Synthesis and Characterization of High-Purity Mesoporous Alumina with Excellent Adsorption Capacity for Congo Red
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Preparation of Mesoporous Alumina Materials
2.3. Characterization of Alumina Materials
- M0: the individual impurities mass ratio of industrial Al(OH)3 shown in Table 1.
- Mt: the individual impurity’s mass ratio of alumina product.
- R: the individual impurity’s removal rate.
2.4. Adsorption Experiments on HPMA Materials
- C0: the initial concentration of Congo Red (mg L−1)
- Ct: the mass content of Congo Red for the corresponding time (mg L−1)
- V: the volume of solution (L)
- m: mass of HPMA materials (g)
- qt: mass of adsorbent adsorbed per unit mass of adsorbent at time t
- C0: the initial concentration of Congo Red (mg L−1)
- Ce: the mass content of Congo Red at adsorption equilibrium (mg L−1)
- V: the volume of solution (L)
- m: mass of HPMA materials (g)
- qm: the maximum adsorbed mass
3. Results and Discussion
3.1. Effects of Various Factors on the Mesoporous Alumina Product
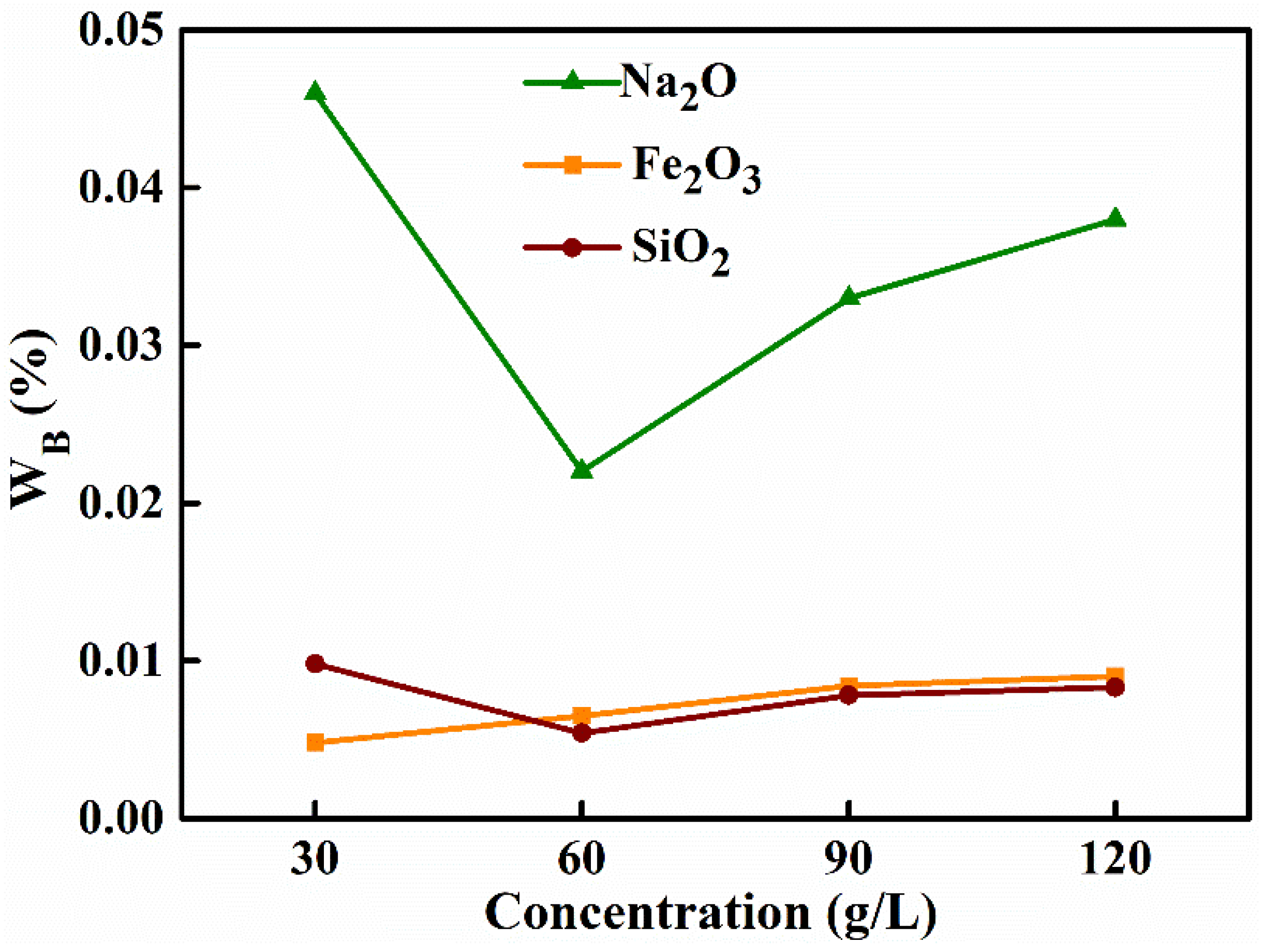
3.1.1. Initial Concentration of NaAl(OH)4 Solution
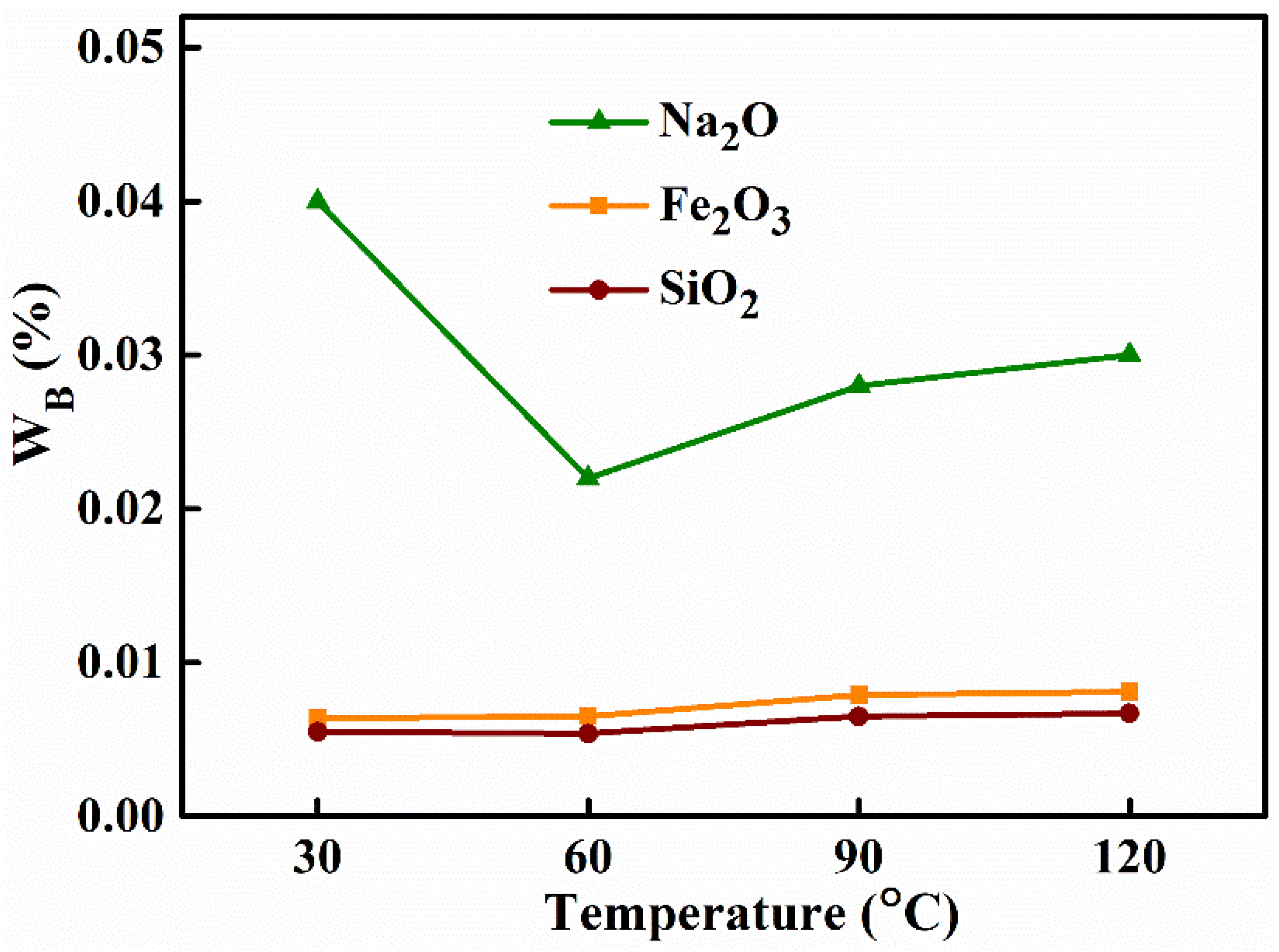
3.1.2. Aging Temperature
3.1.3. Aging Time
3.1.4. Type of Ammonium Salt
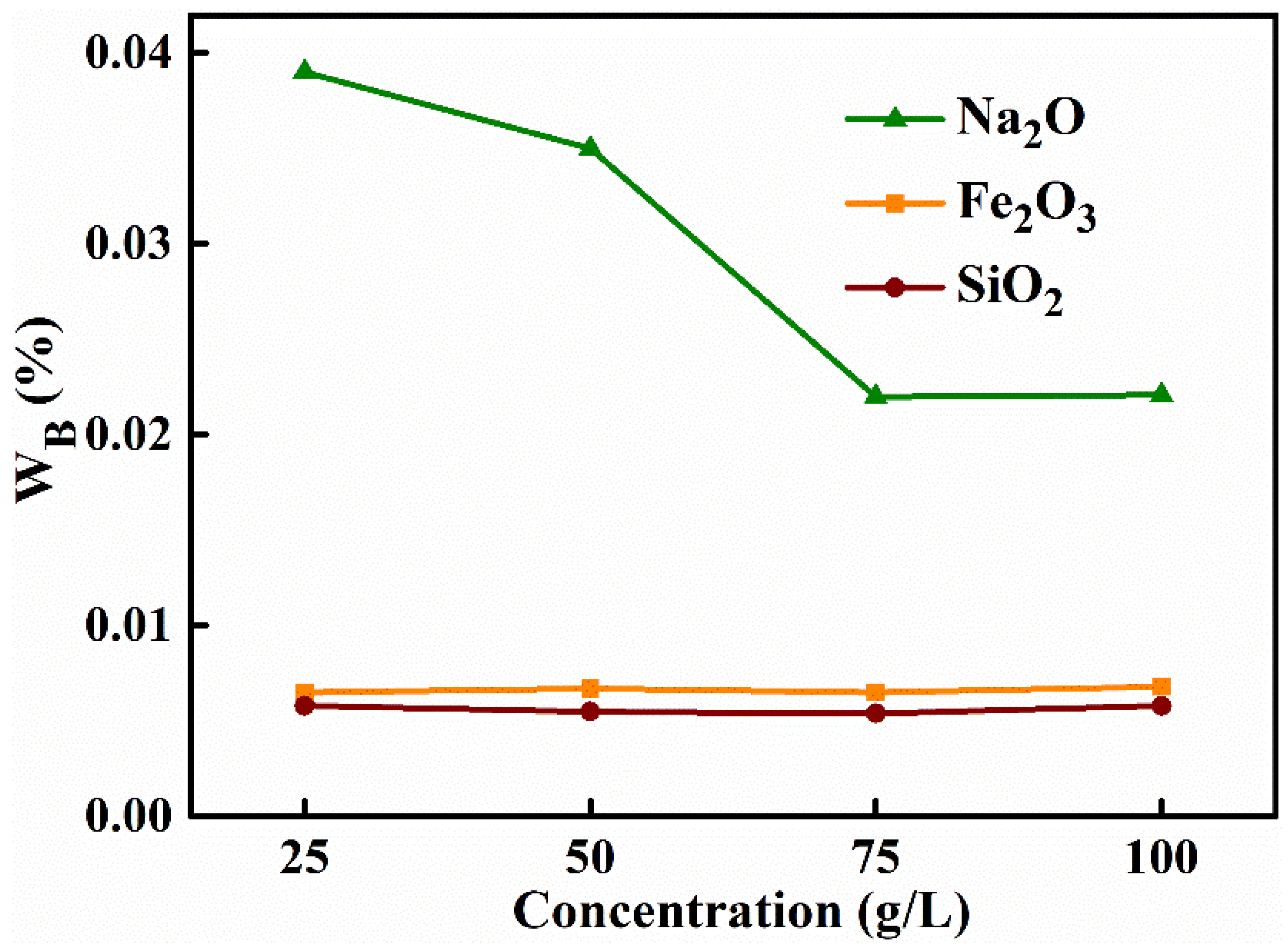
3.1.5. Mass Concentration of (NH4)2CO3 Solution
3.1.6. Roasting Temperature
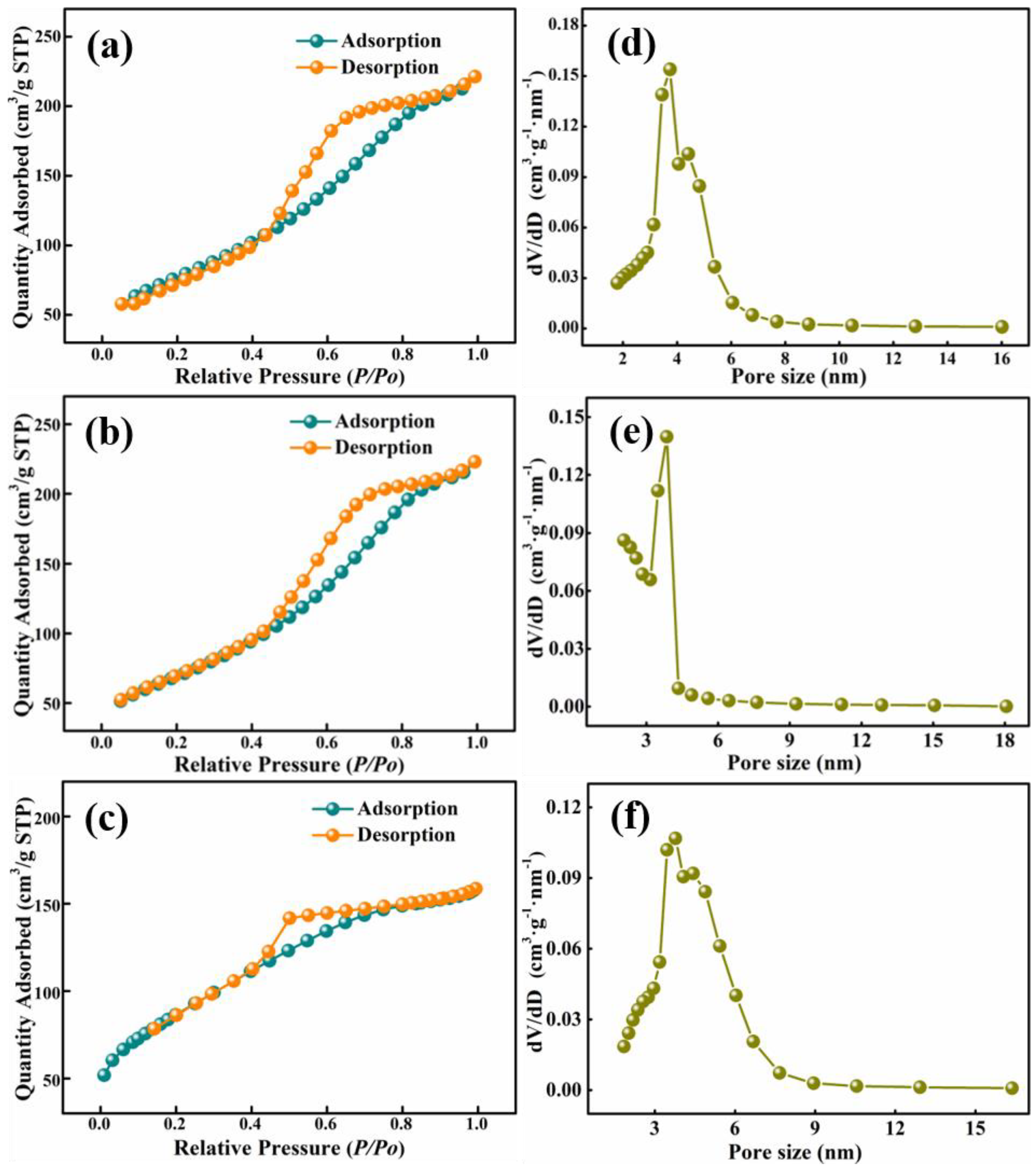
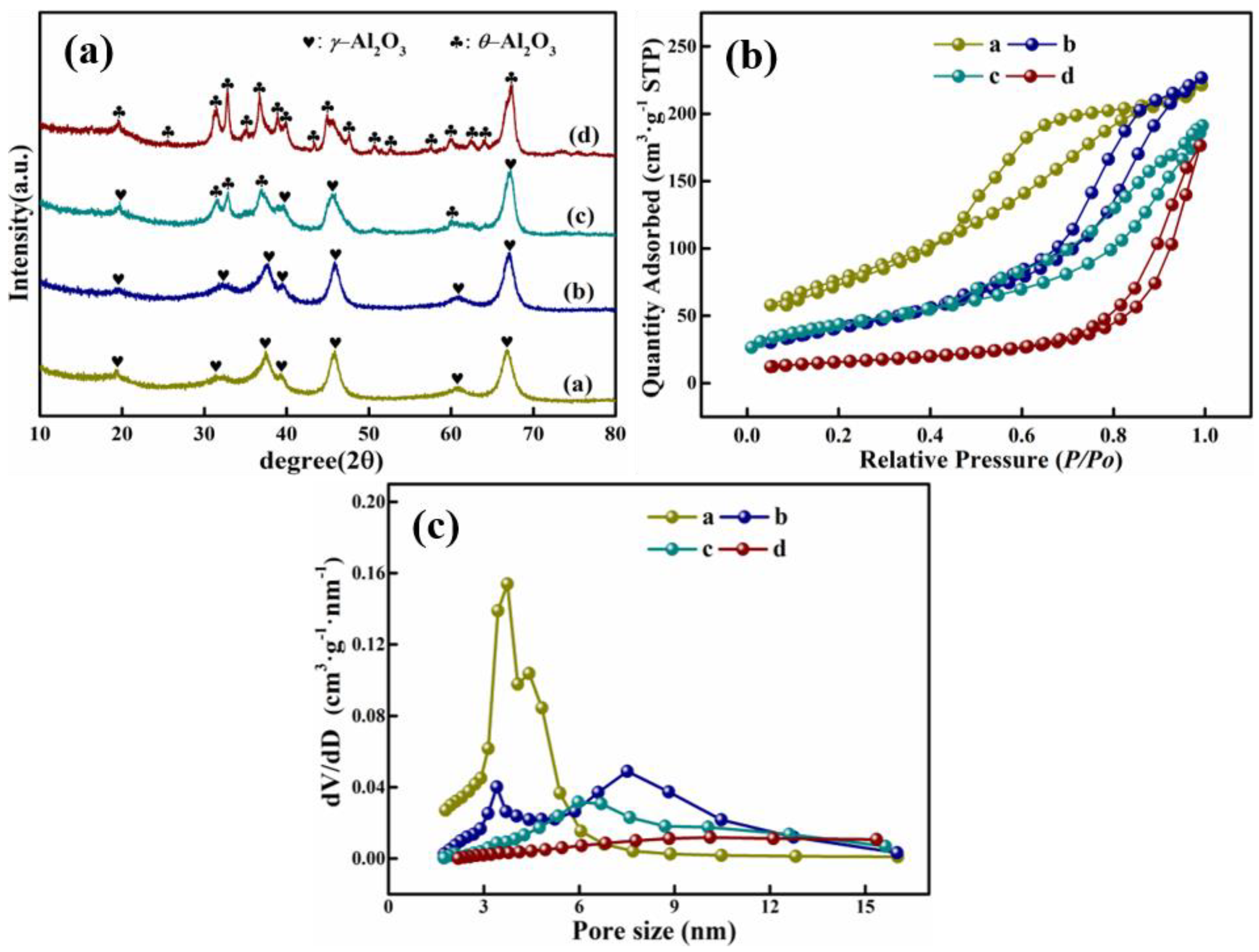
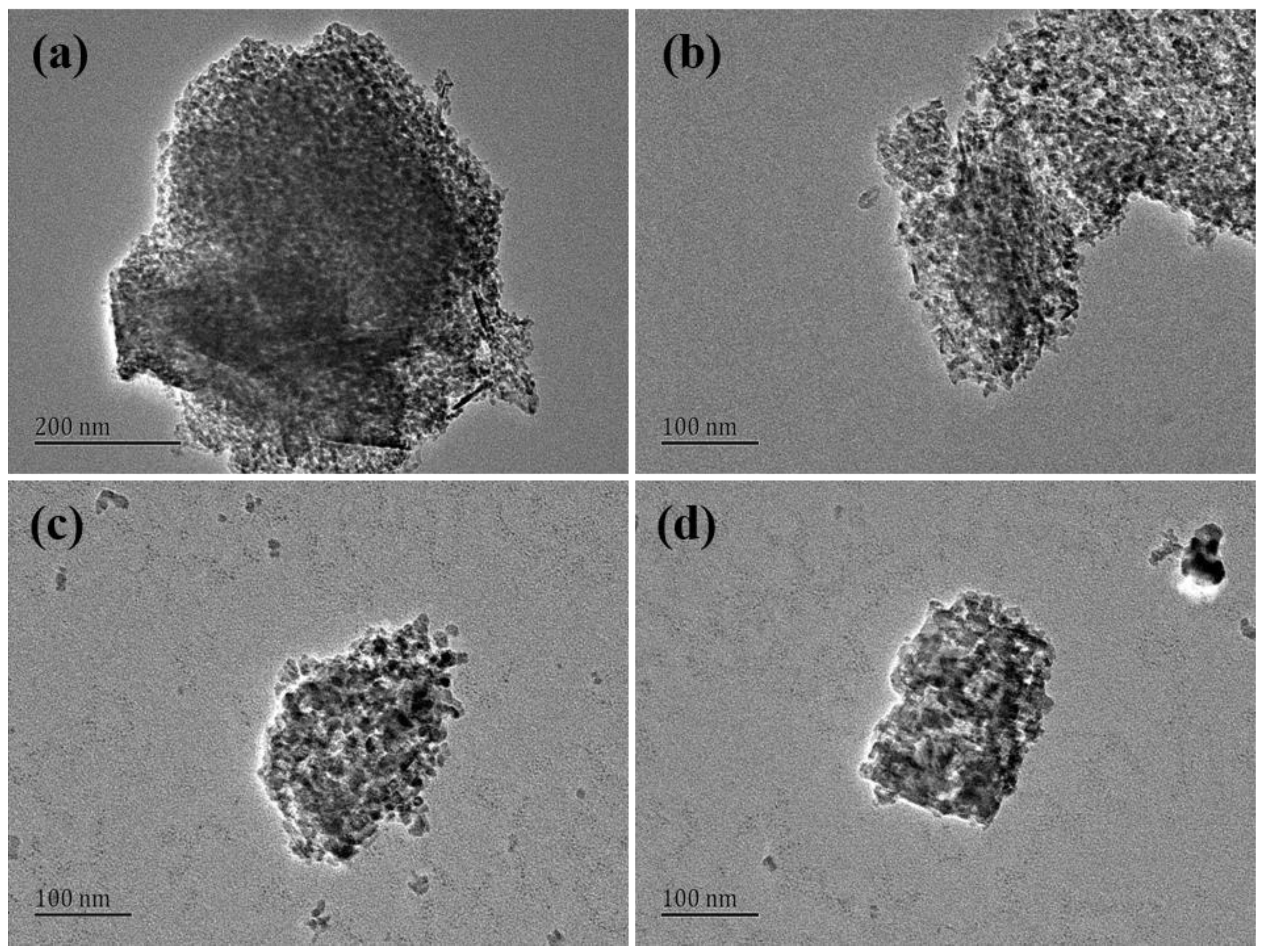
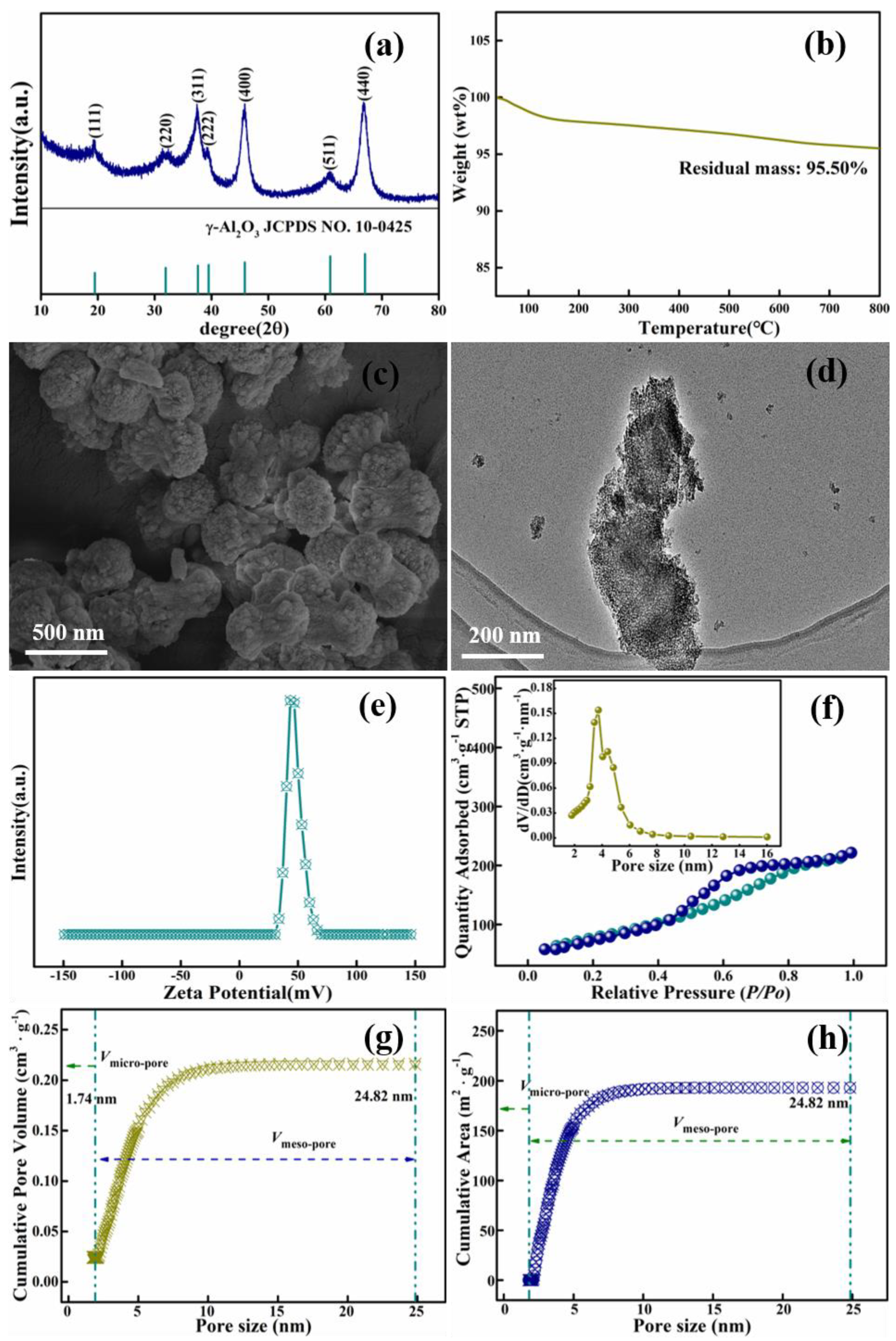
3.2. Characteristics of High Purity Mesoporous Alumina
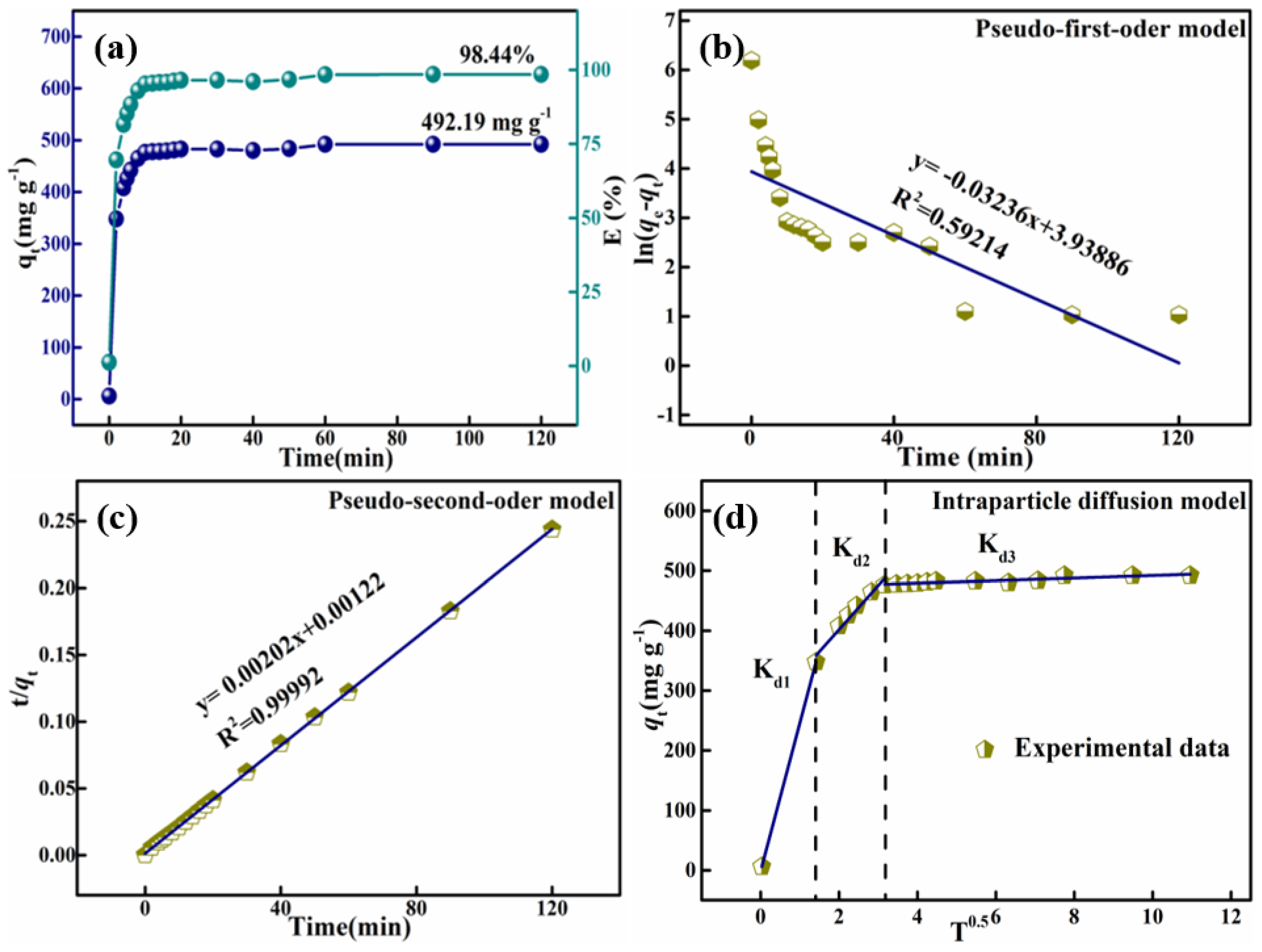
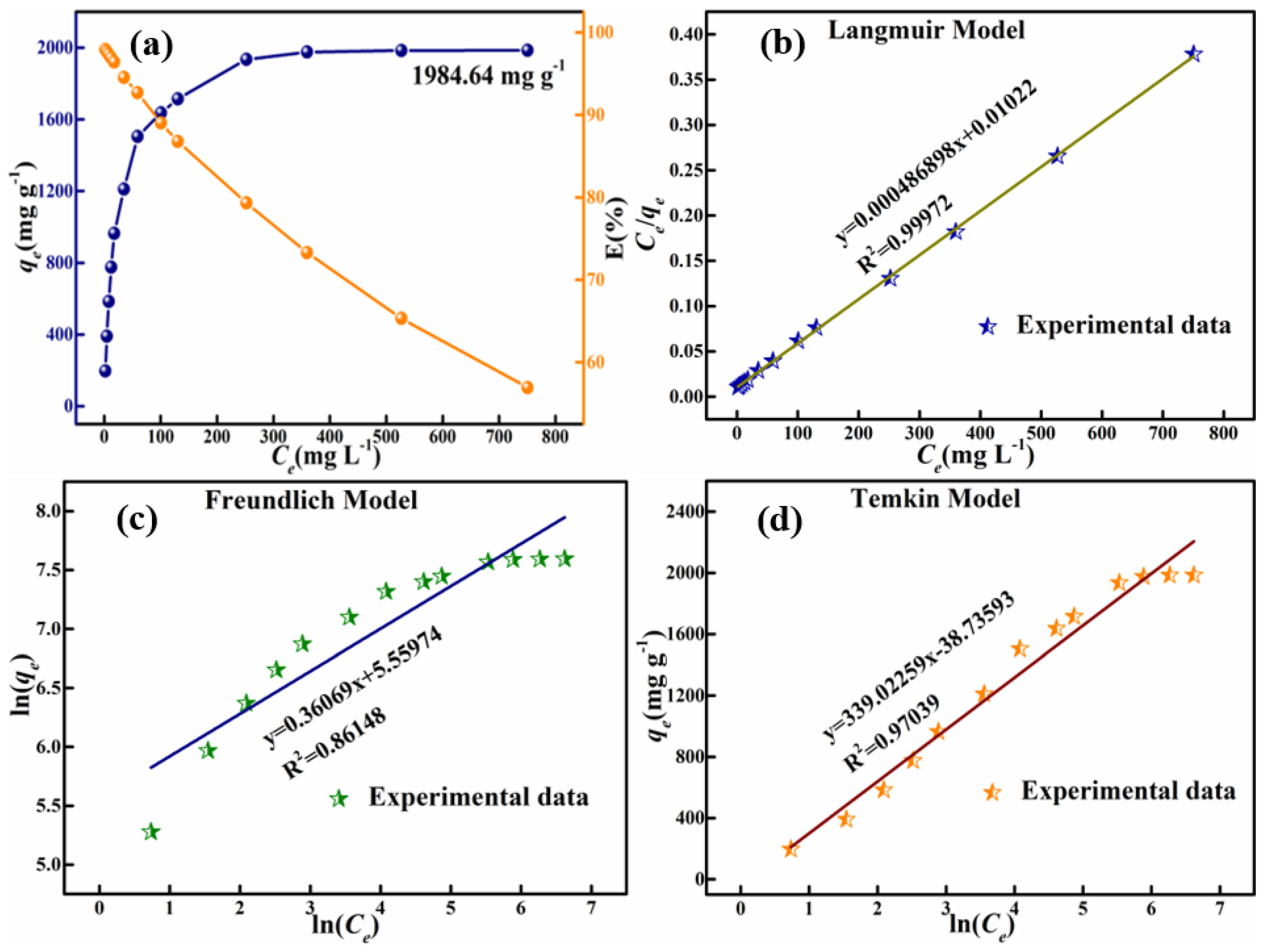
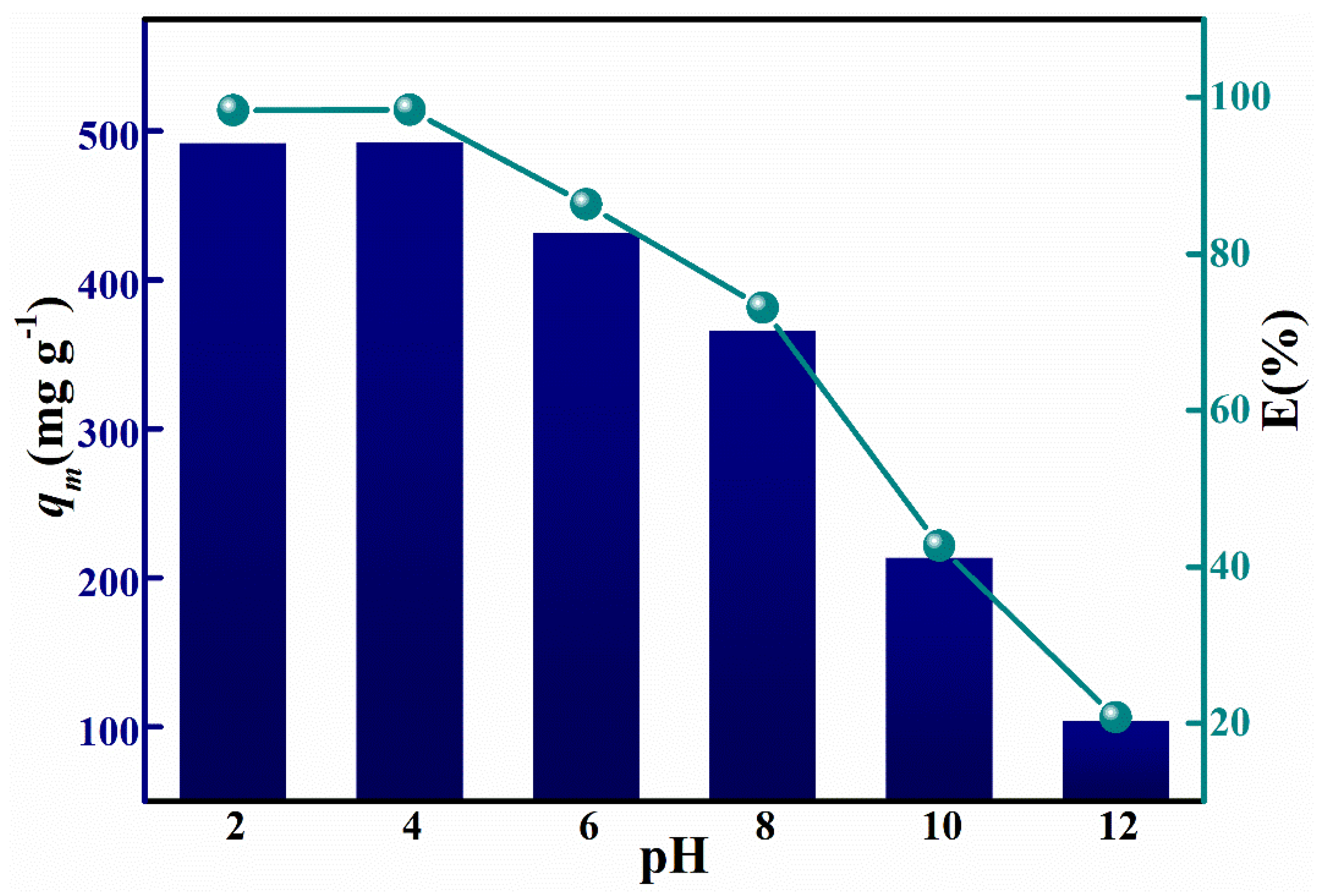
3.3. HPMA Materials Adsorption of Congo Red Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, M.; Hutchings, G.J.; Willock, D.J.; Scott, J.; Taylor, S.H. Zinc promoted alumina catalysts for the fluorination of chlor-ofluorocarbons. J. Catal. 2018, 364, 102–111. [Google Scholar] [CrossRef]
- Wang, F.; Chen, P.; Li, X.; Zhu, B.Q. Effect of micro-spinel powders addition on the microstructure and properties of alumina refractory castables. Ceram. Int. 2019, 45, 2989–2999. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, S.; Liu, Y.; Fan, X.; Zhang, M.L.; Zhai, J.; Jiang, L. Self-Assembled porphyrin nanofiber membrane-decorated alumina channels for enhanced photoelectric response. ACS Nano 2018, 12, 11169–11177. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, K.; Liu, T.T.; Liao, W.H.; Zhang, C.D.; Shao, H. Cracks of alumina ceramics by selective laser melting. Ceram. Int. 2019, 45, 175–184. [Google Scholar] [CrossRef]
