Hexagonal Nano and Micro Boron Nitride: Properties and Lubrication Applications
Abstract
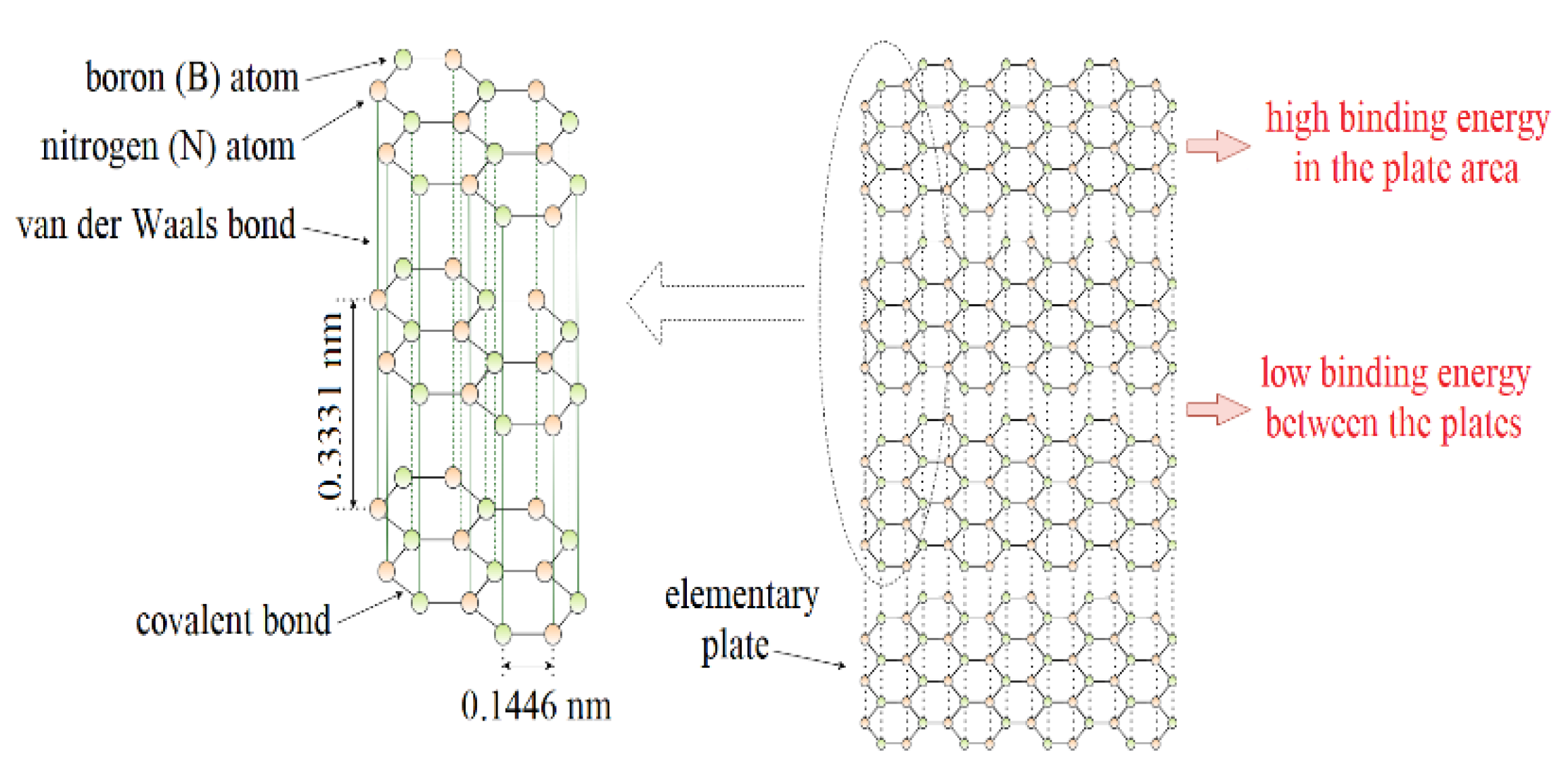
:1. Introduction
2. Hexagonal Boron Nitride as an Additive to Lubricants
3. Experimental Methods
3.1. Crystal Structure Determination—X-ray Diffraction (XRD)
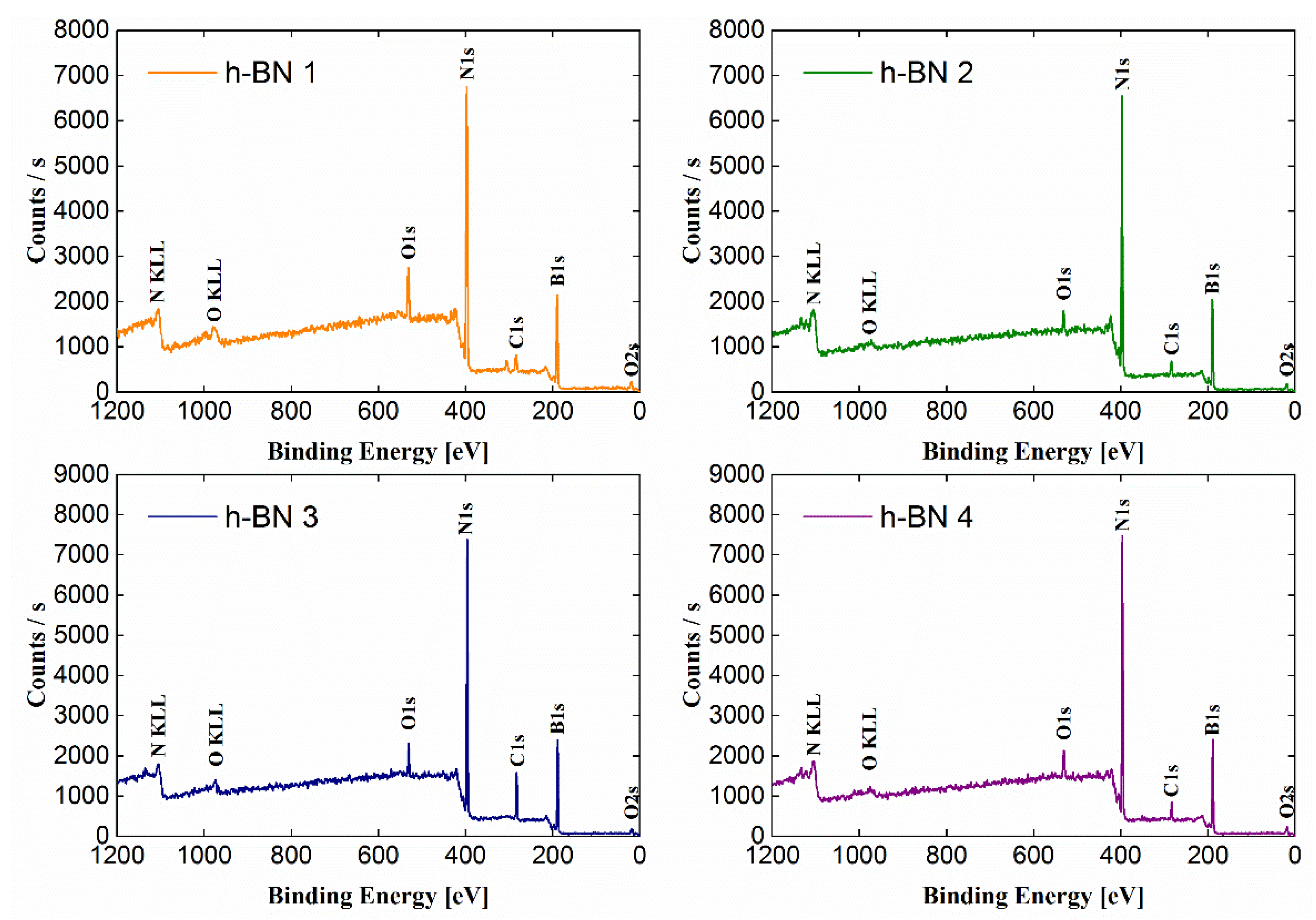
3.2. Quantitative and Qualitative Assessment of the Surface Composition—X-ray Photoelectron Spectroscopy (XPS)
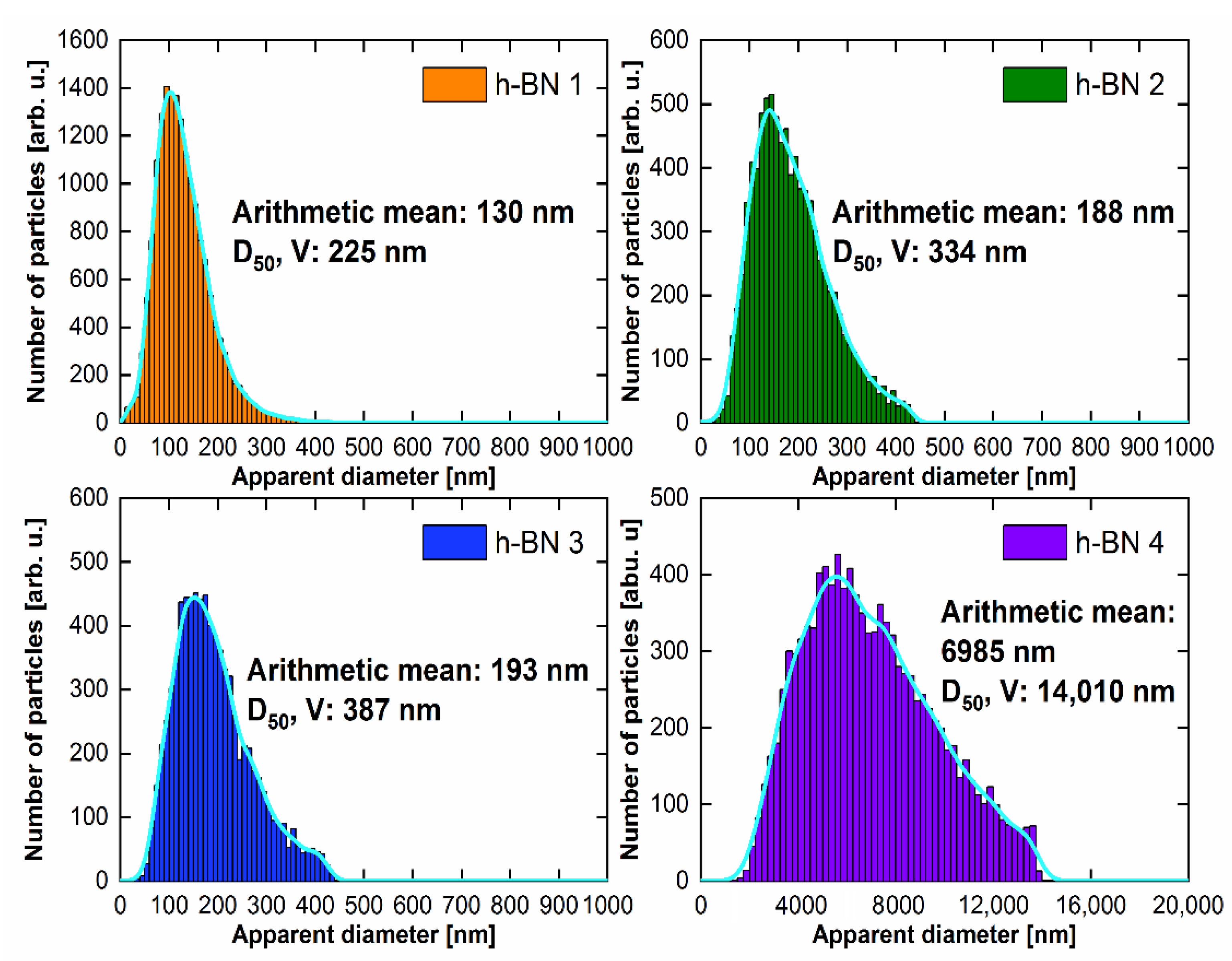
3.3. Imaging and Determination of Particle Size Distribution—Scanning Electron Microscope (SEM)
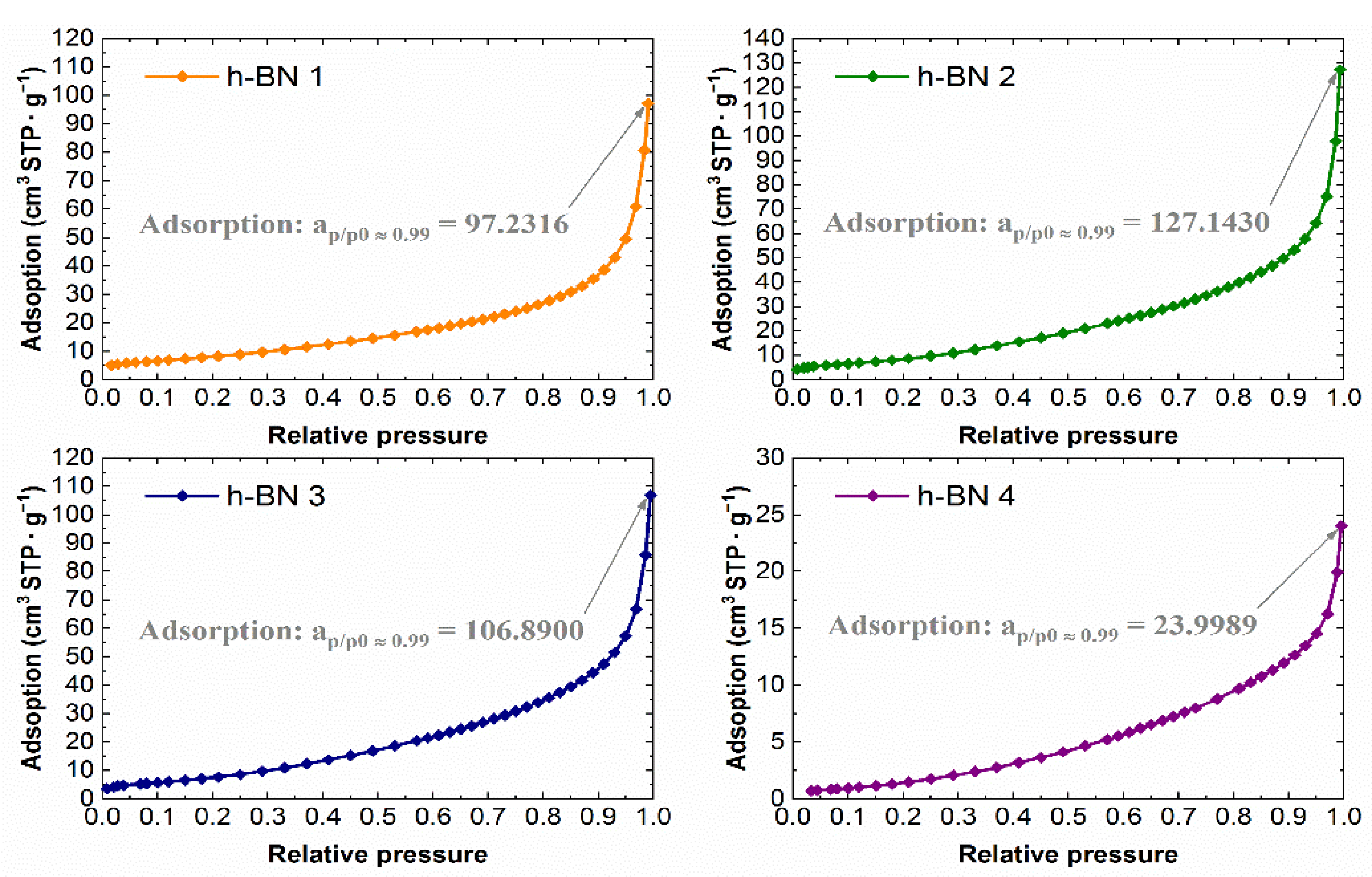
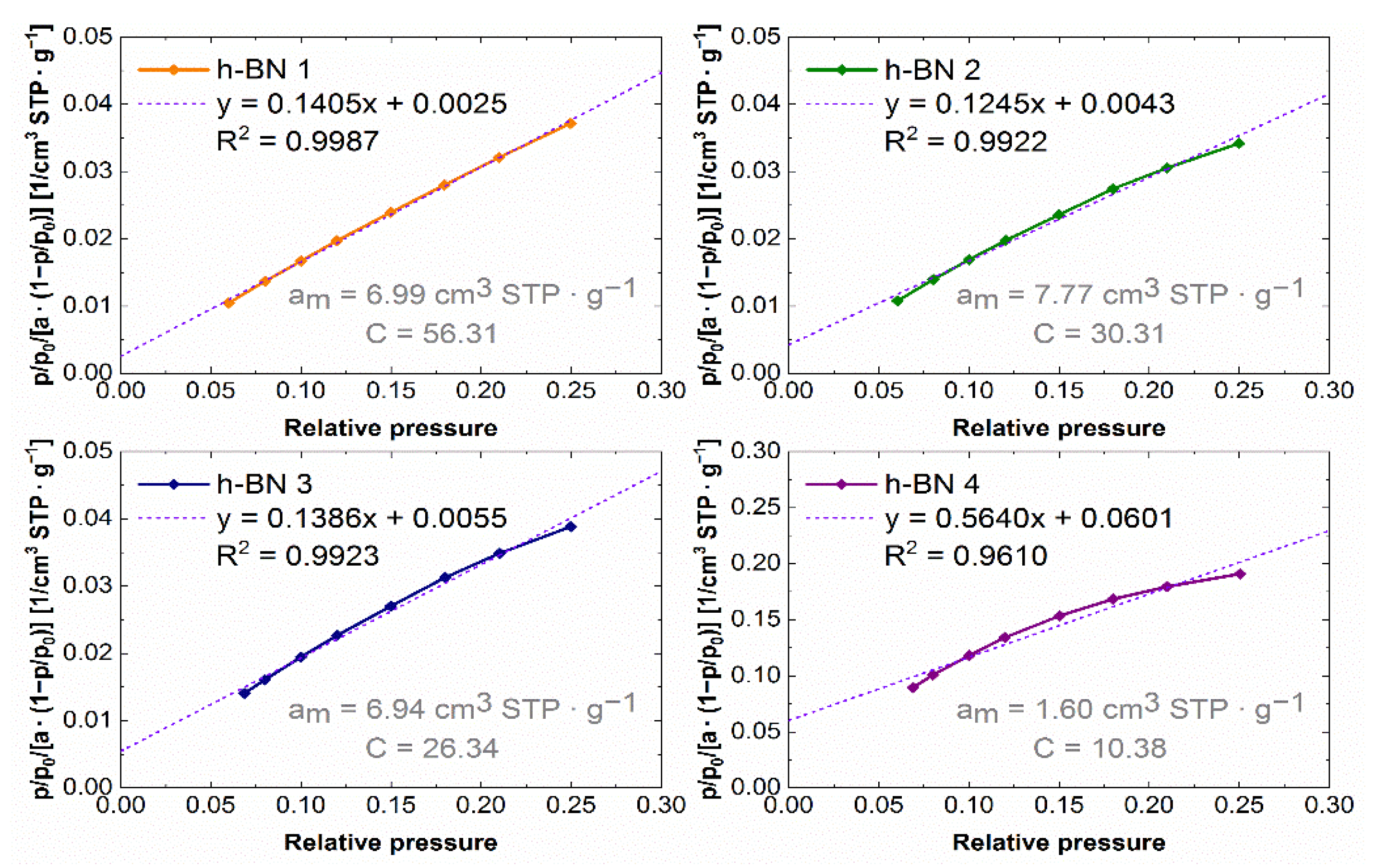
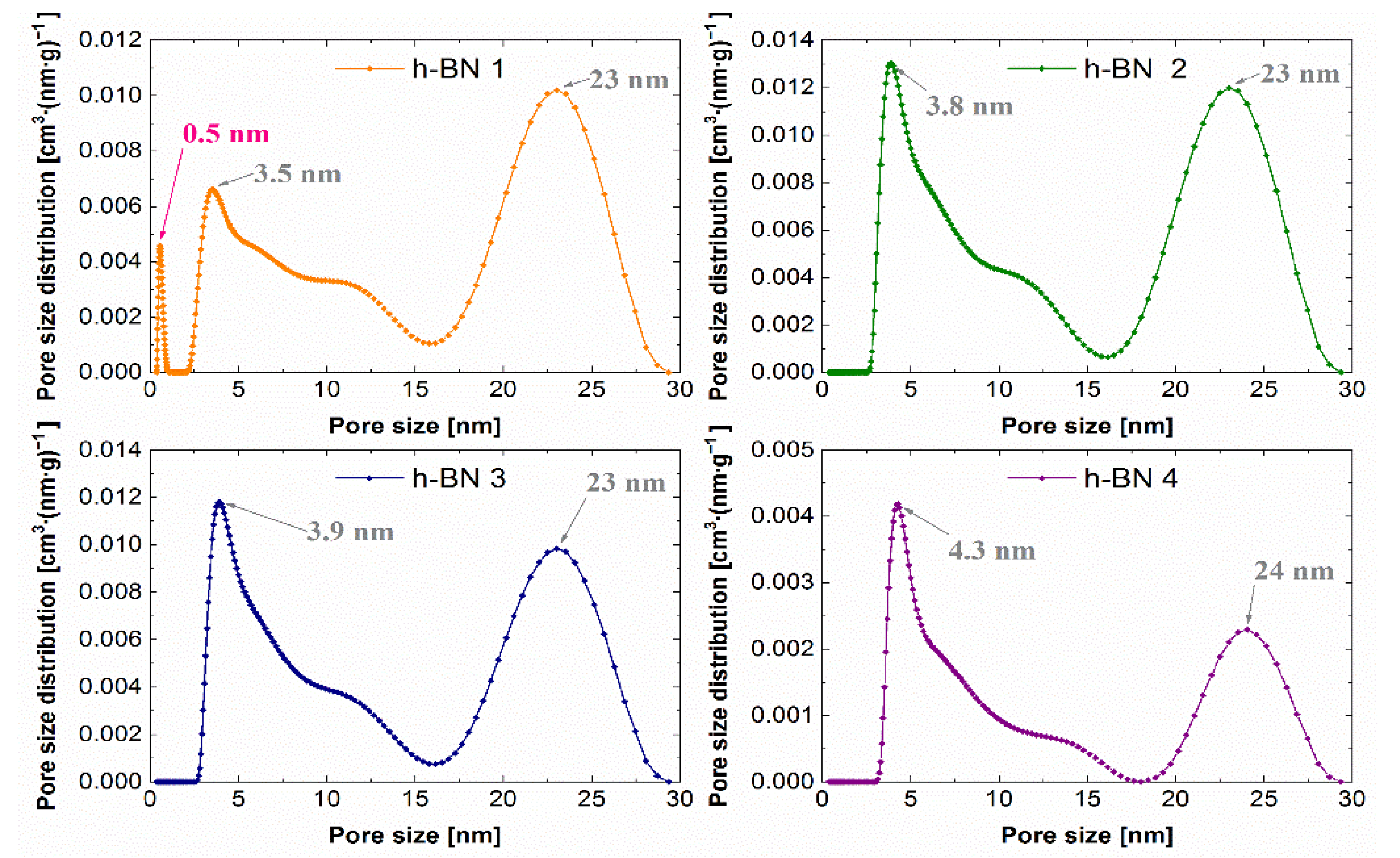
3.4. Determination of the Specific Surface and Characteristics of Porosity
4. Results and Discussion
4.1. Crystal Structure
4.2. Quantitative and Qualitative Assessment of the Surface Composition
4.3. Morphology and Particle Size Distribution
4.4. Specific Surface Area and Characteristics of Porosity
4.5. The Analysis of the Properties in Terms of Lubrication Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Ma, F.; Sun, M. Graphene, Hexagonal Boron Nitride, and Their Heterostructures: Properties and Applications. RSC Adv. 2017, 7, 16801–16822. [Google Scholar] [CrossRef] [Green Version]
- Pawlak, Z.; Kaldonski, T.; Pai, R.; Bayraktar, E.; Oloyede, A. A Comparative Study on the Tribological Behaviour of Hexagonal Boron Nitride (h-BN) as Lubricating Micro-Particles-An Additive in Porous Sliding Bearings for a Car Clutch. Wear 2009, 267, 1198–1202. [Google Scholar] [CrossRef]
- Chkhartishvili, L.; Tabatadze, G.; Nackebia, D.; Bzhalava, T.; Kalandadze, I. Hexagonal Boron Nitride as a Solid Lubricant Additive (An Overview). Nano Stud. 2016, 14, 91–98. [Google Scholar]
