Superhydrophobic Coating Based on Porous Aluminum Oxide Modified by Polydimethylsiloxane (PDMS)
Abstract
:1. Introduction
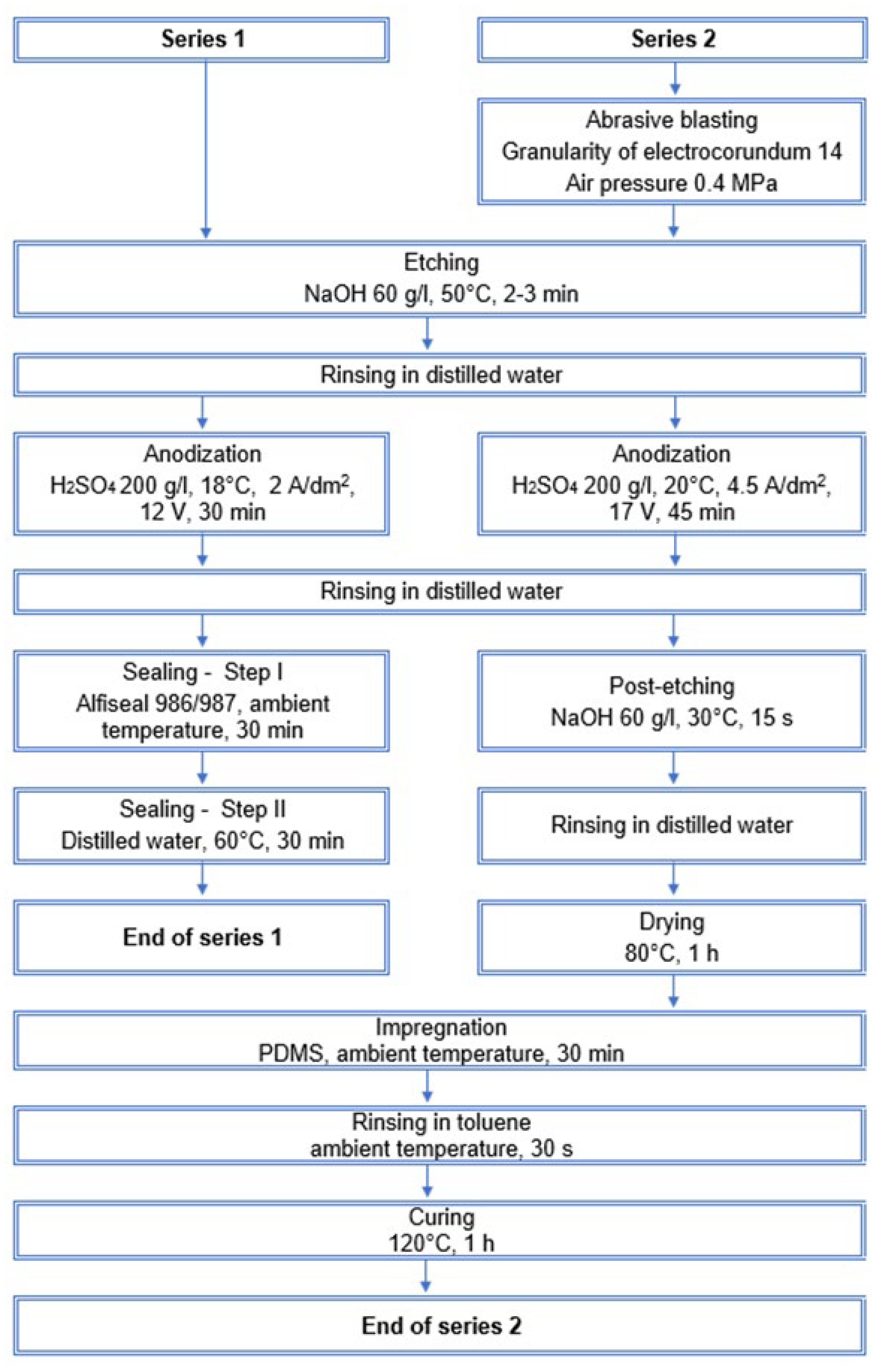
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
