Soil Properties and Maize Yield Improvement with Biochar-Enriched Poultry Litter-Based Fertilizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Poultry Matured Litter Production and Analyses
2.2. Pot Experiment
2.3. Soil Analyses
2.4. Soil Analyses
3. Results
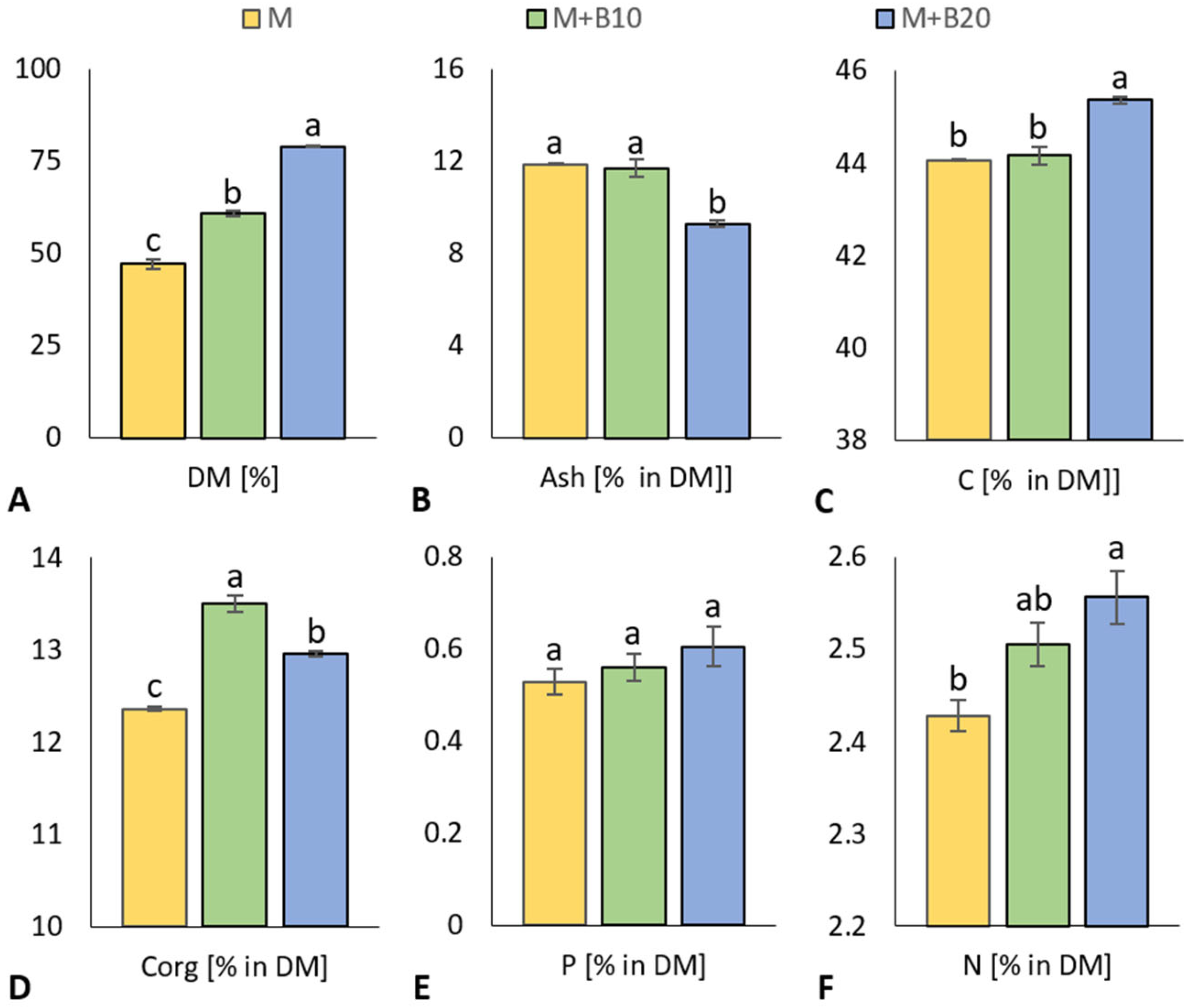
3.1. Poultry Matured Litter Properties
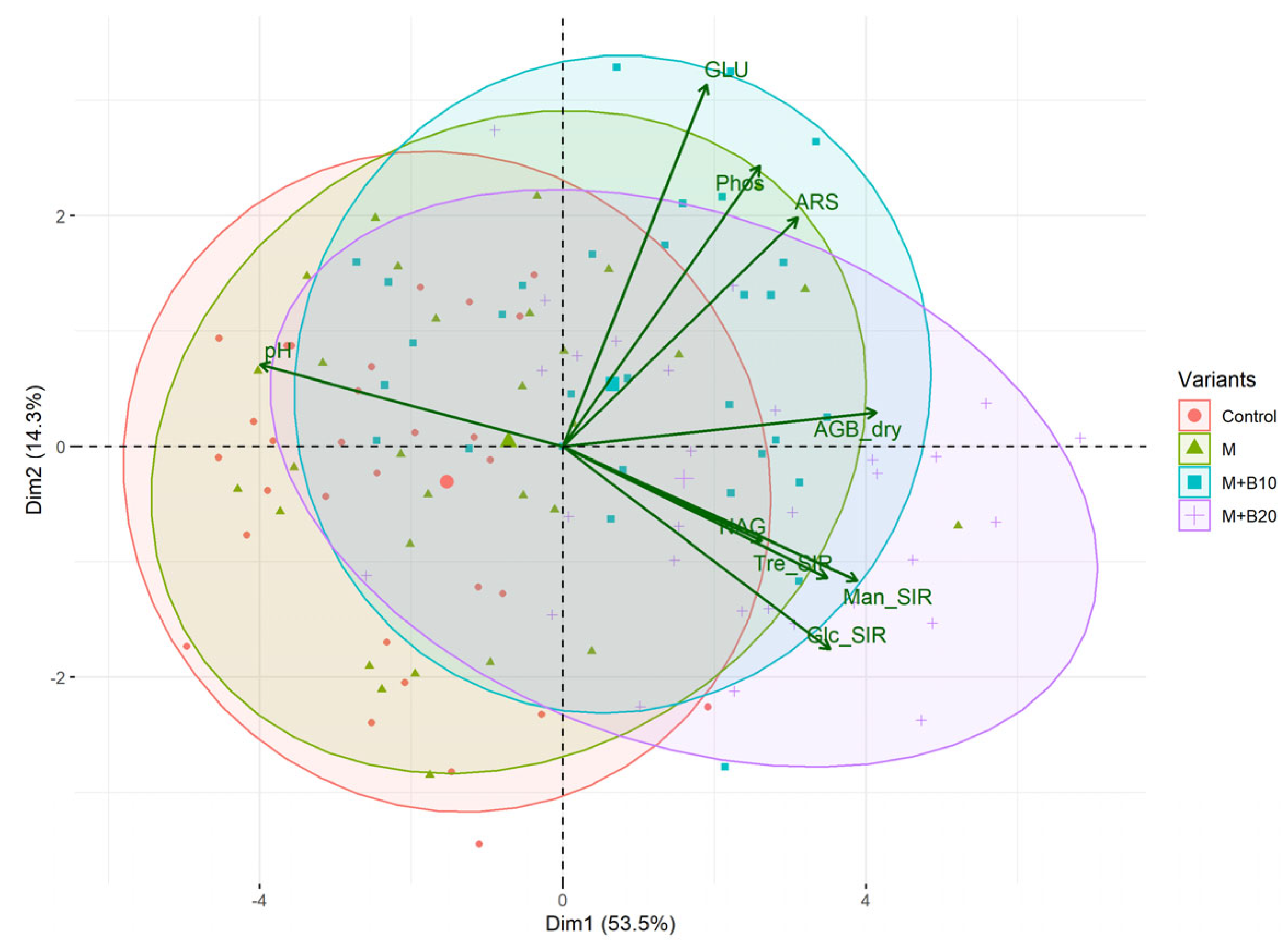
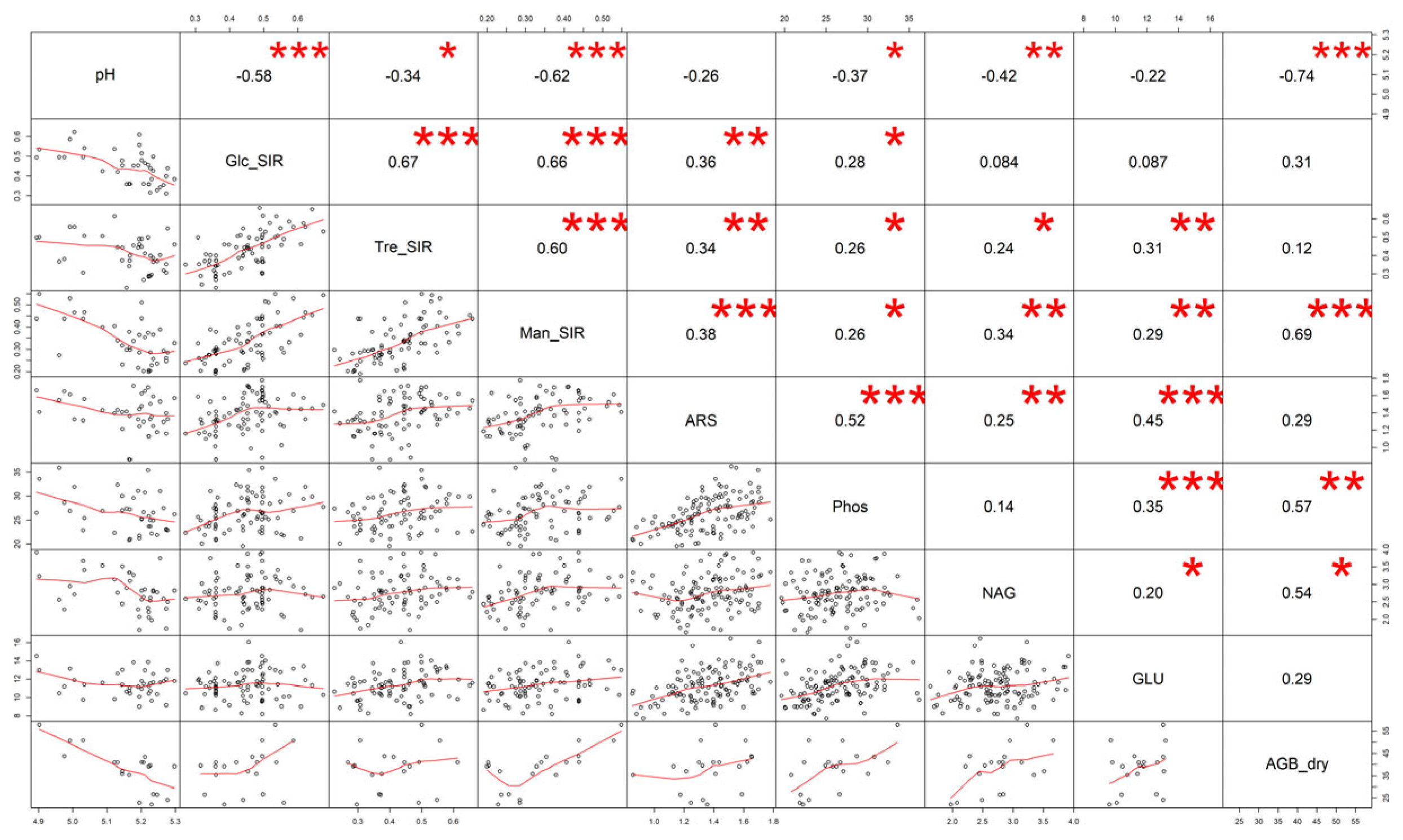
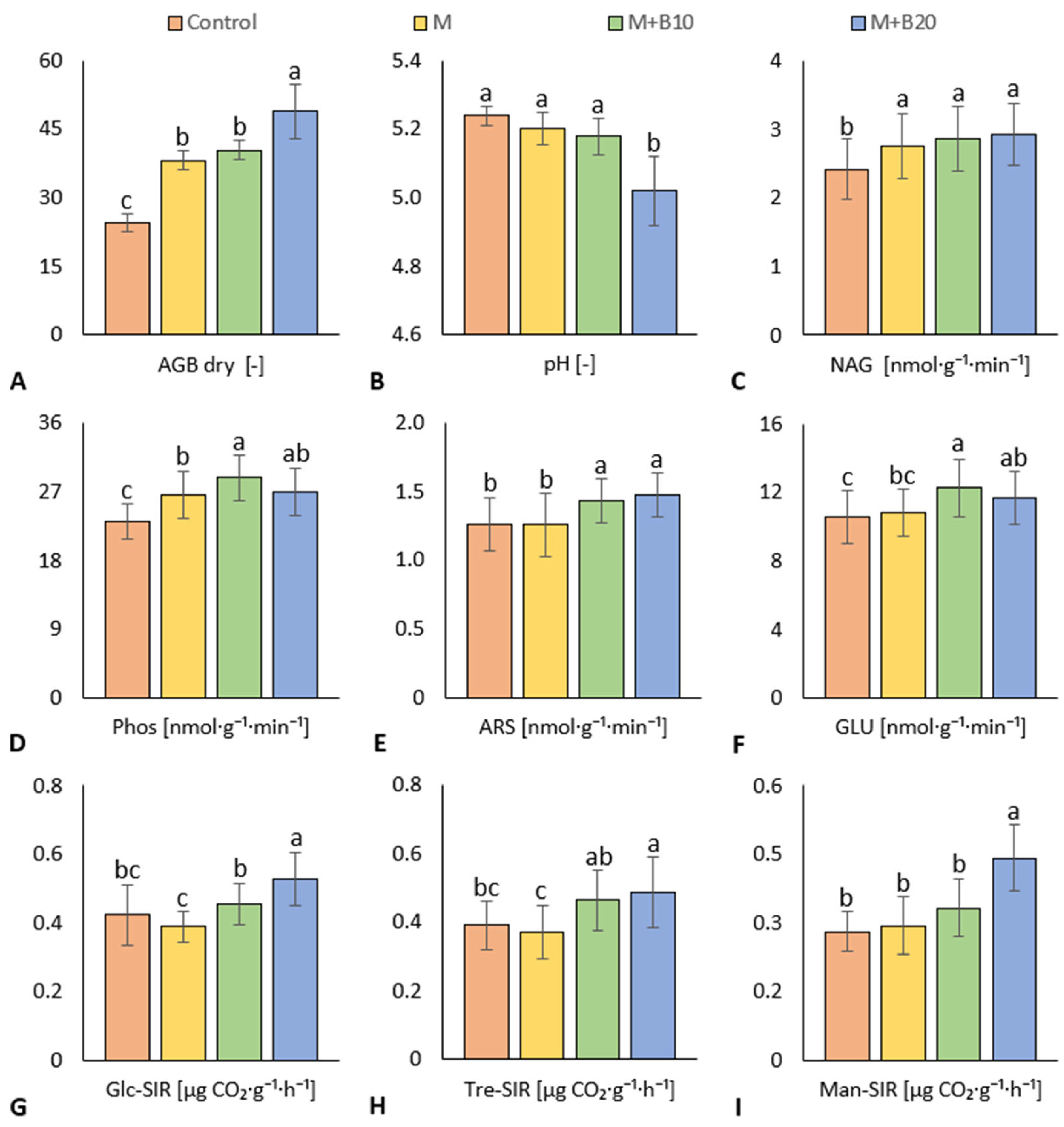
3.2. Soil Properties
4. Discussion
4.1. Poultry Matured Litter Properties
4.2. Soil Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| dry above-ground biomass | AGB dry |
| arylsulfatase | ARS |
| ash | Ash |
| biochar | BC |
| Brunauer–Emmett–Teller specific surface | BET |
| total carbon:nitrogen ratio | C:N) |
| litter carbon content | C |
| organic carbon | Corg |
| dry matter | DM |
| D-glucose | Glc |
| β-glucosidase | GLU |
| D-mannose | Man |
| litter nitrogen content | N |
| N-acetyl-β-D-glucosaminidase | NAG |
| litter phosphorus content | P |
| phosphatase | Phos |
| p-value | p |
| Pearson’s correlation coefficient | r |
| soil organic matter | SOM |
| Substrate-induced respiration | SIR |
| total soil carbon | TC |
| total soil nitrogen | TN |
| D-trehalose | Tre |
Appendix A
Appendix B
References
- Rashad, F.M.; Saleh, W.D.; Moselhy, M.A. Bioconversion of rice straw and certain agro-industrial wastes to amendments for organic farming systems: 1. Composting, quality, stability and maturity indices. Bioresour. Technol. 2010, 101, 5952–5960. [Google Scholar] [CrossRef] [PubMed]
- Higgins, B.T.; Chaump, K.; Wang, Q.; Prasad, R.; Dey, P. Moisture content and aeration control mineral nutrient solubility in poultry litter. J. Environ. Manag. 2021, 300, 113787. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Karthikeyan, S.; Iyappan, P. Trends in patenting and commercial utilisation of poultry farm excreta. Worlds Poult. Sci. J. 2019, 66, 533–572. [Google Scholar] [CrossRef]
- Sousa, F.; Tinoco, I.; Souza, C.; Baptista, F.; Cruz, V.; Silva, J.; Barbari, M.; Coelho, D.; Oliveira, K.; Andrade, R. Methods of treatment and disposal of poultry wastes in Brazil. In Proceedings of the 9th Iberian Congress of Agroengineering, Braganca, Portugal, 4–6 September 2017; pp. 854–861. [Google Scholar]