- Kumari, U.; Behera, S.K.; Meikap, B.C. A novel acid modified alumina adsorbent with enhanced defluoridation property: Kinetics, isotherm study and applicability on industrial wastewater. J. Hazard. Mater. 2019, 365, 868–882. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Tang, D.; D’Acierno, F.; Michal, C.A.; MacLachlan, M.J. Biotemplated light weight γ-Alumina aerogels. Chem. Mater. 2018, 30, 1602–1609. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, X.; Lin, W.; Michal, C.A.; MacLachlan, M.J. Adsorption property and catalytic performance over ordered mesoporous phosphorus-doped Pd-alumina catalysts. Powder Technol. 2018, 338, 869–877. [Google Scholar] [CrossRef]
- Busca, G. The surface of transitional aluminas: A critical review. Catal. Today 2014, 226, 2–13. [Google Scholar] [CrossRef]
- Maruoka, H.; Tomita, A.; Zheng, L.; Kimura, T. Mesopore connectivity improving aerosol-assisted synthesis of mesoporous alumina powders with high surface area. Langmuir 2018, 34, 13781–13787. [Google Scholar] [CrossRef]
- Liu, S.; Liang, X.; Zhang, J.; Chen, B. Temperature sensitive synthesis of γ-Al2O3 support with different morphologies for CoMo/γ-Al2O3 catalysts for hydrodesulfurization of thiophene and 4,6-dimethyldibenzothiophene. Catal. Sci. Technol. 2017, 7, 466–480. [Google Scholar] [CrossRef]
- Wang, X.H.; Ni, H.H.; Cao, Y.; Li, G.; Gao, H.; Yang, J.L. The Reaction Kinetics of Aluminum and Isopropanol. Appl. Mech. Mater. 2012, 164, 21–25. [Google Scholar] [CrossRef]
- Smith, P.; Power, G. High purity alumina-current and future production. Miner. Processing Extr. Metall. Rev. 2021. [Google Scholar] [CrossRef]
- Lee, G.; Chen, C.; Yang, S.T.; Ahn, W.S. Enhanced adsorptive removal of fluoride using mesoporous alumina. Microporous Mesoporous Mater. 2010, 127, 152–156. [Google Scholar] [CrossRef]
- Nalivaiko, A.Y.; Ozherelkov, D.Y.; Pak, V.I.; Kirov, S.S.; Arnautov, A.N.; Gromov, A.A. Preparation of aluminum hydroxide during the synthesis of high purity alumina via aluminum anodic oxidation. Metall. Mater. Trans. B-Prcocess Metall. Mater. Processing Sci. 2020, 51, 1154–1161. [Google Scholar] [CrossRef]
- Kim, S.W.; Jung, Y.M. Nanocrystalline particle coatings on a-alumina powders by a carbonate precipitation and thermal-assisted combustion route. J. Nanosci. Nanotechnol. 2007, 11, 3906–3909. [Google Scholar] [CrossRef] [PubMed]
- Yong-Taeg, O.; Kim, S.W.; Shin, D.C. Fabrication and synthesis of alpha-alumina nanopowders by thermal decomposition of ammonium aluminum carbonate hydroxide (AACH). Colloids Surf. A Physicochem. Eng. Asp. 2008, 313, 415–418. [Google Scholar]
- Ambaryan, G.N.; Vlaskin, M.S.; Buryakovskaya, O.A.; Kislenko, S.; Zhuk, A.; Shkolnikov, E.; Arnautov, A.; Zmanovsky, S.; Osipenkova, A.; Tarasov, V.; et al. Advanced manufacturing process of ultrahigh-purity α-Al2O3. Sustain. Mater. Technol. 2018, 17, e00065. [Google Scholar] [CrossRef]
- Bae, H.C.; Lee, S.J. Study on the fabrication processing of high-purity, high-density alumina green body prepared by a hydrothermal method. J. Ceram. Processing Res. 2015, 16, 319–323. [Google Scholar]
- Suchanek, W.L. Hydrothermal Synthesis of Alpha Alumina (alpha-Al2O3) Powders: Study of the Processing Variables and Growth Mechanisms. J. Am. Ceram. Soc. 2010, 93, 399–412. [Google Scholar] [CrossRef]
