Aging Interfacial Structure and Abnormal Tensile Strength of SnAg3Cu0.5/Cu Solder Joints
Abstract
1. Introduction
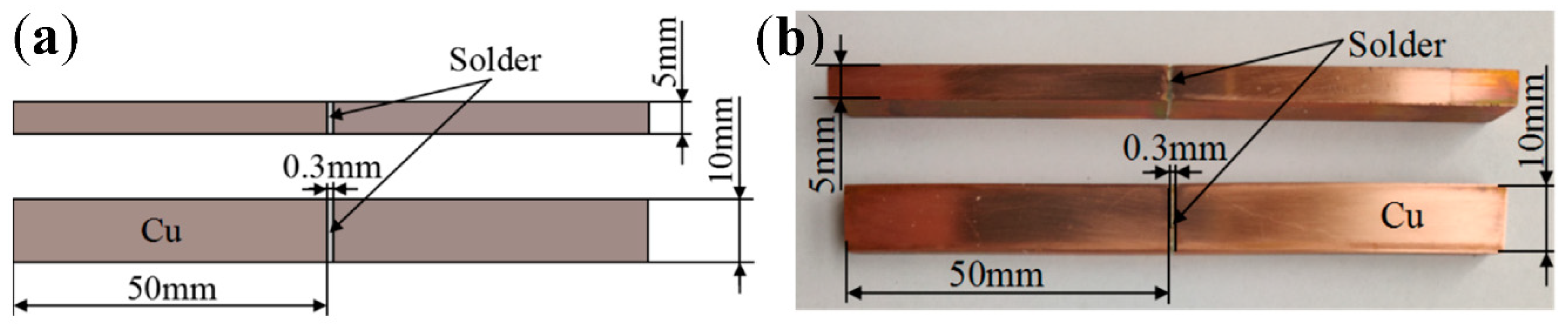
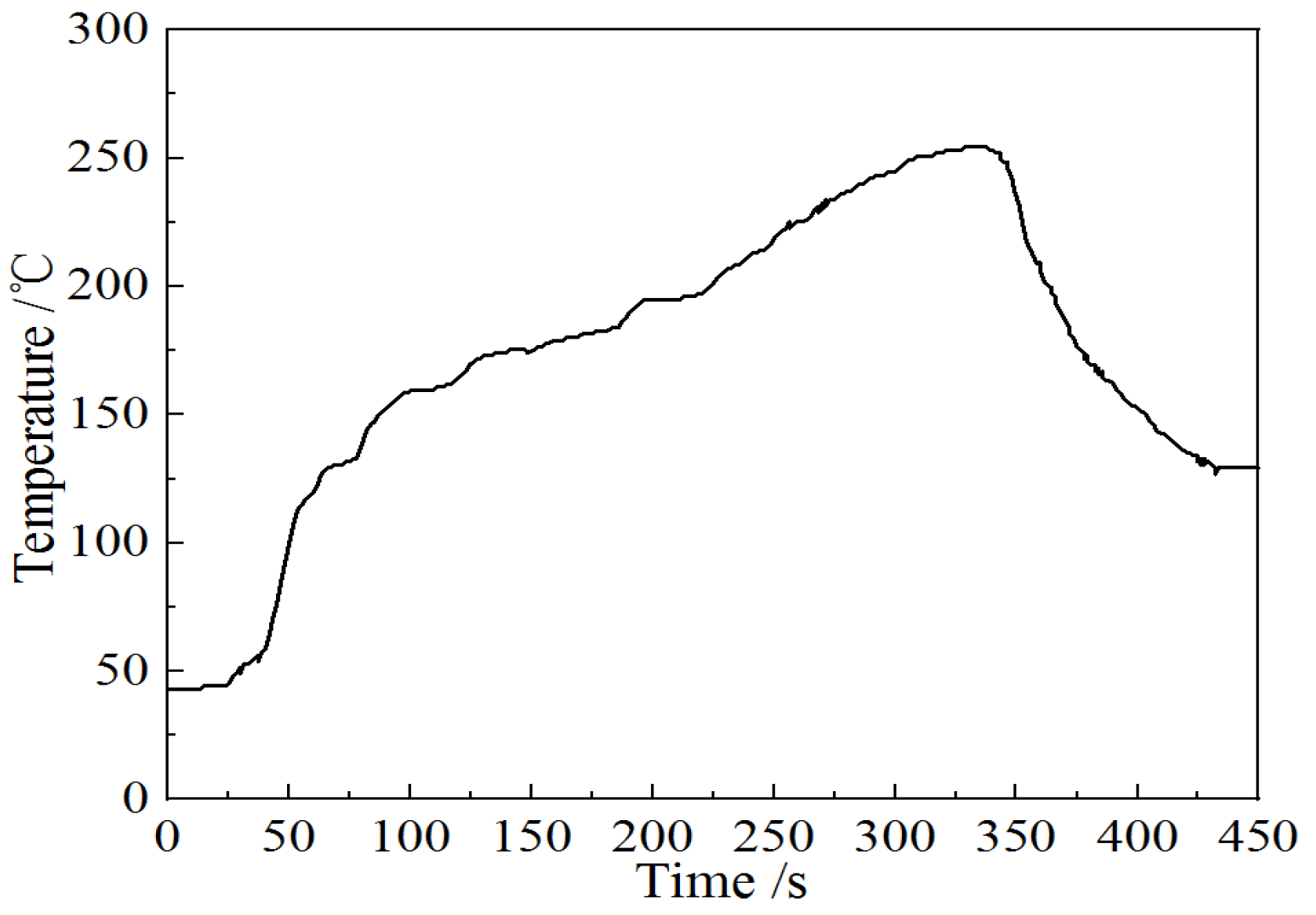
2. Experimental Procedures
