layer growth and tensile properties of low-silver Cu/SACBE/Cu solder joints. Solder. Surf. Mt. Technol. 2012, 24, 249–256. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, L. Properties and Microstructures of Sn-Ag-Cu-X Lead-Free Solder Joints in Electronic Packaging. Adv. Mater. Sci. Eng. 2015, 2015, 639028. [Google Scholar] [CrossRef]
- Sayyadi, R.; Naffakh-Moosavy, H. The Role of Intermetallic Compounds in Controlling the Microstructural, Physical and Mechanical Properties of Cu-[Sn-Ag-Cu-Bi]-Cu Solder Joints. Sci. Rep. 2019, 9, 8389. [Google Scholar] [CrossRef]
- Kotadia, H.R.; Howes, P.; Mannan, S. A review: On the development of low melting temperature Pb-free solders. Microelectron. Reliab. 2014, 54, 1253–1273. [Google Scholar] [CrossRef]
- Nogita, K.; Read, J.; Nishimura, T.; Sweatman, K.; Suenaga, S.; Dahle, A.K. Microstructure Control in Sn–0.7 mass%Cu Alloys. Mater. Trans. 2005, 46, 2419–2425. [Google Scholar] [CrossRef]
- Gourlay, C.; Nogita, K.; McDonald, S.; Nishimura, T.; Sweatman, K.; Dahle, A. A rheological assessment of the effect of trace level Ni additions on the solidification of Sn–0.7Cu. Scr. Mater. 2006, 54, 1557–1562. [Google Scholar] [CrossRef]
- Nogita, K.; McDonald, S.D.; Tsukamoto, H.; Read, J.; Suenaga, S.; Nishimura, T. Inhibiting Cracking of Interfacial Cu6Sn5 by Ni Additions to Sn-based Lead-free Solders. Trans. Jpn. Inst. Electron. Packag. 2009, 2, 46–54. [Google Scholar] [CrossRef]
- Salleh, M.M.; McDonald, S.; Gourlay, C.; Belyakov, S.; Yasuda, H.; Nogita, K. Effect of Ni on the Formation and Growth of Primary Cu6Sn5 Intermetallics in Sn-0.7 wt.%Cu Solder Pastes on Cu Substrates During the Soldering Process. J. Electron. Mater. 2015, 45, 154–163. [Google Scholar] [CrossRef]
- Ventura, T.; Terzi, S.; Rappaz, M.; Dahle, A.K. Effects of Ni additions, trace elements and solidification kinetics on microstructure formation in Sn–0.7Cu solder. Acta Mater. 2011, 59, 4197–4206. [Google Scholar] [CrossRef]
- Silva, B.L.; Cheung, N.; Garcia, A.; Spinelli, J.E. Thermal Parameters, Microstructure, and Mechanical Properties of Directionally Solidified Sn-0.7 wt.%Cu Solder Alloys Containing 0 ppm to 1000 ppm Ni. J. Electron. Mater. 2013, 42, 179–191. [Google Scholar] [CrossRef]
- Gumaan, M.S.; Shalaby, R.M.; Yousef, M.K.M.; Ali, E.A.; Abdel-Hady, E.E. Nickel effects on the structural and some physical properties of the eutectic Sn-Ag lead-free solder alloy. Solder. Surf. Mt. Technol. 2019, 31, 40–51. [Google Scholar] [CrossRef]
- Xian, J.W.; Belyakov, S.A.; Gourlay, C.M. Controlling Bulk Cu6Sn5 Nucleation in Sn0.7Cu/Cu Joints with Al Micro-alloying. J. Electron. Mater. 2015, 45, 69–78. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, P.; Cao, F.; Kang, H.; Chen, Z.; Fu, Y.; Xiao, T.; Huang, W.; Yuan, Q. Growth behavior of Cu6Sn5 in Sn–6.5 Cu solders under DC considering trace Al: In situ observation. Intermetallics 2015, 58, 84–90. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Du, C.; Dai, J.; Zhang, N. Effect of aluminum concentration on the microstructure and mechanical properties of Sn–Cu–Al solder alloy. Microelectron. Reliab. 2015, 55, 596–601. [Google Scholar] [CrossRef]
- Ma, X.; Qian, Y.; Yoshida, F. Effect of La on the Cu–Sn intermetallic compound (IMC) growth and solder joint reliability. J. Alloys Compd. 2002, 334, 224–227. [Google Scholar] [CrossRef]
- McDonald, S.; Nogita, K.; Read, J.; Ventura, T.; Nishimura, T. Influence of Composition on the Morphology of Primary Cu6Sn5 in Sn-4Cu Alloys. J. Electron. Mater. 2013, 42, 256–262. [Google Scholar] [CrossRef]
- Reeve, K.N.; Anderson, I.E.; Handwerker, C.A. Nucleation and Growth of Cu-Al Intermetallics in Al-Modified Sn-Cu and Sn-Ag-Cu Lead-Free Solder Alloys. J. Electron. Mater. 2015, 44, 842–866. [Google Scholar] [CrossRef]