- Kałdoński, T. Tribological Applications of Boron Nitride, 1st ed.; Military University of Technology: Warsaw, Poland, 2006. (In Polish) [Google Scholar]
- Mahathanabodee, S.; Palathai, T.; Raadnui, S.; Tongsri, R.; Sombatsompop, N. Effects of Hexagonal Boron Nitride and Sintering Temperature on Mechanical and Tribological Properties of SS316L/h-BN Composites. Mater. Des. 2013, 46, 588–597. [Google Scholar] [CrossRef]
- Hisakado, T.; Tsukizoe, T.; Yoshikawa, H. Lubrication Mechanism of Solid Lubricants in Oils. J. Tribol. 1983, 105, 245–252. [Google Scholar] [CrossRef]
- Hu, E.Z.; Xu, Y.; Hu, K.H.; Hu, X.G. Tribological Properties of 3 Types of MoS2 Additives in Different Base Greases. Lubr. Sci. 2017, 29, 541–555. [Google Scholar] [CrossRef]
- Bagi, S.D.; Aswath, P.B. Role of MoS2 Morphology on Wear and Friction under Spectrum Loading Conditions. Lubr. Sci. 2015, 27, 429–449. [Google Scholar] [CrossRef]
- Kałdoński, T. The Influence of Boron Nitride on the Performance of Greases for Sliding Bearings. In Materials of the Eight National Congress of Technical Device Maintenance; Institute for Sustainable Technologies—National Research Institute: Krynica Górska, Poland, 23–26 September 1997. (In Polish) [Google Scholar]
- Kałdoński, T. Influence of the Type of Grease and Boron Nitride on the Self-Lubrication Process of a Porous Bearing. In Materials of the Eight National Congress of Technical Device Maintenance; Institute for Sustainable Technologies—National Research Institute: Krynica Górska, Poland, 23–26 September 1997. (In Polish) [Google Scholar]