3. Results and Discussion
3.1. Aging
3.2. Interface Layer Composition
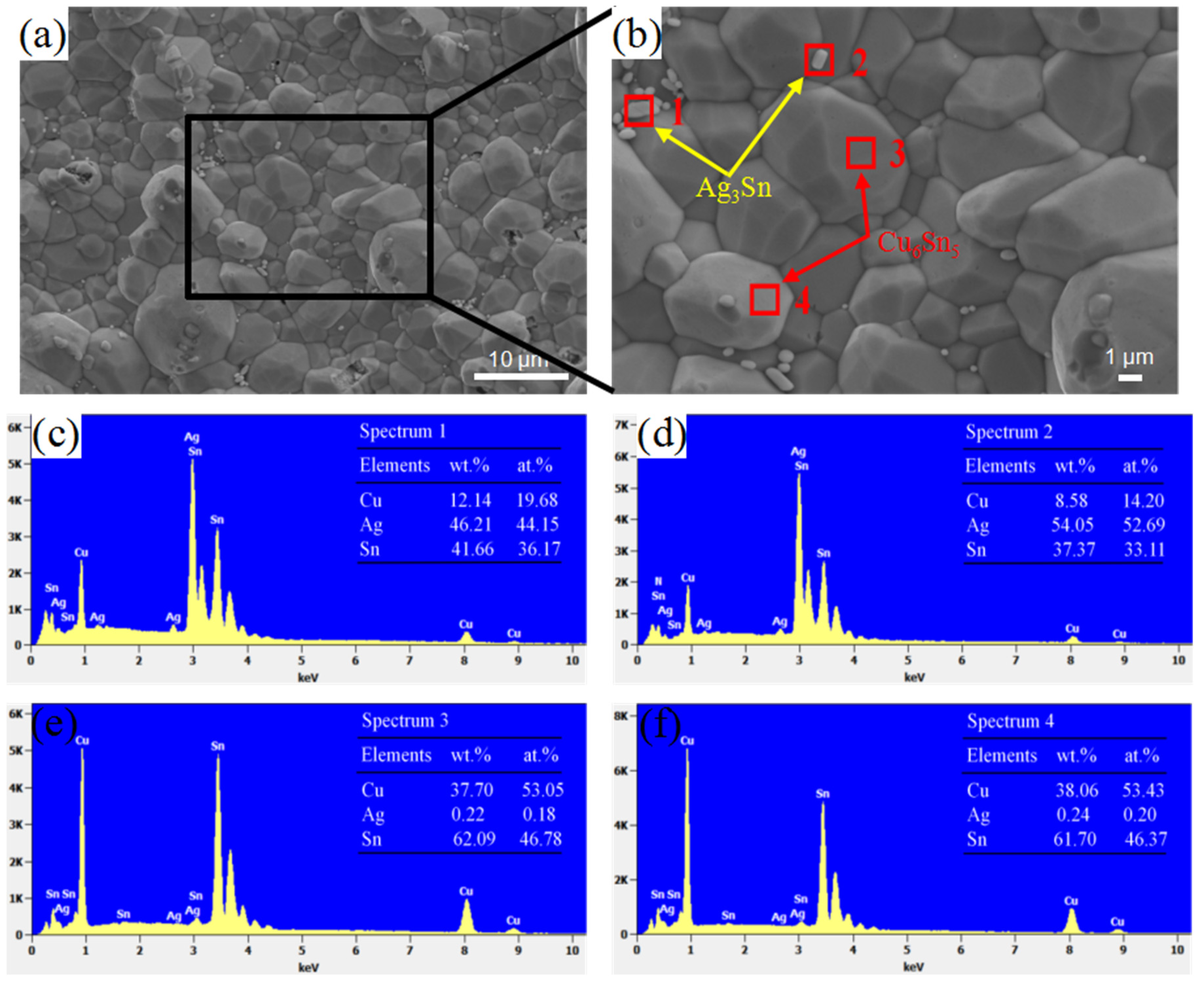
3.3. The IMCs Morphology
3.4. Tensile Strength and Fracture Behavior of the Solder Joints
4. Conclusions
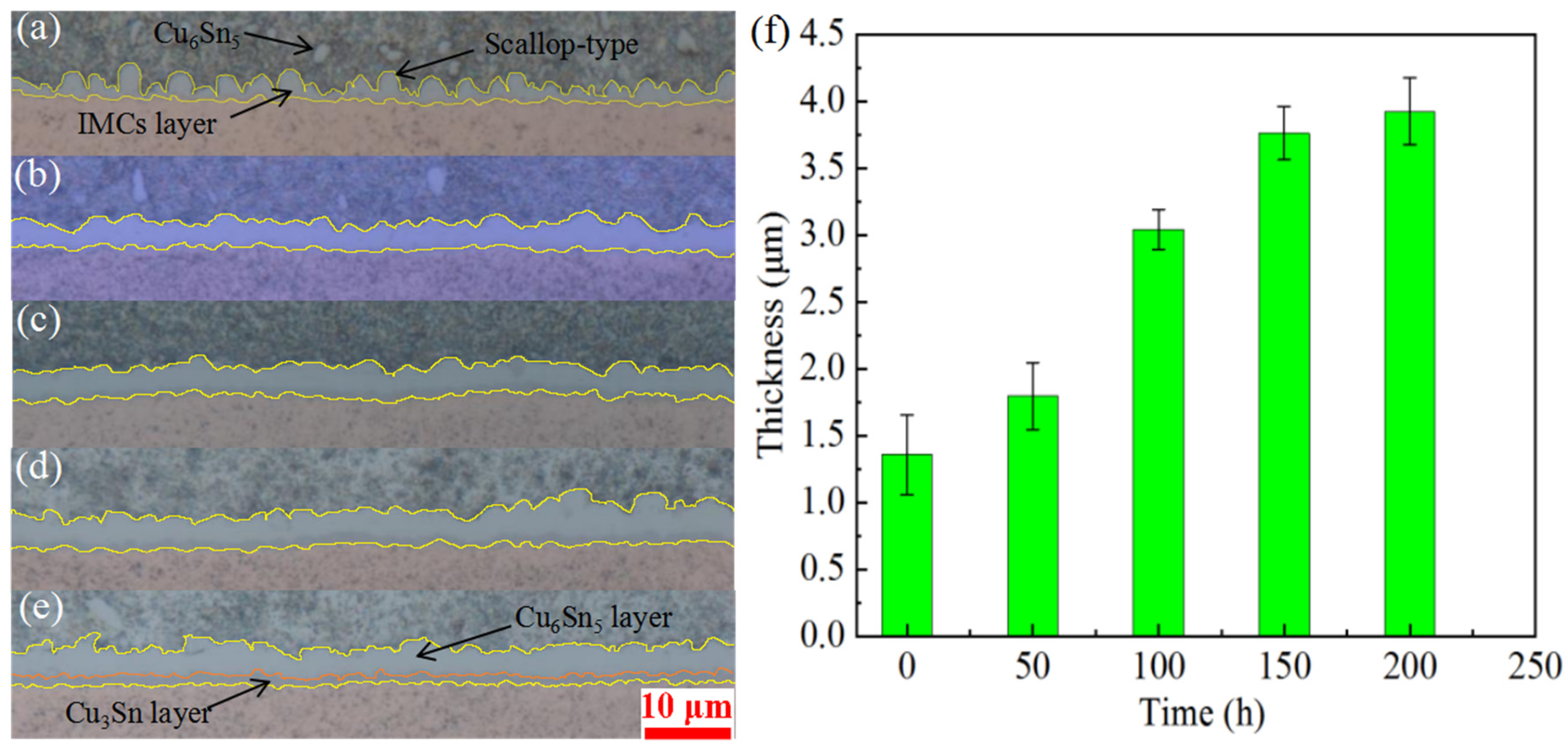
- The IMC layer at the interface after the reflow was thin and scallop-type. After 200 h of aging at 150 °C, the thickness of the interface gradually increased to reach 3.93 μm, and the interface became smooth.
