Preparation of Flexible Liquid Crystal Films with Broadband Reflection Based on PD&SLC
Abstract
1. Introduction
2. Materials and Methods
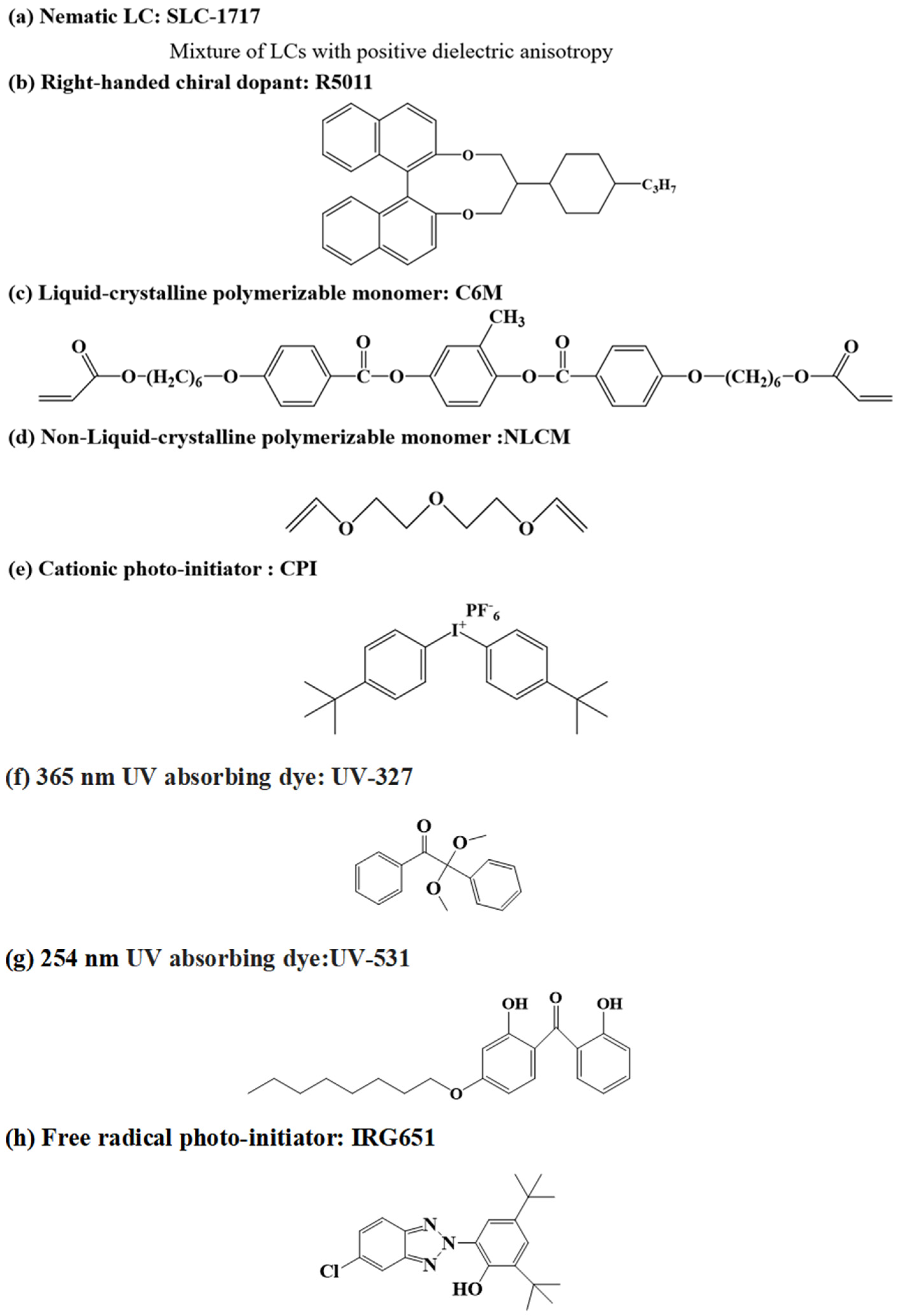
2.1. Materials
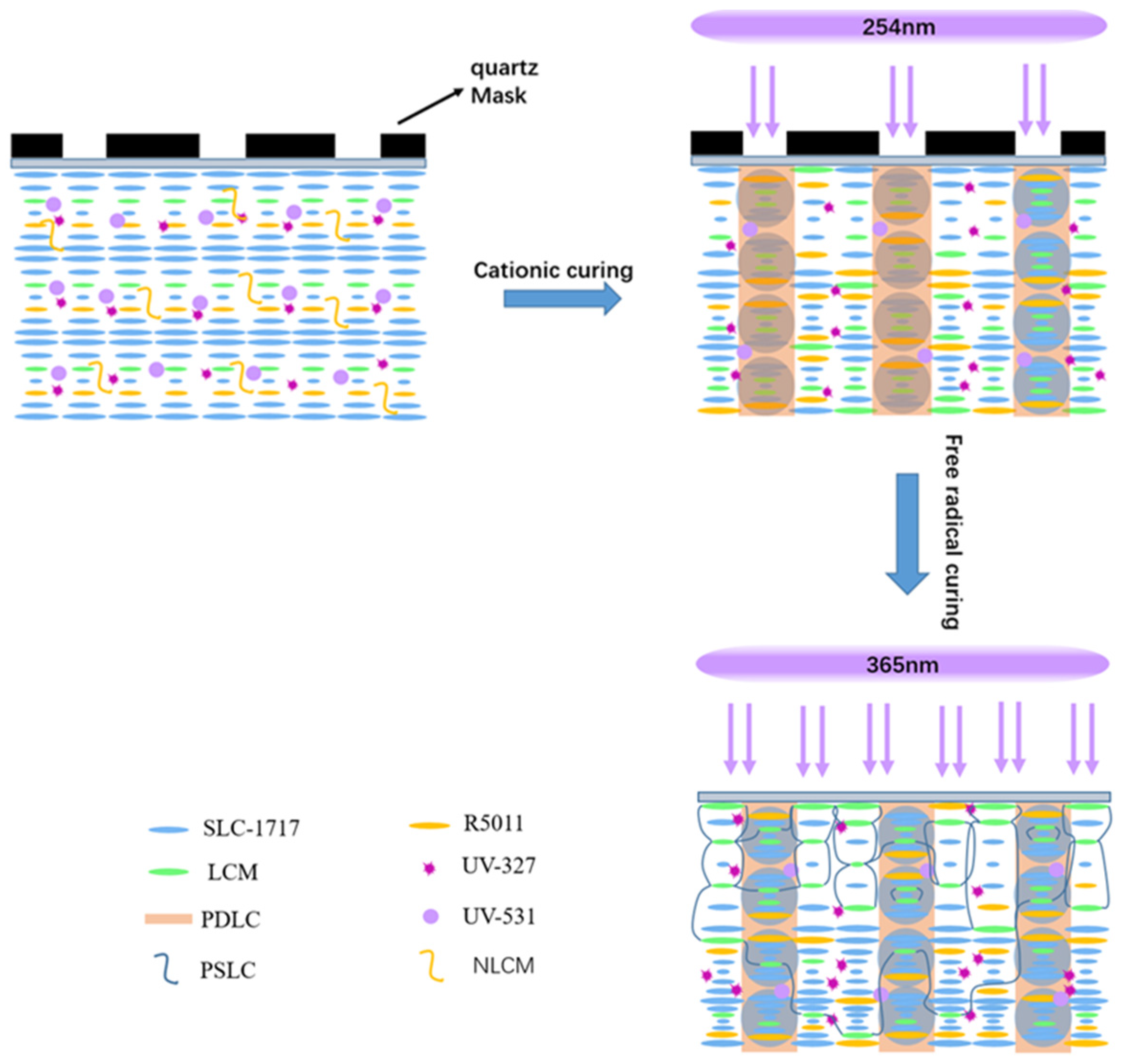
2.2. Preparation of Samples and Cells
2.3. Measurements
3. Results and Discussion
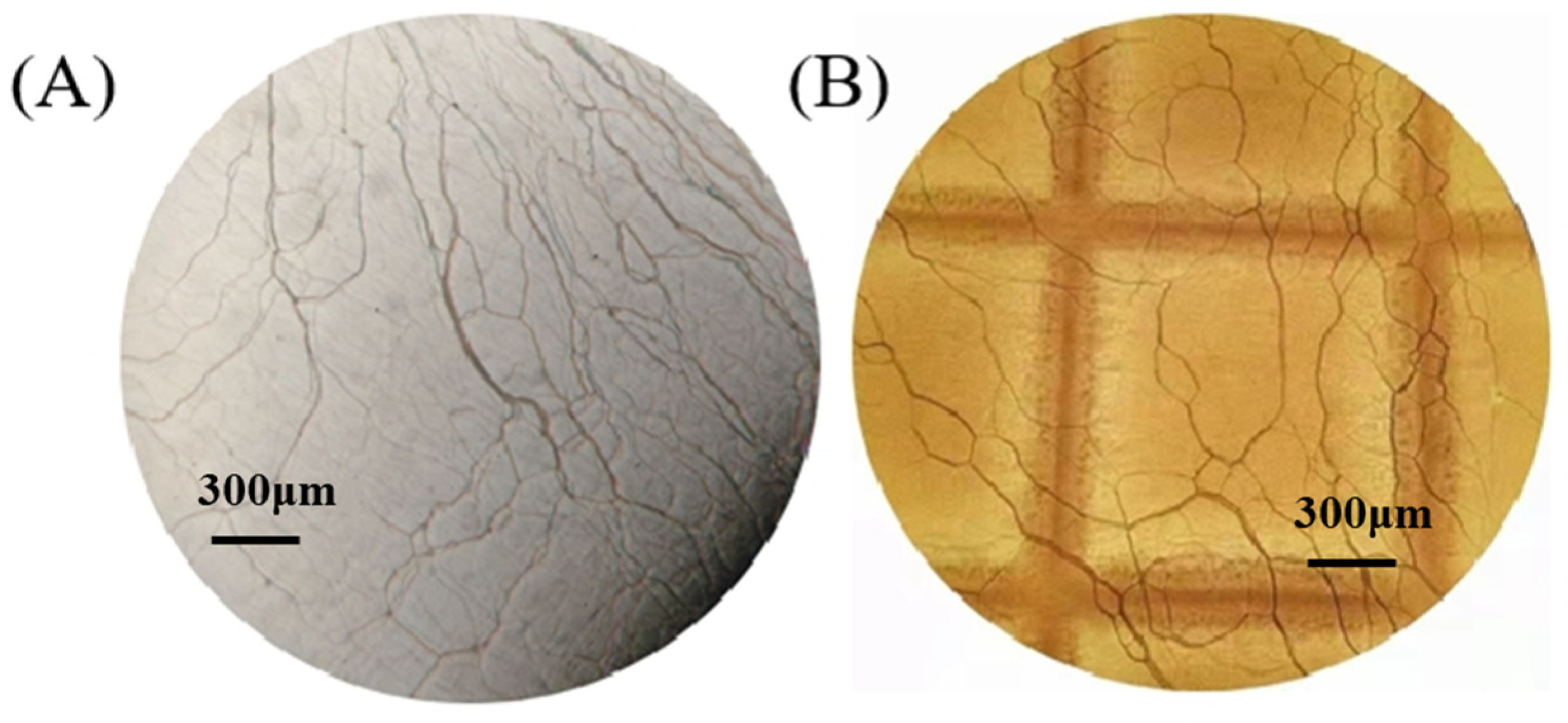
3.1. Texture
3.2. Mechanism of Broadband Reflection
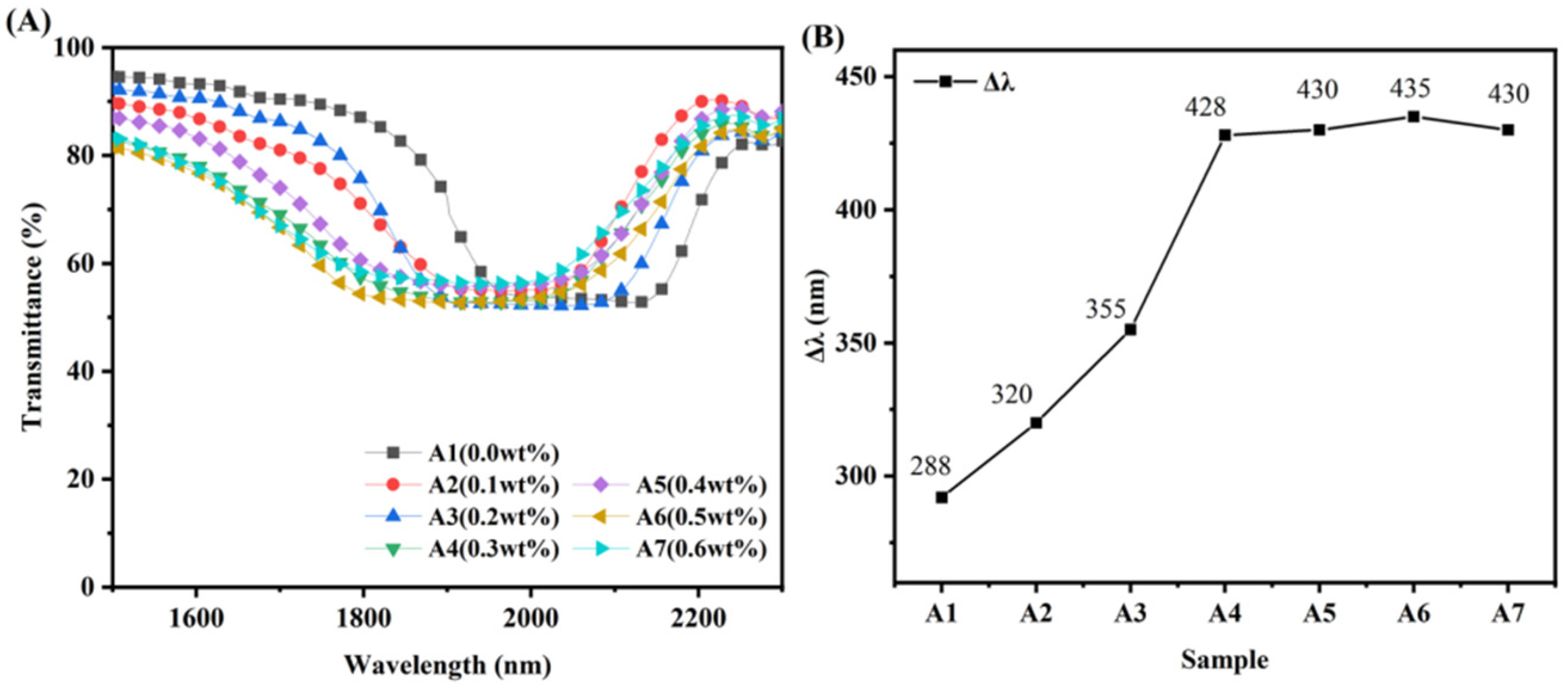
3.3. Broadened Reflection Induced by the Content of UV-531
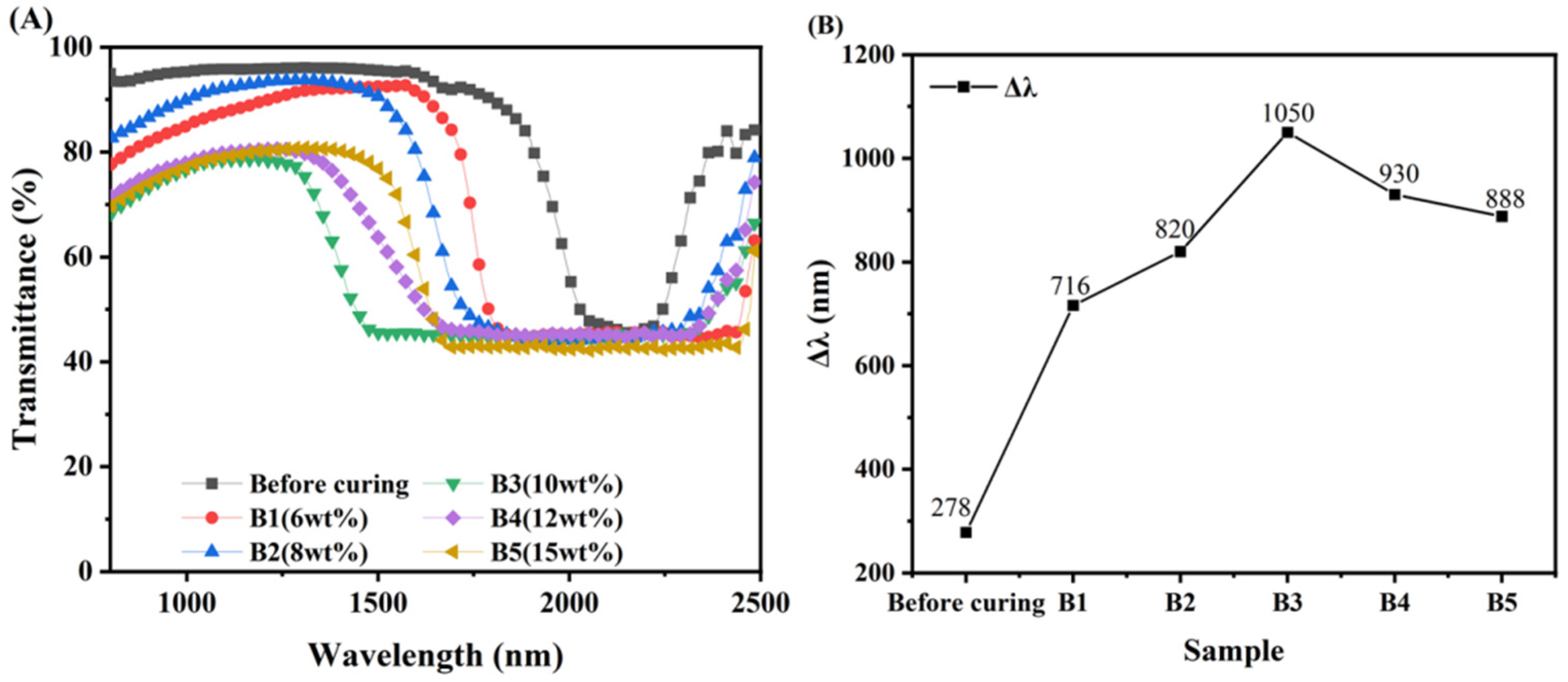
3.4. Broadened Reflection Induced by the Content of C6M
3.5. Broadened Reflection Induced by the Content of R5011
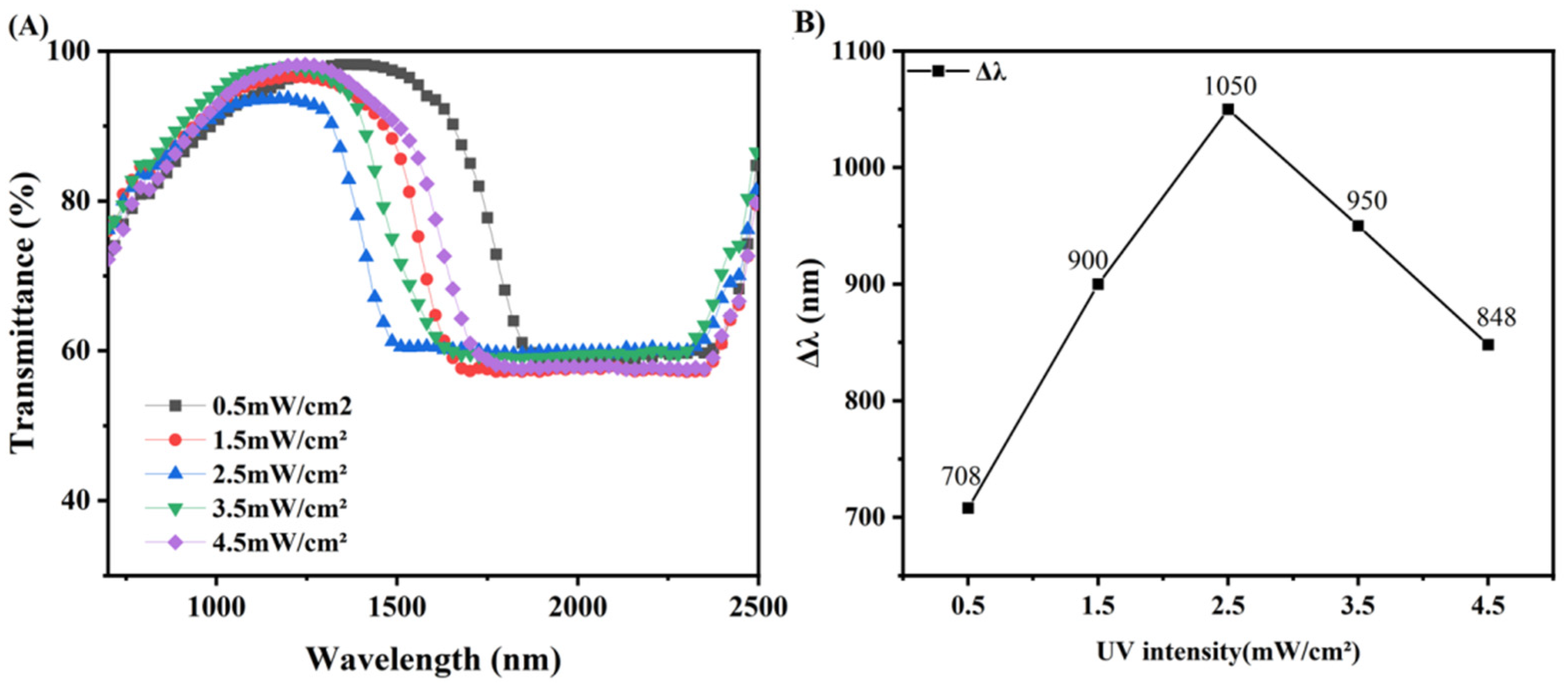
3.6. Broadened Reflection Induced by the UV Intensity
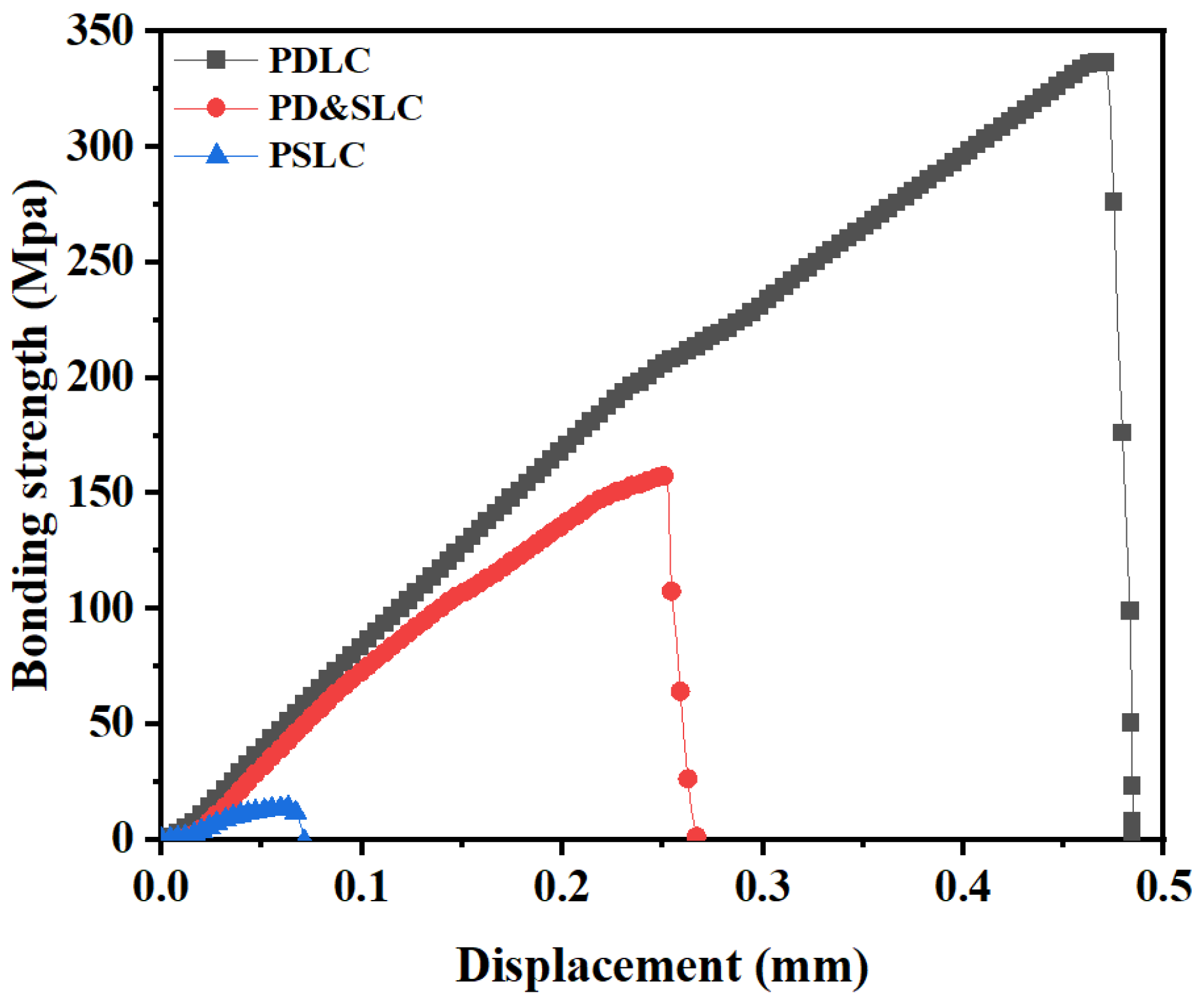
3.7. Comparison of Bonding Strength
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Drzaic, P. Polymer dispersed nematic liquid crystal for large area displays and light valves. J. Appl. Phys. 1986, 60, 2142. [Google Scholar] [CrossRef]
- Dierking, I.; Scalia, G.; Morales, P.; LeClere, D. Aligning and Reorienting Carbon Nanotubes with Nematic Liquid Crystals. Adv. Mater. 2004, 16, 865–869. [Google Scholar] [CrossRef]
- Khandelwal, H.; Schenning, A.H.J.; Debije, M.G. Infrared Regulating Smart Window Based on Organic Materials. Adv. Energy Mater. 2017, 7, 1602209. [Google Scholar] [CrossRef]