- Wu, C.M.L.; Yu, D.Q.; Law, C.M.T.; Wang, L. Microstructure and Mechanical Properties of New Lead-Free Sn-Cu-RE Solder Alloys. J. Electron. Mater. 2002, 31, 928–932. [Google Scholar] [CrossRef]
- Zeng, G.; McDonald, S.; Gu, Q.; Terada, Y.; Uesugi, K.; Yasuda, H.; Nogita, K. The influence of Ni and Zn additions on microstructure and phase transformations in Sn–0.7Cu/Cu solder joints. Acta Mater. 2015, 83, 357–371. [Google Scholar] [CrossRef]
- Zeng, G.; McDonald, S.D.; Gourlay, C.M.; Uesugi, K.; Terada, Y.; Yasuda, H.; Nogita, K. Solidification of Sn-0.7Cu-0.15Zn Solder: In Situ Observation. Met. Mater. Trans. A 2014, 45A, 918–926. [Google Scholar] [CrossRef]
- Laurila, T.; Vuorinen, V.; Kivilahti, J.K. Interfacial reactions between lead-free solders and common base materials. Mater. Sci. Eng. R Rep. 2005, 49, 1–60. [Google Scholar] [CrossRef]
- Nogita, K. Stabilisation of Cu6Sn5 by Ni in Sn-0.7Cu-0.05Ni lead-free solder alloys. Intermetallics 2010, 18, 145–149. [Google Scholar] [CrossRef]
- Hu, X.; Qiu, H.; Jiang, X. Effect of Ni addition into the Cu substrate on the interfacial IMC growth during the liquid-state reaction with Sn–58Bi solder. J. Mater. Sci. Mater. Electron. 2018, 30, 1907–1918. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Dai, J.; Jing, Y.; Ge, J.; Zhang, N. Microstructure, interfacial IMC and mechanical properties of Sn-0.7Cu-xAl (x = 0–0.075) lead-free solder alloy. Mater. Des. 2015, 67, 209–216. [Google Scholar] [CrossRef]
- Liu, P.; Yao, P.; Liu, J. Evolutions of the interface and shear strength between SnAgCu-xNi solder and Cu substrate during isothermal aging at 150 °C. J. Alloys Compd. 2009, 486, 474–479. [Google Scholar] [CrossRef]
- Yao, P.; Liu, P.; Liu, J. Effects of multiple reflows on intermetallic morphology and shear strength of SnAgCu-xNi composite solder joints on electrolytic Ni/Au metallized substrate. J. Alloys Compd. 2008, 462, 73–79. [Google Scholar] [CrossRef]
- Nagy, E.; Kristaly, F.; Gyenes, A.; Gacsi, Z. Investigation of Intermetallic Compounds In Sn-Cu-Ni Lead-Free Solders. Arch. Met. Mater. 2015, 60, 1511–1515. [Google Scholar] [CrossRef]
- Rizvi, M.; Bailey, C.; Chan, Y.; Islam, M.; Lu, H. Effect of adding 0.3wt% Ni into the Sn–0.7wt% Cu solder: Part II. Growth of intermetallic layer with Cu during wetting and aging. J. Alloys Compd. 2007, 438, 122–128. [Google Scholar] [CrossRef]
- Nishikawa, H.; Komatsu, A.; Takemoto, T. Morphology and Pull Strength of Sn-Ag(-Co) Solder Joint with Copper Pad. J. Electron. Mater. 2007, 36, 1137–1143. [Google Scholar] [CrossRef]
- Yen, Y.-W.; Chiang, Y.-C.; Jao, C.-C.; Liaw, D.-W.; Lo, S.-c.; Lee, C. Interfacial reactions and mechanical properties between Sn-4.0 Ag-0.5 Cu and Sn-4.0 Ag-0.5 Cu-0.05 Ni-0.01 Ge lead-free solders with the Au/Ni/Cu substrate. J. Alloys Compd. 2011, 509, 4595–4602. [Google Scholar] [CrossRef]
- Wang, C.-H.; Shen, H.-T. Effects of Ni addition on the interfacial reactions between Sn–Cu solders and Ni substrate. Intermetallics 2010, 18, 616–622. [Google Scholar] [CrossRef]
- Zou, H.; Zhang, Z. Effect of Zn Addition on Interfacial Reactions between Sn-4Ag Solder and Ag Substrates. J. Electron. Mater. 2008, 37, 1119–1129. [Google Scholar] [CrossRef][Green Version]
- Chan, Y.H.; Arafat, M.M.; Haseeb, A.S.M.A. Effects of reflow on the interfacial characteristics between Zn nanoparticles containing Sn-3.8Ag-0.7Cu solder and copper substrate. Solder. Surf. Mt. Technol. 2013, 25, 91–98. [Google Scholar] [CrossRef]
- Zhang, L.; Han, J.g.; He, C.w.; Guo, Y.h. Effect of Zn on properties and microstructure of SnAgCu alloy. J. Mater. Sci. Mater. Electron. 2012, 23, 1950–1956. [Google Scholar] [CrossRef]