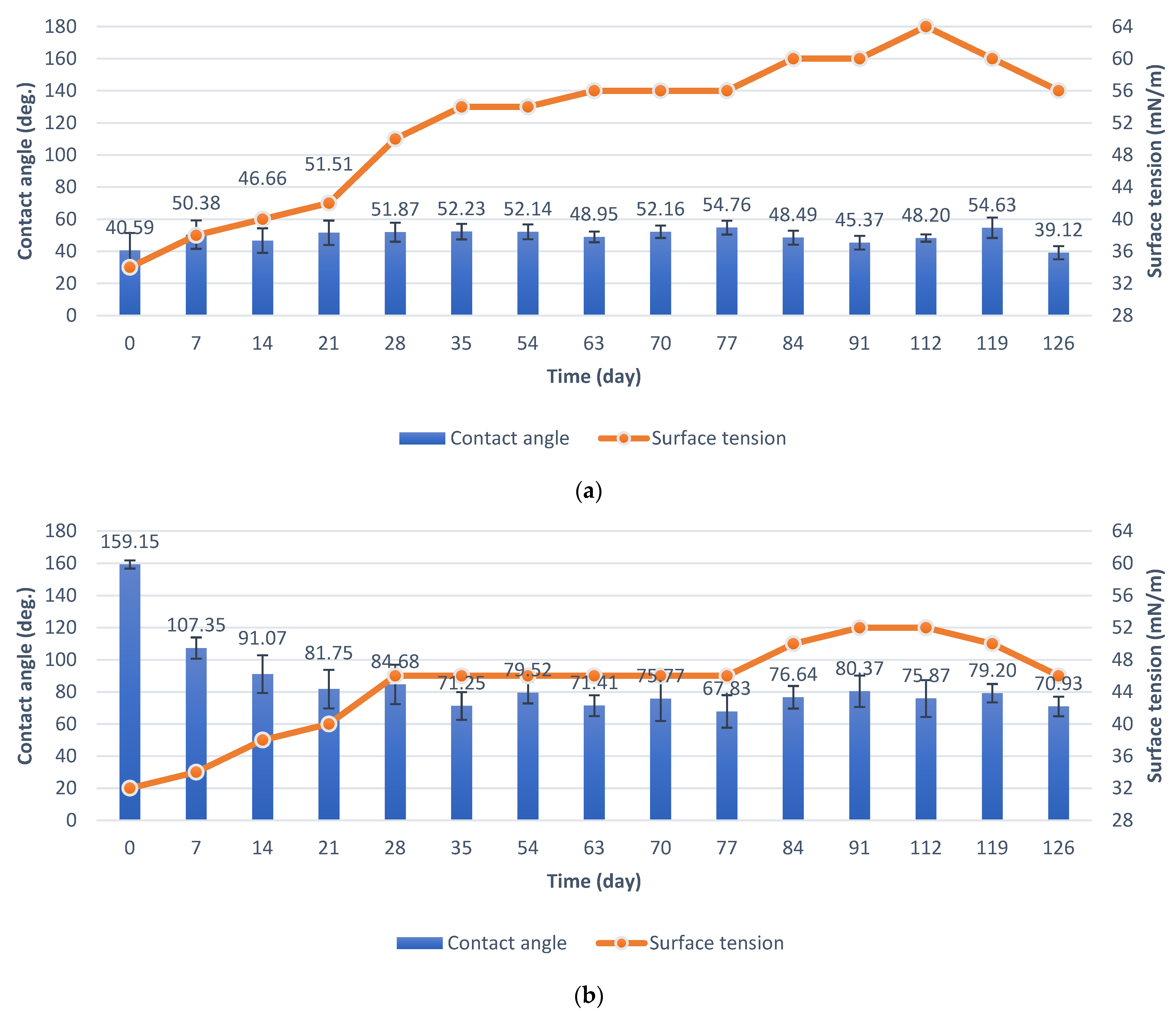
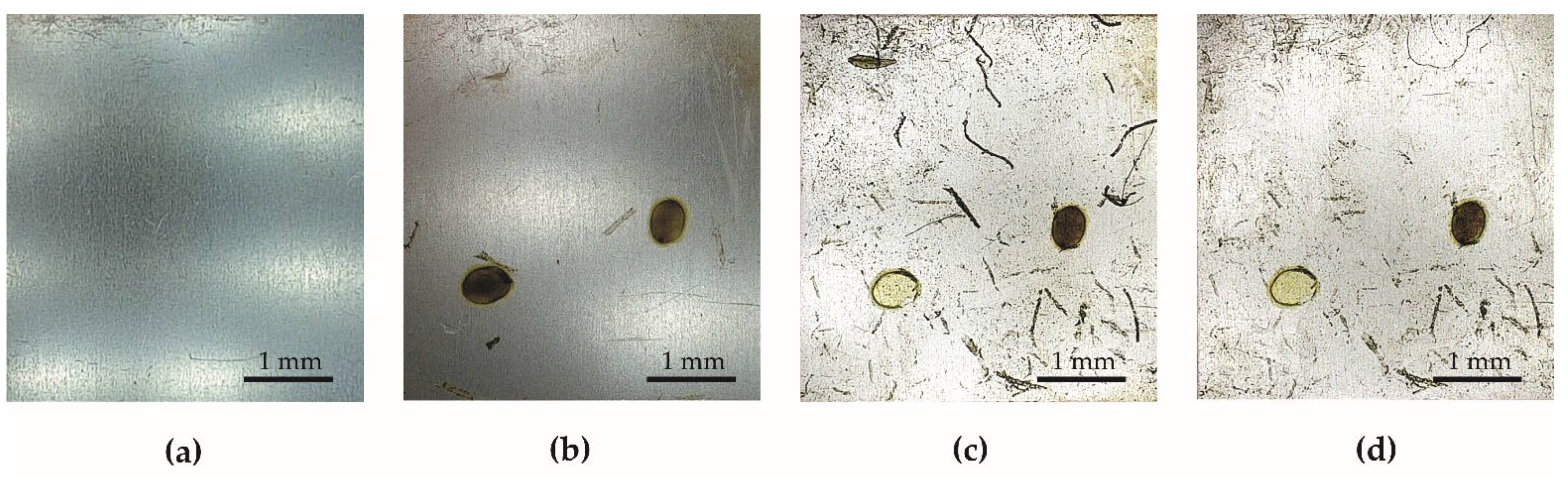
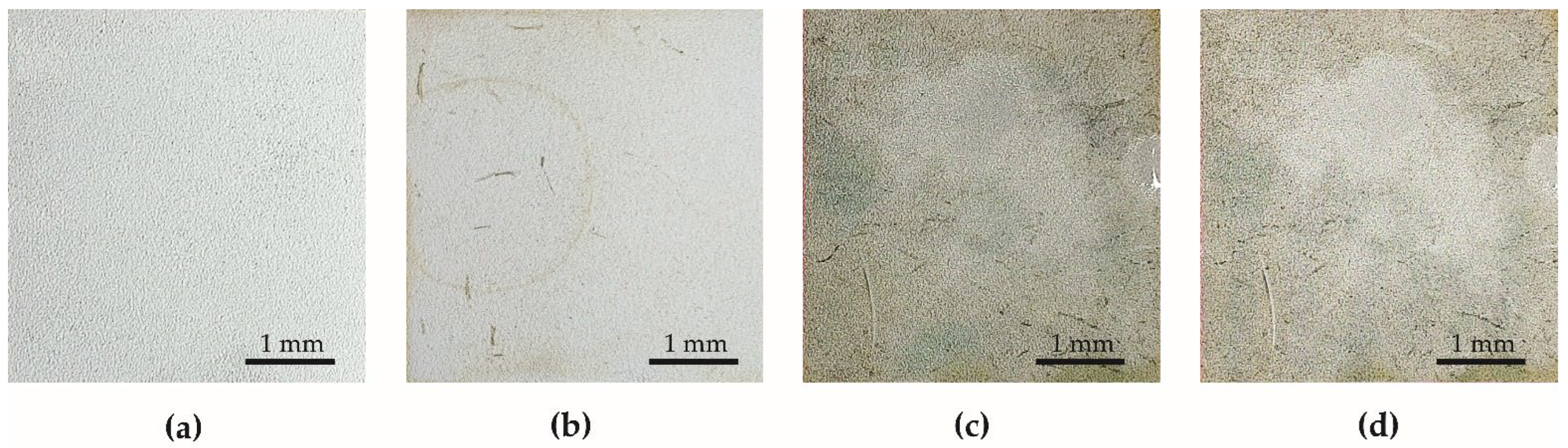
5.1. Durability Test in Natural Conditions
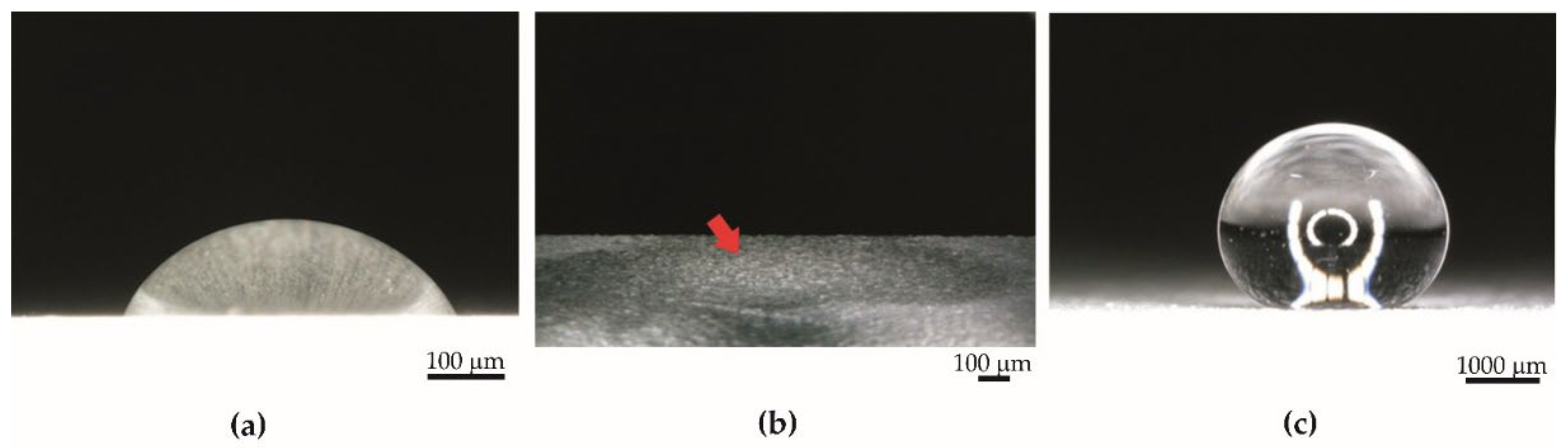