- Pratt, C.; Redding, M.; Hill, J.; Jensen, P.D. Does manure management affect the latent greenhouse gas emitting potential of livestock manures? Waste Manag. 2015, 46, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Matsuda, J. Ammonia emissions from composting livestock manure. In Proceedings of the Symposium on Ammonia and Odour Emissions from Animal Production Facilities, Vinkeloord, The Netherlands, 6–10 October 1997; pp. 145–153. [Google Scholar]
- Wang, Y.-J.; Xing, Z.-X.; Zhang, X.-F.; Hou, Z.-G.; Zhao, X.-S.; Dou, S.; Zhou, M.-P. On-site Detection of Volatile Organic Compounds During Composting Treatment of Livestock and Poultry Manure by GC-MS. Chin. J. Anal. Chem. 2013, 40, 899–903. (In Chinese) [Google Scholar] [CrossRef]
- Mondini, C.; Chiumenti, R.; da Borso, F.; Leita, L.; De Nobili, M. Changes during processing in the organic matter of composted and air-dried poultry manure. Bioresour. Technol. 1996, 55, 243–249. [Google Scholar] [CrossRef]
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Martín-Ramos, P. Reduction of Ammonia Emissions from Laying Hen Manure in a Closed Composting Process Using Gas-Permeable Membrane Technology. Agronomy 2021, 11, 2384. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; Yong, X.; Luo, L.; Wong, J.W.C.; Zhang, Y.; Wu, H.; Zhou, J. Effect of biochar combined with a biotrickling filter on deodorization, nitrogen retention, and microbial community succession during chicken manure composting. Bioresour. Technol. 2022, 343, 126137. [Google Scholar] [CrossRef]
- Abd El-Rahim, M.G.M.; Dou, S.; Xin, L.; Xie, S.; Sharaf, A.; Alio Moussa, A.; Eissa, M.A.; Mustafa, A.-R.A.; Ali, G.A.M.; Hamed, M.H. Effect of biochar addition method on ammonia volatilization and quality of chicken manure compost. Zemdirbyste 2021, 108, 331–338. [Google Scholar] [CrossRef]
- Arif, M.; Ilyas, M.; Riaz, M.; Ali, K.; Shah, K.; Ul Haq, I.; Fahad, S. Biochar improves phosphorus use efficiency of organic-inorganic fertilizers, maize-wheat productivity and soil quality in a low fertility alkaline soil. Field Crop. Res. 2017, 214, 25–37. [Google Scholar] [CrossRef]