- Mahdavifard, M.H.; Sabri, M.F.M.; Shnawah, D.A.; Said, S.M.; Badruddin, I.A.; Rozali, S. The effect of iron and bismuth addition on the microstructural, mechanical, and thermal properties of Sn–1Ag–0.5Cu solder alloy. Microelectron. Reliab. 2015, 55, 1886–1890. [Google Scholar] [CrossRef]
- Zeng, G.; Xue, S.; Zhang, L.; Gao, L.; Dai, W.; Luo, J. A review on the interfacial intermetallic compounds between Sn–Ag–Cu based solders and substrates. J. Mater. Sci. Mater. Electron. 2010, 21, 421–440. [Google Scholar] [CrossRef]
- Shalaby, R.M. Indium, chromium and nickel-modified eutectic Sn–0.7 wt% Cu lead-free solder rapidly solidified from molten state. J. Mater. Sci. Mater. Electron. 2015, 26, 6625–6632. [Google Scholar] [CrossRef]
- Li, L.F.; Cheng, Y.K.; Xu, G.L.; Wang, E.Z.; Zhang, Z.H.; Wang, H. Effects of indium addition on properties and wettability of Sn–0.7Cu–0.2Ni lead-free solders. Mater. Des. 2014, 64, 15–20. [Google Scholar] [CrossRef]
- Lee, H.-T.; Lee, F.-F.; Hong, T.-F.; Chen, H.-W. Effect of in addition on Sn-Ag-Sb lead-free solder system. IEEE 2008, 191–194. [Google Scholar] [CrossRef]
- Li, G.; Shi, Y.; Hao, H.; Xia, Z.; Lei, Y.; Guo, F. Effect of phosphorus element on the comprehensive properties of Sn–Cu lead-free solder. J. Alloys Compd. 2010, 491, 382–385. [Google Scholar] [CrossRef]
- Xian, J.W.; Belyakov, S.A.; Britton, T.B.; Gourlay, C.M. Heterogeneous nucleation of Cu6Sn5 in Sn-Cu-Al solders. J. Alloys Compd. 2015, 619, 345–355. [Google Scholar] [CrossRef]
- Ma, L.; Tai, F.; Xu, G.; Guo, F.; Wang, X. Effects of Processing and Amount of Co Addition on Shear Strength and Microstructual Development in the Sn-3.0Ag-0.5Cu Solder Joint. J. Electron. Mater. 2011, 40, 1416–1421. [Google Scholar] [CrossRef]
- Luo, D.-X.; Xue, S.-B.; Li, Z.-Q. Effects of Ga addition on microstructure and properties of Sn–0.5Ag–0.7Cu solder. J. Mater. Sci. Mater. Electron. 2014, 25, 3566–3571. [Google Scholar] [CrossRef]
- Gao, L.; Xue, S.; Zhang, L.; Sheng, Z.; Zeng, G.; Ji, F. Effects of trace rare earth Nd addition on microstructure and properties of SnAgCu solder. J. Mater. Sci. Mater. Electron. 2010, 21, 643–648. [Google Scholar] [CrossRef]
- Dudek, M.A.; Sidhu, R.S.; Chawla, N.; Renavikar, M. Microstructure and mechanical behavior of novel rare earth-containing Pb-Free solders. J. Electron. Mater. 2006, 35, 2088–2097. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, S.B.; Zeng, G.; Gao, L.L.; Ye, H. Interface reaction between SnAgCu/SnAgCuCe solders and Cu substrate subjected to thermal cycling and isothermal aging. J. Alloys Compd. 2012, 510, 38–45. [Google Scholar] [CrossRef]
- Liu, X.; Huang, M.; Zhao, N.; Wang, L. Liquid-state and solid-state interfacial reactions between Sn-Ag-Cu-Fe composite solders and Cu substrate. J. Mater. Sci. Mater. Electron. 2014, 25, 328–337. [Google Scholar] [CrossRef]
- Haseeb, A.S.M.A.; Leng, T.S. Effects of Co nanoparticle addition to Sn–3.8Ag–0.7Cu solder on interfacial structure after reflow and ageing. Intermetallics 2011, 19, 707–712. [Google Scholar] [CrossRef]
- Haseeb, A.; Arafat, M.; Johan, M.R. Stability of molybdenum nanoparticles in Sn–3.8Ag–0.7Cu solder during multiple reflow and their influence on interfacial intermetallic compounds. Mater. Charact. 2012, 64, 27–35. [Google Scholar] [CrossRef]
- Rizvi, M.; Chan, Y.; Bailey, C.; Lu, H.; Islam, M. Effect of adding 1wt% Bi into the Sn–2.8Ag–0.5Cu solder alloy on the intermetallic formations with Cu-substrate during soldering and isothermal aging. J. Alloys Compd. 2006, 407, 208–214. [Google Scholar] [CrossRef]
- Koo, J.; Lee, C.; Hong, S.J.; Kim, K.-S.; Lee, H.M. Microstructural discovery of Al addition on Sn–0.5Cu-based Pb-free solder design. J. Alloys Compd. 2015, 650, 106–115. [Google Scholar] [CrossRef]