- Kałdoński, T.; Krzemiński, K. The Use of Boron Nitride as an Additive to Grease Filling a Rolling Bearing. In Materials of the Eight National Congress of Technical Device Maintenance; Institute for Sustainable Technologies–National Research Institute: Krynica Górska, Poland, 23–26 September 1997. (In Polish) [Google Scholar]
- Kałdoński, T. (Military University of Technology, Warsaw, Poland). Research, and Technology of Optimal Use of Boron Nitride α-BN as an Additive to Lubricant in Military Equipment. Report on the Implementation of the Research Project PB 0T 00A 014 08; Warszawa, Poland, 1998; (Unpublished work, In Polish). [Google Scholar]
- Kałdoński, T.; Nosal, S.; Zwierzycki, W. Influence of Boron Nitride Content in WAT Production on Antifretting Properties of Lubricants. In Proceedings of the Materials of the IV Poltrib’97 Conference, Białobrzegi, Poland, 10–12 June 1997. (In Polish). [Google Scholar]
- Pawlak, Z.; Kałdoński, T.; Lisewski, M.; Urbaniak, W.; Oloyede, A. The Effect of Hexagonal Boron Nitride Additive on the Effectiveness of Grease-Based Lubrication of a Steel Surface. Ind. Lubr. Tribol. 2012, 64, 84–89. [Google Scholar] [CrossRef]
- Jóźwiak, P.; Siczek, K. The Research on the Influence of the Presence of Boron Nitride in the Grease on the Resistance to Movement of the Steering Rod Pin. Arch. Automot. Eng. 2012, 58, 123–137. (In Polish) [Google Scholar]
- Krawiec, M.; Leśniewski, T. Investigation of Lubrication Efficiency of Lithium Grease Filled with Boron Nitride. Surf. Min. 2013, 54, 82–86. (In Polish) [Google Scholar]
- Podgornik, B.; Kosec, T.; Kocijan, A.; Donik, Č. Tribological Behaviour and Lubrication Performance of Hexagonal Boron Nitride (h-BN) as a Replacement for Graphite in Aluminium Forming. Tribol. Int. 2015, 81, 267–275. [Google Scholar] [CrossRef]