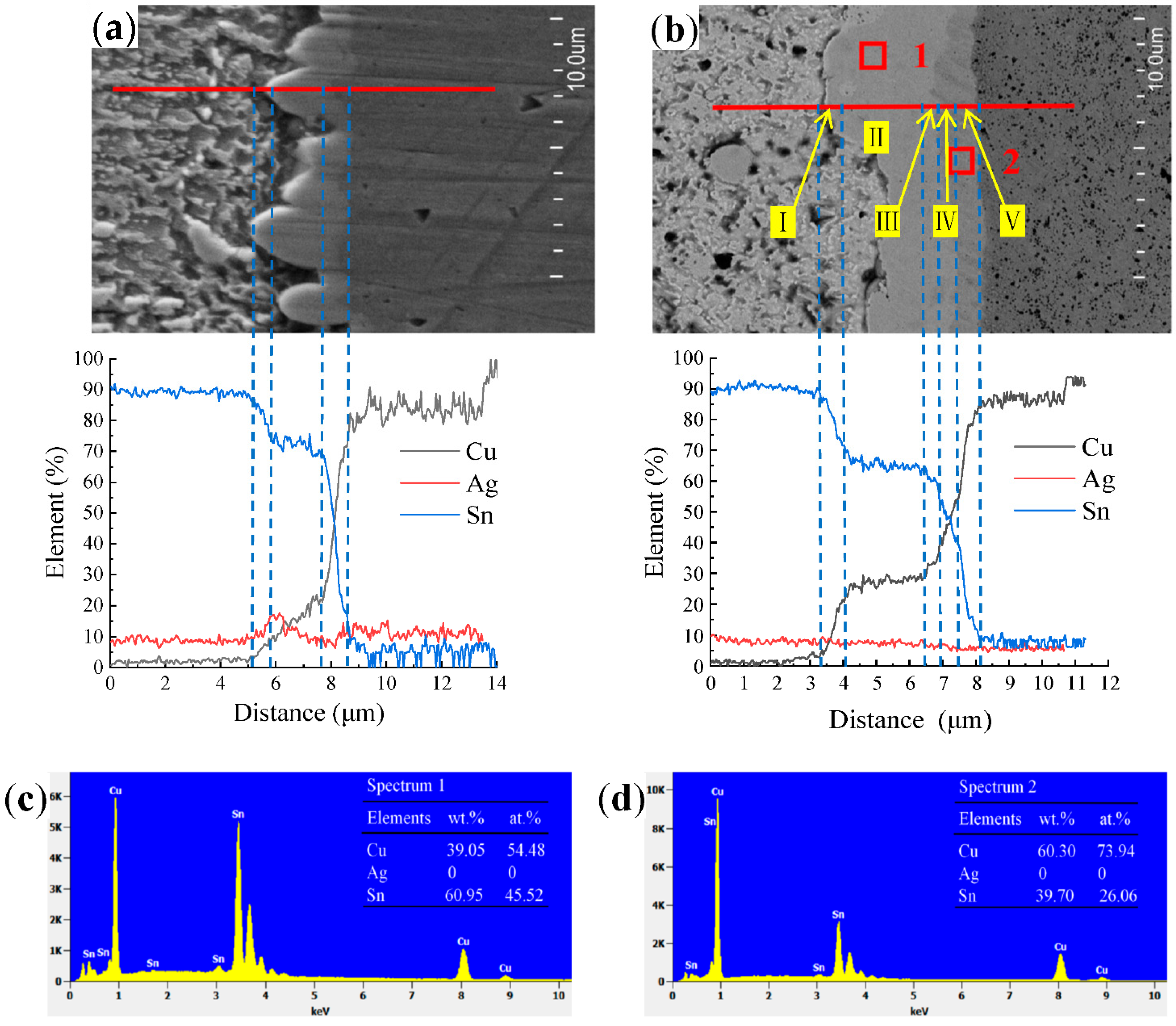
- Analysis of the unaged solder joint interface revealed that it could be divided into three areas: the transition layer between the solder, the Cu6Sn5 layer, and the transition layer from the Cu6Sn5 layer to the Cu plate. The solder joints interface after 200 h of aging at 150 °C could be divided into five areas. Compared with the unaged solder joint interface, this interface had more Cu3Sn in the interfacial layer.
- The top of the IMC that had just been reflowed was relatively smooth, and there was a large gap between the Cu6Sn5 particles. After 200 h of aging at 150 °C, the top of the IMC layer became denser and exhibited a prismatic shape. Ag3Sn particles precipitated in the gap between the Cu6Sn5 particles.
- The tensile strength of the joint immediately after reflow reached 81.93 MPa. When the aging time was 50 h, the tensile strength decreased to 69.58 MPa. With an increase in the aging time, the tensile strength gradually increased. When the aging time was 200 h at 150 °C, the tensile strength was 83.86 MPa, exceeding that of the unaged solder joint.