- Sanchez-Monedero, M.A.; Sanchez-Garcia, M.; Alburquerque, J.A.; Cayuela, M.L. Biochar reduces volatile organic compounds generated during chicken manure composting. Bioresour. Technol. 2019, 288, 121584. [Google Scholar] [CrossRef] [PubMed]
- Agyarko-Mintah, E.; Cowie, A.; Van Zwieten, L.; Singh, B.P.; Smillie, R.; Harden, S.; Fornasier, F. Biochar lowers ammonia emission and improves nitrogen retention in poultry litter composting. Waste Manag. 2017, 61, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, J.; Han, L.; Ma, S.; Sun, X.; Huang, G. Role and multi-scale characterization of bamboo biochar during poultry manure aerobic composting. Bioresour. Technol. 2017, 241, 190–199. [Google Scholar] [CrossRef]
- Sheng, J.; Adeli, A.; Brooks, J.P.; McLaughlin, M.R.; Read, J. Effects of bedding materials in applied poultry litter and immobilizing agents on runoff water, soil properties, and bermudagrass growth. J. Environ. Qual. 2014, 43, 290–296. [Google Scholar] [CrossRef]
- Prasai, T.P.; Walsh, K.B.; Midmore, D.J.; Jones, B.E.H.; Bhattarai, S.P. Manure from biochar, bentonite and zeolite feed supplemented poultry: Moisture retention and granulation properties. J. Environ. Manag. 2018, 216, 82–88. [Google Scholar] [CrossRef]
- Agyarko-Mintah, E.; Cowie, A.; Singh, B.P.; Joseph, S.; Van Zwieten, L.; Cowie, A.; Harden, S.; Smillie, R. Biochar increases nitrogen retention and lowers greenhouse gas emissions when added to composting poultry litter. Waste Manag. 2017, 61, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Czekala, W.; Malinska, K.; Caceres, R.; Janczak, D.; Dach, J.; Lewicki, A. Co-composting of poultry manure mixtures amended with biochar—The effect of biochar on temperature and C-CO2 emission. Bioresour. Technol. 2016, 200, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Rong, R.; Zheng, Y.; Zhang, F.; Yang, L.; Li, Z. The Effects of Different Types of Biochar on Ammonia Emissions during Co-composting Poultry Manure with a Corn Leaf. Pol. J. Environ. Stud. 2019, 28, 3837–3843. [Google Scholar] [CrossRef]
