The Reaction Products of the Al–Nb–B2O3–CuO System in an Al 6063 Alloy Melt and Their Influence on the Alloy’s Structure and Characteristics
Abstract
1. Introduction
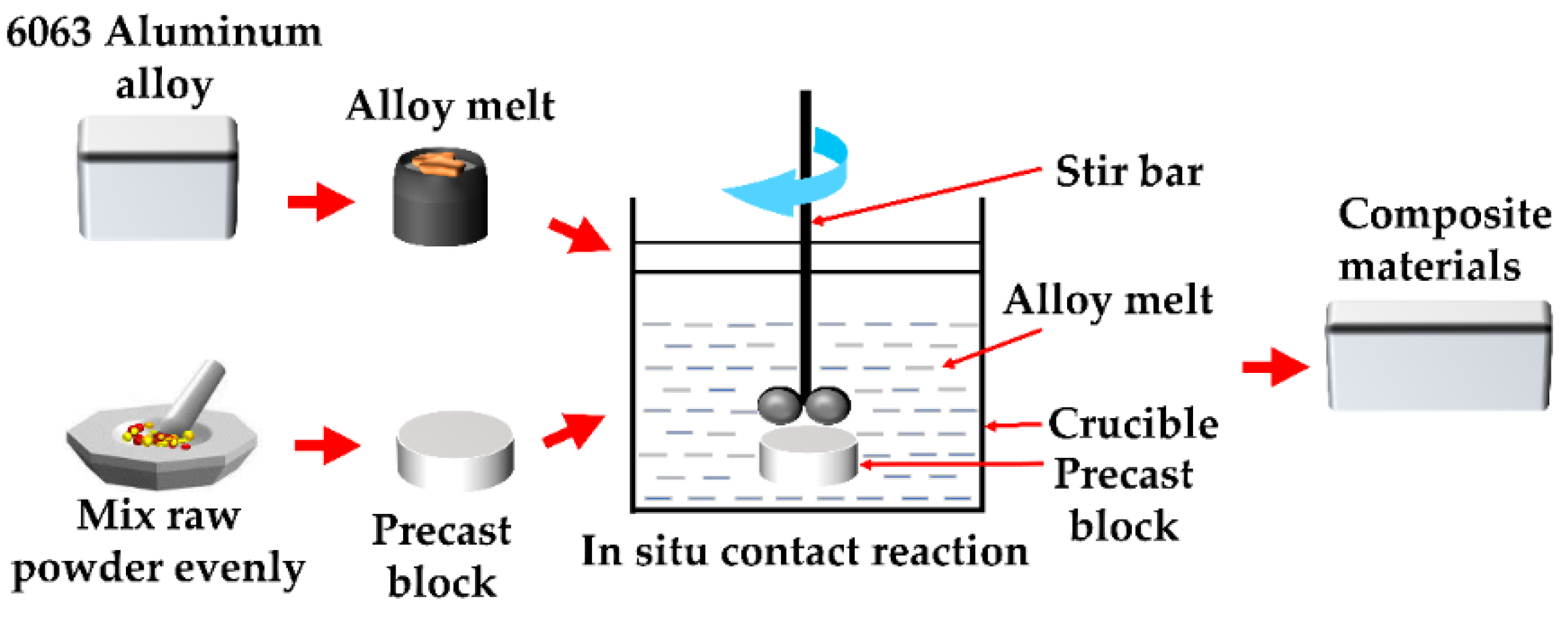
2. Materials and Methods
3. Results and Discussion
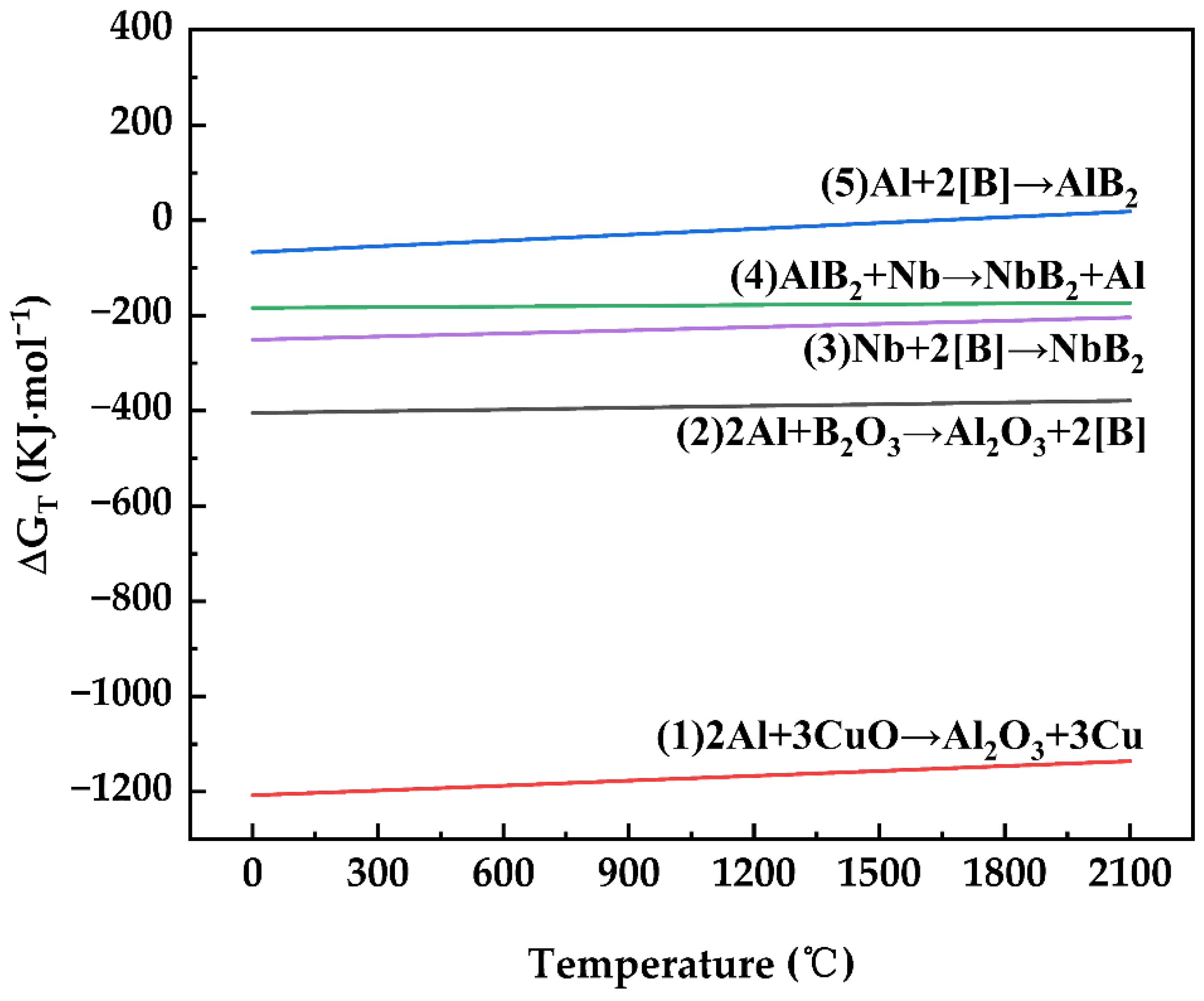
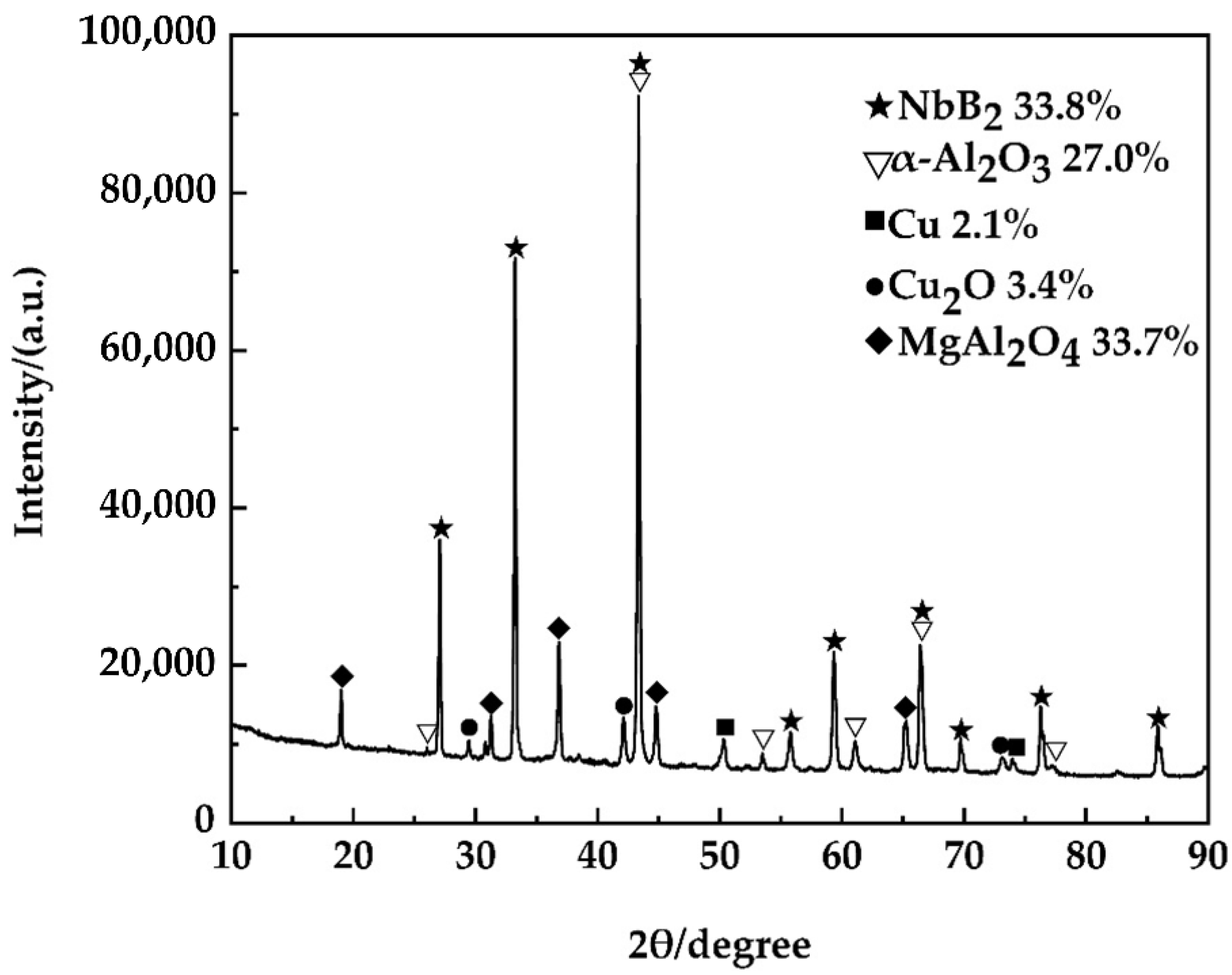
3.1. Thermodynamic Analysis
| Chemical Reaction | Gibbs Free Energy (J/mol) | Number |
|---|---|---|