- Popp, U.; Herbig, R.; Michel, G.; Muller, E.; Oeatreich, C. Properties of nanocrystalline ceramic powders prepared by laser evaporation and recondensation. J. Eur. Ceram. Soc. 1998, 18, 1153–1160. [Google Scholar] [CrossRef]
- Gautam, R.; Mudhoo, A.; Chattopadhyaya, M. Kinetic, equilibrium, thermodynamic tudies and spectroscopic analysis of Alizarin Red S removal by adsorption onto ustard husk. J. Environ. Chem. Eng. 2013, 1, 1283–1291. [Google Scholar] [CrossRef]
- Roosta, M.; Ghaedi, M.; Daneshfar, A.; Sahraei, R. Experimental design-based response surface methodology optimization of ultrasonic assisted adsorption of safaranin O by tin sulfide nanoparticle loaded on activated carbon. Spectrochim. Acta 2014, 122, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Lee, M.; Woo, S. Adsorption of congo red by chitosan hydrogel beads impregnated with carbon nanotubes. Bioresour. Technol. 2010, 101, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Soylak, M.; Yilmaz, E.; Ghaedi, M.; Montazerozohori, M. Cloud point extraction and flame atomic absorption spectrometry determination of lead (II) in environmental and food samples. J. AOAC Int. 2012, 95, 1797–1802. [Google Scholar] [CrossRef]
- Espergham, O.; Ghaedi, M.; Niknam, K.; Kokhdan, S.N. A cloud point extraction methodology for the determination of trace amounts of copper, cobalt, zinc and manganese by flame atomic absorption spectrometry using a new imidazole derivative. Fresenius Environ. Bullet. 2011, 20, 2350–2356. [Google Scholar]
- Ghaedi, M.; Niknam, K.; Niknam, E.; Soylak, M. Application of cloud point extraction for copper, nickel, zinc and iron ions in environmental samples. J. Chin. Chem. Soc. 2013, 56, 981–986. [Google Scholar] [CrossRef]
- Ghaedi, M.; Shokrollahi, A.; Niknam, K.; Niknam, E.; Najibi, A.; Soylak, M. Cloud point extraction and flame atomic absorption spectrometric determination of cadmium (II), lead (II), palladium (II) and silver(I) in environmental samples. J. Hazard. Mater. 2009, 168, 1022–1027. [Google Scholar] [CrossRef]
- Chafi, M.; Gourich, B.; Essadki, A.; Vial, L.; Fabregat, A. Comparison of electrocoagulation using iron and aluminium electrodes with chemical coagulation for the removal of a highly soluble acid dye. Desalination 2011, 281, 285–292. [Google Scholar] [CrossRef]
- Vijayaraghavan, G.; Shanthakumar, S. Performance study on algal alginate as natural coagulant for the removal of Congo red dye. Desal. Water Treat. 2016, 57, 6384–6392. [Google Scholar] [CrossRef]
- Munagapati, V.; Kim, D. Equilibrium isotherms, kinetics, and thermodynamics studies for Congo Red adsorption using calcium alginate beads impregnated with nano-goethite. Ecotox. Environ. Saf. 2017, 141, 226–234. [Google Scholar] [CrossRef]
- Omran, A.; Baiee, M.; Juda, S. Removal of Congo red dye from aqueous solution using a new adsorbent surface developed from aquatic plant (Phragmites australis). Int. J. ChemTech Res. 2016, 9, 334–342. [Google Scholar]