- Dierking, I.; Scalia, G. Liquid crystal–carbon nanotube dispersions. J. Appl. Phys. 2005, 97, 044309. [Google Scholar] [CrossRef]
- Kim, D.J.; Hwang, D.Y.; Park, J.Y.; Kim, H.K. Liquid crystal–Based flexible smart windows on roll-to-roll slot die—Coated Ag nanowire network films. J. Alloys Compd. 2018, 765, 1090–1098. [Google Scholar] [CrossRef]
- Hinojosa, A.; Sharma, S.C. Effects of gold nanoparticles on electro-optical properties of a polymer-dispersed liquid crystal. Appl. Phys. Lett. 2010, 97, 081114. [Google Scholar] [CrossRef]
- Dierking, I. Polymer Network–Stabilized Liquid Crystals. Adv. Mater. 2000, 12, 167–181. [Google Scholar] [CrossRef]
- Mitov, M.; Nouvet, E.; Dessaud, N. Polymer-stabilized cholesteric liquid crystals as switchable photonic broad bandgaps. Eur. Phys. J. E 2004, 15, 413–419. [Google Scholar] [CrossRef]
- Lee, M.H.; Li, X.D.; Kim, Y.J.; Kang, J.; Paek, S.; Kim, J.J. Facile Fabrication of Polymer Waveguide by Using Photosensitive Polyimides. Mol. Cryst. Liq. Cryst. 2002, 377, 7–12. [Google Scholar] [CrossRef]
- Chen, X.W.; Wang, L.; Chen, Y.J.; Li, C.Y.; Hou, G.Y.; Liu, X.; Zhang, X.G.; He, W.L.; Yang, H. Broadband reflection of polymer-stabilized chiral nematic liquid crystals induced by a chiral azobenzene compound. Chem. Commun. 2014, 50, 691–694. [Google Scholar] [CrossRef]