- Yan, C.; Zeng, Q.; Hao, Y.; Xu, Y.; Zhou, M. Friction-Induced Hardening Behaviors and Tribological Properties of 60NiTi Alloy Lubricated by Lithium Grease Containing Nano-BN and MoS2. Tribol. Trans. 2019, 62, 812–820. [Google Scholar] [CrossRef]
- Wang, T.; Li, Z.; Li, J.; He, Q. Impact of Boron Nitride Nanoparticles on the Wear Property of Lithium Base Grease. Journal of Materials Engineering and Performance. J. Mater. Eng. Perform. 2020, 29, 1–10. [Google Scholar] [CrossRef]
- Bajer, J. The Influence of Lubricating Additive on Tribological Characteristics of Low-Temperature Grease. Tribology 2006, 6, 67–80. (In Polish) [Google Scholar]
- Kimura, Y.; Wakabayashi, T.; Okada, K.; Wada, T.; Nishikawa, H. Boron Nitride as a Lubricant Additive. Wear 1999, 232, 199–206. [Google Scholar] [CrossRef]
- Kałdoński, T.; Krzemiński, K.; Kulczycki, A.; Włodarczyk, E. The Influence of Boron Nitride Concentration in Oil on Tribological Properties of a Porous Bearing. Tribology 1995, 6, 715–725. [Google Scholar]
- Kałdoński, T.; Krzemiński, K.; Szczawnicka, E. The Application of Boron Nitride α-BN for Lubrication of Plain Bearings. In Proceedings of the Materials of the National Scientific and Technical Ceonference “Bearing engineering’96”, Gdańsk, Poland, 4–5 June 1996. (In Polish). [Google Scholar]
- Reeves, C.J.; Menezes, P.L.; Lovell, M.R.; Jen, T.C. The Size Effect of Boron Nitride Particles on the Tribological Performance of Biolubricants for Energy Conservation and Sustainability. Tribol. Lett. 2013, 51, 437–452. [Google Scholar] [CrossRef]
- Reeves, C.J.; Menezes, P.L.; Lovell, M.R.; Jen, T.C. The Influence of Surface Roughness and Particulate Size on the Tribological Performance of Bio-Based Multi-Functional Hybrid Lubricants. Tribol. Int. 2015, 88, 40–55. [Google Scholar] [CrossRef]