| 2Al + 3CuO → Al2O3 + 3Cu | = −1,207,712 + 34.16 T | (1) |
| 2Al + B2O3 → Al2O3 + 2[B] | = −404,844 + 12.34 T | (2) |
| Nb + 2[B] → NbB2 | = −251,040 + 22.35 T | (3) |
| AlB2 + Nb → NbB2 + Al | = −184,096 + 5.15 T | (4) |
| Al + 2[B] → AlB2 | = −66,944 + 40.809 T | (5) |
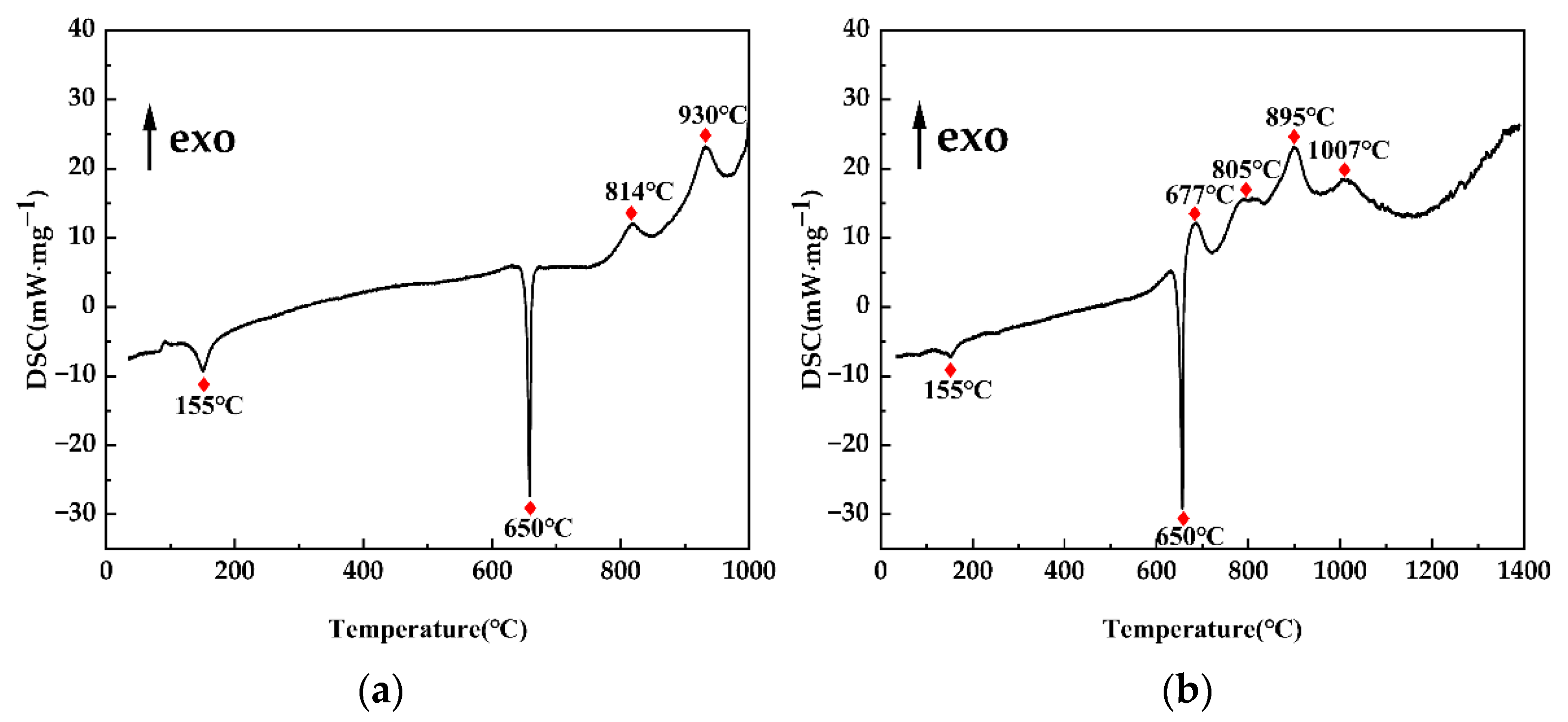
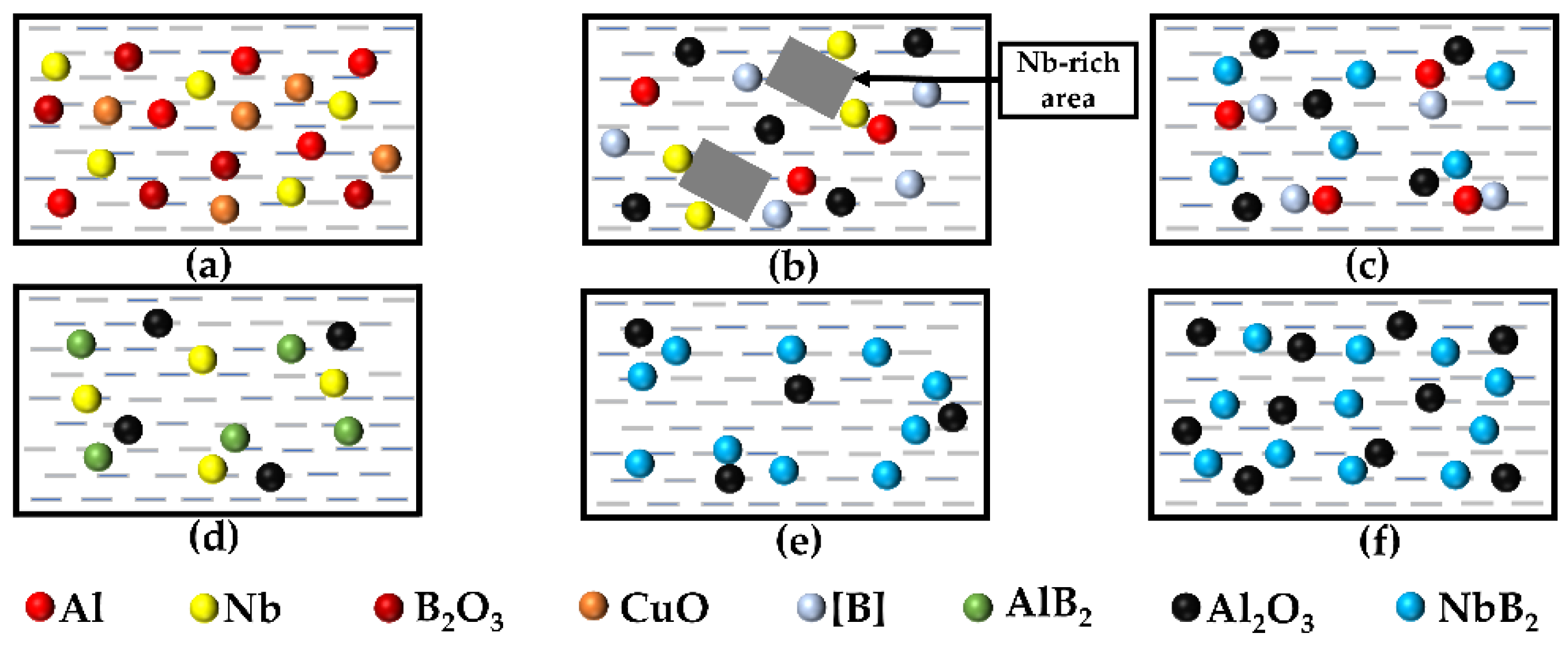
3.2. Mechanistic Analysis of the In Situ Reaction Process
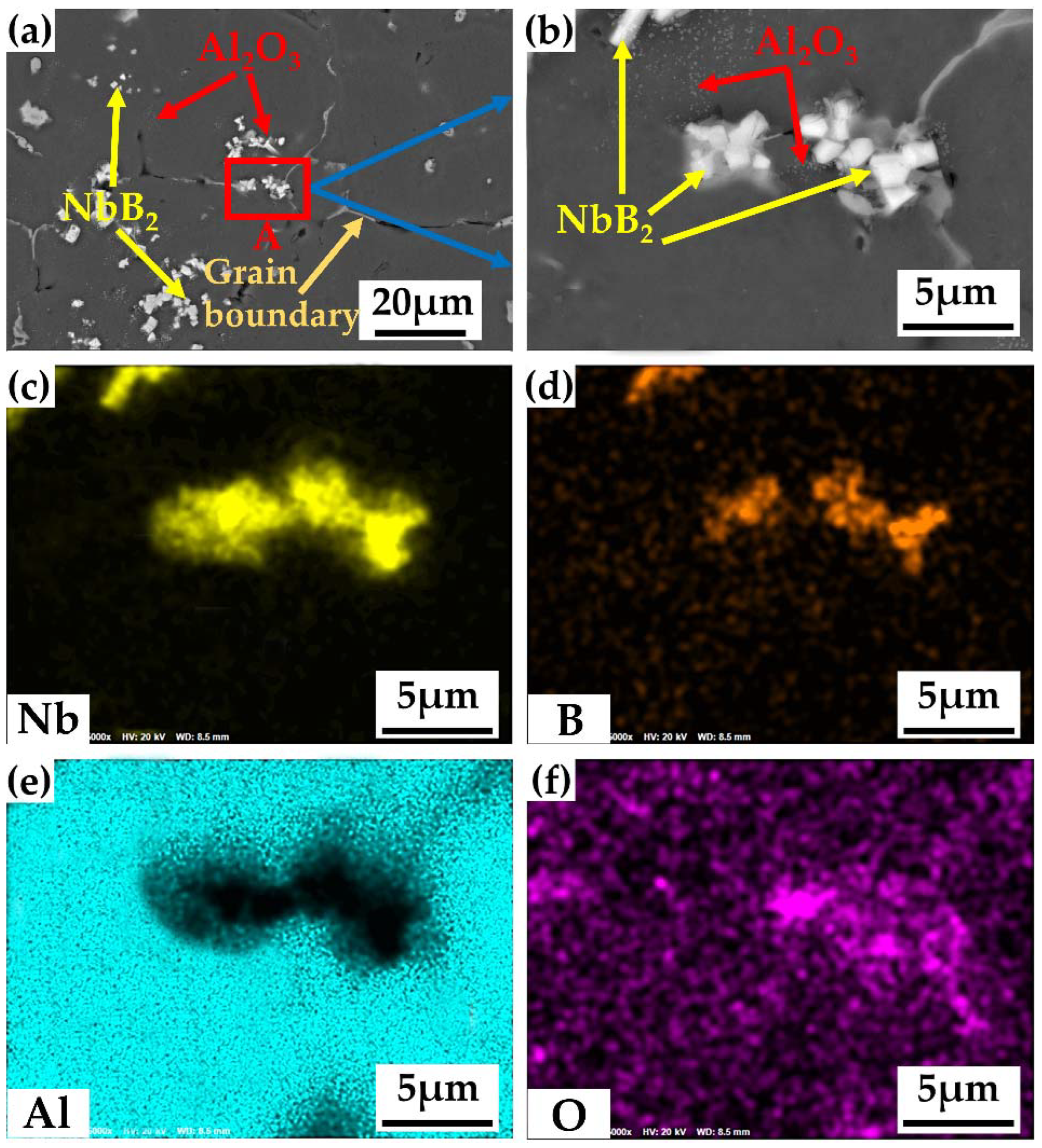
3.3. Distribution of In Situ Particles in the Alloy