- St. John, W.D.; Fritz, W.J.; Lu, Z.J.; Yang, D.K. Bragg reflection from cholesteric liquid crystals. Phys. Rev. E 1995, 51, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Boudet, A.; Binet, C.; Mitov, M.; Bourgerette, C.; Boucher, E. Microstructure of variable pitch cholesteric films and its relationship with the optical properties. Eur. Phys. J. E 2000, 2, 247–253. [Google Scholar] [CrossRef]
- Guo, J.B.; Sun, J.; Zhang, L.P.; Li, K.X.; Cao, H.; Yang, H.; Zhu, S.Q. Broadband reflection in polymer stabilized cholesteric liquid crystal cells with chiral monomers derived from cholesterol. Polym. Adv. Technol. 2008, 19, 1504–1512. [Google Scholar] [CrossRef]
- Broer, D.J.; Lub, J.; Mol, G.N. Photo-controlled diffusion in reacting liquid crystals: A new tool for the creation of complex molecular architectures. Macromol. Symp. 1997, 117, 33–44. [Google Scholar] [CrossRef]
- Broer, D.J.; Mol, G.N.; Haaren, J.M.M.; Lub, J. Photo-induced diffusion in polymerizing chiral-nematic media. Adv. Mater. 1999, 11, 573–578. [Google Scholar] [CrossRef]