- Satheesh, R.; Vignesh, K.; Rajarajan, M.; Suganthi, A.; Sreekantan, S.; Knag, M.; Kwak, B.S. Removal of Congo red from water using quercetin modified α-Fe2O3 nanoparticles as effective nanoadsorbent. Mater. Chem. Phys. 2016, 180, 53–65. [Google Scholar] [CrossRef]
- Solísabbba, M. Microbial decolouration of azo dyes: A review. Process Biochem. 2012, 47, 1723–1748. [Google Scholar]
- Liu, M.; Chen, Q.; Lu, K.; Huang, W.; Lu, Z.; Zhou, C.; Yu, S.; Gao, C. High efficient removal of dyes from aqueous solution through nanofiltration using diethanolamine-modified polyamide thin-film composite membrane. Sep. Pur. Technol. 2017, 173, 135–143. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, C.; Cheng, B.; Yu, J.; Jaroniec, M. Rattle-type carbon-alumina core-shell spheres: Synthesis and application for adsorption of organic dyes. ACS Appl. Mater. Inter. 2012, 4, 2174–2179. [Google Scholar] [CrossRef]
- Roy, T.; Mondal, N. Biosorption of Congo Red from aqueous solution onto burned root of Eichhornia crassipes biomass. Appl. Water Sci. 2017, 7, 1841–1854. [Google Scholar] [CrossRef] [Green Version]
- Carmen, A.; Ghinea, C.; Alves, M.; Gavrilescu, M. Removal of Erythrosine B dye from water effluents using crop waste pumpkin seed hulls as adsorbent. Desal. Water Treat. 2016, 57, 22585–22608. [Google Scholar] [CrossRef] [Green Version]
- Fang, B.; Bao, Z.; Lu, L.; Zhao, L.; Wang, H. Preparation of a hierarchical flower-like γ-Al2O3@C composite exhibiting enhanced adsorption performance for congo red by high temperature transformation of γ-AlOOH@ C precursors. RSC Adv. 2016, 6, 61–64. [Google Scholar] [CrossRef]
- Liu, S.; Cai, Y.; Cai, X.; Li, H.; Zhang, F.; Mu, Q.; Wang, Y.; Wang, D. Catalytic photodegradation of Congo red in aqueous solution by Ln(OH)3, (Ln = Nd, Sm, Eu, Gd, Tb, and Dy) nanorods. Appl. Catal. A 2013, 453, 45–53. [Google Scholar] [CrossRef]
- Ong, C.; Mohammad, A.; Rohani, R.; Ba-Abbad, M.M. Solar photocatalytic degradation of hazardous Congo red using low-temperature synthesis of zinc oxide nanoparticles. Process Saf. Environ. 2016, 104, 549–557. [Google Scholar] [CrossRef]
- Thomas, M.; Naikoo, G.; Sheikh, M.; Bano, M.; Khan, F. Effective photocatalytic degradation of Congo red dye using alginate/carboxymethyl cellulose/TiO2 nanocomposite hydrogel under direct sunlight irradiation. J. Photochem. Photobio. A 2016, 327, 33–43. [Google Scholar] [CrossRef]
- Tanwar, R.; Kumar, S.; Mandal, U. Photocatalytic activity of PANI/Fe0 doped BiOCl under visible light-degradation of Congo red dye. J. Photochem. Photobio. A 2017, 333, 105–116. [Google Scholar] [CrossRef]
- Rengaraj, S.; Moona, S.; Sivabalan, R.; Arabindoo, B.; Murugesan, V. Removal of phenol from aqueous solution and resin manufacturing industry wastewater using an agricultural waste: Rubber seed coat. J. Hazard. Mater. 2002, 89, 185–196. [Google Scholar] [CrossRef]