- Ramli, M.; Salleh, M.M.; Yasuda, H.; Chaiprapa, J.; Nogita, K. The effect of Bi on the microstructure, electrical, wettability and mechanical properties of Sn-0.7Cu-0.05Ni alloys for high strength soldering. Mater. Des. 2020, 186, 108281. [Google Scholar] [CrossRef]
- Shen, C.; Hai, Z.; Zhao, C.; Zhang, J.; Evans, J.L.; Bozack, M.J.; Suhling, J.C. Packaging Reliability Effect of ENIG and ENEPIG Surface Finishes in Board Level Thermal Test under Long-Term Aging and Cycling. Materials 2017, 10, 451. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Yang, S.; Lee, P.; Lee, C.; Chen, C.; Kuo, T. IMC microstructure modification and mechanical reinforcement of Sn–Ag–Cu/Cu microelectronic joints through an advanced surface finish technique. J. Mater. Res. Technol. 2021, 11, 1895–1910. [Google Scholar] [CrossRef]
- Nogita, K.; Nishimura, T. Nickel-stabilized hexagonal (Cu,Ni)6Sn5 in Sn–Cu–Ni lead-free solder alloys. Scr. Mater. 2008, 59, 191–194. [Google Scholar] [CrossRef]
- Liu, S.; Yang, C.; Ling, H.; Hu, A.; Hang, T.; Gao, L.; Li, M. Inhibiting effects of the Ni barrier layer on the growth of porous Cu3Sn in 10-μm microbumps. J. Mater. Sci. Mater. Electron. 2021, 32, 17655–17661. [Google Scholar] [CrossRef]
- Seo, W. Effect of Electroless Ni Plating Bath Conditions on the Impact Reliability of Sn-Ag-Cu Solder Joints. Ph.D. Thesis, Hanyang University, Seoul, Korea, 2020. [Google Scholar]
- Yoon, J.-W.; Noh, B.-I.; Jung, S.-B. Effects of third element and surface finish on interfacial reactions of Sn-Ag-xCu (or Ni)/(Cu or ENIG) solder joints. J. Alloys Compd. 2010, 506, 331–337. [Google Scholar] [CrossRef]
- Zheng, Y.; Hillman, C.; McCluskey, P. Effect of PWB Plating on the Microstructure and Reliability of SnAgCu Solder Joints. In Proceedings of the AESF SUR/FIN 2002: Annual International Technical Conference, Chicago, IL, USA, 24–17 June 2002; pp. 24–27. [Google Scholar]
- Ourdjini, A.; Hanim, M.A.A.; Aisha, I.S.R.; Chin, Y.T. Effect of surface finish metallurgy on intermetallic compounds during soldering with tin-silver-copper solders. In Proceedings of the 2008 33rd IEEE/CPMT International Electronics Manufacturing Technology Conference (IEMT); Institute of Electrical and Electronics Engineers (IEEE), Penang, Malaysia, 4–6 November 2008; pp. 1–4. [Google Scholar]
- Ha, S.-S.; Park, J.; Jung, S.-B. Effect of Pd Addition in ENIG Surface Finish on Drop Reliability of Sn-Ag-Cu Solder Joint. Mater. Trans. 2011, 52, 1553–1559. [Google Scholar] [CrossRef]
- Kim, Y.M.; Park, J.-Y. Effect of Pd Thickness on the Interfacial Reaction and Shear Strength in Solder Joints between Sn-3.0Ag-0.5Cu Solder and Electroless Nickel/Electroless Palladium/Immersion Gold (ENEPIG) Surface Finish. J. Electron. Mater. 2012, 41, 763–773. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Kim, S.-W.; Jung, S.-B. Effect of reflow time on interfacial reaction and shear strength of Sn–0.7Cu solder/Cu and electroless Ni–P BGA joints. J. Alloys Compd. 2004, 385, 192–198. [Google Scholar] [CrossRef]
- Yin, H.G.; Shen, J.; Tang, Q. Wetting of Sn-O 7Cu Solder Alloy on Different Substrates at Different Temperatures. In Proceedings of the International Conference on Electronic Packaging Technology & High Density Packaging, Guilin, China, 13–16 August 2012; pp. 280–284. [Google Scholar]
- Aisha, I.S.R.; Ourdjini, A.; Astuty, A.; Saliza, O. Effect of Surface Finish On Intermetallic Compound Formation During Soldering With Ni-Doped Solders. Solid State Sci. Technol. 2011, 19, 319–329. [Google Scholar]
- Yoon, J.-W.; Noh, B.-I.; Jung, S.-B. Comparative Study of ENIG and ENEPIG as Surface Finishes for a Sn-Ag-Cu Solder Joint. J. Electron. Mater. 2011, 40, 1950–1955. [Google Scholar] [CrossRef]
- Sun, P.; Andersson, C.; Wei, X.; Cheng, Z.; Shangguan, D.; Liu, J. Intermetallic compound formation in Sn-Co-Cu, Sn-Ag-Cu and eutectic Sn-Cu solder joints on electroless Ni(P) immersion Au surface finish after reflow soldering. Mater. Sci. Eng. B 2006, 135, 134–140. [Google Scholar] [CrossRef]