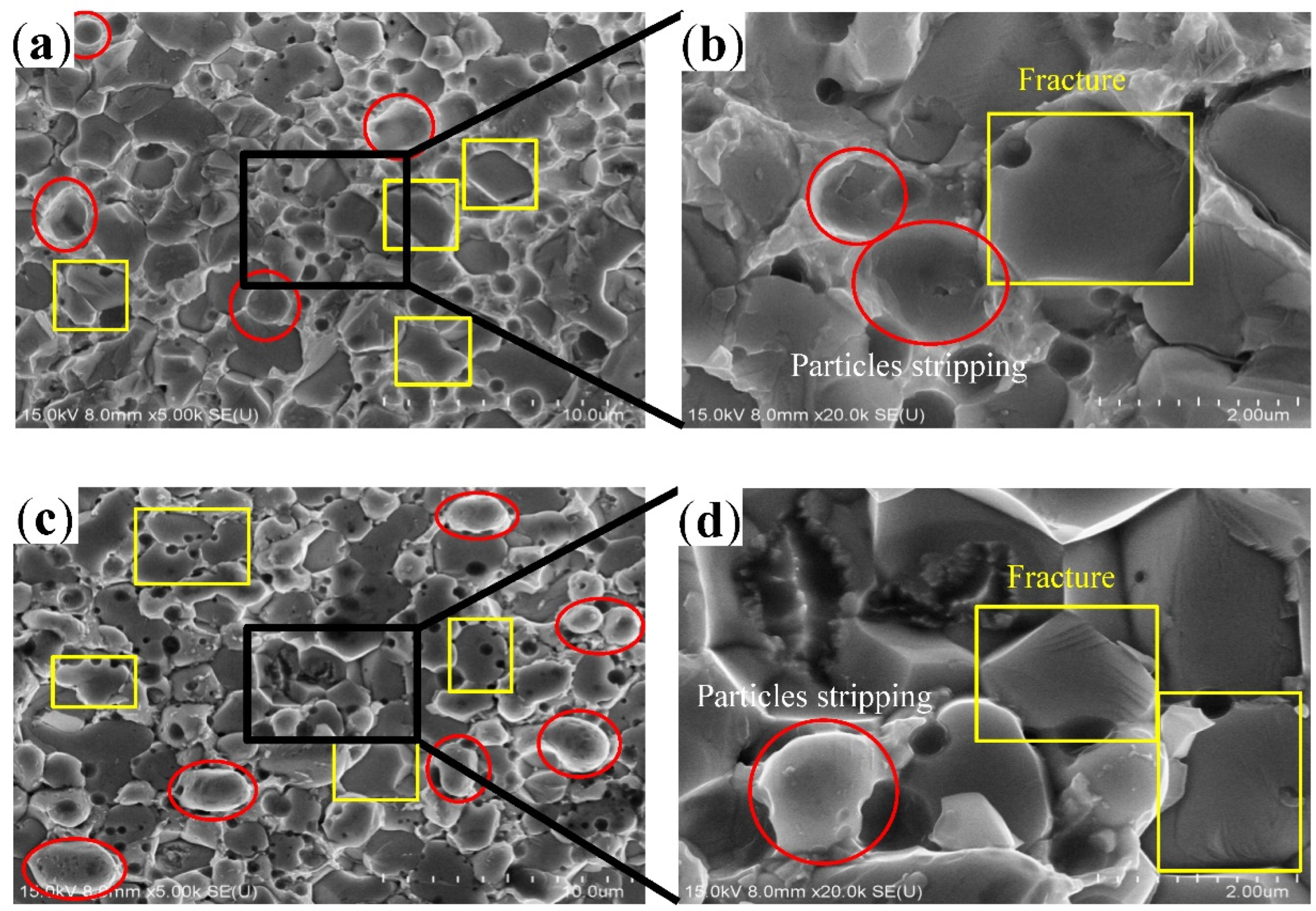
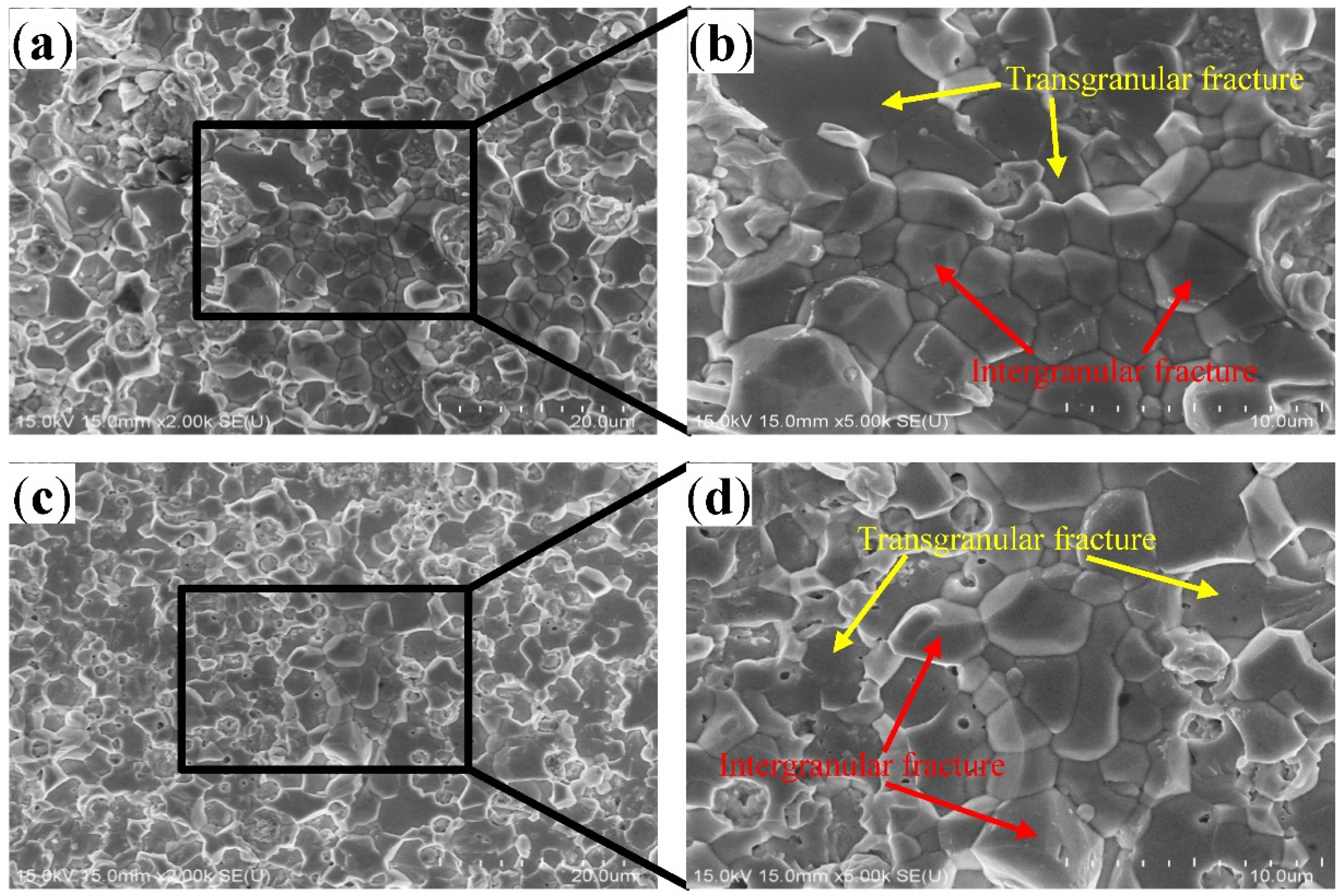
- During the tensile fracture of the joint that had just been reflowed, fracture occurred at the interface between the IMC layer and solder, and some Cu6Sn5 particles broke, while some Cu6Sn5 particles peeled off from the solder joint interface. After 200 h of aging at 150 °C, fracture occurred in the IMC layer, where some Cu6Sn5 particles underwent intergranular fracture, and some underwent transgranular fracture.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Šebo, P.; Švec, P.; Janičkovič, D.; Illeková, E.; Plevachuk, Y. Interface between Sn–Sb–Cu solder and copper substrate. Mater. Sci. Eng. A 2011, 528, 5955–5960. [Google Scholar] [CrossRef]
- Zou, H.F.; Yang, H.J.; Zhang, Z.F. Coarsening mechanisms, texture evolution and size distribution of Cu6Sn5 between Cu and Sn-based solders. Mater. Chem. Phys. 2011, 131, 190–198. [Google Scholar] [CrossRef]
- Suh, J.; Tu, K.; Lutsenko, G.; Gusak, A. Size distribution and morphology of Cu6Sn5 scallops in wetting reaction between molten solder and copper. Acta Mater. 2008, 56, 1075–1083. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, R.; Hang, C.; Niu, L.; Wang, C. Relationship between morphologies and orientations of Cu6Sn5 grains in Sn3.0Ag0.5Cu solder joints on different Cu pads. Mater. Charact. 2014, 88, 58–68. [Google Scholar] [CrossRef]
- Gusak, A.M.; Tu, K.N. Kinetic theory of flux-driven ripening. Phys. Rev. B 2002, 66, 115403. [Google Scholar] [CrossRef]
- Xu, R.; Liu, Y.; Wei, C.; Wang, X.; Gao, Z. Evolution of intermetallic compounds layers in the soldered Sn–3.7Ag–1.0In–0.9Zn/Cu interface. J. Alloys Compd. 2009, 468, 203–208. [Google Scholar] [CrossRef]
- Kim, H.K.; Tu, K.N. Kinetic analysis of the soldering reaction between eutectic SnPb alloy and Cu accompanied by ripening. Phys. Rev. B 1996, 53, 16027–16034. [Google Scholar] [CrossRef]
- Park, M.; Stephenson, M.; Shannon, C.; Díaz, L.C.; Hudspeth, K.; Gibbons, S.; Muñoz-Saldaña, J.; Arróyave, R. Experimental and computational study of the morphological evolution of intermetallic compound (Cu6Sn5) layers at the Cu/Sn interface under isothermal soldering conditions. Acta Mater. 2012, 60, 5125–5134. [Google Scholar] [CrossRef]