- Malik, P. Dye removal from wastewater using activated carbon developed from sawdust: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2004, 113, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, J.; Zhang, H.; Yang, S.; Qi, J.; Wang, Z.; Xu, H. Use of rice husk-based porous carbon for adsorption of rhodamine B from aqueous solutions. Dye. Pigm. 2005, 66, 123–128. [Google Scholar] [CrossRef]
- Rehman, M.; Kim, I.; Han, J. Adsorption of methylene blue dye from aqueous solution by sugar extracted spent rice biomass. Carbohyd. Polym. 2012, 90, 1314–1322. [Google Scholar] [CrossRef]
- Dubey, S.; Singh, A.; Nim, B.; Singh, I.B. Optimization of molar concentration of AlCl3 salt in the sol-gel synthesis of nanoparticles of gamma alumina and their application in the removal of fluoride of water. J. CFCSol-Gel Sci. Techn. 2017, 82, 468–477. [Google Scholar] [CrossRef]
- Xu, N.; Liu, Z.; Dong, Y.; Hong, T.; Dang, L.; Li, W. Controllable synthesis of mesoporous alumina with large surface area for high and fast fluoride removal. Ceram. Int. 2016, 42, 15253–15260. [Google Scholar] [CrossRef]
- Zhu, W.; He, D.; Han, C.; He, S.; Liao, M.; Shan, X.; Luo, Y. Optimization of As (V) removal from contaminated water with mesoporous alumina: Effects of pH, contact time, concentration and temperature. J. Environ. Eng. 2017, 143, 9. [Google Scholar] [CrossRef]
- Lanas, S.; Valiente, M.; Aneggi, E.; Trovarelli, A.; Tolazzi, M.; Melchior, A. Efficient fluoride adsorption by mesoporous hierarchical alumina microspheres. RSC Adv. 2016, 6, 42288–42296. [Google Scholar] [CrossRef]
- Xu, N.; Liu, Z.; Bian, S.; Dong, Y.; Li, W. Preparation of MnO2–Al2O3 adsorbent with large specific surface area for fluoride removal. Particuology 2016, 27, 66–71. [Google Scholar] [CrossRef]
- Padmaja, P.; Pillai, P.K.; Warrier, K.G.K.; Padmanabhan, M. Adsorption isotherm and pore characteristics of nano alumina derived from sol-gel boehmite. J. Porous Mater. 2004, 11, 147–155. [Google Scholar] [CrossRef]
- Wu, Y.S.; Ma, J.; Hu, F.; Li, M.C. Synthesis and Characterization of Mesoporous Alumina via a Reverse Precipitation Method. J. Mater. Sci. Technol. 2012, 28, 572–576. [Google Scholar] [CrossRef]
- Jian-hong, Y.; You-yi, S.; Jian-feng, G.; Chun-yan, X. Synthesis of crystalline γ-Al2O3 with high purity. Trans. Nonferrous Met. Soc. China 2009, 19, 1237–1242. [Google Scholar]
- Zaitan, H.; Bianchi, D.; Achak, O.; Chafik, T.A. comparative study of the adsorption and desorption of o-xylene onto bentonite clay and alumina. J. Hazard. Mater. 2008, 153, 852–859. [Google Scholar] [CrossRef]
- Cai, W.Q.; Li, H.Q.; Zhang, Y. Azeotropic distillation-assisted preparation of macro-mesostructured γ-Al2O3 nanofibres of crumpled sheet-like morphology. Mater. Chem. Phys. 2006, 96, 136–139. [Google Scholar] [CrossRef]
- Chanda, S.C.; Manna, A.; Vijavan, V.; Nayak, P.; Ashok, M.; Acharya, H.N. PIXE & XRD analysis of nanocrystals of Fe, Ni and Fe2O3. Mater. Lett. 2007, 61, 5059–5062. [Google Scholar]
- Li, D.; Schubert, B.; Norman, J.W. Characterization of gationic polyelectrolytes adsorption to an anionic emulsion via zeta-potential and microcalorimetry. J. Surfactants Deterg. 2014, 4, 655–667. [Google Scholar] [CrossRef]