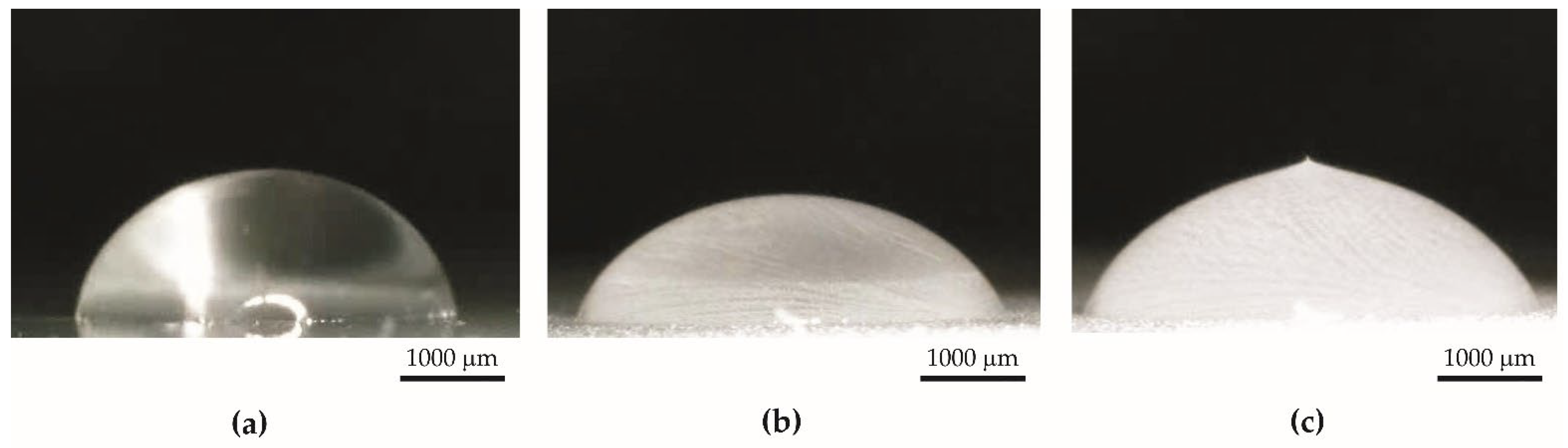
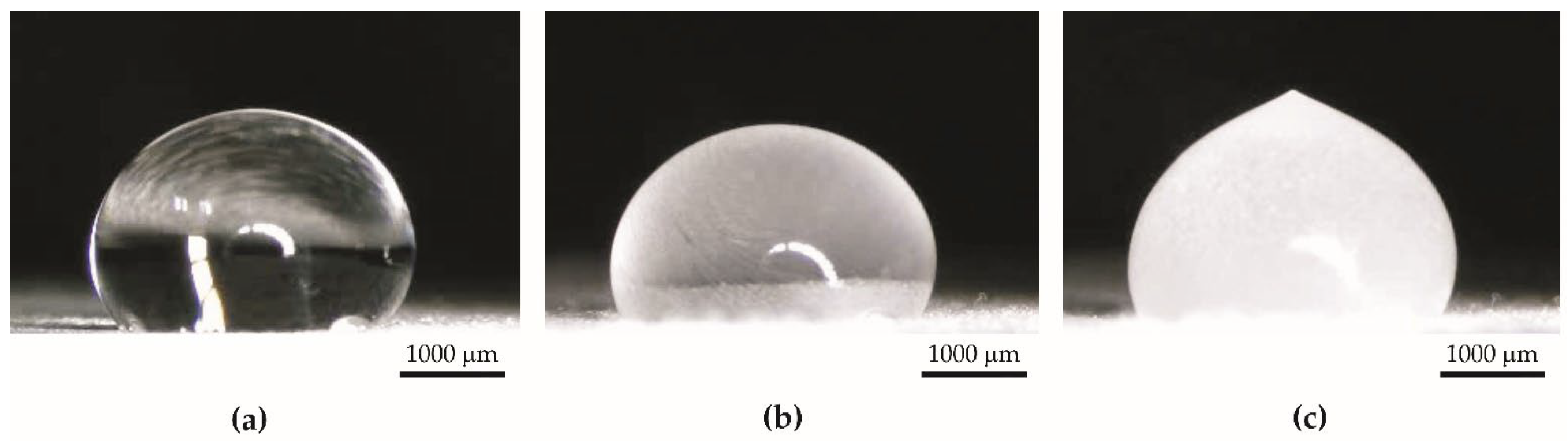
- The contact angle of aluminum anodized by the basic process increased from 40.59° to 53.07°. Surface tension increased from 34 mN/m to 52 mN/m.