- Lashari, M.S.; Liu, Y.; Li, L.; Pan, W.; Fu, J.; Pan, G.; Zheng, J.; Zheng, J.; Zhang, X.; Yu, X. Effects of amendment of biochar-manure compost in conjunction with pyroligneous solution on soil quality and wheat yield of a salt-stressed cropland from Central China Great Plain. Field Crops Res. 2013, 144, 113–118. [Google Scholar] [CrossRef]
- Lashari, M.S.; Ye, Y.; Ji, H.; Li, L.; Kibue, G.W.; Lu, H.; Zheng, J.; Pan, G. Biochar-manure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from central China: A 2-year field experiment. J. Sci. Food Agric. 2015, 95, 1321–1327. [Google Scholar] [CrossRef]
- Lu, H.; Lashari, M.S.; Liu, X.; Ji, H.; Li, L.; Zheng, J.; Kibue, G.W.; Joseph, S.; Pan, G. Changes in soil microbial community structure and enzyme activity with amendment of biochar-manure compost and pyroligneous solution in a saline soil from Central China. Eur. J. Soil Biol. 2015, 70, 67–76. [Google Scholar] [CrossRef]
- Ahmad, S.; Ghaffar, A.; Rahman, M.H.U.; Hussain, I.; Iqbal, R.; Haider, G.; Khan, M.A.; Ikram, R.M.; Hussnain, H.; Bashir, M.S. Effect of Application of Biochar, Poultry and Farmyard Manures in Combination with Synthetic Fertilizers on Soil Fertility and Cotton Productivity under Arid Environment. Commun. Soil Sci. Plant Anal. 2021, 52, 2018–2031. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M.; Aboyeji, C.M.; Dunsin, O.; Simeon, V.T. Biochar and poultry manure effects on soil properties and radish (Raphanus sativus L.) yield. Biol. Agric. Hortic. 2018, 35, 33–45. [Google Scholar] [CrossRef]
- Agbede, T.M.; Adekiya, A.O.; Odoja, A.S.; Bayode, L.N.; Omotehinse, P.O.; Adepehin, I. Effects of biochar and poultry manure on soil properties, growth, quality, and yield of cocoyam (Xanthosoma sagittifolium Schott) in degraded tropical sandy soil. Exp. Agric. 2020, 56, 528–543. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Taskin, M.B.; Sahin, O.; Kaya, E.C.; Atakol, A.; Goss, M. Effect of phosphorus-enriched biochar and poultry manure on growth and mineral composition of lettuce (Lactuca sativa L. cv.) grown in alkaline soil. Soil Use Manag. 2014, 30, 182–188. [Google Scholar] [CrossRef]