- Reeves, C.J.; Menezes, P.L. Evaluation of Boron Nitride Particles on the Tribological Performance of Avocado and Canola Oil for Energy Conservation and Sustainability. Int. J. Adv. Manuf. Technol. 2017, 89, 3475–3486. [Google Scholar] [CrossRef]
- Çelik, O.N.; Ay, N.; Göncü, Y. Effect of Nano Hexagonal Boron Nitride Lubricant Additives on the Friction and Wear Properties of AISI 4140 Steel. Part. Sci. Technol. 2013, 31, 501–506. [Google Scholar] [CrossRef]
- Charoo, M.S.; Wani, M.F. Tribological Properties of H-BN Nanoparticles as Lubricant Additive on Cylinder Liner and Piston Ring. Lubr. Sci. 2017, 29, 241–254. [Google Scholar] [CrossRef]
- Wan, Q.; Jin, Y.; Sun, P.; Ding, Y. Tribological Behaviour of a Lubricant Oil Containing Boron Nitride Nanoparticles. Procedia Eng. 2015, 102, 1038–1045. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.K.; Bijwe, J.; Padhan, M. Role of Size of Hexagonal Boron Nitride Particles on Tribo-Performance of Nano and Micro Oils. Lubr. Sci. 2018, 30, 441–456. [Google Scholar] [CrossRef]
- Afzal, O.; Shafi, W.K.; Charoo, M.S. Effect of H-BN Nanoparticles on the Tribological and Rheological Properties of API-Group I Oils. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–17. [Google Scholar] [CrossRef]
- Chinas-Castillo, F.; Spikes, H.A. Mechanism of Action of Colloidal Solid Dispersions. J. Tribol. 2003, 125, 552–557. [Google Scholar] [CrossRef]
- Bondarev, A.; Kovalskii, A.; Firestein, K.; Loginov, P.; Sidorenko, D.; Shvindina, N.; Sukhorukova, I.; Shtansky, D. Hollow spherical and nanosheet-base BN nanoparticles as perspective additives to oil lubricants: Correlation between large-scale friction behavior and in situ TEM compression testing. Ceram. Int. 2018, 44, 6801–6809. [Google Scholar] [CrossRef]