- The contact angle of the superhydrophobic coating with PDMS decreased from 159.15° to 131.55°. A significant reduction in the contact angle occurred after 63 days of the test. Surface tension increased from 32 mN/m to 34 mN/m. The coating had sufficient self-cleaning properties, despite the loss of superhydrophobicity.
5.2. Anti-Fouling Test in the Pond
- The contact angle of aluminum anodized by the basic process decreased from 40.59° to 39.12°. Surface tension increased from 34 mN/m to 56 mN/m.
- The contact angle of superhydrophobic coating with PDMS decreased from 159.15° to 70.93°. Surface tension increased from 32 mN/m to 44 mN/m. The specimens with PDMS had better anti-fouling properties.
5.3. Anti-Icing Test
- Icing delay time for aluminum anodized by the basic process was 146 s ± 10 s. For the superhydrophobic coating with PDMS, the icing delay time was 328 s ± 52 s.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohamed, A.M.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef] [Green Version]
- Fürstner, R.; Barthlott, W.; Neinhuis, C.; Walzel, P. Wetting and self-cleaning properties of artificial superhydrophobic surfaces. Langmuir 2005, 21, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhan, Y.; Yu, S. Applications of superhydrophobic coatings in anti-icing: Theory, mechanisms, impact factors, challenges and perspectives. Prog. Org. Coat. 2021, 152, 106117. [Google Scholar] [CrossRef]
- Chapman, J.; Regan, F. Nanofunctionalized superhydrophobic antifouling coatings for environmental sensor applications—Advancing deployment with answers from nature. Adv. Eng. Mater. 2012, 14, B175–B184. [Google Scholar] [CrossRef]
- Qin, D. A facile approach for the fabrication of superhydrophobic surface with candle smoke particles. Physicochem. Probl. Miner. Process. 2015, 51, 501–510. [Google Scholar]
- Liang, C.J.; Liao, J.D.; Li, A.J.; Chen, C.; Lin, H.Y.; Wang, X.J.; Xu, Y.H. Relationship between wettabilities and chemical compositions of candle soots. Fuel 2014, 124, 422–427. [Google Scholar] [CrossRef]
- Pagels, J.; Wierzbicka, A.; Nilsson, E.; Isaxon, C.; Dahl, A.; Gudmundsson, A.; Swietlicki, E.; Bohgard, M. Chemical composition and mass emission factors of candle smoke particles. J. Aerosol Sci. 2009, 40, 193–208. [Google Scholar] [CrossRef] [Green Version]
- Ruan, M.; Li, W.; Wang, B.; Luo, Q.; Ma, F.; Yu, Z. Optimal conditions for the preparation of superhydrophobic surfaces on al substrates using a simple etching approach. Appl. Surf. Sci. 2012, 258, 7031–7035. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, M.; Li, J.; Ye, X.; Li, G.; Cai, L. Superhydrophobic surfaces fabricated by microstructuring of stainless steel using a femtosecond laser. J. Taiwan Inst. Chem. Eng. 2009, 256, 61–66. [Google Scholar] [CrossRef]
- Baldacchini, T.; Carey, J.E.; Zhou, M.; Mazur, E. Superhydrophobic surfaces prepared by microstructuring of silicon using a femtosecond laser. Langmuir 2006, 22, 4917–4919. [Google Scholar] [CrossRef]
- Dunn, A.; Wasley, T.J.; Li, J.; Kay, R.W.; Stringer, J.; Smith, P.J.; Esenturk, E.; Connaughton, C.; Shephard, J.D. Laser textured superhydrophobic surfaces and their applications for homogeneous spot deposition. Appl. Surf. Sci. 2016, 365, 153–159. [Google Scholar]
- Öner, D.; McCarthy, T.J. Ultrahydrophobic surfaces. Effects of topography length scales on wettability. Langmuir 2000, 16, 7777–7782. [Google Scholar] [CrossRef]
- Choi, H.J.; Choo, S.; Shin, J.H.; Kim, K.I.; Lee, H. Fabrication of superhydrophobic and oleophobic surfaces with overhang structure by reverse nanoimprint lithography. J. Phys. Chem. C 2013, 117, 24354–24359. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Fujishima, A. Superhydrophobic surfaces developed by mimicking hierarchical surface morphology of lotus leaf. Molecules 2014, 19, 4256–4283. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Li, S.; Wang, L. Fabrication of artificial super-hydrophobic lotus-leaf-like bamboo surfaces through soft lithography. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 389–395. [Google Scholar] [CrossRef]
- Chang, K.C.; Lu, H.I.; Peng, C.W.; Lai, M.C.; Hsu, S.C.; Hsu, M.H.; Tsai, Y.K.; Chang, C.H.; Hung, W.I.; Wei, Y.; et al. Nanocasting technique to prepare lotus-leaf-like superhydrophobic electroactive polyimide as advanced anticorrosive coatings. ACS Appl. Mater. Interfaces 2013, 5, 1460–1467. [Google Scholar] [CrossRef]
- Liu, B.; He, Y.; Fan, Y.; Wang, X. Fabricating Super-Hydrophobic Lotus-Leaf-Like Surfaces through Soft-Lithographic Imprinting. Macromol. Rapid Commun. 2006, 27, 1859–1864. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, X.; Bin, J.; Peng, C.; Xing, S.; Wang, M.; Xiao, J.; Zeng, J.; Xie, Y.; Fu, X.; et al. A novel fabrication of a superhydrophobic surface with highly similar hierarchical structure of the lotus leaf on a copper sheet. Appl. Surf. Sci. 2013, 285, 205–210. [Google Scholar] [CrossRef]
- Osuchowska, E.; Buczko, Z.; Olkowicz, K. The effect of surface formation on the wettability of Zn-Cr coatings. Przemysł Chem. 2019, 98, 630–633. [Google Scholar]
- Osuchowska, E.; Buczko, Z.; Olkowicz, K. Zn-Cr alloy coatings: Electrodeposition and properties. J. KONBiN 2021, 51, 29–41. [Google Scholar] [CrossRef]
- Haghdoost, A.; Pitchumani, R. Fabricating superhydrophobic surfaces via a two-step electrodeposition technique. Langumir 2014, 30, 4183–4191. [Google Scholar] [CrossRef] [PubMed]
- Buczko, Z.; Olkowicz, K.; Krasucki, J.; Grabowiecki, K.; Osuchowska, E.; Tomassi, P. Superhydrophobic properties of aluminium produced by surface abrasive blasting, anodic oxidation and fatty acid impregnation. Trans. IMF 2021, 99, 73–79. [Google Scholar] [CrossRef]