- Adawiyah, M.R.; Azlina, O.S. Comparative study on the isothermal aging of bare Cu and ENImAg surface finish for Sn-Ag-Cu solder joints. J. Alloys Compd. 2018, 740, 958–966. [Google Scholar] [CrossRef]
- Vidyatharran, K.; Hanim, M.A.; Dele-Afolabi, T.; Matori, K.; Azlina, O.S. Microstructural and shear strength properties of GNSs-reinforced Sn-1.0Ag-0.5Cu (SAC105) composite solder interconnects on plain Cu and ENIAg surface finish. J. Mater. Res. Technol. 2021, 15, 2497–2506. [Google Scholar] [CrossRef]
- Ab Rahim, R.A.A.; Zulkifli, M.N.; Mohd Afdzaluddin, A.; Shyong, K.S. Effect of isothermal aging and copper substrate roughness on the SAC305 solder joint intermetallic layer growth of high temperature storage (HTS). Sains Malays. 2020, 49, 3045–3054. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Jung, S.-B. Effect of surface finish on interfacial reactions of Cu/Sn–Ag–Cu/Cu(ENIG) sandwich solder joints. J. Alloys Compd. 2008, 448, 177–184. [Google Scholar] [CrossRef]
- Seo, W.; Kim, K.-H.; Kim, Y.-H.; Yoo, S. Effect of Ni-P Plating Temperature on Growth of Interfacial Intermetallic Compound in Electroless Nickel Immersion Gold/Sn-Ag-Cu Solder Joints. J. Electron. Mater. 2017, 47, 110–116. [Google Scholar] [CrossRef]
- Su, S.; Jian, M.; Hamasha, S. Effects of Surface Finish on the Shear Fatigue of SAC-Based Solder Alloys. IEEE Trans. Compon. Packag. Manuf. Technol. 2019, 10, 457–466. [Google Scholar] [CrossRef]
- Amli, S.F.N.M.; Salleh, M.A.A.M.; Ramli, M.I.I.; Razak, N.R.A.; Yasuda, H.; Chaiprapa, J.; Nogita, K. Effects of Surface Finish on Sn-3.0Ag-0.5Cu Solder Joint Microstructure and Strength. J. Electron. Mater. 2021, 50, 855–868. [Google Scholar] [CrossRef]
- Azlina, O.S.; Ourdjini, A.; Ibrahim, M.H.I.; Sallehuddin, Y.M. Comparison between SAC405 Lead-Free Solders and EN(P)EPIG and EN(B)EPIG Surface Finishes. Appl. Mech. Mater. 2015, 773, 232–236. [Google Scholar] [CrossRef]
- Amli, S.F.M.; Salleh, M.A.A.M.; Said, R.M.; Razak, N.R.A.; Wahab, J.A.; Ramli, M.I.I. Effect of surface finish on the wettability and electrical resistivity of Sn-3.0 Ag-0.5 Cu solder. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Akkara, F.; Abueed, M.; Rababah, M.; Zhao, C.; Su, S.; Suhling, J.; Evans, J. Effect of surface finish and high bi solder alloy on component reliability in thermal cycling. In Proceedings of the 2018 IEEE 68th Electronic Components and Technology Conference (ECTC) IEEE, San Diego, CA, USA, 29 May–1 June 2018. [Google Scholar]
- Wu, C.M.L.; Yu, D.Q.; Law, C.M.T.; Wang, L. Properties of lead-free solder alloys with rare earth element additions. Mater. Sci. Eng. R Rep. 2004, 44, 1–44. [Google Scholar] [CrossRef]
- Sivakumar, P.; O’Donnell, K.; Cho, J. Effects of bismuth and nickel on the microstructure evolution of Sn-Ag-Cu (SAC)-based solders. Mater. Today Commun. 2021, 26, 101787. [Google Scholar] [CrossRef]
- Mookam, N.; Kanlayasiri, K. Evolution of Intermetallic Compounds between Sn-0.3Ag-0.7Cu Low-silver Lead-free Solder and Cu Substrate during Thermal Aging. J. Mater. Sci. Technol. 2012, 28, 53–59. [Google Scholar] [CrossRef]
- Noor, E.E.M.; Abdullah, M.M.A.B. Effect of Thermal Aging on the Interfacial of Sn-Zn and Sn-Zn-Bi Solders Joint on Cu Substrate. Key Eng. Mater. 2016, 700, 113–122. [Google Scholar]
- Chuang, T.-H.; Yen, S.-F.; Wu, H.-M. Intermetallic formation in Sn3Ag0.5Cu and Sn3Ag0.5Cu0.06Ni0.01Ge solder BGA packages with immersion Ag surface finish. J. Electron. Mater. 2006, 35, 310–318. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Kim, S.-W.; Jung, S.-B. Interfacial reaction and mechanical properties of eutectic Sn–0.7Cu/Ni BGA solder joints during isothermal long-term aging. J. Alloys Compd. 2005, 391, 82–89. [Google Scholar] [CrossRef]