- Bagi, S.D.; Aswath, P.B. Mechanism of Friction and Wear in MoS2 and ZDDP/F-PTFE Greases under Spectrum Loading Conditions. Lubricants 2015, 3, 687–711. [Google Scholar] [CrossRef] [Green Version]
- Gulzar, M.; Masjuki, H.H.; Kalam, M.A.; Varman, M.; Zulkifli, N.W.M.; Mufti, R.A.; Zahid, R. Tribological Performance of Nanoparticles as Lubricating Oil Additives. J. Nanopart. Res. 2016, 18, 1–25. [Google Scholar] [CrossRef]
- He, Q.; Li, A.; Guo, Y.; Liu, S.; Zhang, Y.; Kong, L. Tribological Properties of Nanometer Cerium Oxide as Additives in Lithium Grease. J. Rare Earths 2018, 36, 209–214. [Google Scholar] [CrossRef]
- He, Q.; Li, A.; Guo, Y.; Liu, S.; Kong, L.H. Effect of Nanometer Silicon Dioxide on the Frictional Behavior of Lubricating Grease. Nanomater. Nanotechnol. 2017, 7, 1847980417725933. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.; Komai, K. Tribofilm formation and mild wear by tribo-sintering of nanometer-sized oxide particles on rubbing steel surfaces. Wear 2007, 262, 36–41. [Google Scholar] [CrossRef]
- Kurzydłowski, K.; Lewandowska, M. Engineering, Construction and Functional Nanomaterials; Polish Scientific Publishers: Warsaw, Poland, 2010. (In Polish) [Google Scholar]
- Chkhartishvili, L. Correlation between Surface Specific Area and Particles Average Size: Hexagonal Boron Nitride Nano-Powders. Nano Stud. 2012, 6, 65–76. [Google Scholar]