- Wu, R.; Chao, G.; Jiang, H.; Hu, Y.; Pan, A. The superhydrophobic aluminum surface prepared by different methods. Mater. Lett. 2015, 142, 176–179. [Google Scholar] [CrossRef]
- Leese, H.; Bhurtun, V.; Lee, K.P.; Mattia, D. Wetting behaviour of hydrophilic and hydrophobic nanostructured porous anodic alumina. Colloids Surf. A Physicochem. Eng. Asp. 2013, 420, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Hwang, W.; Park, H.C.; Lee, K.H. Superhydrophobic nanostructures based on porous alumina. Curr. Appl. Phys. 2008, 8, 770–773. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2022, 13, 2. [Google Scholar] [CrossRef]
- Atthi, N.; Sripumkhai, W.; Pattamang, P.; Thongsook, O.; Meananeatra, R.; Saengdee, P.; Srihapat, A.; Supadech, J.; Janseng, T.; Maneesong, A.; et al. Superhydrophobic and superoleophobic properties enhancement on PDMS micro-structure using simple flame treatment method. Microelectron. Eng. 2020, 230, 111362. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Chen, F.; Zhang, D.; Du, G.; Bian, H.; Si, J.; Yun, F.; Hou, X. Superhydrophobic PDMS surfaces with three-dimensional (3D) pattern-dependent controllable adhesion. Appl. Surf. Sci. 2014, 288, 579–583. [Google Scholar] [CrossRef]
- Martin, S.; Bhushan, B. Transparent, wear-resistant, superhydrophobic and superoleophobic poly(dimethylsiloxane) (PDMS) surfaces. J. Colloid Interface Sci. 2017, 488, 118–126. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Li, L.; Zhang, H.; Qiu, C. Drag-reductive and anti-corrosive superhydrophobic surface fabricated on aluminum with thin PDMS/SiO2 coating. Mater. Res. Express 2019, 6, 1065a8. [Google Scholar] [CrossRef]
- Sun, W.; Wang, L.; Yang, Z.; Li, S.; Wu, T.; Liu, G. Fabrication of polydimethylsiloxane-derived superhydrophobic surface on aluminium via chemical vapour deposition technique for corrosion protection. Corros. Sci. 2017, 128, 176–185. [Google Scholar] [CrossRef]
- Tong, W.; Karthik, N.; Li, J.; Wang, N.; Xiong, D. Superhydrophobic surface with stepwise multilayered micro-and nanostructure and an investigation of its corrosion resistance. Langmuir 2019, 35, 15078–15085. [Google Scholar] [CrossRef]
- Wang, M.; Ye, X.; Feng, J. Fabrication of length-controlled polymer nanopillars using poly (dimethylsiloxane) filled anodised aluminium oxide templates. Micro Nano Lett. 2013, 8, 713–717. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, H.U.; Kim, N.H.; Lee, K.H.; Cho, D.W.; Kwon, T.H. Fabrication of microchannel containing nanopillar arrays using micromachined AAO (anodic aluminum oxide) mold. Microelectron. Eng. 2007, 84, 1532–1535. [Google Scholar] [CrossRef]
- Barthwal, S.; Lee, B.; Lim, S.H. Fabrication of robust and durable slippery anti-icing coating on textured superhydrophobic aluminum surfaces with infused silicone oil. Appl. Surf. Sci. 2019, 496, 143677. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Chen, J.; Gu, H.; Liu, J.; Wang, R.; Zhang, B.; Zhang, H.; Zhang, Q. Design and preparation of bioinspired slippery liquid-infused porous surfaces with anti-icing performance via delayed phase inversion process. Colloids Surf. A Physicochem. Eng. Asp. 2020, 588, 124384. [Google Scholar] [CrossRef]
- Wen, S.F.; Wang, Y.M.; Zhang, Z.M.; Liu, Y.L. Application of anti-icing coating based on adsorption of functional substances by microporous sphere. Prog. Org. Coat. 2019, 137, 105320. [Google Scholar] [CrossRef]
- Hong, S.; Wang, R.; Huang, X.; Liu, H. Facile one-step fabrication of PHC/PDMS anti-icing coatings with mechanical properties and good durability. Prog. Org. Coat. 2019, 135, 263–269. [Google Scholar] [CrossRef]
- Yang, C.; Wang, F.; Li, W.; Ou, J.; Li, C.; Amirfazli, A. Anti-icing properties of superhydrophobic ZnO/PDMS composite coating. Appl. Phys. A 2016, 122, 1–10. [Google Scholar] [CrossRef]
- Xie, Q.; Hao, T.; Zhang, J.; Wang, C.; Zhang, R.; Qi, H. Anti-Icing Performance of a Coating Based on Nano/Microsilica Particle-Filled Amino-Terminated PDMS-Modified Epoxy. Coatings 2019, 9, 771. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zi, Y.; Zhu, J.; Huang, W.; Zhang, Z.; Zhang, H. Construction of super-hydrophobic PDMS@ MOF@ Cu mesh for reduced drag, anti-fouling and self-cleaning towards marine vehicle applications. Chem. Eng. J. 2021, 417, 129265. [Google Scholar] [CrossRef]
- Lin, Y.T.; Ting, Y.S.; Chen, B.Y.; Cheng, Y.W.; Liu, T.Y. Bionic shark skin replica and zwitterionic polymer brushes functionalized PDMS membrane for anti-fouling and wound dressing applications. Surf. Coat. Technol. 2020, 391, 125663. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Pan, Y.; Liu, G.; Wu, H.; Jiang, M.; Jin, W. Fluorinated PDMS membrane with anti-biofouling property for in-situ biobutanol recovery from fermentation-pervaporation coupled process. J. Membr. Sci. 2020, 609, 118225. [Google Scholar] [CrossRef]
- Rahimi, M.; Fojan, P.; Gurevich, L.; Afshari, A. Aluminium alloy 8011: Surface characteristics. Appl. Mech. Mater. 2015, 719, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.L.; Lian, Z.X.; Lou, J.; Yu, H.D. Fabrication and coupling mechanism of Super-hydrophobical aluminium alloy surface. China Surf. Eng. 2014, 27, 112–116. [Google Scholar]
- Polini, W.; Sorrentino, L. Improving the wettability of 2024 aluminium alloy by means of cold plasma treatment. Appl. Surf. Sci. 2003, 214, 232–242. [Google Scholar] [CrossRef]
- Meteoblue. Available online: https://www.meteoblue.com/ (accessed on 25 November 2021).