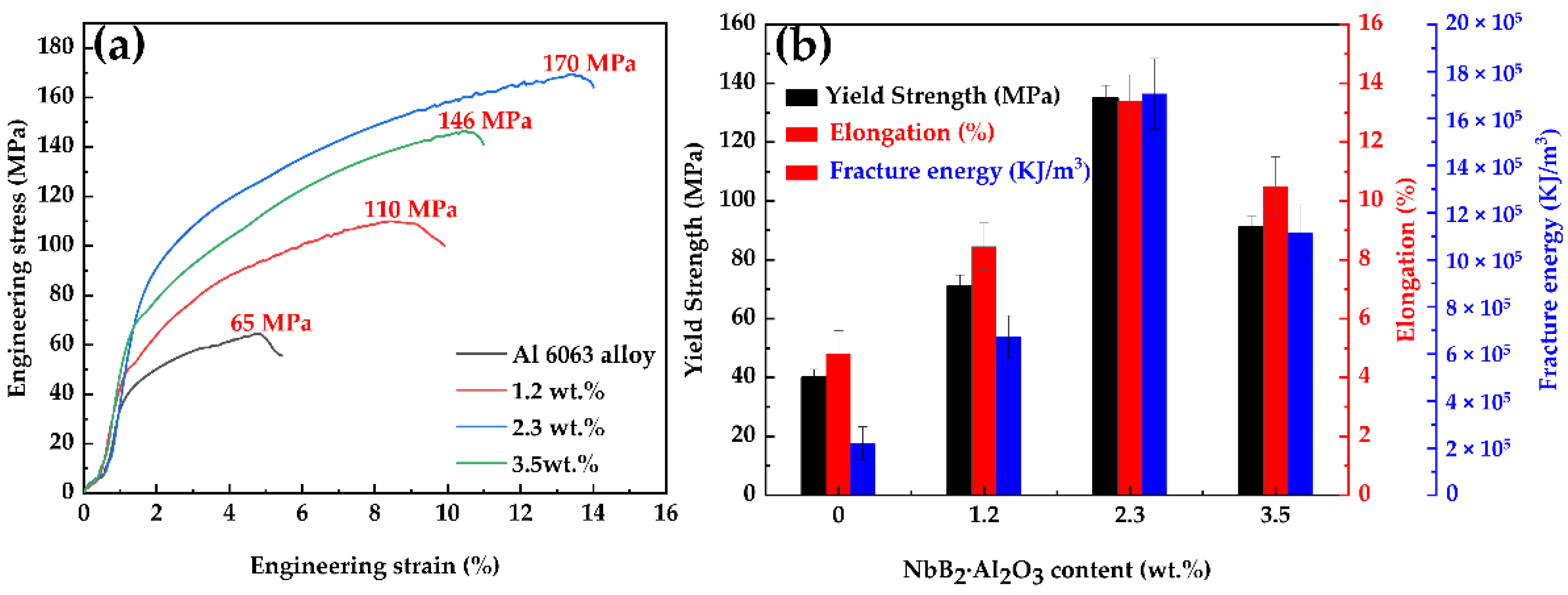
3.4. Effect of In Situ Particles on the Grain Structure and Properties of Alloys
4. Conclusions
- The aluminothermic interaction of Al–B2O3 and Al–CuO during SHS created Al2O3 particles and displaced [B] atoms, increasing the wettability of the B in the Al, which nucleated to form NbB2 and AlB2 once a threshold [B] concentration surrounding the Nb and the Al was achieved. Furthermore, at high temperatures, the exothermic reaction between the Al and the CuO promoted the interaction of the AlB2 with the Nb to generate NbB2. At the same time, due to the high solubility of [B] in this melt, the high [B] concentration promoted stoichiometric NbB2 production.
- The Al2O3 particles were dispersed throughout the crystal, while the NbB2 particles were primarily dispersed throughout the crystal with a tiny number near the grain boundaries. The intracrystalline particles stimulated nucleation, whereas the particles near the grain borders inhibited grain development, resulting in a very tiny microstructure.
- The grain size of each composite reduced and subsequently grew when the NbB2 and Al2O3 content of each composite increased, and when the complex-phase particle content was 2.3 wt.%, the grain size of each composite was the minimal value of 22 μm.
- The ultimate tensile strength, the yield strength, the elongation, and the fracture energy of the composites increased and then declined as the concentration of NbB2 and Al2O3 in each composite increased. When the complex-phase particle concentration was 2.3 wt.%, the ultimate tensile strength, yield strength, elongation, and fracture energy were 170 MPa, 135 MPa, 13.4%, and 17.05 × 105 KJ/m3, respectively. These values were 161.5%, 237.5%, 179%, and 671% more, respectively, than the values of those qualities in the 6063 aluminum alloy.