- Janczak, D.; Malinska, K.; Czekala, W.; Caceres, R.; Lewicki, A.; Dach, J. Biochar to reduce ammonia emissions in gaseous and liquid phase during composting of poultry manure with wheat straw. Waste Manag. 2017, 66, 36–45. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.; Alburquerque, J.A.; Sanchez-Monedero, M.A.; Roig, A.; Cayuela, M.L. Biochar accelerates organic matter degradation and enhances N mineralisation during composting of poultry manure without a relevant impact on gas emissions. Bioresour. Technol. 2015, 192, 272–279. [Google Scholar] [CrossRef]
- Zainudin, M.H.; Mustapha, N.A.; Maeda, T.; Ramli, N.; Sakai, K.; Hassan, M. Biochar enhanced the nitrifying and denitrifying bacterial communities during the composting of poultry manure and rice straw. Waste Manag. 2020, 106, 240–249. [Google Scholar] [CrossRef]
- Dias, B.O.; Silva, C.A.; Higashikawa, F.S.; Roig, A.; Sanchez-Monedero, M.A. Use of biochar as bulking agent for the composting of poultry manure: Effect on organic matter degradation and humification. Bioresour. Technol. 2010, 101, 1239–1246. [Google Scholar] [CrossRef]
- Jindo, K.; Sánchez-Monedero, M.A.; Matsumoto, K.; Sonoki, T. The Efficiency of a Low Dose of Biochar in Enhancing the Aromaticity of Humic-Like Substance Extracted from Poultry Manure Compost. Agronomy 2019, 9, 248. [Google Scholar] [CrossRef]
- Jindo, K.; Sonoki, T.; Matsumoto, K.; Canellas, L.; Roig, A.; Sanchez-Monedero, M.A. Influence of biochar addition on the humic substances of composting manures. Waste Manag. 2016, 49, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Jindo, K.; Sanchez-Monedero, M.A.; Hernandez, T.; Garcia, C.; Furukawa, T.; Matsumoto, K.; Sonoki, T.; Bastida, F. Biochar influences the microbial community structure during manure composting with agricultural wastes. Sci. Total Environ. 2012, 416, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Jindo, K.; Suto, K.; Matsumoto, K.; Garcia, C.; Sonoki, T.; Sanchez-Monedero, M.A. Chemical and biochemical characterisation of biochar-blended composts prepared from poultry manure. Bioresour. Technol. 2012, 110, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.; Das, K.C.; Melear, N.; Lakly, D. Reducing nitrogen loss during poultry litter composting using biochar. J. Environ. Qual. 2010, 39, 1236–1242. [Google Scholar] [CrossRef]