- Stanton, M.M.; Ducker, R.E.; MacDonald, J.C.; Lambert, C.R.; McGimpsey, W.G. Super-hydrophobic, highly adhesive, polydimethylsiloxane (PDMS) surfaces. J. Colloid Interface Sci. 2012, 367, 502–508. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Sun, S.; Zhang, H.; Zhou, R.; Li, Y.; Liang, Y.; Wang, J. Preparation of a temperature-sensitive superhydrophobic self-cleaning SiO2-TiO2@ PDMS coating with photocatalytic activity. Surf. Coat. Technol. 2021, 408, 126853. [Google Scholar] [CrossRef]
- Barthwal, S.; Barthwal, S.; Singh, B.; Singh, N.B. Multifunctional and fluorine-free superhydrophobic composite coating based on PDMS modified MWCNTs/ZnO with self-cleaning, oil-water separation, and flame retardant properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124776. [Google Scholar] [CrossRef]
- Buczko, Z.; Olkowicz, K.; Tomassi, P.; Zolciak, T. Coatings with carbon nanostructures with hydrophobic properties made by anodizing and the CVD method on stainless steel. Inzynieria Powierzchni 2018, 23, 30–37. [Google Scholar]
- Alam, K.; Khan, K.I.; Ullah, A.; Ullah, A.; Ali, S.; Ullah, S.; Ali, A.; Hussain, S. Fabrication of superhydrophillic and graded index antireflective double layer coating for solar photovoltaics module using aerosol impact deposition assembly. Thin Solid Films 2021, 721, 138518. [Google Scholar] [CrossRef]
- Young, T. III. An essay on the cohesion of fluids. Philos. Trans. Ro. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Miwa, M.; Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watanabe, T. Effects of the surface roughness on sliding angles of water droplets on superhydrophobic surfaces. Langmuir 2000, 16, 5754–5760. [Google Scholar] [CrossRef]
- Marmur, A. Wetting on hydrophobic rough surfaces: To be heterogeneous or not to be? Langmuir 2003, 19, 8343–8348. [Google Scholar] [CrossRef]

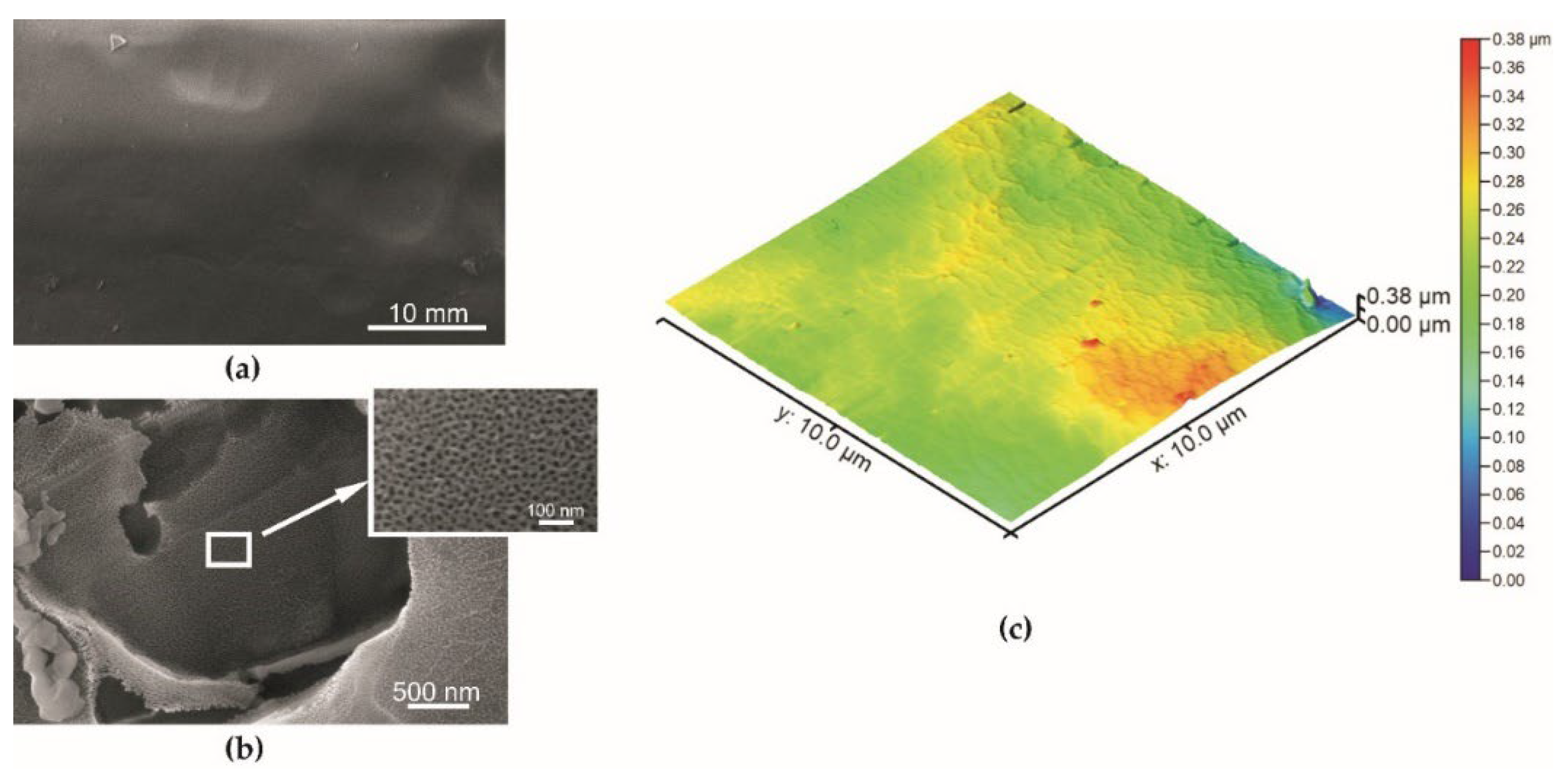
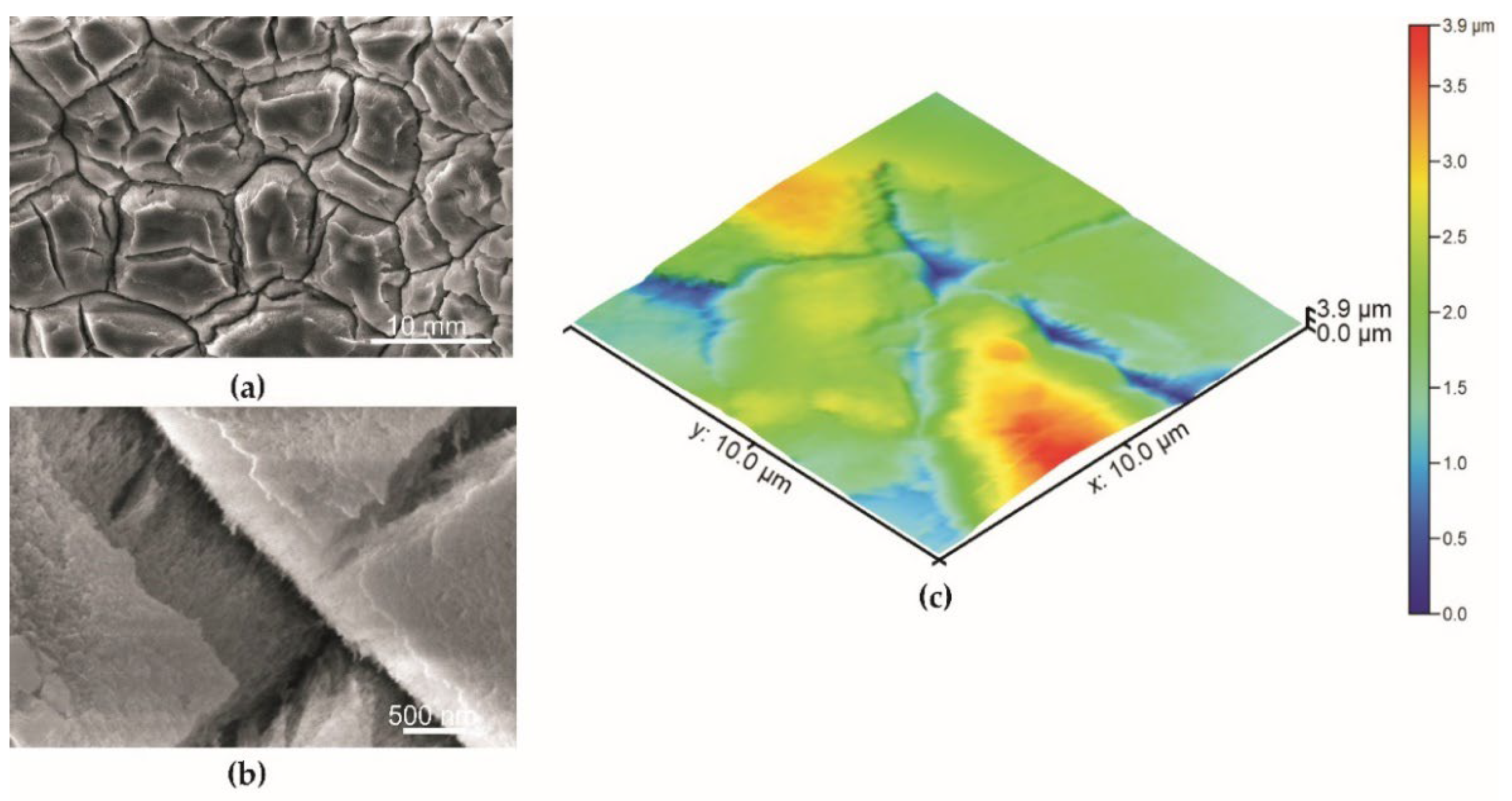
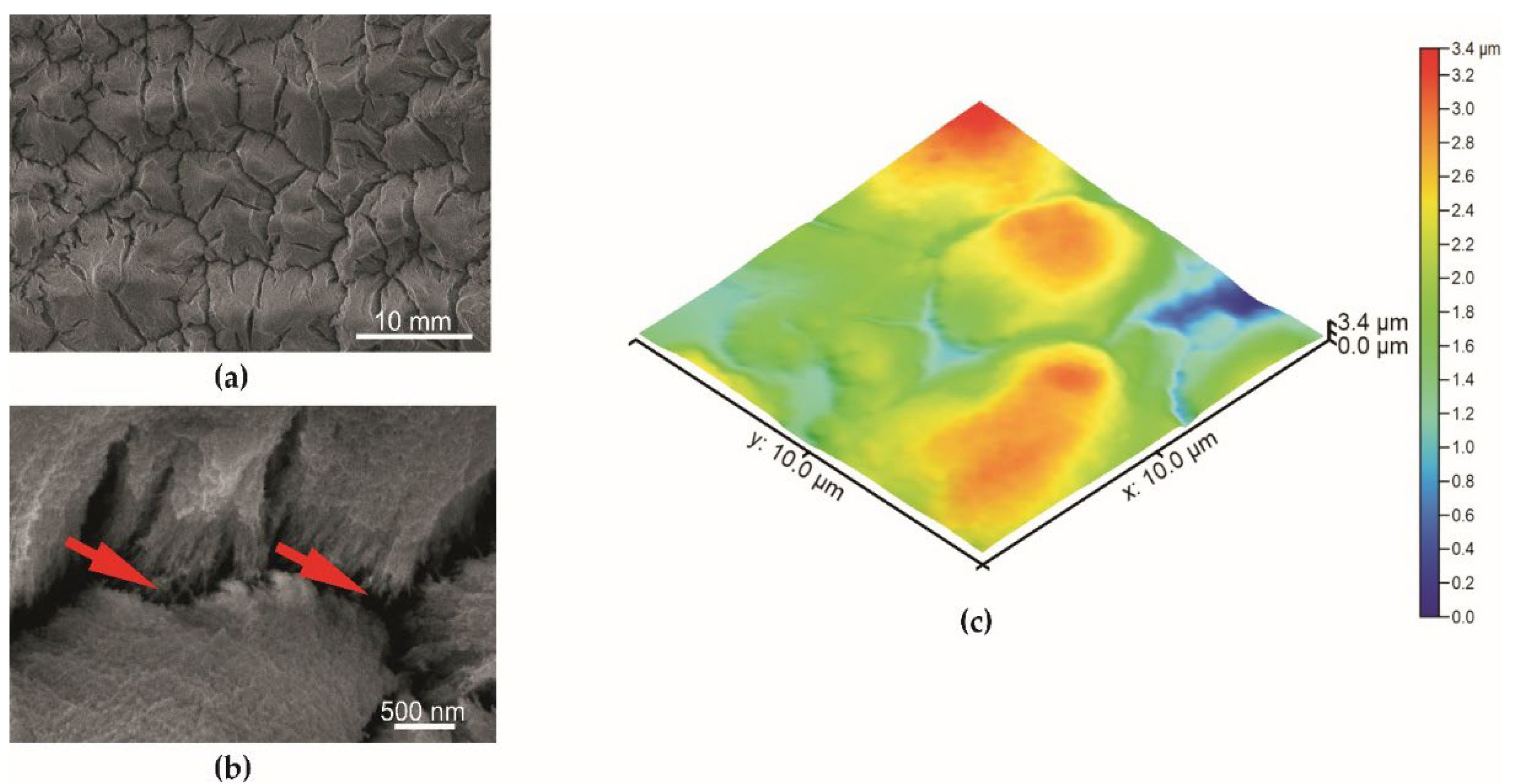
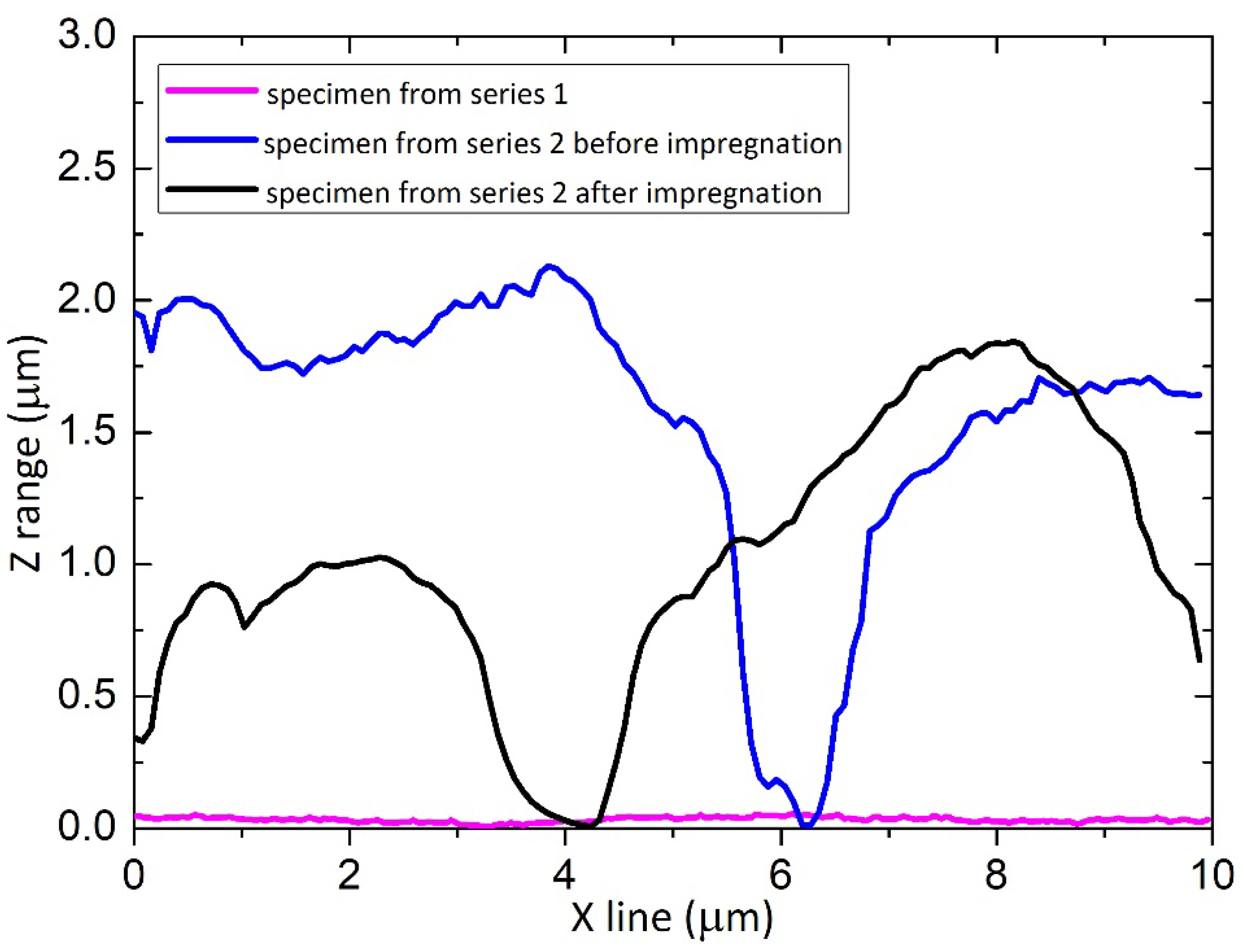

| Specimen | Sa—Average Surface Roughness (nm) |
|---|---|
| Series 1 | 25.5 ± 3.5 |
| Series 2 before impregnation | 484.5 ± 42.4 |