- Hu, W.; Chen, M.; Wang, Q.; Zhang, L.Y.; Yuan, X.T.; Chen, F.W.; Yang, H. Broadband reflection in polymer-stabilized cholesteric liquid crystals via thiol–acrylate chemistry. Angew. Chem. Int. Ed. 2019, 58, 6698–6702. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Zhang, L.Y.; He, Z.M.; Chen, G.; Wang, D.; Zhang, H.Q.; Yang, H. Photoinduced polymer-stabilised chiral nematic liquid crystal films reflecting both right- and left-circularly polarised light. Liq. Cryst. 2015, 42, 1120–1123. [Google Scholar] [CrossRef]
- Guo, J.B.; Cao, H.; Wei, J.; Zhang, D.W.; Liu, F.; Pan, G.H.; Zhao, D.Y.; He, W.L.; Yang, H. Polymer stabilized liquid crystal films reflecting both right- and left-circularly polarized light. Appl. Phys. Lett. 2008, 93, 201901. [Google Scholar] [CrossRef]
- Guo, J.; Yang, H.; Li, R.; Ji, N.; Dong, X.M.; Wu, H.; Wei, J. Effect of Network concentration on the performance of polymer-stabilized cholesteric liquid crystals with a double-handed circularly polarized light reflection band. J. Phys. Chem. C 2009, 113, 16538–16543. [Google Scholar] [CrossRef]
- Guo, J.; Liu, F.; Chen, F.J.; Wei, J.; Yang, H. Realisation of cholesteric liquid-crystalline materials reflecting both right- and left-circularly polarised light using the wash-out/refill technique. Liq. Cryst. 2010, 37, 171–178. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.J.; Luo, D. Optical thermal sensor based on cholesteric film refilled with mixture of toluene and ethanol. Opt. Express 2017, 25, 