- EN_15934. Sludge, Treated Biowaste, Soil and Waste—Calculation of Dry Matter Fraction after Determination of Dry Residue or Water Content. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2012.
- EN_15169. Characterization of Waste—Determination of Loss on Ignition in Waste, Sludge and Sediment. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2007.
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Schroder, J.L.; Zhang, H.; Richards, J.R.; Payton, M.E. Interlaboratory Validation of the Mehlich 3 Method as a Universal Extractant for Plant Nutrients. J. AOAC Int. 2009, 92, 995–1008. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 2008, 15, 1409–1416. [Google Scholar] [CrossRef]
- ISO 11261:1995. Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 1995.
- ISO 10390:2005. Soil Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 20130:2018. Soil Quality—Measurement of Enzyme Activity Patterns in Soil Samples Using Colorimetric Substrates in Micro-Well Plates. International Organization for Standardization: Geneva, Switzerland, 2018.
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef]
- R_Core_Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Sun, X.; Shan, R.; Li, X.; Pan, J.; Liu, X.; Deng, R.; Song, J. Characterization of 60 types of Chinese biomass waste and resultant biochars in terms of their candidacy for soil application. GCB Bioenergy 2017, 9, 1423–1435. [Google Scholar] [CrossRef]
- Jien, S.-H.; Wang, C.-C.; Lee, C.-H.; Lee, T.-Y. Stabilization of Organic Matter by Biochar Application in Compost-amended Soils with Contrasting pH Values and Textures. Sustainability 2015, 7, 3317. [Google Scholar] [CrossRef]
- Ch’Ng, H.Y.; Ahmed, O.H.; Majid, N.M.A. Improving Phosphorus Availability, Nutrient Uptake and Dry Matter Production of Zea Mays L. On a Tropical Acid Soil Using Poultry Manure Biochar and Pineapple Leaves Compost. Exp. Agric. 2015, 52, 447–465. [Google Scholar] [CrossRef]