- Shirley, D.A. High-Resolution X-ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Sanchez, M.A. GrainSizeTools: A Python Script for Grain Size Analysis and Paleopiezometry Based on Grain Size. J. Open Source Softw. 2018, 3, 863. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W.; Salzberg, H.W. Adsorption Surface Area, and Porosity. J. Electrochem. Soc. 1967, 114, 279C. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. 2D-NLDFT Adsorption Models for Carbon Slit-Shaped Pores with Surface Energetical Heterogeneity and Geometrical Corrugation. Carbon 2013, 55, 70–80. [Google Scholar] [CrossRef]
- Hahn, T. International Tables for Crystallography. Volume A: Space-Group Symmetry, 5th ed.; Springer for the International Union of Crystallography: Dordrecht, The Netherland, 2005. [Google Scholar]
- Trzaski Durski, Z.; Trzaska Durska, H. Fundamentals of Crystallography; Publishing House of the Warsaw University of Technology: Warsaw, Poland, 2003. (In Polish) [Google Scholar]
- Bachmann, P.; Düll, F.; Späth, F.; Bauer, U.; Steinrück, H.P.; Papp, C. A HR-XPS Study of the Formation of h-BN on Ni(111) from the Two Precursors, Ammonia Borane and Borazine. J. Chem. Phys. 2018, 149, 164709. [Google Scholar] [CrossRef]
- Guimon, C.; Gonbeau, D.; Pfister-Guillouzo, G.; Dugne, O.; Guette, A.; Naslain, R.; Lahaye, M. XPS Study of BN Thin Films Deposited by CVD on SiC Plane Substrates. Surf. Interface Anal. 1990, 16, 440–445. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High-Resolution XPS of Organic Polymers: The Scienta ESCA300 Database. J. Chem. Educ. 1993, 70, A25. [Google Scholar] [CrossRef]
- Rouxhet, P.G.; Genet, M.J. XPS Analysis of Bio-Organic Systems. Surf. Interface Anal. 2011, 43, 1453–1470. [Google Scholar] [CrossRef]
- Kaczyński, Ł. The Effect of Contamination in X-Ray Microanalysis. Electron. Mater. 1990, 3, 45–48. (In Polish) [Google Scholar]