- Yang, M.; Li, M.; Wang, C. Interfacial reactions of eutectic Sn3.5Ag and pure tin solders with Cu substrates during liquid-state soldering. Intermetallics 2012, 25, 86–94. [Google Scholar] [CrossRef]
- Gao, F.; Takemoto, T.; Nishikawa, H. Effects of Co and Ni addition on reactive diffusion between Sn–3.5Ag solder and Cu during soldering and annealing. Mater. Sci. Eng. A 2006, 420, 39–46. [Google Scholar] [CrossRef]
- Li, Z.; Tang, Y.; Guo, Q.; Li, G. A diffusion model and growth kinetics of interfacial intermetallic compounds in Sn-0.3Ag-0.7Cu and Sn-0.3Ag-0.7Cu-0.5CeO2 solder joints. J. Alloys Compd. 2019, 818, 152893. [Google Scholar] [CrossRef]
- Li, M.-L.; Zhang, L.; Jiang, N.; Zhong, S.-J.; Zhang, L. Influences of silicon carbide nanowires’ addition on IMC growth behavior of pure Sn solder during solid–liquid diffusion. J. Mater. Sci. Mater. Electron. 2021, 32, 18067–18075. [Google Scholar] [CrossRef]
- Wang, F.-J.; Yu, Z.-S.; Qi, K. Intermetallic compound formation at Sn–3.0Ag–0.5Cu–1.0Zn lead-free solder alloy/Cu interface during as-soldered and as-aged conditions. J. Alloys Compd. 2007, 438, 110–115. [Google Scholar] [CrossRef]
- Liu, P.; Yao, P.; Liu, J. Evolutions of the interface and shear strength between SnAgCu–xNi solder and Cu substrate during isothermal aging at 150 °C. J. Alloys Compd. 2009, 486, 474–479. [Google Scholar] [CrossRef]
- Sun, Y.; Pang, J.H. Experimental and numerical investigations of near-crack-tip deformation in a solder alloy. Acta Mater. 2008, 56, 537–548. [Google Scholar] [CrossRef]
- Kuna, M.; Wippler, S. A cyclic viscoplastic and creep damage model for lead free solder alloys. Eng. Fract. Mech. 2010, 77, 3635–3647. [Google Scholar] [CrossRef]
- Lai, Y.; Hu, X.; Jiang, X.; Li, Y. Effect of Ni Addition to Sn0.7Cu Solder Alloy on Thermal Behavior, Microstructure, and Mechanical Properties. J. Mater. Eng. Perform. 2018, 27, 6564–6576. [Google Scholar] [CrossRef]
- Thambi, J.; Tetzlaff, U.; Schiessl, A.; Lang, K.-D.; Waltz, M. High cycle fatigue behaviour and generalized fatigue model development of lead-free solder alloy based on local stress approach. Microelectron. Reliab. 2016, 66, 98–105. [Google Scholar] [CrossRef]
- Ying, D.; Chunqing, W.; Mingyu, L.; Han-Sur, B. In-situ SEM observation on fracture behaviors of Sn-based solder alloys. J. Mater. Sci. 2005, 40, 1993–2001. [Google Scholar] [CrossRef]