- Under the action of double particles, the composite developed more comprehensive characteristics in the as-cast condition as the NbB2 and Al2O3 concentrations in the 6063 aluminum alloy increased.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Yang, S.; Du, Z. Micromechanical modeling of damage evolution and mechanical behaviors of CF/Al composites under transverse and longitudinal tensile loadings. Materials 2019, 12, 3133. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, N.; Hanim, M.; Sarraf, M. Microstructural, tribology and corrosion properties of optimized Fe3O4-SiC reinforced aluminum matrix hybrid nanofiller composite fabricated through powder metallurgy method. Materials 2020, 13, 4090. [Google Scholar] [CrossRef] [PubMed]
- Rahul, G.; Tarun, N.; Pandey, O.P. Comparison of wear behaviour of LM13 Al-Si alloy based composites reinforced with synthetic(B4C) and natural(ilmenite) ceramic particles. Trans. Nonferrous Met. Soc. 2021, 31, 3613–3625. [Google Scholar]
- Zhang, Q.; Gu, J.; Wei, S. Differences in dry sliding wear behavior between Al–12Si–CuNiMg Alloy and it’s composite reinforced with Al2O3 Fibers. Materials 2019, 12, 1749. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, R.A. Effect of silicon nitride and graphene nanoplatelets on the properties of aluminum metal matrix composites. Materials 2021, 14, 1898. [Google Scholar]
- Wang, H.W. Preparation and application of in-Situ ceramic particles reinforced Al matrix composites. Aeron. Manuf. Technol. 2021, 64, 14–26. [Google Scholar]
- Ma, Y.; Addad, A.; Ji, G.; Zhang, M.X.; Ji, V. Atomic-scale investigation of the interface precipitation in a TiB2 nanoparticle reinforced Al-Zn-Mg-Cu matrix composite. Acta Mater. 2019, 185, 287–299. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.M.; Cui, Z.S. Compressive response and microstructural evolution of in-situ TiB2 particle-reinforced 7075 aluminum matrix composite. Trans. Nonferrous Met. Soc. 2021, 31, 1235–1248. [Google Scholar] [CrossRef]
- Dang, C.; Liu, H.M.; Feng, S.; Xinba, Y.E.; Wang, J.; Shi, Z.M. Effect of La2O3 content on microstructure and properties of Al2O3/Cu matrix composites. Rare Met. Mater. Eng. 2020, 49, 4341–4347. [Google Scholar]
- Ma, K.; Liu, Z.Y.; Liu, B.S. Improving ductility of bimodal carbon nanotube/2009Al composites by optimizing coarse grain microstructure via hot extrusion. Compos. Part A 2021, 140, 106198. [Google Scholar] [CrossRef]
- Kumar, N.; Gautam, G.; Gautam, R.K.; Mohan, A.; Mohan, S. Synthesis and characterization of TiB2 reinforced aluminum matrix composites: A review. J. Inst. Eng. 2016, 97, 233–253. [Google Scholar]
- Mohammad, S.E. In situ synthesis of NbB2–Al2O3 nanocomposite powder by mechanochemical processing. Adv. Appl. Ceram. 2017, 116, 173–179. [Google Scholar] [CrossRef]
- Jin, S.B.; Shen, P.; Li, Y.J.; Zhou, D.S. Synthesis of spherical NbB2-x particles by controlling the stoichiometry. CrystEngComm 2012, 14, 1925–1928. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.B.; Cai, Q. Distribution and engulfment behavior of TiB2 particles or clusters in wedge-shaped copper casting ingot. Trans. Nonferrous Met. Soc. 2015, 25, 54–60. [Google Scholar] [CrossRef]
- Li, Z.; Pan, W.L.; Wang, W. Investigation of the microstructure and mechanical properties of NbB2 particle reinforced aluminum matrix composites. Int. J. Mater. Res. 2019, 110, 240–246. [Google Scholar] [CrossRef]
- Ding, J.H.; Cui, C.X.; Sun, Y.J.; Shi, J.J.; Cui, S.; Ma, Q. Preparation of in situ Al3Nb-NbB2-NbC/Al inoculant and its effect on microstructures and properties of weldable Al-Cu-Mn alloy. Mater. Sci. Eng. A 2018, 738, 273–282. [Google Scholar] [CrossRef]
- Jiang, J.C.; Liu, H.M.; Hu, Q.L. Effect of Al–Nb–B2O3–CuO system reaction products on microstructure and wear resistance of A356 alloy. Mater. Res. Express 2021, 8, 016543. [Google Scholar]
- Zheng, C.; Liu, H.M.; Feng, S.; Xin, B.Y.E.; Wang, J.; Shi, Z.M. In situ reaction mechanism of Cu–Ti–CuO system in pure copper melts. Mater. Res. Express 2021, 8, 106506. [Google Scholar]
- Yeh, C.L.; Chen, Y.C. Combustion synthesis of NbB2–spinel MgAl2O4 composites from MgO-Added thermite-based reactants with excess boron. Crystals 2020, 10, 210. [Google Scholar] [CrossRef]
- Zhu, H.; Hua, B.; Cui, T. Microwave combustion synthesis of in situ Al2O3 and Al3Zr reinforced aluminum matrix composites. Mater. Res. Bull. 2015, 68, 283–288. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Liu, B.; Luo, Q.; Hu, B.; Li, Q. Understanding grain refining and anti-Si-poisoning effect in Al-10Si/Al-5Nb-B system. J. Mater. Sci. Technol. 2021, 65, 190–201. [Google Scholar] [CrossRef]