- Alyassin, Y.; Sayed, E.G.; Mehta, P.; Ruparelia, K.; Arshad, M.S.; Rasekh, M.; Shepherd, J.; Kucuk, L.; Wilson, P.B.; Singh, N. Application of mesoporous silica nanoparticles as drug delivery carriers for chemotherapeutic agents. Drug Discov. Today 2020, 25, 1513–1520. [Google Scholar] [CrossRef]
- Nicola, R.; Muntean, S.G.; Nistor, M.A.; Putz, A.M.; Almasy, L.; Sacarescu, L. Highly efficient and fast removal of colored pollutants from single and binary systems, using magnetic mesoporous silica. Chemosphere 2020, 261, 127737. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, L.; Zhou, W.; Lu, R.; Gao, H.; Zhang, S. Superior adsorption capacity of Fe3O4@nSiO2@mSiO2 core-shell microspheres for removal of Congo red from aqueous solution. J. Mol. Liq. 2016, 219, 88–94. [Google Scholar] [CrossRef]
- Weber, W.; Morris, J. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Pal, S.; Patra, A.; Ghorai, S.; Sarkar, A.; Mahato, V.; Sarkar, S.; Singh, R. Efficient and rapid adsorption characteristics of templating modified guar gum and silica nanocomposite toward removal of toxic reactive blue and Congo red dyes. Bioresour. Technol. 2015, 191, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Kirthika, K. Equilibrium and kinetic study of adsorption of nickel from aqueous solution onto Bael tree leaf powder. J. Eng. Sci. Technol. 2009, 4, 351–363. [Google Scholar]
- Assche, T.R.; Baron, G.V.; Denayer, J.F. An explicit multicomponent adsorption isotherm model: Accounting for the size-effect for components with Langmuir adsorption behavior. Adsorption 2018, 24, 517–530. [Google Scholar] [CrossRef]
- Darvishi, R.; Soltani, C.; Hassani, A.; Karaca, S.; Khataee, A. Preparation of cetyltrimethylammonium bromide modified montmorillonite nanomaterial for adsorption of a textile dye. J. Taiwan Inst. Chem. Eng. 2014, 45, 2565–2577. [Google Scholar]
- Samad, A.; Din, M.I.; Ahmed, M.; Ahmad, S. Synthesis of zinc oxide nanoparticles reinforced clay and their applications for removal of Pb (Ⅱ) ions from aqueous media. Chin. J. Chem. Eng. 2021, 32, 454–461. [Google Scholar] [CrossRef]
- Hao, T.; Yang, C.; Rao, X.; Wang, J.; Niu, C.; Su, X. Facile additive-free synthesis of iron oxide nanoparticles for efficient adsorptive removal of Congo red and Cr(Ⅵ). Appl. Surf. Sci. 2014, 292, 174–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Y.; Zhou, X.; Liu, Z.; Zhu, G.; Li, D.; Li, X. Monodispersed hollow aluminosilica microsphere@hierarchical γ-AlOOH deposited with or without Fe(OH)3 nanoparticles for efficient adsorption of organic pollutants. J. Mater. Chem. A 2016, 4, 838–846. [Google Scholar] [CrossRef]
- Zeng, B.; Yang, L.; Zheng, W.; Zhu, J.; Ma, X.; Liu, X.; Yuan, C.; Xu, Y.; Dai, L. Analysis of the formation process and performance of magnetic Fe3O4@Poly(4-vinylpyridine) absorbent prepared by in-situ synthesis. J. Mater. Sci. Technol. 2018, 34, 999–1007. [Google Scholar] [CrossRef]