- Hodulova, E.; Palcut, M.; Lechovic, E.; Simekova, B.; Ulrich, K. Kinetics of intermetallic phase formation at the interface of Sn-Ag-Cu-X (X = Bi, In) solders with Cu substrate. J. Alloys Compd. 2011, 509, 7052–7059. [Google Scholar] [CrossRef]
- Lejuste, C.; Hodaj, F.; Petit, L. Solid state interaction between a Sn-Ag-Cu-In solder alloy and Cu substrate. Intermetallics 2013, 36, 102–108. [Google Scholar] [CrossRef]
- Wu, A.T.; Chen, M.-H.; Siao, C.-N. The Effects of Solid-State Aging on the Intermetallic Compounds of Sn-Ag-Bi-In Solders on Cu Substrates. J. Electron. Mater. 2008, 38, 252–256. [Google Scholar] [CrossRef]
- Kanlayasiri, K.; Sukpimai, K. Effects of indium on the intermetallic layer between low-Ag SAC0307-xIn lead-free solders and Cu substrate. J. Alloys Compd. 2016, 668, 169–175. [Google Scholar] [CrossRef]
- Wang, F.; Ma, X.; Qian, Y. Improvement of microstructure and interface structure of eutectic Sn–0.7Cu solder with small amount of Zn addition. Scr. Mater. 2005, 55, 699–702. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Tu, C.; Kao, C. Effects of minor Fe, Co, and Ni additions on the reaction between SnAgCu solder and Cu. J. Alloys Compd. 2009, 478, 121–127. [Google Scholar] [CrossRef]
- Dong, W.; Shi, Y.; Xia, Z.; Lei, Y.; Guo, F. Effects of Trace Amounts of Rare Earth Additions on Microstructure and Properties of Sn-Bi-Based Solder Alloy. J. Electron. Mater. 2008, 37, 982–991. [Google Scholar] [CrossRef]
- Song, Q.; Yang, W.; Li, Y.; Mao, J.; Qin, W.; Zhan, Y. Interfacial reaction and mechanical properties of Sn58Bi-XCr solder joints under isothermal aging conditions. Vacuum 2021, 194, 110559. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, B.; Xue, Y.; Zhou, M.; Cao, R.; Chen, P.; Zeng, X. Microstructure and mechanical behavior of SnBi-xAg and SnBi-xAg@P-Cu solder joints during isothermal aging. Microelectron. Reliab. 2021, 127, 114388. [Google Scholar] [CrossRef]
- Tang, Y.; Li, G.Y.; Chen, D.Q.; Pan, Y.C. Influence of TiO2 nanoparticles on IMC growth in Sn-3.0Ag-0.5Cu-xTiO2 solder joints during isothermal aging process. J. Mater. Sci. Mater. Electron. 2014, 25, 981–991. [Google Scholar] [CrossRef]
- Yang, L.; Quan, S.; Liu, C.; Shi, G. Aging resistance of the Sn-Ag-Cu solder joints doped with Mo nanoparticles. Mater. Lett. 2019, 253, 191–194. [Google Scholar] [CrossRef]
- Zeng, G.; McDonald, S.; Mu, D.; Terada, Y.; Yasuda, H.; Gu, Q.; Salleh, M.M.; Nogita, K. The influence of ageing on the stabilisation of interfacial (Cu,Ni)6(Sn,Zn)5 and (Cu,Au,Ni)6Sn5 intermetallics in Pb-free Ball Grid Array (BGA) solder joints. J. Alloys Compd. 2016, 685, 471–482. [Google Scholar] [CrossRef]
- Hasnine, M.; Vahora, N. Microstructural and mechanical behavior of SnCu–Ge solder alloy subjected to high temperature storage. J. Mater. Sci. Mater. Electron. 2018, 29, 8904–8913. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, S.; Li, Z.; Hou, C.; Li, G. Morphological Evolution and Growth Kinetics of Interfacial Cu6Sn5 and Cu3Sn Layers in Low-Ag Sn-0.3Ag-0.7Cu-xMn/Cu Solder Joints During Isothermal Ageing. J. Electron. Mater. 2018, 47, 5913–5929. [Google Scholar] [CrossRef]
- Mahdavifard, M.H.; Sabri, M.F.M.; Said, S.M.; Rozali, S. High stability and aging resistance Sn-1Ag-0.5Cu solder alloy by Fe and Bi minor alloying. Microelectron. Eng. 2019, 208, 29–38. [Google Scholar] [CrossRef]
- Wang, Y. Effects of surface diffusion and solder volume on porous-type Cu3Sn in Cu/Sn/Cu microjoints. Mater. Chem. Phys. 2021, 275, 125307. [Google Scholar] [CrossRef]
- Park, H.-P.; Seo, G.; Kim, S.; Kim, Y.-H. Effects of Solder Volume and Reflow Conditions on Self-Alignment Accuracy for Fan-Out Package Applications. J. Electron. Mater. 2018, 47, 133–141. [Google Scholar] [CrossRef]