- Chang, T.-C.; Hon, M.-H.; Wang, M.-C. Intermetallic compounds formation and interfacial adhesion strength of Sn–9Zn–0.5Ag solder alloy hot-dipped on Cu substrate. J. Alloys Compd. 2003, 352, 168–174. [Google Scholar] [CrossRef]
- Lai, Z.; Ye, D. Microstructure and fracture behavior of non eutectic Sn–Bi solder alloys. J. Mater. Sci. Mater. Electron. 2016, 27, 3182–3192. [Google Scholar] [CrossRef]
- Ye, D.; Du, C.; Wu, M.; Lai, Z. Microstructure and mechanical properties of Sn–xBi solder alloy. J. Mater. Sci. Mater. Electron. 2015, 26, 3629–3637. [Google Scholar] [CrossRef]
- Lai, Z.; Ye, D. Microstructure and Properties of Sn-10Bi-xCu Solder Alloy/Joint. J. Electron. Mater. 2016, 45, 3702–3711. [Google Scholar] [CrossRef]
- Li, X.P.; Xia, J.M.; Zhou, M.B.; Ma, X.; Zhang, X.P. Solder Volume Effects on the Microstructure Evolution and Shear Fracture Behavior of Ball Grid Array Structure Sn-3.0Ag-0.5Cu Solder Interconnects. J. Electron. Mater. 2011, 40, 2425–2435. [Google Scholar] [CrossRef]
- Lee, H.-T.; Hu, S.-Y.; Hong, T.-F.; Chen, Y.-F. The Shear Strength and Fracture Behavior of Sn-Ag-xSb Solder Joints with Au/Ni-P/Cu UBM. J. Electron. Mater. 2008, 37, 867–873. [Google Scholar] [CrossRef]
- Goh, Y.; Haseeb, A.S.; Liew, H.L.; Sabri, M.F. Deformation and fracture behaviour of electroplated Sn–Bi/Cu solder joints. J. Mater. Sci. 2015, 50, 4258–4269. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Kim, S.-W.; Jung, S.-B. IMC Growth and Shear Strength of Sn-Ag-Bi-In/Au/Ni/Cu BGA Joints During Aging. Mater. Trans. 2004, 45, 727–733. [Google Scholar] [CrossRef]
- Tang, Y.; Li, G.; Pan, Y. Influence of TiO2 nanoparticles on IMC growth in Sn–3.0Ag–0.5Cu–xTiO2 solder joints in reflow process. J. Alloys Compd. 2013, 554, 195–203. [Google Scholar] [CrossRef]
- Li, G.Y.; Bi, X.D.; Chen, Q.; Shi, X.Q. Influence of Dopant on Growth of Intermetallic Layers in Sn-Ag-Cu Solder Joints. J. Electron. Mater. 2011, 40, 165–175. [Google Scholar] [CrossRef]
- Tang, Y.; Li, G.Y.; Chen, D.Q.; Pan, Y.C. Influence of TiO2 nanoparticles on IMC growth in Sn–3.0Ag–0.5Cu–xTiO2 solder joints during isothermal aging process. J. Mater. Sci. Mater. Electron. 2013, 25, 981–991. [Google Scholar] [CrossRef]