26349–26355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Pan, J.K.; Liu, Y.H.; Xu, Y.; Zhang, A.M. NIR–UV responsive actuator with graphene oxide/microchannel-induced liquid crystal bilayer structure for biomimetic devices. ACS Appl. Mater. Interfaces 2020, 12, 6727–6735. [Google Scholar] [CrossRef]
- Hwang, H.; Kang, M.S.; Han, J.H.; Shin, K.; Cho, J.H. Photo-crosslinkable NIR-absorbing window with environmental stability. Pigm. Resin. Technol. 2013, 42, 170–174. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.A.; Ambulo, C.P.; Lee, H.B.; Kim, S.H.; Naik, V.V.; Haines, C.S.; Aliev, A.E.; Ovalle-Robles, R.; Baughman, R.H.; et al. Intelligently actuating liquid crystal elastomer-carbon nanotube composites. Adv. Funct. Mater. 2019, 29, 1905063. [Google Scholar] [CrossRef]
- Bakker, L.G.; Brouwer, A.H.; Babuska, R. Integrated smart control of heating, cooling, ventilation, day-lighting and electrical lighting in buildings. J. Sol. Energy 2001, 21, 203–217. [Google Scholar]
- Kolvir, H.R.; Domola, H.M. The study of environmental psychology in tall buildings with sustainable architecture approach. Toxicol. Appl. Pharm. 2015, 128, 45–49. [Google Scholar]
- Liang, X.; Chen, M.; Wang, Q.; Guo, S.J.; Yang, H. Ethanol-Precipitable, Silica-Passivated perovskite nanocrystals incorporated into polystyrene microspheres for long-term storage and reusage. Angew. Chem. Int. Ed. 2019, 131, 2825–2829. [Google Scholar] [CrossRef]