- Hoover, N.L.; Law, J.Y.; Long, L.A.M.; Kanwar, R.S.; Soupir, M.L. Long-term impact of poultry manure on crop yield, soil and water quality, and crop revenue. J. Environ. Manag. 2019, 252, 109582. [Google Scholar] [CrossRef] [PubMed]
- Toor, G.S. Enhancing Phosphorus Availability in Low-Phosphorus Soils by Using Poultry Manure and Commercial Fertilizer. Soil Sci. 2009, 174, 358–364. [Google Scholar] [CrossRef]
- Das, S.K.; Avasthe, R.K.; Singh, R.; Babu, S. Biochar as carbon negative in carbon credit under changing climate. Curr. Sci. 2014, 107, 1090–1091. [Google Scholar]
- Mechler, M.A.A.; Jiang, R.W.; Silverthorn, T.K.; Oelbermann, M. Impact of biochar on soil characteristics and temporal greenhouse gas emissions: A field study from southern Canada. Biomass Bioenergy 2018, 118, 154–162. [Google Scholar] [CrossRef]
- Jena, M.; Das, S.K.; Mishra, S.K.; Swain, R.K.; Dehuri, P.K. Mineral profile of feeds, fodders and cattle in north-eastern coastal plain zone of Odisha. Indian J. Anim. Sci. 2011, 81, 1143–1147. [Google Scholar]
- Amin, A.E.-E.A.Z. Amelioration of calcareous sandy soil productivity via incorporation between biochar and some organic manures. Arab. J. Geosci. 2018, 11, 10. [Google Scholar] [CrossRef]
- Inal, A.; Gunes, A.; Sahin, O.; Taskin, M.B.; Kaya, E.C. Impacts of biochar and processed poultry manure, applied to a calcareous soil, on the growth of bean and maize. Soil Use Manag. 2015, 31, 106–113. [Google Scholar] [CrossRef]
- Ameloot, N.; Sleutel, S.; Das, K.C.; Kanagaratnam, J.; de Neve, S. Biochar amendment to soils with contrasting organic matter level: Effects on N mineralization and biological soil properties. GCB Bioenergy 2015, 7, 135–144. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, R.; Nielsen, S.; Joseph, S.D.; Huang, D.; Thomas, T. A Combination of Biochar-Mineral Complexes and Compost Improves Soil Bacterial Processes, Soil Quality, and Plant Properties. Front. Microbiol. 2016, 7, 372. [Google Scholar] [CrossRef]
- Romero, C.M.; Li, C.; Owens, J.; Ribeiro, G.O.; McAllister, T.A.; Okine, E.; Hao, X. Nutrient cycling and greenhouse gas emissions from soil amended with biochar-manure mixtures. Pedosphere 2021, 31, 289–302. [Google Scholar] [CrossRef]