- Xie, H.; Chawla, N. Mechanical shock behavior of Sn–3.9Ag–0.7Cu and Sn–3.9Ag–0.7Cu–0.5Ce solder joints. Microelectron. Reliab. 2013, 53, 733–740. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z. Fracture mechanism and strength-influencing factors of Cu/Sn–4Ag solder joints aged for different times. J. Alloys Compd. 2009, 485, 853–861. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, Q.; Bai, H.; Zhao, L.; Yan, J. Investigations on deformation and fracture behaviors of the multi-alloyed SnAgCu solder and solder joint by in-situ observation. Microelectron. Reliab. 2022, 135, 114574. [Google Scholar] [CrossRef]
- Yu, J.; Kim, J. Effects of residual S on Kirkendall void formation at Cu/Sn–3.5Ag solder joints. Acta Mater. 2008, 56, 5514–5523. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, J.; Zhang, Z.F. Fracture behaviors and strength of Cu6Sn5 intermetallic compounds by indentation testing. J. Appl. Phys. 2011, 110, 014502. [Google Scholar] [CrossRef]
- Kim, K.; Huh, S.; Suganuma, K. Effects of intermetallic compounds on properties of Sn–Ag–Cu lead-free soldered joints. J. Alloys Compd. 2003, 352, 226–236. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z. In-situ observations on fracture behaviors of Cu–Sn IMC layers induced by deformation of Cu substrates. Mater. Sci. Eng. A 2011, 530, 452–461. [Google Scholar] [CrossRef]
- Zhang, Q.K.; Zou, H.F.; Zhang, Z.F. Tensile and Fatigue Behaviors of Aged Cu/Sn-4Ag Solder Joints. J. Electron. Mater. 2009, 38, 852–859. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Qin, J.; Zhang, X.; Liang, D.; Bai, H.; Yi, J.; Yan, J. Aging Interfacial Structure and Abnormal Tensile Strength of SnAg3Cu0.5/Cu Solder Joints. Materials 2022, 15, 9004. https://doi.org/10.3390/ma15249004
Chen D, Qin J, Zhang X, Liang D, Bai H, Yi J, Yan J. Aging Interfacial Structure and Abnormal Tensile Strength of SnAg3Cu0.5/Cu Solder Joints. Materials. 2022; 15(24):9004. https://doi.org/10.3390/ma15249004
Chicago/Turabian StyleChen, Dongdong, Junhu Qin, Xin Zhang, Dongcheng Liang, Hailong Bai, Jianhong Yi, and Jikang Yan. 2022. "Aging Interfacial Structure and Abnormal Tensile Strength of SnAg3Cu0.5/Cu Solder Joints" Materials 15, no. 24: 9004. https://doi.org/10.3390/ma15249004
APA StyleChen, D., Qin, J., Zhang, X., Liang, D., Bai, H., Yi, J., & Yan, J. (2022). Aging Interfacial Structure and Abnormal Tensile Strength of SnAg3Cu0.5/Cu Solder Joints. Materials, 15(24), 9004. https://doi.org/10.3390/ma15249004