- Liang, X.; Chen, M.; Wang, Q.; Guo, S.M.; Zhang, L.Y.; Yang, H. Active and passive modulation of solar light transmittance in a hybrid thermochromic soft-matter system for energy-saving smart window applications. J. Mater. Chem. C 2018, 6, 7054–7062. [Google Scholar] [CrossRef]
- Guo, S.M.; Liang, X.; Zhang, C.H.; Chen, M.; Shen, C.; Zhang, L.Y.; Yang, X.; He, B.F.; Yang, H. Preparation of a thermally light-transmittance-controllable film from a coexistent system of polymer-dispersed and polymer-stabilized liquid crystals. ACS Appl. Mater. Interfaces 2017, 9, 2942–2947. [Google Scholar] [CrossRef]
- Wang, F.F.; Li, K.X.; Song, P.; Wu, X.J.; Cao, H.; Yang, H. Photoinduced pitch gradients and the reflection behaviour of the broadband films: Influence of dye concentration, light intensity, temperature and monomer concentration. Liq. Cryst. 2012, 39, 707–714. [Google Scholar] [CrossRef]
- Zhang, X.T.; Shi, W.T.; Han, R.; Li, H.; Cao, H.; Chen, Y.J.; Yang, Z.; Wang, D.; He, W.L. Self-diffusion method for broadband reflection in polymer-stabilized cholesteric liquid crystal films. Liq. Cryst. 2022, 49, 494–503. [Google Scholar] [CrossRef]
- Guillard, H.; Sixou, P. Active broadband polymer stabilized liquid crystals. Liq. Cryst. 2001, 28, 933–944. [Google Scholar] [CrossRef]