- Gangwar, J.; Gupta, B.K.; Tripathi, S.K.; Srivastava, A.K. Phase dependent thermal and spectroscopic responses of Al2O3 nanostructures with different morphogenesis. Nanoscale 2015, 7, 13313–13344. [Google Scholar] [CrossRef] [PubMed]
- Ao, M.; Liu, H.M.; Dong, C.F. The effect of La2O3 addition on intermetallic-free aluminum matrix composites reinforced with TiC and Al2O3 ceramic particles. Ceram. Int. 2019, 45, 12001–12009. [Google Scholar] [CrossRef]
- Liu, R.; Elleuch, O.; Wan, Z.Y.; Zuo, P. Phase selection and structure of low-defect-density γ-Al2O3 created by epitaxial crystallization of amorphous Al2O3. ACS. Appl. Mater. Inter. 2020, 12, 57598–57608. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.W.; Nie, J.F.; Ma, X.; Liu, X.F. Increasing the ductility of heat-resistant AlNp/Al composites by submicron Al2O3 particles. Mater. Charact. 2020, 170, 110672. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, H.Y.; Wang, Q.D. Influence of nanoparticles on microstructural evolution and mechanical properties of Sr-modified Al-10Si alloys. Mater. Sci. Eng. A 2016, 666, 264–268. [Google Scholar] [CrossRef]
- Cheng, X.; Han, J.; Guo, Y.U. Effect of Ce on Microstructure and Properties of 6063 Aluminum Alloy for Electric Purpose. Hot Work. Technol. 2018, 47, 38–41. [Google Scholar]
- Tekoglu, E.; Agaogullari, D.; Mertdinc, S. Microstructural characterizations and mechanical properties of NbB2 and VB particulate-reinforced eutectic Al-12.6 wt% Si composites via powder metallurgy method. Adv. Powder Technol. 2018, 29, 2070–2081. [Google Scholar] [CrossRef]
- Wu, Q.L.; Guo, Y.C.; Li, J.P. Effect of Al-Nb-B intermediate alloy on the organization and properties of Al-12Si-4Cu-2Ni-1Mg alloy. Hot Work. Technol. 2023, 5, 36–40. [Google Scholar]
- Bolzoni, L.; Nowak, M.; Hari, B.N. Grain refining potency of Nb–B inoculation on Al–12Si–0.6Fe–0.5Mn alloy. J. Alloys Compd. 2015, 623, 79–82. [Google Scholar] [CrossRef]
- Nowak, M.; Yeoh, W.K.; Bolzoni, L.; Hari, B.N. Development of Al-Nb-B master alloys using Nb and KBF4 powders. Mater. Des. 2015, 34, 40–46. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Ma, K.; Fu, Y.N.; Guo, E.Y.; Chen, Z.N.; Gu, Q.F. In-situ observation of grain refinement dynamics of hypoeutectic Al-Si alloy inoculated by Al-Ti-Nb-B alloy. Scripta Mater. 2020, 187, 142–147. [Google Scholar] [CrossRef]
- Sharma, V.K.; Kumar, V. Development of rare-earth oxides based hybrid AMCs reinforced with SiC/Al2O3: Mechanical & metallurgical characterization. J. Mater. Res. Technol. 2019, 8, 1971–1981. [Google Scholar]
- Ao, M.; Liu, H.M.; Dong, C.F.; Feng, S.; Liu, J.C. Degradation mechanism of 6063 aluminum matrix composite reinforced with TiC and Al2O3 particles. J. Alloys Compd. 2020, 859, 157838. [Google Scholar] [CrossRef]
- Wwa, B.; Jz, C.; Nq, B. Effects of minor rare earth on the microstructure and properties of Cu-Cr-Zr alloy. J. Alloys Compd. 2020, 847, 155762. [Google Scholar]
- Gao, X.; Zhao, Y.T.; Kai, X.Z.; Qian, W.; Jin, L.W. Characteristics on microstructure and mechanical performances of 6111Al influenced by Ce-containing precipitates. J. Rare Earths 2020, 40, 153–160. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Ao, M.; Zhai, J.; Shi, Z.; Liu, H. The Reaction Products of the Al–Nb–B2O3–CuO System in an Al 6063 Alloy Melt and Their Influence on the Alloy’s Structure and Characteristics. Materials 2022, 15, 8898. https://doi.org/10.3390/ma15248898
Zhang C, Ao M, Zhai J, Shi Z, Liu H. The Reaction Products of the Al–Nb–B2O3–CuO System in an Al 6063 Alloy Melt and Their Influence on the Alloy’s Structure and Characteristics. Materials. 2022; 15(24):8898. https://doi.org/10.3390/ma15248898
Chicago/Turabian StyleZhang, Chenggong, Min Ao, Jingyu Zhai, Zhiming Shi, and Huimin Liu. 2022. "The Reaction Products of the Al–Nb–B2O3–CuO System in an Al 6063 Alloy Melt and Their Influence on the Alloy’s Structure and Characteristics" Materials 15, no. 24: 8898. https://doi.org/10.3390/ma15248898
APA StyleZhang, C., Ao, M., Zhai, J., Shi, Z., & Liu, H. (2022). The Reaction Products of the Al–Nb–B2O3–CuO System in an Al 6063 Alloy Melt and Their Influence on the Alloy’s Structure and Characteristics. Materials, 15(24), 8898. https://doi.org/10.3390/ma15248898