- Genet, M.J.; Dupont-Gillain, C.C.; Rouxhet, P.G. XPS Analysis of Biosystems and Biomaterials. In Medical Applications of Colloids; Springer: New York, NY, USA, 2008. [Google Scholar]

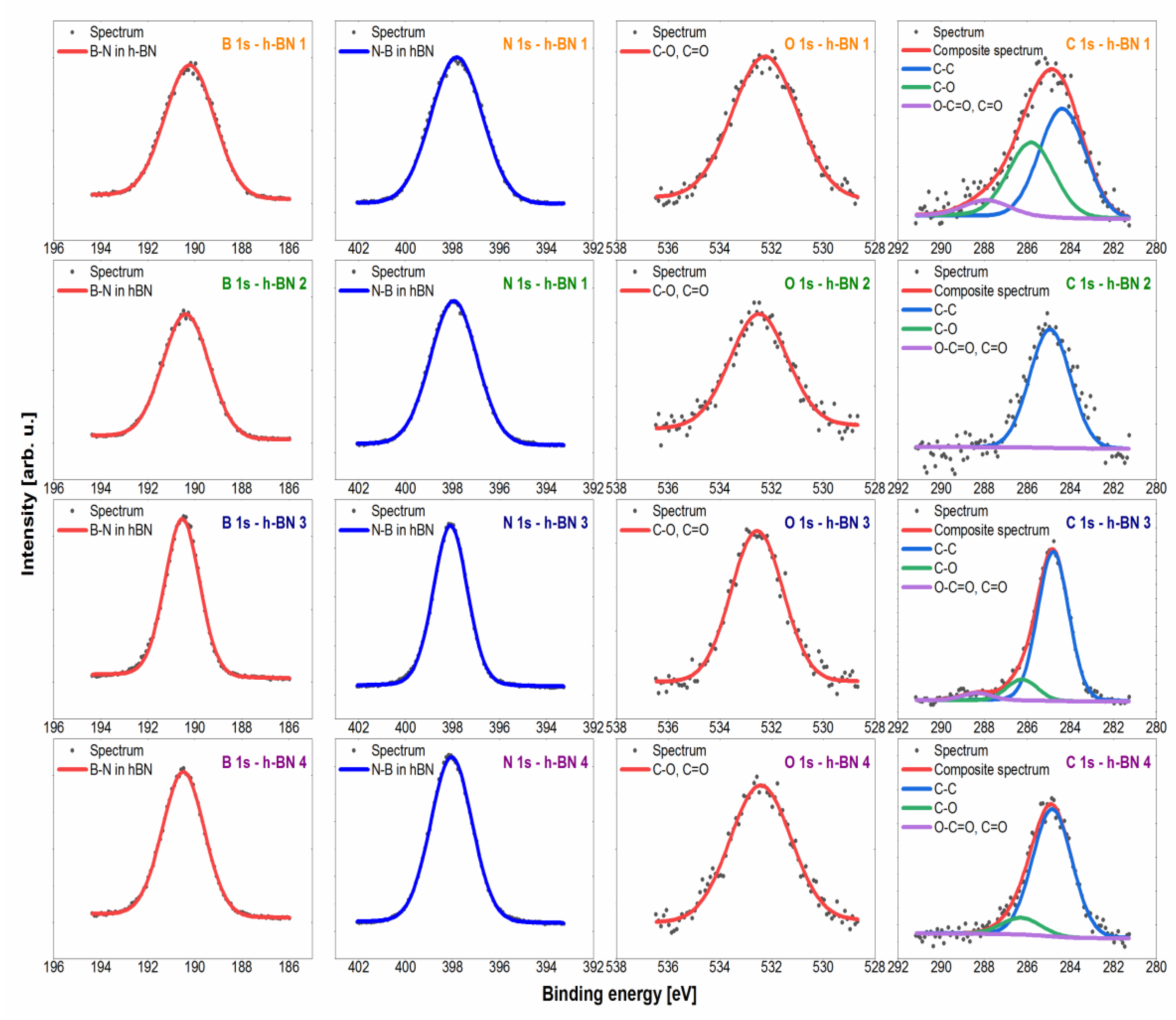

| Chemical Element | B | C | N | O | ||
|---|---|---|---|---|---|---|
| Binding energy (eV) | 190.3 | 284.8 | 286.3 | 288.3 | 379.9 | 532.5 |
| Chemical environment | h-BN | C–C | C–O | O–C=O | N–B | O–C O=C |
| h-BN 1 | 43.9 | 3.0 | 2.1 | 0.5 | 44.8 | 5.7 |
| h-BN 2 | 45.7 | 2.9 | 0.0 | 0.0 | 48.9 | 2.5 |
| h-BN 3 | 41.8 | 10.5 | 1.4 | 0.5 | 42.1 | 3.7 |
| h-BN 4 | 45.3 | 4.8 | 0.6 | 0.0 | 46.2 | 3.1 |
| Sample | Specific Surface Area SBET (m2·g−1) | Total Pore Volume Vt (cm3·g−1) |
|---|---|---|
| h-BN 1 | 30 | 0.15 |
| h-BN 2 | 33 | 0.20 |
| h-BN 3 | 30 | 0.16 |
| h-BN 4 | 7 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senyk, S.; Chodkiewicz, A.; Gocman, K.; Szczęśniak, B.; Kałdoński, T. Hexagonal Nano and Micro Boron Nitride: Properties and Lubrication Applications. Materials 2022, 15, 955. https://doi.org/10.3390/ma15030955
Senyk S, Chodkiewicz A, Gocman K, Szczęśniak B, Kałdoński T. Hexagonal Nano and Micro Boron Nitride: Properties and Lubrication Applications. Materials. 2022; 15(3):955. https://doi.org/10.3390/ma15030955
Chicago/Turabian StyleSenyk, Szymon, Arkadiusz Chodkiewicz, Krzysztof Gocman, Barbara Szczęśniak, and Tadeusz Kałdoński. 2022. "Hexagonal Nano and Micro Boron Nitride: Properties and Lubrication Applications" Materials 15, no. 3: 955. https://doi.org/10.3390/ma15030955
APA StyleSenyk, S., Chodkiewicz, A., Gocman, K., Szczęśniak, B., & Kałdoński, T. (2022). Hexagonal Nano and Micro Boron Nitride: Properties and Lubrication Applications. Materials, 15(3), 955. https://doi.org/10.3390/ma15030955