- Guillard, H.; Sixou, P.; Reboul, L.; Perichaud, A. Electrooptical characterizations of polymer stabilized cholesteric liquid crystals. Polymer 2001, 42, 9753–9762. [Google Scholar] [CrossRef]
- Shi, W.T.; Zhang, X.T.; Han, R.; Li, H.; Cao, H.; Chen, Y.J.; Wang, D.; Yang, Z.; He, W.L. Preparation of cholesteric polymer networks with broadband reflection memory effect. Liq. Cryst. 2022, 49, 153–161. [Google Scholar] [CrossRef]



| Sample Number | SLC-1717 (wt%) | C6M (wt%) | NLCM (wt%) | UV-327 (wt%) | UV-531 (wt%) | IRG651 (wt%) | CPI (wt%) | R5011 (wt%) |
|---|---|---|---|---|---|---|---|---|
| A1 | 72.5 | 10 | 16 | 0.3 | 0 | 0.2 | 0.2 | 0.8 |
| A2 | 72.4 | 10 | 16 | 0.3 | 0.1 | 0.2 | 0.2 | 0.8 |
| A3 | 72.3 | 10 | 16 | 0.3 | 0.2 | 0.2 | 0.2 | 0.8 |
| A4 | 72.2 | 10 | 16 | 0.3 | 0.3 | 0.2 | 0.2 | 0.8 |
| A5 | 72.1 | 10 | 16 | 0.3 | 0.4 | 0.2 | 0.2 | 0.8 |
| A6 | 72 | 10 | 16 | 0.3 | 0.5 | 0.2 | 0.2 | 0.8 |
| A7 | 71.9 | 10 | 16 | 0.3 | 0.6 | 0.2 | 0.2 | 0.8 |
| B1 | 76.2 | 6 | 16 | 0.3 | 0.3 | 0.2 | 0.2 | 0.8 |
| B2 | 74.2 | 8 | 16 | 0.3 | 0.3 | 0.2 | 0.2 | 0.8 |
| B3 | 72.2 | 10 | 16 | 0.3 | 0.3 | 0.2 | 0.2 | 0.8 |
| B4 | 70.2 | 12 | 16 | 0.3 | 0.3 | 0.2 | 0.2 | 0.8 |
| B5 | 67.2 | 15 | 16 | 0.3 | 0.3 | 0.2 | 0.2 | 0.8 |