- Cai, W.; Yu, J.; Jaroniec, M. Template-free synthesis of hierarchical spindle-like γ-Al2O3 materials and their adsorption affinity towards organic and inorganic pollutants in water. J. Mater. Chem. 2010, 20, 4587–4594. [Google Scholar] [CrossRef]
- Zhang, E.L.; Sun, X.J.; Liu, X.T.; Wang, Q.D. Morphology controlled synthesis of α-FeOOH crystals and their shape-dependent adsorption for decontamination of Congo red dye. Mater. Res. Innov. 2015, 19, 385–391. [Google Scholar] [CrossRef]
- Cai, W.; Hu, Y.; Chen, J.; Zhang, G.; Xia, T. Synthesis of nanorod-like mesoporous γ-Al2O3 with enhanced affinity towards Congo red removal: Effects of anions and structure-directing agents. Crystengcomm 2012, 14, 972–977. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhai, J.; Zhou, M.; Dong, S. Ordered magnetic core-manganese oxide shell nanostructures and their application in water treatment. J. Mater. Chem. 2009, 19, 7030–7035. [Google Scholar] [CrossRef]

| HPA Purity (by Weight) | 3N (99.9%) | 4N (99.99%) | 5N (99.999%) |
|---|---|---|---|
| approx. market price range (US$/tonne) | $5000–$15,000 | $15,000–$30,000 | $30,000–$50,000 |
| Component Content (wB/%) | |||
|---|---|---|---|
| SiO2 | Fe2O3 | Na2O | Al(OH)3 |
| 1.25 | 1.16 | 0.58 | 97.01 |
| Concentration (g L−1) | Removal Efficiency (%) | Alumina Purity (%) | |||
|---|---|---|---|---|---|
| Fe2O3 | SiO2 | Na2O | Total Removal Rate | ||
| 30 | 99.58 | 99.21 | 92.06 | 97.97 | 99.9393 |
| 60 | 99.44 | 99.57 | 96.21 | 98.86 | 99.9661 |
| 90 | 99.28 | 99.37 | 94.31 | 98.36 | 99.9509 |
| 120 | 99.22 | 99.33 | 93.45 | 98.15 | 99.9447 |
| Aging Temperature (°C) | Removal Efficiency (%) | Alumina Purity (%) | |||
|---|---|---|---|---|---|
| Fe2O3 | SiO2 | Na2O | Total Removal Rate | ||
| 30 | 99.45 | 99.56 | 93.10 | 98.26 | 99.9481 |
| 60 | 99.44 | 99.57 | 96.21 | 98.86 | 99.9661 |
| 90 | 99.32 | 99.48 | 95.17 | 98.58 | 99.9576 |
| 120 | 99.30 | 99.52 | 94.83 | 98.53 | 99.9552 |
| Aging Time (h) | Removal Efficiency (%) | Alumina Purity (%) | |||
|---|---|---|---|---|---|
| Fe2O3 | SiO2 | Na2O | Total Removal Rate | ||
| 1 | 99.42 | 99.55 | 93.45 | 98.31 | 99.9497 |
| 1.5 | 99.44 | 99.54 | 94.31 | 98.48 | 99.9547 |
| 2 | 99.44 | 99.57 | 96.21 | 98.86 | 99.9661 |
| 2.5 | 99.37 | 99.50 | 94.14 | 98.41 | 99.9526 |
| 3 | 99.32 | 99.42 | 92.41 | 98.03 | 99.9410 |
| 3.5 | 99.32 | 99.43 | 92.24 | 97.99 | 99.9401 |
| 4 | 99.32 | 99.44 | 91.72 | 97.89 | 99.9371 |
| Desodium Agent | Content (wB/%) | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) | |||
|---|---|---|---|---|---|---|---|
| Fe2O3 | SiO2 | Na2O | Al2O3 | ||||
| (NH4)2CO3 | 0.0065 | 0.0054 | 0.022 | 99.9661 | 312.43 | 0.43 | 3.80 |
| CH3COONH4 | 0.0063 | 0.0057 | 0.054 | 99.9340 | 276.85 | 0.32 | 3.86 |
| (NH4)2C2O4 | 0.0066 | 0.0054 | 0.050 | 99.9380 | 252.20 | 0.33 | 4.15 |
| Concentration (g L−1) | Removal Efficiency (%) | Alumina Purity (%) | Specific Surface Are (m2 g−1) | |||
|---|---|---|---|---|---|---|