- Sriperumbudur, S.S.; Meilunas, M.; Anselm, M. Solder paste volume effects on assembly yield and reliability for bottom terminated components. Solder. Surf. Mt. Technol. 2017, 29, 99–109. [Google Scholar] [CrossRef]
- Wang, S.; Yao, Y.; Long, X. Critical Review of Size Effects on Microstructure and Mechanical Properties of Solder Joints for Electronic Packaging. Appl. Sci. 2019, 9, 227. [Google Scholar] [CrossRef]
- Yang, S.C.; Chang, C.C.; Tsai, M.H.; Kao, C.R. Effect of Cu concentration, solder volume, and temperature on the reaction between SnAgCu solders and Ni. J. Alloys Compd. 2010, 499, 149–153. [Google Scholar] [CrossRef]
- Chang, C.; Lin, Y.; Wang, Y.; Kao, C. The effects of solder volume and Cu concentration on the consumption rate of Cu pad during reflow soldering. J. Alloys Compd. 2010, 492, 99–104. [Google Scholar] [CrossRef]
- Azlina, O.S.; Ourdjini, A.; Amrin, A.; Siti Rabiatull Aisha, I. Effect of Solder Volume on Interfacial Reaction between SAC405 Solders and EN (B) EPIG Surface Finish. In Advanced Materials Research. Trans. Tech. Publ. 2014, 845, 76–80. [Google Scholar]
- Liu, L.; Huang, M.; Yang, F.; Liu, T. Effect of solder volume on interfacial reaction between Sn-3Ag-0.5Cu solder ball and Cu Pad after multiple reflows. In Proceedings of the 2010 11th International Conference on Electronic Packaging Technology & High Density Packaging; Institute of Electrical and Electronics Engineers (IEEE), Shanghai, China, 8–11 August 2011; pp. 1–6. [Google Scholar]
- Ourdjini, A.; Hanim, M.A.; Koh, S.J.; Aisha, I.S.; Tan, K.; Chin, Y. Effect of Solder Volume on Interfacial Reactions between Eutectic Sn-Pb and Sn-Ag-Cu Solders and Ni(P)-Au Surface Finish. In Proceedings of the 2006 Thirty-First IEEE/CPMT International Electronics Manufacturing Technology Symposium; Institute of Electrical and Electronics Engineers (IEEE), Petaling Yaya, Malaysia, 8–10 November 2006; pp. 437–442. [Google Scholar]
- Huang, M.; Yang, F.; Zhao, N.; Liu, X. (Invited) Solder Volume Effect on Interfacial Reaction between Sn-3.0Ag-0.5Cu Solder Balls and Cu Substrates-Experiment and Simulation. ECS Trans. 2013, 52, 753–758. [Google Scholar] [CrossRef]


| Solder Alloy | Element | Thickness of IMC | Ref |
|---|---|---|---|
| Sn-Ag-0.5Cu | Fe | Unchanged | [49] |
| Sn-3.0Ag-0.5Cu | Sb | Unchanged | [38] |
| Sn-3.0Ag-0.5Cu | Fe | Unchanged | [38] |
| Sn-3.0Ag-0.5Cu | In | Unchanged | [38] |
| Sn-3.0Ag-0.5Cu | Ge | Increase | [38] |
| Sn-3.9Ag-0.7Cu | La | Decrease | [47] |
| Sn-3.8Ag-0.7Cu | Nd | Decrease | [46] |
| Sn-2.8Ag-0.5Cu | Bi | Unchanged | [52] |
| Mo | Decrease | [50] | |
| Sn-3.8Ag-0.7Cu | Co | Decrease | [51] |
| Zn | Decrease | [35] | |
| Sn-0.5Ag-0.7Cu | Ga | Decrease | [45] |
| Sn-3.0Ag-0.5Cu | Co | Decrease | [44] |
| Sn-3.8Ag-0.7Cu | Zn | Decrease | [36] |
| Sn-4Ag | Zn | Decrease | [34] |
| Sn-0.7Cu | Al | Decrease | [43] |
| Sn-0.5Cu | Al | Decrease | [53] |
| Sn-0.7Cu | P | Increased | [42] |
| Sn-0.7Cu | In, Cr and Ni | Decrease | [39] |
| Sn-0.7Cu | Ni | Decrease | [26] |
| Sn-0.7Cu | Ni | Decrease | [30] |
| Sn-Cu | Ni | Decrease | [24] |
| Sn-0.7Cu-0.05Ni | Bi | Unchanged | [54] |
| Sn-0.7Cu-0.2Ni | In | Increased | [40] |
| Surface Finish | Solder Alloy | IMC Thickness, µm | IMC Formation | Reflow Peak Temperature, °C | Dwell Time, Min | Ref |
|---|---|---|---|---|---|---|
| Cu-OSP | Sn-xAg-0.5Cu (x = 3.0, 4.0) | 4–5 | Cu6Sn5, Ag3Sn, Cu3Sn | 230 | 20 | [70] |
| Sn-1.0Ag-0.5Cu | 3–5 | Cu6Sn5, Ag3Sn, Cu3Sn | 250 | - | [71] | |
| Sn-3.0Ag-0.5Cu | 2.5–3 | Cu6Sn5, Ag3Sn, Cu3Sn | 300 | - | [72] | |
| Sn-3.8Ag-0.7Cu | 1.0–2.3 | Cu6Sn5, Ag3Sn, Cu3Sn | 270 | 2 | [51] | |
| Sn-3.8Ag-0.7Cu | 2.0 | Cu6Sn5, Ag3Sn, Cu3Sn | 244 | 1 | [61] | |