| C1 | 72.3 | 10 | 16 | 0.3 | 0.3 | 0.2 | 0.2 | 0.7 |
| C2 | 72.2 | 10 | 16 | 0.3 | 0.3 | 0.2 | 0.2 | 0.8 |
| C3 | 72 | 10 | 16 | 0.3 | 0.3 | 0.2 | 0.2 | 1 |
| C4 | 71.8 | 10 | 16 | 0.3 | 0.3 | 0.2 | 0.2 | 1.2 |
| C5 | 71.5 | 10 | 16 | 0.3 | 0.3 | 0.2 | 0.2 | 1.5 |
| C6 | 71.2 | 10 | 16 | 0.3 | 0.3 | 0.2 | 0.2 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Han, R.; Li, H.; Zhao, X.; Cao, H.; Chen, Y.; Yang, Z.; Wang, D.; He, W. Preparation of Flexible Liquid Crystal Films with Broadband Reflection Based on PD&SLC. Materials 2022, 15, 8896. https://doi.org/10.3390/ma15248896
Zhang X, Han R, Li H, Zhao X, Cao H, Chen Y, Yang Z, Wang D, He W. Preparation of Flexible Liquid Crystal Films with Broadband Reflection Based on PD&SLC. Materials. 2022; 15(24):8896. https://doi.org/10.3390/ma15248896
Chicago/Turabian StyleZhang, Xuetao, Rui Han, Hui Li, Xiaohui Zhao, Hui Cao, Yinjie Chen, Zhou Yang, Dong Wang, and Wanli He. 2022. "Preparation of Flexible Liquid Crystal Films with Broadband Reflection Based on PD&SLC" Materials 15, no. 24: 8896. https://doi.org/10.3390/ma15248896
APA StyleZhang, X., Han, R., Li, H., Zhao, X., Cao, H., Chen, Y., Yang, Z., Wang, D., & He, W. (2022). Preparation of Flexible Liquid Crystal Films with Broadband Reflection Based on PD&SLC. Materials, 15(24), 8896. https://doi.org/10.3390/ma15248896