| Fe2O3 | SiO2 | Na2O | Total Removal Rate | |||
| 25 | 99.44 | 99.54 | 93.28 | 98.28 | 99.9478 | 205.86 |
| 50 | 99.42 | 99.56 | 93.97 | 98.42 | 99.9528 | 276.54 |
| 75 | 99.44 | 99.57 | 96.21 | 98.87 | 99.9661 | 312.43 |
| 100 | 99.41 | 99.54 | 96.21 | 98.84 | 99.9653 | 311.59 |
| Temperature | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Diameter (nm) |
|---|---|---|---|
| 500 °C | 312.43 | 0.48 | 3.80 |
| 700 °C | 199.35 | 0.36 | 5.36 |
| 900 °C | 144.34 | 0.19 | 8.66 |
| 1100 °C | 88.52 | 0.08 | 10.03 |
| Impurities | Fe2O3 | SiO2 | Na2O | Total Values | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|---|---|---|---|
| Content (wB/%) | 0.0065 | 0.0054 | 0.022 | 0.0339 | |||
| Remove rate (%) | 99.44 | 99.57 | 96.21 | 98.87 | 312.43 | 0.48 | 3.80 |
| qe-exp (mg g−1) | 495 | ||
|---|---|---|---|
| pseudo-first-oder model | qe-cal (mg g−1) | K1 (min−1) | R2 |
| 51.36 | 0.03236 | 0.59214 | |
| pseudo-second-oder model | qe-cal (mg g−1) | K2 (min−1) | R2 |
| 495.05 | 0.00334459 | 0.99992 | |
| Kd1 (min−1) | C1 | R12 |
| 247.343 | −1.82167 | 1 |
| Kd2 (min−1) | C2 | R22 |
| 73.47937 | 254.92449 | 0.95626 |
| Kd3 (min−1) | C3 | R32 |
| 2.11518 | 470.82288 | 0.82684 |
| Langmuir model | qm (mg g−1) | KL | R2 |
| 2053.818 | 0.0476 | 0.99972 | |
| Freundlich model | KF (mg1−(1/n) L1/ng−1) | n | R2 |
| 259.755 | 2.882 | 0.86148 | |
| Temkin model | KT (L mg−1) | b | R2 |
| 1.021 | 7.1853 | 0.97039 |
| Adsorbents | qm(mg g−1) | References |
|---|---|---|
| CR | ||
| HPMA nanopowder | 1984.64 | This study |
| a-Fe2O3 nanoparticles and nanowhiskers | 254.00 | [68] |
| HAM@ γ-AlOOH/Fe(OH)3 | 252.53 | [69] |
| Fe3O4@P4-VP nanospheres | 151.50 | [70] |
| spindle-like γ-Al2O3 | 176.70 | [71] |
| α-FeOOH nanorods | 104.2 | [72] |
| nanorod-like mesoporous γ-Al2O3 | 83.80 | [73] |
| magnetic core-manganes oxide shell | 43.00 | [74] |
| Fe2O3@mSiO2 | 72.22 | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, D.; Lv, F.; Chen, J.; Wu, C.; Li, Y.; Shen, J.; Li, Y. Synthesis and Characterization of High-Purity Mesoporous Alumina with Excellent Adsorption Capacity for Congo Red. Materials 2022, 15, 970. https://doi.org/10.3390/ma15030970
Li Z, Wang D, Lv F, Chen J, Wu C, Li Y, Shen J, Li Y. Synthesis and Characterization of High-Purity Mesoporous Alumina with Excellent Adsorption Capacity for Congo Red. Materials. 2022; 15(3):970. https://doi.org/10.3390/ma15030970
Chicago/Turabian StyleLi, Zhonglin, Ding Wang, Fengcheng Lv, Junxue Chen, Chengzhi Wu, Yuping Li, Jialong Shen, and Yibing Li. 2022. "Synthesis and Characterization of High-Purity Mesoporous Alumina with Excellent Adsorption Capacity for Congo Red" Materials 15, no. 3: 970. https://doi.org/10.3390/ma15030970
APA StyleLi, Z., Wang, D., Lv, F., Chen, J., Wu, C., Li, Y., Shen, J., & Li, Y. (2022). Synthesis and Characterization of High-Purity Mesoporous Alumina with Excellent Adsorption Capacity for Congo Red. Materials, 15(3), 970. https://doi.org/10.3390/ma15030970