| ENIG | Sn-4.0Ag-0.5Cu | <2.0 | Ni3Sn4, Ni3Sn2, Cu6Sn5, (Cu, Ni)6Sn5 Ag3Sn, (Ni, Cu)3Sn4, Ag3Sn | 250 | 1 | [62] |
| Sn-3.5Ag-0.7Cu | <2.0 | Ni3Sn4, Ni3Sn2, Cu6Sn5, (Cu, Ni)6Sn5, Ag3Sn, (Ni, Cu)3Sn4, Ag3Sn | 255 | 1 | [73] | |
| Sn-3.0Ag-0.5Cu | 1.5–2 | Ni3Sn4, Ni3Sn2, Cu6Sn5, (Cu, Ni)6Sn5, Ag3Sn, (Ni, Cu)3Sn4, Ag3Sn | 250 | - | [74] | |
| Sn-3.0Ag-0.5Cu | 2–3 | Ni3Sn4, Ni3Sn2, Cu6Sn5, (Cu, Ni)6Sn5, Ag3Sn, (Ni, Cu)3Sn4, Ag3Sn | 245 | 1 | [75] | |
| ENEPIG | Sn-3.0Ag-0.5Cu | 2.3 | Ni3Sn4, Ni3Sn2, PdSn4, AuSn4, Cu6Sn5, (Cu, Ni)6Sn5, Ag3Sn, (Ni, Cu)3Sn4 | 250 | 1 | [76] |
| Sn-3.0Ag-0.5Cu | 1.3–2.5 | Ni3Sn4, Ni3Sn2, PdSn4, AuSn4, Cu6Sn5, (Cu, Ni)6Sn5, Ag3Sn, (Ni, Cu)3Sn4 | 260 | 1 | [63] | |
| Sn-4.0Ag-0.5Cu | 1.0–2.5 | Ni3Sn4, Ni3Sn2, PdSn4, AuSn4, Cu6Sn5, (Cu, Ni)6Sn5, Ag3Sn, (Ni, Cu)3Sn4 | 230 | - | [77] | |
| Im-Ag | Sn-3.0Ag-0.5Cu | 2–3 | Cu6Sn5, Cu3Sn, Ag3Sn | 250 | 1 | [78] |
| Sn-3.8Ag-0.7Cu-0.15Ni- 1.4Sb-3.0Bi, Sn-3.4Ag- 0.5Cu-3.3Bi, and Sn-3.8Ag- 0.8Cu-3.0Bi. | 10–12 | Cu6Sn5, Cu3Sn, Ag3Sn | 250 | 1 | [79] |
| Solder | Element | Aging Temperature, °C | Aging Time, h | Rate Constant of IMC Growth (µm/Day) | IMC Formation | Ref |
|---|---|---|---|---|---|---|
| Sn-3.0Ag-0.4Cu | In | 100–180 | 1506 | 0.13 | Cu6Sn5, Ag3Sn, Cu3Sn | [87] |
| Sn-3Ag-3Bi | In | 120, 150 and 180 | 960 | 0.2 | Cu6Sn5, Ag3Sn, Cu3Sn | [88] |
| Sn-2.8Ag-0.5Cu | Bi | 150 | 336 | 0.5 | Cu6Sn5, Ag3Sn, Cu3Sn | [52] |
| Sn-58Bi | Ce, La | 80 | 168 | 0.5–0.79 | Cu6Sn5, Cu3Sn | [92] |
| Sn-58Bi | Cr | 100 | 240 | 0.19 | Cu6Sn5, Cu3Sn | [93] |
| Sn-Bi | Ag | 100 | 600 | 0.1 | Cu6Sn5, Cu3Sn, Ag3Sn | [94] |
| Sn-2.5Ag-0.8Cu | Fe, Co and Ni | 160 | 2000 | 0.44 | Cu6Sn5, Ag3Sn, Cu3Sn | [91] |
| Sn-3.0Ag-0.5Cu | TiO2 | 190 | 720 | 0.37 | Cu6Sn5, Ag3Sn, Cu3Sn | [95] |
| Sn-0.7Cu | Ni | 170 | 2400 | 0.09 | Cu6Sn5, Cu3Sn, (Ni, Cu)6Sn5 | [85] |
| Sn-3.0Ag-0.5Cu | Bi and Er | 150 | 500 | 0.14 | Cu6Sn5, Ag3Sn, Cu3Sn | [3] |
| Sn-3.7Ag-0.7Cu | Bi and In | 150 | 400 | 2.5 | Cu6Sn5, Ag3Sn, Cu3Sn, (Ni, Cu)3Sn4, | [86] |
| Sn-3.0Ag-0.5Cu | Mo | 180 | 480 | 0.7 | Cu6Sn5, Ag3Sn, Cu3S | [96] |
| Sn-0.7Cu | Zn | 150 | 480 | 0.35 | Cu6Sn5, Ag3Sn, Cu3Sn, CuZn, Cu5Zn8 | [90] |
| Sn-0.7Cu-0.06Zn | Ni | 150 | 500 | 0.25 | Cu6Sn5, Ag3Sn, Cu3Sn, (Ni, Cu)3Sn4, CuZn, (Ni, Cu)6Sn5 | [97] |
| Sn99.3Cu0.7 | Ge | 150 | 720 | 0.22 | Cu6Sn5, Cu3Sn | [98] |
| Sn-0.3Ag-0.7Cu | Mn | 190 | 1152 | 0.10 | Cu6Sn5, Ag3Sn, Cu3Sn | [99] |
| Sn-1.0Ag-0.5Cu | Fe and Bi | 125 | 720 | 0.23 | Cu6Sn5, Ag3Sn, Cu3Sn, FeSn2 | [100] |
| Sn-4.0Ag-0.5Cu | Bi and Ni | 175 | 2000 | 0.3 | Cu6Sn5, Ag3Sn, Cu3Sn, (Ni, Cu)3Sn4, (Ni, Cu)6Sn5 | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramli, M.I.I.; Salleh, M.A.A.M.; Abdullah, M.M.A.B.; Zaimi, N.S.M.; Sandu, A.V.; Vizureanu, P.; Rylski, A.; Amli, S.F.M. Formation and Growth of Intermetallic Compounds in Lead-Free Solder Joints: A Review. Materials 2022, 15, 1451. https://doi.org/10.3390/ma15041451
Ramli MII, Salleh MAAM, Abdullah MMAB, Zaimi NSM, Sandu AV, Vizureanu P, Rylski A, Amli SFM. Formation and Growth of Intermetallic Compounds in Lead-Free Solder Joints: A Review. Materials. 2022; 15(4):1451. https://doi.org/10.3390/ma15041451
Chicago/Turabian StyleRamli, Mohd Izrul Izwan, Mohd Arif Anuar Mohd Salleh, Mohd Mustafa Al Bakri Abdullah, Nur Syahirah Mohamad Zaimi, Andrei Victor Sandu, Petrica Vizureanu, Adam Rylski, and Siti Farahnabilah Muhd Amli. 2022. "Formation and Growth of Intermetallic Compounds in Lead-Free Solder Joints: A Review" Materials 15, no. 4: 1451. https://doi.org/10.3390/ma15041451
APA StyleRamli, M. I. I., Salleh, M. A. A. M., Abdullah, M. M. A. B., Zaimi, N. S. M., Sandu, A. V., Vizureanu, P., Rylski, A., & Amli, S. F. M. (2022). Formation and Growth of Intermetallic Compounds in Lead-Free Solder Joints: A Review. Materials, 15(4), 1451. https://doi.org/10.3390/ma15041451