- Mohammadi-Aragh, M.K.; Stokes, C.E.; Street, J.T.; Linhoss, J.E. Effects of Loblolly Pine Biochar and Wood Vinegar on Poultry Litter Nutrients and Microbial Abundance. Animals 2021, 11, 2209. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Awasthi, S.K.; Liu, T.; Chen, H.; Zhang, Z.; Wang, Q.; Ren, X.; Tu, Z.; Awasthi, M.K.; Taherzadeh, M.J. Dynamics of fungal diversity and interactions with environmental elements in response to wheat straw biochar amended poultry manure composting. Bioresour. Technol. 2019, 274, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Solaiman, Z.M.; Shafi, M.I.; Beamont, E.; Anawar, H.M. Poultry Litter Biochar Increases Mycorrhizal Colonisation, Soil Fertility and Cucumber Yield in a Fertigation System on Sandy Soil. Agriculture 2020, 10, 480. [Google Scholar] [CrossRef]
- Rousk, J.; Baath, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holatko, J.; Hammerschmiedt, T.; Kucerik, J.; Baltazar, T.; Radziemska, M.; Havlicek, Z.; Kintl, A.; Jaskulska, I.; Malicek, O.; Brtnicky, M. Soil Properties and Maize Yield Improvement with Biochar-Enriched Poultry Litter-Based Fertilizer. Materials 2022, 15, 9003. https://doi.org/10.3390/ma15249003
Holatko J, Hammerschmiedt T, Kucerik J, Baltazar T, Radziemska M, Havlicek Z, Kintl A, Jaskulska I, Malicek O, Brtnicky M. Soil Properties and Maize Yield Improvement with Biochar-Enriched Poultry Litter-Based Fertilizer. Materials. 2022; 15(24):9003. https://doi.org/10.3390/ma15249003
Chicago/Turabian StyleHolatko, Jiri, Tereza Hammerschmiedt, Jiri Kucerik, Tivadar Baltazar, Maja Radziemska, Zdenek Havlicek, Antonin Kintl, Iwona Jaskulska, Ondrej Malicek, and Martin Brtnicky. 2022. "Soil Properties and Maize Yield Improvement with Biochar-Enriched Poultry Litter-Based Fertilizer" Materials 15, no. 24: 9003. https://doi.org/10.3390/ma15249003
APA StyleHolatko, J., Hammerschmiedt, T., Kucerik, J., Baltazar, T., Radziemska, M., Havlicek, Z., Kintl, A., Jaskulska, I., Malicek, O., & Brtnicky, M. (2022). Soil Properties and Maize Yield Improvement with Biochar-Enriched Poultry Litter-Based Fertilizer. Materials, 15(24), 9003. https://doi.org/10.3390/ma15249003