| Series 2 after impregnation | 474.0 ± 38.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olkowicz, K.; Buczko, Z.; Nasiłowska, B.; Kowalczyk, K.; Czwartos, J. Superhydrophobic Coating Based on Porous Aluminum Oxide Modified by Polydimethylsiloxane (PDMS). Materials 2022, 15, 1042. https://doi.org/10.3390/ma15031042
Olkowicz K, Buczko Z, Nasiłowska B, Kowalczyk K, Czwartos J. Superhydrophobic Coating Based on Porous Aluminum Oxide Modified by Polydimethylsiloxane (PDMS). Materials. 2022; 15(3):1042. https://doi.org/10.3390/ma15031042
Chicago/Turabian StyleOlkowicz, Klaudia, Zofia Buczko, Barbara Nasiłowska, Kamil Kowalczyk, and Joanna Czwartos. 2022. "Superhydrophobic Coating Based on Porous Aluminum Oxide Modified by Polydimethylsiloxane (PDMS)" Materials 15, no. 3: 1042. https://doi.org/10.3390/ma15031042
APA StyleOlkowicz, K., Buczko, Z., Nasiłowska, B., Kowalczyk, K., & Czwartos, J. (2022). Superhydrophobic Coating Based on Porous Aluminum Oxide Modified by Polydimethylsiloxane (PDMS). Materials, 15(3), 1042. https://doi.org/10.3390/ma15031042







